Abstract
Potocki–Lupski syndrome (PTLS; MIM #610883), characterized by neurobehavioral abnormalities, intellectual disability and congenital anomalies, is caused by a 3.7-Mb duplication in 17p11.2. Neurobehavioral studies determined that ∼70–90% of PTLS subjects tested positive for autism or autism spectrum disorder (ASD). We previously chromosomally engineered a mouse model for PTLS (Dp(11)17/+) with a duplication of a 2-Mb genomic interval syntenic to the PTLS region and identified consistent behavioral abnormalities in this mouse model. We now report extensive phenotyping with behavioral assays established to evaluate core and associated autistic-like traits, including tests for social abnormalities, ultrasonic vocalizations, perseverative and stereotypic behaviors, anxiety, learning and memory deficits and motor defects. Alterations were identified in both core and associated ASD-like traits. Rearing this animal model in an enriched environment mitigated some, and even rescued selected, neurobehavioral abnormalities, suggesting a role for gene-environment interactions in the determination of copy number variation-mediated autism severity.
INTRODUCTION
Autism and other syndromes encompassing an endophenotype within the autism spectrum disorders (ASDs) are characterized by three categories of behavioral abnormalities: (i) impairments in social interaction, (ii) qualitative impairments in social communication, and (iii) restricted and/or repetitive interests, behaviors or activities (1–3). Secondary behavioral anomalies associated with autism include motor deficits, aggressive behavior, abnormal sleep patterns, gastrointestinal symptoms, seizures and mental retardation (3–5).
Autistic-like behaviors in the mouse have been identified and objectively quantified by behavioral assays allowing systematic investigations that may provide insights into autism (6–9). To date, the majority of mouse models of autistic-like behaviors have focused on monogenic aberrations, such as loss-of-function mutation in Fmr1, Mecp2, or Ube3a (10), which account for ∼5% of autism cases. In contrast, specific autism-associated rare copy number variants (CNVs) have been estimated to cause ∼10–20% of autism cases (10–12). Limited mouse models for CNV-induced autistic-like behavior in mice include 15q11-13 and 16p11.2 duplications and deletion mice (13,14), underscoring the need for additional well-characterized chromosome-engineered mouse models of CNV-induced complex autistic-like behavior. These models represent mechanistically similar genetic alterations analogous to the autism-associated CNVs observed in human patients (targeted duplication/deletion syntenic to human critical interval), they are polygenic, they equate with common clinically described features and they may be utilized to study the complex effects of environmental factors and to assess interventional therapies (15).
We previously used chromosome engineering to generate mouse models for Smith–Magenis syndrome (SMS) Potocki–Lupski syndrome (PTLS) (16). PTLS (MIM 610883) results from an interstitial duplication of 17p11.2, and the reciprocal 17p11.2 deletion is associated with SMS (MIM 182290). PTLS is characterized by a non-specific developmental delay, intellectual disability (ID), infantile hypotonia and failure to thrive, dysmorphic features, congenital heart disease, sleep-disordered breathing, electro-encephalographic abnormalities, behavioral disturbances and autism or ASD, though not all features are present in every patient (17–20). Although ASD is not an absolute feature of PTLS, it has been shown that children with PTLS do experience difficulties with atypicality, withdrawal, hyperactivity, inattention and anxiety (18). Furthermore, six of seven PTLS patients studied by objective psychological testing (ADOS/ADIR) had findings that were compatible with an autism diagnosis.
Autism (or ASD) is known to be highly variable and is hypothesized to be dependent on both genetic and environmental factors, the latter including pre-term birth, age of parents, diet, infection, xenobiotics and pesticide exposure (21–23). Environmental exposures are amenable to systematic studies using mouse models. A recent study of a mouse model of Rett syndrome demonstrated that early environmental enrichment was able to reverse the cortical long-term potentiation deficit, ameliorate motor coordination, memory defects and anxiety-like behaviors, indicating gene × environment involvement in the phenotypic manifestation (24). Previous studies of the PTLS mouse identified altered behavioral phenotypes, including abnormalities of motor coordination, activity levels, social interactions, learning and memory and anxiety (25–28). We now perform an extensive analysis of the core and associated autistic-like phenotypes of these mice. Furthermore, we examine the plasticity of these phenotypes by investigating the effects of environmental enrichment. Our findings suggest the potential mitigation of selected CNV-associated neurobehavioral traits by rearing in an enriched environment.
RESULTS
Neurobehavioral abnormalities in Dp(11)17/+ mice
Previous studies of the behavioral phenotypes of Dp(11)17/+ mice on both mixed (129S5/C57BL/6J) (27,28) and congenic (N > 10 C57BL/6J) (25) backgrounds demonstrated abnormalities in the following assays: open field analysis (OFA), elevated-plus maze (EPM), conditioned fear (CF), rotarod and tube test. These assays represent surrogate measures for the human traits of activity, anxiety, learning and memory, motor coordination and social function. The identification of autistic-like behaviors, including hyperactivity, anxiety-like behaviors, learning and memory defects, impairment in motor coordination and social abnormalities, together with the known autistic features of PTLS patients, suggest that this chromosome-engineered mouse model is a potential model for neurobehavioral traits associated with ASD. We first confirmed abnormal behaviors for Dp(11)17/+ mice compared with their wild-type (WT) littermates on a congenic background (N > 10 C57BL/6J), documenting that the behavioral phenotypes are robust, present in multiple laboratory settings, and occur in both mixed and congenic backgrounds. We then sought to investigate the more comprehensive range of potential autistic-like behaviors utilizing additional rodent behavioral tests.
Environmental enrichment mitigates the autistic-like abnormal behavioral phenotypes
Gene × environment interactions are hypothesized to be important in trait manifestation for autism and ASD, as well as many other complex disorders (22,29). To determine the extent of a potential environmental contribution to autistic-like phenotypes, we also concurrently investigated the effect of environmental home-cage enrichment on the objectively characterized behaviors identified in Dp(11)17/+ mice. For all experiments, environmental enrichment was initiated at the time of weaning and maintained until the implementation of the behavioral assay on adult animals.
Social abnormalities and repetitive stereotypic behavior in the PTLS mouse model
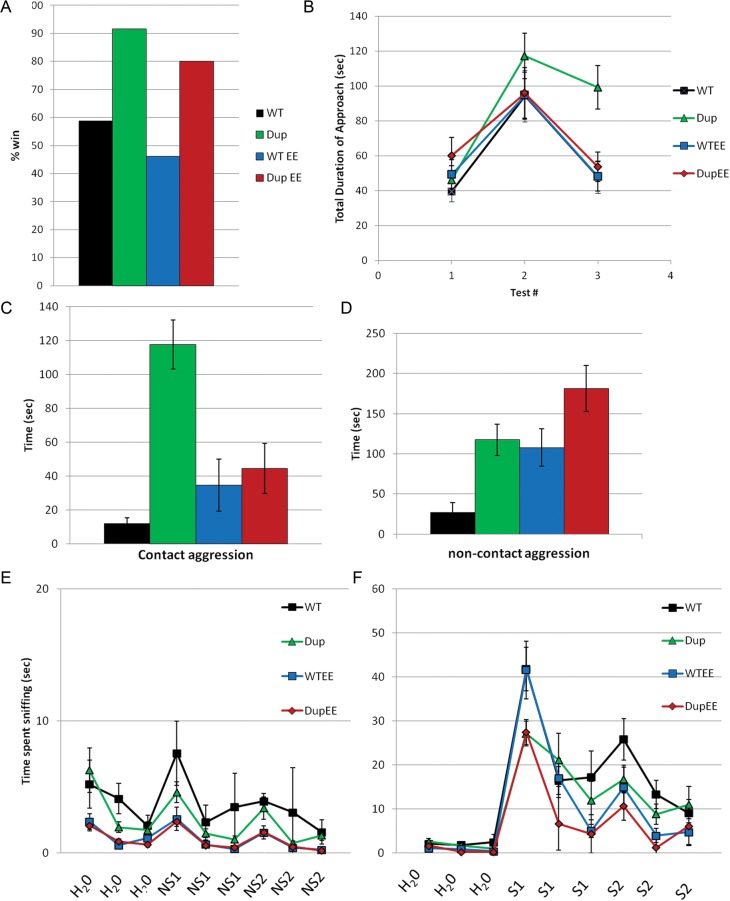
We evaluated social behaviors in Dp(11)17/+ mice to further explore phenotypes important in the diagnosis of autism. We confirmed previous studies that demonstrated social dominance in the tube test (Fig. 1A; P = 0.001) (25,26). When the tube test was performed on mice under enriched housing conditions, the level of dominance was slightly, although not significantly, reduced for both Dp(11)17/+ (80 win compared with 91.7%; P = 0.1986, Fisher's exact test) and WT (46.1 compared with 58.8%; P = 0.2881) mice compared with similar mice under the standard housing condition. Dominance was still observed in Dp(11)17/+ mice (P = 0.0024), indicating that there was no significant effect of environmental enrichment on social dominance.
Figure 1.
Dp(11)17/+ mice display abnormal social behaviors. (A) Dp(11)17/+ mice (green bar, Dup) demonstrate increased social dominance in the tube test compared with WT littermates (black bar) in either standard (n = 11 for Dup, n = 13 for WT, P = 0.0012) or enriched [n = 11 for WTEE (red bar, wild-type in enriched environment), n = 12 for DupEE (blue bar, Dup from enriched environment), P = 0.0024] housing conditions. (B) WT (black squares, n = 10) and Dp(11)17/+ (Dup, green triangle, n = 13) mice from standard housing and enriched housing (WTEE, red squares, n = 15; DupEE, blue circle, n = 12) demonstrate an increased interest in a novel partner during test 2 compared with baseline levels in test 1 of the partition test (P < 0.001). Their interest in the familiar partner returns to baseline for test 3, with the exception of Dp(11)17/+ mice from standard housing, which maintain an elevated interest. All values are shown ±standard error of the mean (SEM). (C and D) Dp(11)17/+ mice (n = 12) showed increased contact (P = 0.001) and non-contact aggression (P = 0.002) in the social interaction test compared with WT littermates (n = 11). Contact and non-contact aggression is reduced following enrichment (n = 10 for DupEE, n = 13 for WTEE; gene x environment interaction (GXE), P = 0.001 and 0.032, respectively). (E) No difference between genotypes for interest in non-social (NS) odors (three presentations each of two different non-social odors) or water control (H2O). (F) Decreased interest in social odors (first presentation of S1, but not second or third presentation or second social odor) are observed in Dp(11)17/+ mice in standard and enriched housing.
The three-chamber test for social preference found no difference between Dp(11)17/+ and WT mice (data not shown), confirming normal levels of social interest in these mice (25). To further investigate social phenotypes, we performed the partition and olfactory habituation/dishabituation tests, which are used to assess social interest and recognition in mice in the absence of direct physical contact, as well as a direct social interaction test (30). We observed a significant effect of the behavioral test number on the amount of time spent in contact with the partition for Dp(11)17/+ mice and their WT littermates in the partition test; both Dp(11)17/+ mice and their WT littermates from the standard housing showed an increased interest to the new unfamiliar mouse (test 2) relative to the familiar partner (test 1), indicating a similar interest in social novelty (Fig. 1B; P < 0.001). However, Dp(11)17/+ mice did not appear to demonstrate the recognition of the returning familiar partner, as evidenced by their elevated interest and time spent at the partition compared with baseline levels, which did not reach overall statistical significance by analysis of variance (ANOVA) with repeated measures. This potentially indicates a reduced ability to recognize/discriminate a familiar partner from an unfamiliar mouse, which may be due to an underlying learning and memory defect. It may also be an effect of elevated aggression or over-stimulation in these mice; however, in these cases, we would expect an increased interest in the initial baseline test. There was no difference between the genotypes for first and second tests, indicating that Dp(11)17/+ mice have normal levels of social interest and independently confirming the result from the three-chamber test. In the same test, Dp(11)17/+ mice from enriched housing and their WT littermates are able to recognize the familiar partner and their interest dropped to near baseline levels (Fig. 1B), suggesting that the abnormal social discrimination response in Dp(11)17/+ mice can be altered by environmental influences.
To further assess abnormal social behaviors in Dp(11)17/+ mice, direct social interactions were evaluated. We observed and scored direct social interactions without a partition in mice that had been housed across a partition from a control mouse for two consecutive overnights. Increased aggression was found in Dp(11)17/+ mice in the direct social interaction test compared with WT littermates when we analyzed the levels of either direct contact aggression (i.e. biting, aggression, grooming, wrestling, boxing, mounting; genotype, P < 0.001; Fig. 1C) or non-contact aggression (aggressive circling, unrest, tail-rattling; P = 0.002; Fig. 1D). Other social behaviors were not able to be scored for Dp(11)17/+ mice from standard cages, as the aggression was so severe that these behaviors dominated the entire social interaction (see Supplementary Material, Fig. S1 for a sample video recording of the behavior). These results suggest that the social dominance seen in the tube test may be linked to underlying aggressive social cues.
Aggression has been widely identified as an associated trait in autistic patients and patients with ID (4,5,31), as well as in mouse models of autism (7,32). Interestingly, in our test for a direct social interaction, the level of direct contact aggression was remarkably decreased in Dp(11)17/+ mice from enriched housing conditions compared with those in standard cages [Fig. 1C; gene x environment interaction (GXE), P = 0.001; see Supplementary Material, Fig. S2 for sample video recording]. However, the level of non-contact aggression was increased for both Dp(11)17/+ and WT mice in enriched housing (Fig. 1D; GXE, P = 0.032). These results suggest that enrichment may enable these mice to control their reaction to intruder mice (reduced contact aggression), despite the persistent tendency toward aggressive or dominant behavior, as evidenced by increased non-contact aggression and social dominance in the tube test.
The olfactory habituation/dishabituation task was used to assess olfaction, as well as interest in and recognition of social odors, which are both important components of rodent social interaction. For the non-social odor test, no difference was observed between genotypes in their interest in odors (Fig. 1E). Both Dp(11)17/+ and WT mice demonstrated dishabituation to non-social odors (P < 0.05) with the exception of wild-type in enriched environment (WTEE) mice for the first non-social odor (P = 0.079) and WT mice for the second non-social odor (P = 0.253). For the social odor test, both genotypes were able to detect the first novel odor (P < 0.001 for all; Fig. 1F). However, dishabituation was not observed in WTEE mice (P = 0.068) and Dup mice (P = 0.306) for the first presentation of the second social odor. WT mice, but not Dp(11)17/+ mice, showed an increased interest upon the first presentation of a social odor, but not for the first presentation of the second novel social odor. These observations indicate intact olfaction in Dp(11)17/+ mice and support the notion that Dp(11)17/+ mice have decreased interest in social stimulus odors compared with WT mice. Furthermore, WT, but not Dp(11)17/+, mice from enriched housing demonstrated an increased interest upon the first presentation of a social odor, showing similar results to those for mice from standard housing (Fig. 1D). Overall, these results underscore the specificity of environmental influences on social behavior in mice, as enrichment was able to alter the phenotype seen in the partition and direct social interaction tests, but not in the tube test or the olfactory habituation/dishabituation test.
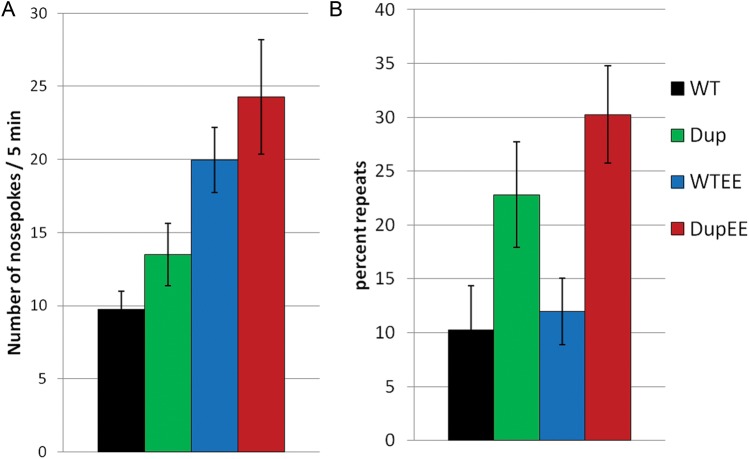
Next, we utilized the automated nose-poke/holeboard (HB) assay as a measure of restrictive, repetitive and stereotypic behaviors in Dp(11)17/+ mice. We did not see a significant increase in the total number of nose-pokes in a 5-min session in Dp(11)17/+ mice compared with WT littermates (P = 0.342, Fig. 2A). However, when we analyzed the percentage of repeats (i.e. the percentage of the time the same hole was poked in succession), we found that Dp(11)17/+ mice had an increased percentage of repetitive nose-pokes compared with WT mice (genotype, P = 0.001, Fig. 2B), suggesting the presence of repetitive or compulsive behavior in Dp(11)17/+ mice. In the same assay, there was a significant effect of enrichment, resulting in increased nose-pokes compared with those mice in standard housing (Fig. 2A; environment, P = 0.004), possibly due to the increased activity and reduced anxiety (Fig. 3D and E). Enriched Dp(11)17/+ mice also had an increased percentage of repetitive nose-pokes compared with WT mice, similar to those in standard housing. This effect was slightly exaggerated in mice from enriched housing, and although there was no significant main interaction of GXE (Fig. 2B), these results suggest that environmental enrichment may exacerbate the pervasiveness of this stereotypic behavior.
Figure 2.
Stereotypic, repetitive behavior in PTLS mice. (A) Enrichment causes an increase in the total number of nose-pokes in Dp(11)17/+ (DupEE, red bars, n = 18) and WT (WTEE, blue bars, n = 12) compared with their counterparts in standard housing (Dup, green bar, n = 13; WT, black bar, n = 11; P = 0.004) during a 5-min session of the HB test. (B) Dp(11)17/+ mice also demonstrate an increased percentage of repeated nose pokes compared with WT littermates in standard and enriched housing conditions (P = 0.001). Values are shown ±SEM.
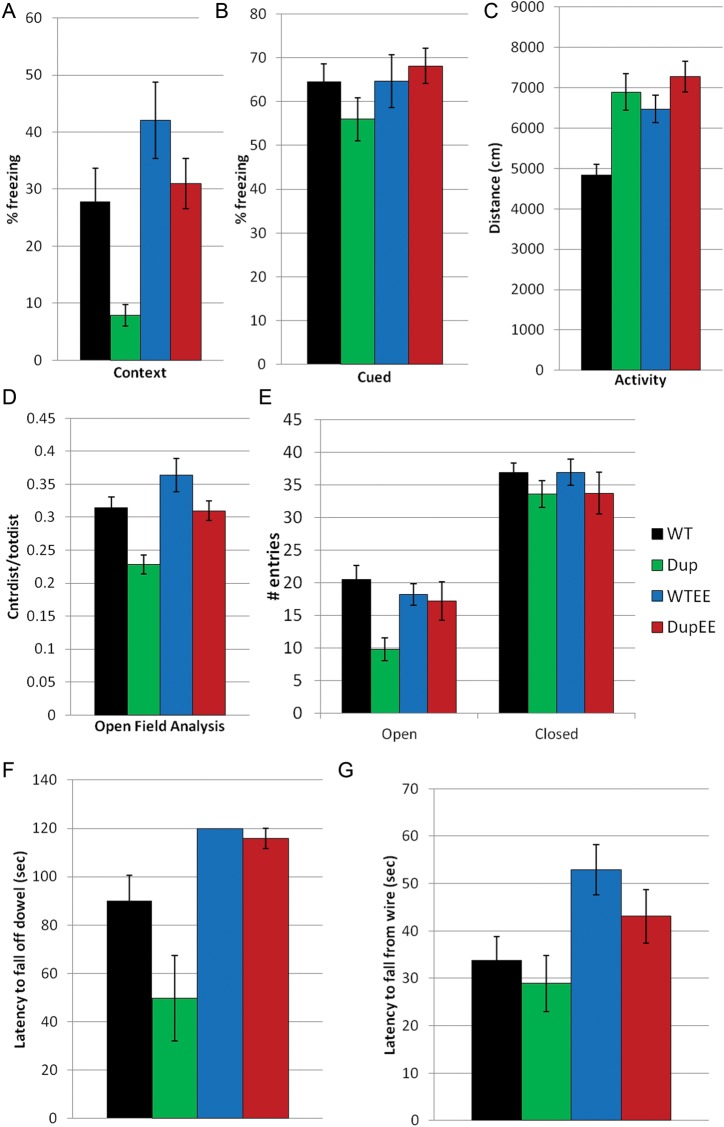
Figure 3.
Abnormal autism-associated behaviors, including learning and memory deficits, hyperactivity, anxiety, motor defects and aggression. Error bars for all figures represent the SEM. (A) Contextual and (B) cued stimulus portions of the conditioned fear (CF) test. (C) Activity and (D) anxiety measures of the open field analysis (OFA). (E) Elevated plus maze (EPM). (F) Dowel walking test. (G) Wire hang test. (A) Dp(11)17/+ mice (Dup, green bars, n = 12) show reduced freezing (P = 0.003) compared with WT littermates (WT, black bars, n = 12) in standard housing in the CF test. Dp(11)17/+ mice (DupEE, red bars, n = 15) in enriched housing are raised to similar levels as WT littermates (WTEE, blue bars, n = 9; P = 0.001 for environment). (B) No differences are seen in the cued test between any of the groups (P > 0.05). (C) Open field activity levels in Dp(11)17/+ mice (Dup, n = 12) are increased compared with WT littermates (WT, n = 12, P < 0.001). Enrichment has a significant effect on activity levels in Dp(11)17/+ (DupEE, n = 15) and WT littermates (WTEE, n = 9, environment, P = 0.012) in the open field test. (D) Dp(11)17/+ mice have reduced center:total distance traveled ratio (genotype, P < 0.001) and enrichment decreases anxiety (environment, P < 0.001). (E) In the EPM, Dp(11)17/+ mice (Dup, n = 9) also have fewer entries into the open arm compared with WT littermates (WT, n = 15; genotype, P = 0.014). Enrichment rescues this anxiety phenotype in Dp(11)17/+ mice (DupEE, n = 10) and WT (WTEE, n = 12, GXE P = 0.038) from enriched cages. (F) Dp(11)17/+ mice (Dup, n = 9) show reduced latency to fall off a raised 9-mm dowel compared with WT littermates (WT, n = 16, P < 0.001). Enrichment improved performance in Dp(11)17/+ mice (DupEE, n = 15) and WT (WTEE, n = 9) mice. (G) Enrichment also improves performance in the wire hang test (environment, P = 0.012).
Evidence of autism-associated abnormal neurobehavioral traits in Dp(11)17/+ mice
Learning and memory deficits are commonly associated with autism and ASD in humans (3). The conditioned fear test assesses learning and memory in rodents and previously identified abnormalities in Dp(11)17/+ mice on both mixed (27,28) and congenic genetic backgrounds (25). We confirmed decreased contextual fear (decreased percent time freezing; genotype, P = 0.003, Fig. 3A), indicating a robust defect in learning and memory. There were no significant differences in the cued test between any of the groups (Fig. 3B). We extended the contextual fear study to include animals in enriched housing conditions and found that this learning deficit was improved by housing in an enriched environment (environment, P = 0.001); Dp(11)17/+ mice from enriched housing displayed a similar freezing behavior to WT mice in standard housing conditions (Fig. 3A).
We confirmed increased activity and anxiety-like behaviors in Dp(11)17/+ mice using the OFA and EPM. The OFA measures both activity and anxiety-like behaviors in mice. We observed increased activity (total distance traveled, P < 0.0001, Fig. 3C) and increased anxiety (decreased center distance/total distance, P = 0.001, Fig. 3D) in Dp(11)17/+ mice compared with their WT littermates, confirming previous results on a mixed (129S5/C57BL/6J) background (28). The activity level in Dp(11)17/+ mice and their WT littermates from enriched cages was increased compared with those from standard housing; however, the increase was not significant in Dp(11)17/+ mice, possibly due to a ceiling effect, as the upper limit for total activity in 30 min for these mice may have been reached (Fig. 3C). The anxiety level (center/total distance) in Dp(11)17/+ was reduced back to non-enriched WT levels (Fig. 3D); however, Dp(11)17/+ mice still showed increased levels of anxiety-like behavior when compared with their enriched WT littermates (environment, P = 0.001), as WT animals also demonstrated reduced anxiety compared with their counterparts in standard housing conditions (GXE, P = 0.361).
We performed the EPM experiment as a secondary measure of anxiety-like behavior and found that Dp(11)17/+ mice had a decreased number of entries into the open arms compared with their WT littermates (genotype, P = 0.014; Fig. 3E), further confirming the previously established ‘anxiety-like’ phenotype in these mice (25). These anxiety-like phenotypes were also rescued by environmental enrichment, as Dp(11)17/+ mice in enriched housing demonstrated a similar number of entries into the open arms as WT mice in standard or enriched housing (GXE, P = 0.038; Fig. 3E).
To evaluate motor ability, we challenged our PTLS mouse model with the dowel-walking and wire-hang tests. We analyzed our data from the dowel walking and wire hang tests using the non-parametric Kruskal–Wallis test, as the majority of the mice from enrichment cages were able to master the task, resulting in a variance nearing zero. Dp(11)17/+ mice have a decreased latency to fall off a 9-mm dowel compared with WT littermates; however, this effect was not significant as determined by the Kruskal–Wallis test (P > 0.7; Fig. 3F). Similarly, there was not a significant difference in the wire hang test (P > 0.5; Fig. 3G). Comparing the motor ability of animals from enriched housing to those in standard cages, we found that both Dp(11)17/+ and WT mice showed improvement following environmental enrichment, although it was not significant. A more challenging test would likely be more informative, as many animals were able to master the test and remain on the dowel or wire for the entirety of the test period (120 s for the dowel test and 60 s for the wire hang; Fig. 3F and G).
Previously published reports identified abnormal performance in the rotarod, but not the dowel walking or wire hang tests for Dp(11)17/+ mice (25). Our results are distinct from previous reports, as we did not find any significant difference in the rotarod (data not shown). However, these differences may be due to differing statistical analysis protocols (t-test for each trial analyzed separately as opposed to an ANOVA with repeated measures). Nevertheless, both studies suggest a trend toward a specific motor deficit in Dp(11)17/+ mice. Overall, these results indicate a benefit of environmental enrichment for motor coordination.
Further support for Dp(11)17/+ as a mouse model of autism
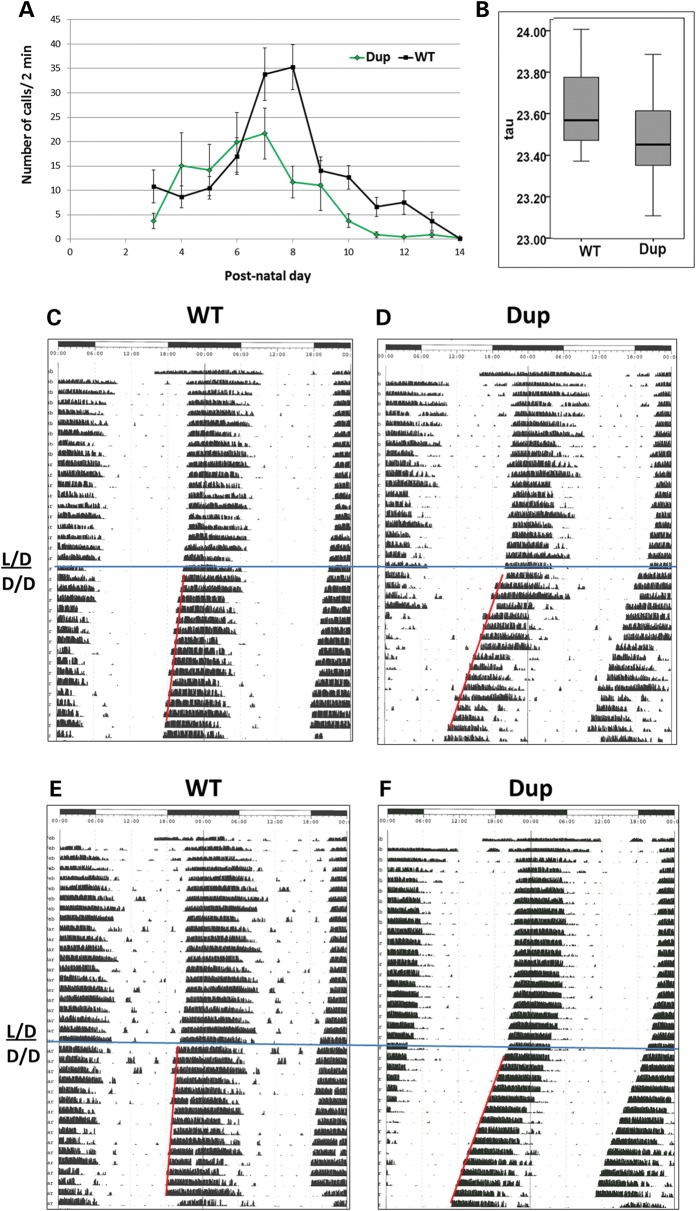
We studied separation-induced ultrasonic vocalizations (USVs) in Dp(11)17/+ pups and their WT littermates as a measure of developmentally-regulated vocalization. Both groups of mice showed a normal progression of USVs until post-natal day (PND) 6, at which point the Dp(11)17/+ mice displayed an altered communication pattern (Fig. 4A). Dp(11)17/+ mice had peak USVs at PNDs 6–7, whereas WT animals peaked at PND 7–8. Furthermore, Dp(11)17/+ mice had decreased peak USVs and continued to emit fewer vocalizations until PND 13 (P = 0.0001–0.015). Decreased levels of USVs have been anticipated for mouse models of autism; however, the opposite phenotype has also been observed (7,8). In aggregate with a reduced interest in social odors, the altered communication pattern and decreased peak USVs strongly indicate a deficit in communication in Dp(11)17/+ mice.
Figure 4.
Further evidence for Dp(11)17/+ mice as a mouse model of autism. (A) Dp(11)17/+ mice (Dup, n = 34, in green) have reduced peak USVs (P = 0.0001, PND 8) and continue to have fewer USVs from PND 10–12 (P = 0.002, PND 10; P = 0.015, PND 11; P = 0.004, PND 12) compared with WT littermates (n = 38). All error bars represent ±SEM. (B) The free running period length (12/12 h dark/dark cycle) of female Dp(11)17/+ mice is shortened (23.46 ± 0.043, N = 29) compared with WT mice (23.662 ± 0.040, N = 22; P = 0.010). Representative actigrams for WT (C and E) and Dp(11)17/+ (D and F) mice are double-plotted (above and below). Dark bars above the figure represent dark cycle (active period) and light bars indicate the light cycle. D/D, dark/dark; L/D, light/dark. Red bar demarcating the slope of the line represents the period length or tau for animals housed in 12/12 h dark/dark cycle demarcated by the blue horizontal line.
The circadian rhythms of adult female Dp(11)17/+ mice entrained to a 12 h light/12 h dark cycle were evaluated as an indicator of potential sleep abnormalities. Female mice were used for this study in order to reduce the total number of animals used, as male mice were already being utilized for other behavioral analyses. When these mice were challenged with a 12 h dark/12 h dark cycle, they had a significantly decreased period length (Fig. 4B; period = 23.46 ± 0.043, N = 29) compared with WT littermates (23.662 ± 0.040, N = 22; P = 0.01). Two representative actigrams for WT (Fig. 4C and 4E) and Dp(11)17/+ (Fig. 4D and 4F) mice are shown in Figure 4. A previous report did not identify any significant circadian abnormalities in Dp(11)17/+ mice, but did find differences in the mouse model harboring the reciprocal deletion CNV (Df(11)17/+) (27). However, that study examined male mice on a mixed (129S5/C57BL/6J) background. We believe that this type of short change in period length can be quite significant; studies of the circadian phenotypes of casein kinase 1 (Ck1Є) null mutants also showed only small (but significant) changes in period length (24 versus 23.6 h), whereas tau mutants demonstrate much more dramatic phenotypes (22 versus 24 h) (33). Our results further implicate one or more genes in this genomic interval in the control of circadian clock regulation.
Altered neurotransmitter levels in Dp(11)17/+ mice
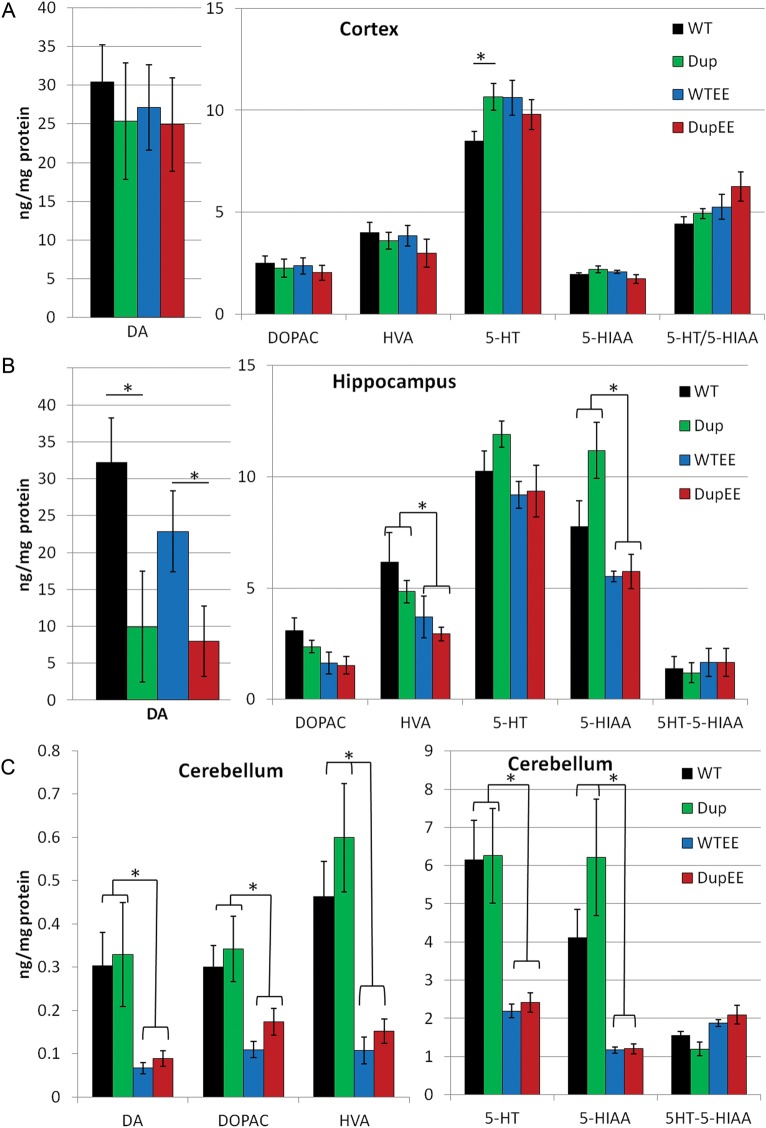
We analyzed the levels of serotonin, dopamine (DA) and their metabolites by high-performance liquid chromatography (HPLC) in the region of the frontal lobe containing the primary somatosensory cortex (Fig. 5A), the hippocampus (Fig. 5B), the hypothalamus (not shown) and the cerebellum (Fig. 5C) from Dp(11)17/+ and WT mice. The levels of serotonin (or 5-hydroxytryptamine, 5-HT) were increased in the primary somatosensory cortex of Dp(11)17/+ mice (GXE, P = 0.039; Fig. 5A). There was no significant difference in the level of the metabolite of serotonin, 5-hydroxyindoleacetic acid (5-HIAA), suggesting intact serotonin metabolism, which is also supported by an unaltered 5-HT/HIAA ratio. The levels of DA and its metabolite (dihydroxyphenlanine, DOPAC) were not significantly altered. Monoamine oxidase catalyzes the degradation of DOPAC to homovanillic acid (HVA), the levels of which were also unaltered in the primary somatosensory cortex. The levels of all neurotransmitters analyzed in the hypothalamus were not significantly altered (P > 0.05 for all; data not shown). In the hippocampus, the level of DA was significantly reduced in Dp(11)17/+ mice (genotype, P = 0.036; Fig. 5B). Similar to the hypothalamus, there was no significant difference between Dp(11)17/+ and WT mice in the cerebellum (Fig. 5C). However, there was an enrichment-specific neurotransmitter signature specific to the cerebellum; for all of the neurotransmitters analyzed, there was a significant decrease in neurotransmitters from enriched animals compared with their standard-housed counterparts (P = 0.0003–0.02; Fig. 5C), potentially suggesting an important role for the cerebellum in the underlying source of the altered behaviors identified in enriched animals.
Figure 5.
Neurochemical alterations in the brain are affected by housing in enriched environments. (A) Analysis of the primary somatosensory cortex indicates that Dp(11)17/+ mice (Dup, green bars, n = 7) are hyperserotonemic (5-HT) compared with WT littermates (WT, black bars, n = 7), which can be rescued by enrichment (GXE, P = 0.039; DupEE, red bars, n = 8; WT, blue bars, n = 6, P = 0.407). There were no differences between Dp(11)17/+ and their littermates for levels of 5-HIAA or the ratio of 5-HT/5-HIAA, however, enrichment led to a significant decrease in 5-HIAA in Dup animals (P = 0.05) compared with their counterparts from enriched housing. (B) Analysis of the hippocampus indicates decreased DA in Dp(11)17/+ mice (genotype, P = 0.036). There was also an effect of enrichment on the level of 5-HIAA (environment, P = 0.001) and HVA (P = 0.019). (C) An enrichment-specific decrease in neurotransmitter levels was identified in the cerebellum (environment, P < 0.001 for all neurotransmitters listed). Error bars represent the SEM for all figures.
Environmental enrichment was able to correct the altered serotonin levels identified in the primary somatosensory cortex of Dp(11)17/+ mice from standard housing (Fig. 5A). However, WT animals from enriched housing did have elevated serotonin compared with WT mice from standard housing, indicating that serotonin levels alone cannot explain the behavioral changes seen between mice from standard versus enriched cages. Enrichment had a significant effect on the levels of HVA (environment, P = 0.019) and 5-HIAA (P = 0.001; Fig. 5B) in the hippocampus, but not the decreased DA levels. Taken together, these results suggest a potential link between the benefits of environmental enrichment and the underlying changes in the serotonergic and dopaminergic pathways. Furthermore, they underscore the differential effects of this duplication CNV and of environmental enrichment on different regions of the brain.
DISCUSSION
The chromosome-engineered Dp(11)17/+ mice harbor a duplication CNV syntenic to the genomic interval that when duplicated in the human genome causes PTLS and associated autistic features. We show that our animal model displays all of the core autistic behaviors typically utilized to diagnose autism and ASD in humans, including abnormal social interactions, impaired communication and the presence of restrictive or repetitive behavior. Specifically, these mice show abnormal social interactions in the form of defects in social recognition, social dominance and reduced interest in social odors, all highly important in rodent social interaction. They also have alterations in developmentally regulated communication and display compulsive repetitive behaviors. In addition, these mice manifest several autism-associated features that are secondary to the diagnosis, but often found in patients with autism, including increased anxiety, defects in learning and memory, defects in motor coordination, aggression and sleep abnormalities (summarized in Table 1). Further, this model also replicates one of the few well-established physiological markers for autism, namely alteration in the levels of serotonin.
Table 1.
A summary of the results from the behavioral tests performed on Dp(11)17/+ mice
| Human trait | Mouse behavioral assay | Results in Dp(11)17/+ | Observation after EE | Geno P-value | Env P-value | GXE P-value | Figure |
|---|---|---|---|---|---|---|---|
| Social dominance | Tube test | Socially dominant | No change | 0.001 | 0.198 | N/A | 1 |
| Social recognition | Partition test | Decreased social recognition | Improvement | 0.066 | 0.569 | 0.092 GxExT | 1 |
| Aggression | Social interaction | Increased aggression | Rescued contact, but increased non-contact aggression | 0.001/0.002 | 0.064/0.008 | 0.001/0.032 | 1 |
| Response to social cues | Olfactory habituation/dishabituation | Decreased interest in social odors | No change | 0.045 | 0.167 | 0.565 | 1 |
| Communication | Pup USVs | Decreased peak USVs and shifted pattern of communication | N/A | 0.001 | N/A | N/A | 4 |
| Repetitive/sterotypic behavior | Holeboard test | Increased percentage of repeated nosepokes (NP) | No change | 0.001 | 0.126 (#NP-0.004) | 0.777 | 2 |
| Learning and memory | Conditioned fear | Decreased freezing behavior | Improved | 0.003 | 0.001 | 0.371 | 3 |
| Anxiety | Open field/elevated plus tests | Increased anxiety | Reduced/rescued | 0.001/0.014 | 0.001/0.264 | 0.361/0.038 | 3 |
| Motor coordination | Wire hang/dowel walking | Abnormal motor coordination | Improvement | 0.291/<0.001 | 0.012/>0.3 | 0.551/N/A | 3 |
| Sleep abnormality | Circadian period length | Decreased period length | N/A | 0.01 | N/A | N/A | 4B-F |
USV, ultrasonic vocalization. Bold type indicates phenotypes that were improved by environmental enrichment. Italicized type indicates significant gene x environment interactions.
Hyperserotonemia has been consistently identified in the serum of a proportion of patients with autism and some of their first-degree relatives, although the underlying cause of the increase in 5-HT and whether serum levels of serotonin are representative of levels in the brain are not understood (34–39). The efficacy of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, in the treatment of some features of autism, including aggression, anxiety and obsessive/compulsive behaviors, provides further evidence for a potential role of serotonin disturbances in the pathogenesis of autism (31,38). A link between serotonin, social hierarchy formation and aggressive behavior has been demonstrated in several species (40–45), indicating that the complex role for 5-HT in a large number of physiological processes is highly conserved throughout evolution. Indeed, 5-HT signaling is an important component in the regulation of early brain development, acting as a growth factor in the immature brain; it also plays a role in adult neuroplasticity, including cell proliferation, migration, differentiation and synaptogenesis (42). As a result, 5-HT is involved in the regulation of emotion, mood, cognition, circadian rhythms, motor function, endocrinological function, food intake, thermoregulation and perception of pain throughout the lifespan (46).
The cerebellum has long been known to be important in balance and motor coordination, but it has recently been shown to also play a role in ‘cerebellar neurocognition’, which may be highly relevant to autism and ASD (47). Indeed, several structural and cellular changes have been identified in the brains of patients with ASD (48). The cerebellum-specific neurotransmitter signature identified here suggests the need for further studies, investigating a potential link between environmental enrichment and the cerebellum. Our results suggest the possibility that DA is partly responsible for the genetic effects, with the largest effect of genotype in the hippocampus, whereas serotonin is more affected by environmental factors. Furthermore, this mouse model may provide an experimental system to further investigate hyperserotonemia and manipulation of the serotonergic neurotransmitter pathway in autism and other developmental disorders.
Our results document gene-environment interactions in the etiology of the behavioral phenotypes caused by Dp(11)17/+; selected behavioral abnormalities were either mitigated or even completely rescued by implementing rearing in an enriched environment. Specifically, enrichment was able to ameliorate motor defects, improve learning and memory defects, reduce aggressive behavior and relieve anxiety in Dp(11)17/+ mice. Similar benefits have been seen in rodent models for other disorders, including those for Rett syndrome, Alzheimer disease and drug addiction (24,49,50). Some of these phenotypic changes are also accompanied by alterations in the underlying endophenotypes, including structural changes within the brain, improvement in synaptic deficits and neurochemical changes (49,51–57). The specificity of the effects of environmental enrichment suggests that certain phenotypes (and their underlying pathomechanisms) may be pliable and influenced by environmental factors, while others are influenced more by genetic determinants and constant regardless of environmental context, such as social dominance, and interest in social odors. Alternatively, the environmental factors that might influence such genetically determined behavioral traits remain to be elucidated, and the possibility remains that standard rearing conditions may be harmful to neurodevelopment, resulting in an exacerbation of the phenotype in enriched housing.
Interestingly, enrichment exacerbated stereotypic, repetitive behavior in both Dp(11)17/+ and WT mice. These observations suggest that this phenotype is sensitive to over-stimulation, as in the case of enriched housing. Although this type of enrichment is beneficial in the treatment of many behavioral abnormalities, it may not be optimal for every type of behavior, suggesting a need for individualized behavioral enrichment for specific types of behavioral abnormalities. Additionally, future work will focus on dissecting out the particular types of enrichment to systematically determine which environmental elements are responsible for specific behavioral effects. Future studies could assay additional environmental conditions, including treatment with drugs that target or alter the serotonergic pathway (e.g. SSRIs, serotonin agonists) or antianxiolytics. This mouse model will not only serve as a tool for the development of therapeutics and behavioral interventions relevant to the treatment of the symptoms and underlying physiological causes of CNV-related autism, but it will also enhance further studies of forward genetics of developmental brain disorders.
MATERIALS AND METHODS
Behavioral analyses
Heterozygous Dp(11)17/+ mice were maintained on a pure C57BL/6Tyrc-Brd background (>N10). Mice were housed in a 12 h light:12 h dark cycle and provided standard mouse chow and water ad libitum. All research and animal care procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Behavior tests were performed on male mice from 8 to 12 weeks of age for each genotype with the exception of the isolation-induced pup USV determination, which used newborn pups of both genders, and circadian rhythm studies, that used female mice. Behavioral tests were performed in four batteries with four separate groups of mice. Only one test was performed per day, except where indicated. The first battery was OFA–light dark analysis–dowel/wire test–CF testing. The second battery was EPM–tube test–partition test–social interaction. The third battery was HB–olfactory habituation/dishabituation. The fourth group underwent only USV analysis.
Tube test of social dominance
Social dominance was evaluated by the tube test (30). Briefly, one test mouse (Dp(11)17/+ or WT littermate) was placed the head first at the opposite end of a clear plastic tube (3.7 cm inner diameter and 30.5 cm length) to a standard control (unrelated, WT C57BL/6Tyrc-Brd) mouse and released simultaneously. The test ended when one mouse completely retreated from the tube; this mouse was determined to be the loser, and the dominant mouse that did not back out was the winner. Each mouse (test and control) underwent exactly three matches with three different partners. Results were analyzed by a Fisher exact test.
Three-chamber test
The three-chamber test was performed as described with some modification (58). Briefly, the test mouse was allowed to freely explore a three-chambered box divided by small doorways. After habituation, the mouse was enclosed in the center chamber and an unfamiliar mouse (129S5 male) was placed in one of the chambers in a wire cage. An inanimate object was placed in the opposite cage. The test mouse was allowed to explore the entire chamber freely for 10 min in order to test for sociability, and the time spent in each chamber was recorded by the automated ANY-maze system.
Partition test
The partition test was implemented as described (30). On the day before the partition test, Dp(11)17/+ and WT littermates were housed in the same cage on the opposite side of a partition from a weight-matched control male 129S5 mouse. We utilized three phases for this test, and testing commenced 24 h after initiation of partitioned housing. First, social interaction across the partition as measured by the number of approaches to the partition and time spent at the partition by the experimental mouse in a 5-min trial were measured with the original overnight partner using a hand-held Psion computer in conjunction with Noldus Observer software. Second, the behavior of the experimental animal was scored with a new, unrelated, control partner in a similar manner to the first test, again for 5 min. Third, the behavior of the experimental animal was scored with the original partner as in the first trial.
Social interaction test
After two overnights in partitioned housing, and as a follow-up to the partition test, mice were given a direct social interaction test (59). This test occurred 24 h after the partition test (after a total of 48 h in partitioned housing with the same control partner throughout the experiment). Social interaction between the mice was videotaped following the removal of the partition for 10 min, and behavioral responses were scored from the videotapes by an observer using a Psion hand-held computer and Noldus Observer software. Behavioral data were analyzed by a two-way ANOVA (gene × environment) for each parameter (number of events, duration and mean time per event) for both active (boxing, mounting, wrestling and aggressive contact/grooming) and non-active (unrest, tail-rattling) aggressive behaviors, as well as for non-aggressive behaviors.
Olfactory detection and discrimination
Olfactory discrimination for non-social and social odors was investigated using a slightly modified habituation/dishabituation protocol (8). Adult mice were separated into individual cages and acclimated to holding cages and a non-odored cotton tip for 30 min. Next, in order to assess habituation, they were given three 2-min presentations of each: water, first non-social (NS1) odor and second non-social odor (NS2). Non-social odors were imitation banana and almond extracts (1:1000 dilution). On the following day of testing, non-social odors were replaced by social odors (swabbed dirty cage bedding from two unfamiliar mouse home-cages, S1 and S2). Olfactory odors were alternated within each test. Time spent sniffing was measured and used to quantify the initial investigation of novel odors, the habituation to the previously presented odor (during consecutive presentations of an odor) and the dishabituation of response (time spent sniffing) upon presentation of a novel odor compared with the immediately preceding familiar odor. Time spent sniffing was analyzed using a three-way ANOVA (genotype × odor × presentation) with repeated measures.
Pup separation-induced vocalization measure
Separation-induced USVs were evaluated on PND 3–14 (8). Each litter was removed from its home cage with nesting material and placed in a holding container. Single pups were isolated and placed in a pseudo-randomized order into a sound-attenuating chamber for 2 min, while isolation-induced USVs in the 70-kHz range were recorded (Noldus Ultravox Bat detector mini-3). Pups were identified by markings placed on the plantar surface of the foot following USV reading. Markings were replaced daily as needed. Body masses were recorded daily immediately following the USV measurement. Data were analyzed using a one-way ANOVA with repeated measures. Follow-up analysis was used to examine PND × genotype interactions for a number of calls on each PND.
Automated holeboard exploration test
A modified HB exploration test was performed to assess stereotypic, repetitive and restricted behaviors (60). Nose-poke responses were measured during a 5-min trial in the open field chamber. A floorboard with 16 equidistant holes was placed on the bottom of the chamber, and the number, time, location and sequence of nose pokes were determined by automated detection by the Pokemon system (Accuscan Instruments).
Pavlovian conditioned fear
A modified CF protocol was utilized to evaluate learning and memory in Dp(11)17/+ mice (27). Briefly, the mice were placed in a test chamber and allowed to acclimate for 2 min before presentation with an auditory stimulus (conditioned stimulus; CS) followed by a mild foot shock (unconditioned stimulus; US). Two minutes later another CS–US pairing was presented. Freezing behavior was recorded and measured by an automated Med Associates/Actimetrics system. Twenty-four hours later, the mouse was placed back into the test chamber for 5 min and freezing behavior was recorded as above. One to two hours later, environmental and contextual cues were altered and the mouse was again tested for freezing behavior upon presentation with the CS after 3 min of acclimation to the new context.
Open field activity
The OFA was used to test mice for anxiety-like responses and exploratory activity according to a standard protocol (59). Briefly, mice were placed into a clear Plexiglass (40 cm × 40 cm × 30 cm) open field arena and allowed to freely explore for 30 min. Data were collected by a computer-operated Digiscan optical animal activity system (Accuscan Electronics). General activity levels were determined by the total distance traveled during the 30 min test, and the level of anxiety was determined by dividing the center distance by the total distance to determine the center:total distance ratio.
Elevated-plus maze for anxiety-like behaviors
A modified version of the EPM was performed to determine anxiety-like behaviors (58). The maze had two closed arms and two open arms, and it was elevated 50 cm from the floor. Animals were placed in the center section and allowed to freely explore the maze for a 10-min trial. ANY-maze software was used to track the position of the mouse, time spent in the open arms and the number of transitions into the open arms. The number of transitions into the light arms was used as a measure of anxiety-related behaviors.
Analysis of free-running circadian rhythm
We used the Mini Mitter circadian rhythm wheel-running system (Respironics) along with VitalView and ActiView software to analyze free-running period lengths in adult female Dp(11)17/+ mice. Mice were housed individually in cages with running wheels under the standard light/dark (12/12 h) cycle for 2 weeks in order to acclimatize the mice to the circadian cages and to evaluate their circadian rhythms with external (light) cues. Food and water were given ad libitum. We then changed the light cycle to a dark/dark (12/12 h) cycle for an additional 2 weeks to analyze the free-running period length. Tau (period length) values were calculated from graphs generated from activity data (number of wheel turns) throughout the day. Data were analyzed using Student's t-test.
Wire hang and dowel-walking test for motor ability
Wire hanging and dowel walking abilities were assessed on the same day to determine motor ability; at least 30 min elapsed between tests. First, the test mouse was placed on a raised wire to hang by its front limbs. The latency to fall onto a soft surface was recorded with a maximum hang time of 60 s. For the dowel-walking test, the mouse was placed on a raised 9 mm wooden dowel and allowed to walk along the dowel. Latency to fall was recorded for each mouse, with a maximum trial time of 2 min.
Environmental enrichment
Mice were weaned at 3 weeks of age from the standard mating cages into enriched cages. Enriched cages were larger (19.7 cm × 16.5 cm × 30.5 cm compared with 27.3 cm × 22.6 cm × 48.9 cm, width × height × length) and contained enrichment items that were replaced and moved within the cage weekly. One of each of the following enrichment items were added to enhance activity (running wheels), climbing (hanging hook and tube), nesting (nestlet, Enrich-o-cob bedding), chewing (chew toy) and social behavior (increased number of mice/cage—4–5 mice in standard cages compared with 7–8 mice in enriched cages and huts for group housing).
Analysis of neurotransmitter levels
Dp(11)17/+ mice and their WT littermates were euthanized by cervical dislocation. Immediately following euthanasia, the brain was extracted and the region of the frontal cortex containing the primary somatosensory complex was isolated using a stereotactic adult rodent brain matrix (Ted Pella, Inc.). The tissues were immediately frozen in liquid nitrogen and stored at −80°C until being shipped for the neurotransmitter assay. Neurotransmitters levels [5-HT (or serotonin) and its metabolite 5-HIAA, adrenaline, noradrenaline, DA, HVA and DOPAC] were analyzed using HPLC by an experimenter blinded to the genotypes in the Neurochemistry Core Lab, Vanderbilt University School of Medicine, Nashville, TN.
Statistical analyses
For the CF, OFA, EPM, partition, wire hang and social interaction tests, data were analyzed by a two-way ANOVA (gene × environment), and significant interactions were followed up with the simple effect test. The three-chamber test and circadian rhythm tau values were analyzed using Student's t-test. USV data were analyzed using a one-way ANOVA with repeated measures using the Greenhouse–Geisser correction for sphericity and follow-up for PND × genotype interaction for the number of calls on each PND. Olfactory habituation/dishabituation data were analyzed using a three-way ANOVA (genotype × odor × presentation) with repeated measures, also utilizing the Greenhouse–Geisser correction for sphericity and follow-up test for odor × genotype interaction. The tube test was analyzed using the Fisher' exact test comparing between genotypes and environments. The dowel test was analyzed using the Kruskal–Wallis test for non-parametric one-way ANOVA comparing between genotypes and environments. For all experiments, results were considered significant when P < 0.05. P-values for behavioral data are summarized in Table 1.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported in part by National Institute of Neurological Disorders and Stroke; National Institutes of Health (R01 NS058529); the inaugural research grant from the SMS Foundation to J.R.L; the Institutional Program Unifying Population and Laboratory Based Sciences Award from the Burroughs Wellcome Fund held by the University of Texas-Houston Health Science Center (fellowship to M.L.) and a BCM IDDRC grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5P30HD024064-23). (The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.)
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge our funding sources for support of this research.
Conflict of Interest statement. J.R.L. is a consultant for Athena Diagnostics, has stock ownership in 23andMe and Ion Torrent Systems and is a coinventor on multiple United States and European patents for DNA diagnostics. The Department of Molecular and Human Genetics derives revenue from clinical testing by high-resolution human genome analysis.
REFERENCES
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders 4th Edn (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.WHO. Mental Disorders: a Glossary and Guide to their Classification in Accordance with the 10th Revision of the International Classification of Diseases: Research Diagnostic Criteria. Geneva: World Health Organization; 1993. [Google Scholar]
- 3.Miles J.H. Autism spectrum disorders—a genetics review. Genet. Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- 4.Brosnan J., Healy O. A review of behavioral interventions for the treatment of aggression in individuals with developmental disabilities. Res. Dev. Disabil. 2011;32:437–446. doi: 10.1016/j.ridd.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Bronsard G., Botbol M., Tordjman S. Aggression in low functioning children and adolescents with autistic disorder. PLoS One. 2011;5:e14358. doi: 10.1371/journal.pone.0014358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawley J.N. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 7.Crawley J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton S.M., Spencer C.M., Harrison W.R., Yuva-Paylor L.A., Graham D.F., Daza R.A., Hevner R.F., Overbeek P.A., Paylor R. Multiple autism-like behaviors in a novel transgenic mouse model. Behav. Brain Res. 2011;218:29–41. doi: 10.1016/j.bbr.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penagarikano O., Abrahams B.S., Herman E.I., Winden K.D., Gdalyahu A., Dong H., Sonnenblick L.I., Gruver R., Almajano J., Bragin A., et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moy S.S., Nadler J.J. Advances in behavioral genetics: mouse models of autism. Mol. Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 11.Jacquemont M.L., Sanlaville D., Redon R., Raoul O., Cormier-Daire V., Lyonnet S., Amiel J., Le Merrer M., Heron D., de Blois M.C., et al. Array-based comparative genomic hybridisation identifies high frequency of cryptic chromosomal rearrangements in patients with syndromic autism spectrum disorders. J. Med. Genet. 2006;43:843–849. doi: 10.1136/jmg.2006.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celestino-Soper P.B., Shaw C.A., Sanders S.J., Li J., Murtha M.T., Ercan-Sencicek A.G., Davis L., Thomson S., Gambin T., Chinault A.C., et al. Use of array CGH to detect exonic copy number variants throughout the genome in autism families detects a novel deletion in TMLHE. Hum. Mol. Genet. 2011;20:4360–4370. doi: 10.1093/hmg/ddr363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatani J., Tamada K., Hatanaka F., Ise S., Ohta H., Inoue K., Tomonaga S., Watanabe Y., Chung Y.J., Banerjee R., et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horev G., Ellegood J., Lerch J.P., Son Y.E., Muthuswamy L., Vogel H., Krieger A.M., Buja A., Henkelman R.M., Wigler M., et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl Acad. Sci. USA. 108:17076–17081. doi: 10.1073/pnas.1114042108. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch L.G., Britton S.L. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity. 2008;16(Suppl. 3):S28–S32. doi: 10.1038/oby.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walz K., Caratini-Rivera S., Bi W., Fonseca P., Mansouri D.L., Lynch J., Vogel H., Noebels J.L., Bradley A., Lupski J.R. Modeling del(17)(p11.2p11.2) and dup(17)(p11.2p11.2) contiguous gene syndromes by chromosome engineering in mice: phenotypic consequences of gene dosage imbalance. Mol. Cell Biol. 2003;23:3646–3655. doi: 10.1128/MCB.23.10.3646-3655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potocki L., Bi W., Treadwell-Deering D., Carvalho C.M., Eifert A., Friedman E.M., Glaze D., Krull K., Lee J.A., Lewis R.A., et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am. J. Hum. Genet. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treadwell-Deering D.E., Powell M.P., Potocki L. Cognitive and behavioral characterization of the Potocki-Lupski syndrome (duplication 17p11.2) J. Dev. Behav. Pediatr. 2010;31:137–143. doi: 10.1097/DBP.0b013e3181cda67e. [DOI] [PubMed] [Google Scholar]
- 19.Soler-Alfonso C., Motil K.J., Turk C.L., Robbins-Furman P., Friedman E.M., Zhang F., Lupski J.R., Fraley J.K., Potocki L. Potocki-Lupski syndrome: a microduplication syndrome associated with oropharyngeal dysphagia and failure to thrive. J. Pediatr. 2010;158:655–659. doi: 10.1016/j.jpeds.2010.09.062. e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferies J.L., Pignatelli R.H., Martinez H.R., Robbins-Furman P.J., Liu P., Gu W., Lupski J.R., Potocki L. Cardiovascular findings in duplication 17p11.2 syndrome. Genet. Med. 2011;14:90–94. doi: 10.1038/gim.0b013e3182329723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts E.M., English P.B., Grether J.K., Windham G.C., Somberg L., Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ. Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis L.T., Patel K. Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): a review. J. Altern. Complement. Med. 2008;14:79–85. doi: 10.1089/acm.2007.0610. [DOI] [PubMed] [Google Scholar]
- 23.Geier D.A., Kern J.K., Garver C.R., Adams J.B., Audhya T., Nataf R., Geier M.R. Biomarkers of environmental toxicity and susceptibility in autism. J. Neurol. Sci. 2009;280:101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Lonetti G., Angelucci A., Morando L., Boggio E.M., Giustetto M., Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol. Psychiatry. 2010;67:657–665. doi: 10.1016/j.biopsych.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Ricard G., Molina J., Chrast J., Gu W., Gheldof N., Pradervand S., Schutz F., Young J.I., Lupski J.R., Reymond A., et al. Phenotypic consequences of copy number variation: insights from Smith-Magenis and Potocki-Lupski syndrome mouse models. PLoS Biol. 2010;8:e1000543. doi: 10.1371/journal.pbio.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina J., Carmona-Mora P., Chrast J., Krall P.M., Canales C.P., Lupski J.R., Reymond A., Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum. Mol. Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- 27.Walz K., Spencer C., Kaasik K., Lee C.C., Lupski J.R., Paylor R. Behavioral characterization of mouse models for Smith-Magenis syndrome and dup(17)(p11.2p11.2) Hum. Mol. Genet. 2004;13:367–378. doi: 10.1093/hmg/ddh044. [DOI] [PubMed] [Google Scholar]
- 28.Walz K., Paylor R., Yan J., Bi W., Lupski J.R. Rai1 duplication causes physical and behavioral phenotypes in a mouse model of dup(17)(p11.2p11.2) J. Clin. Invest. 2006;116:3035–3041. doi: 10.1172/JCI28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elder J.H., Shankar M., Shuster J., Theriaque D., Burns S., Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J. Autism Dev. Disord. 2006;36:413–420. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 30.Spencer C.M., Alekseyenko O., Serysheva E., Yuva-Paylor L.A., Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 31.Robb A.S. Managing irritability and aggression in autism spectrum disorders in children and adolescents. Dev. Disabil. Res. Rev. 2010;16:258–264. doi: 10.1002/ddrr.118. [DOI] [PubMed] [Google Scholar]
- 32.Sala M., Braida D., Lentini D., Busnelli M., Bulgheroni E., Capurro V., Finardi A., Donzelli A., Pattini L., Rubino T., et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Meng Q.J., Logunova L., Maywood E.S., Gallego M., Lebiecki J., Brown T.M., Sladek M., Semikhodskii A.S., Glossop N.R., Piggins H.D., et al. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makkonen I., Riikonen R., Kokki H., Airaksinen M.M., Kuikka J.T. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev. Med. Child Neurol. 2008;50:593–597. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 35.Cook E.H., Leventhal B.L. The serotonin system in autism. Curr. Opin. Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Cross S., Kim S.J., Weiss L.A., Delahanty R.J., Sutcliffe J.S., Leventhal B.L., Cook E.H., Jr, Veenstra-Vanderweele J. Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism. Neuropsychopharmacology. 2008;33:353–360. doi: 10.1038/sj.npp.1301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janusonis S., Anderson G.M., Shifrovich I., Rakic P. Ontogeny of brain and blood serotonin levels in 5-HT receptor knockout mice: potential relevance to the neurobiology of autism. J. Neurochem. 2006;99:1019–1031. doi: 10.1111/j.1471-4159.2006.04150.x. [DOI] [PubMed] [Google Scholar]
- 38.Neuhaus E., Beauchaine T.P., Bernier R. Neurobiological correlates of social functioning in autism. Clin. Psychol. Rev. 2010;30:733–748. doi: 10.1016/j.cpr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Scott M.M., Deneris E.S. Making and breaking serotonin neurons and autism. Int. J. Dev. Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Alekseyenko O.V., Lee C., Kravitz E.A. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:11. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Audet M.C., Anisman H. Neuroendocrine and neurochemical impact of aggressive social interactions in submissive and dominant mice: implications for stress-related disorders. Int. J. Neuropsychopharmacol. 2009;13:361–372. doi: 10.1017/S1461145709990174. [DOI] [PubMed] [Google Scholar]
- 42.Lewejohann L., Kloke V., Heiming R.S., Jansen F., Kaiser S., Schmitt A., Lesch K.P., Sachser N. Social status and day-to-day behaviour of male serotonin transporter knockout mice. Behav. Brain Res. 2010;211:220–228. doi: 10.1016/j.bbr.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Miller-Butterworth C.M., Kaplan J.R., Barmada M.M., Manuck S.B., Ferrell R.E. The serotonin transporter: sequence variation in Macaca fascicularis and its relationship to dominance. Behav. Genet. 2007;37:678–696. doi: 10.1007/s10519-007-9162-3. [DOI] [PubMed] [Google Scholar]
- 44.Cubitt K.F., Winberg S., Huntingford F.A., Kadri S., Crampton V.O., Overli O. Social hierarchies, growth and brain serotonin metabolism in Atlantic salmon (Salmo salar) kept under commercial rearing conditions. Physiol. Behav. 2008;94:529–535. doi: 10.1016/j.physbeh.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzi V., Carpenter R.E., Summers C.H., Earley R.L., Grober M.S. Serotonin, social status and sex change in the bluebanded goby Lythrypnus dalli. Physiol. Behav. 2009;97:476–483. doi: 10.1016/j.physbeh.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lesch K.P. Linking emotion to the social brain. The role of the serotonin transporter in human social behaviour. EMBO Rep. 2007;8(Spec No):S24–S29. doi: 10.1038/sj.embor.7401008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillig P.M., Sanders R.D. Psychiatry, neurology, and the role of the cerebellum. Psychiatry (Edgmont.) 2010;7:38–43. [PMC free article] [PubMed] [Google Scholar]
- 48.Palmen S.J., van Engeland H., Hof P.R., Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- 49.Herring A., Lewejohann L., Panzer A.L., Donath A., Kroll O., Sachser N., Paulus W., Keyvani K. Preventive and therapeutic types of environmental enrichment counteract beta amyloid pathology by different molecular mechanisms. Neurobiol. Dis. 2011;42:530–538. doi: 10.1016/j.nbd.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 50.El Rawas R., Thiriet N., Lardeux V., Jaber M., Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl.) 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- 51.Naka F., Shiga T., Yaguchi M., Okado N. An enriched environment increases noradrenaline concentration in the mouse brain. Brain Res. 2002;924:124–126. doi: 10.1016/s0006-8993(01)03257-7. [DOI] [PubMed] [Google Scholar]
- 52.Branchi I., D'Andrea I., Fiore M., Di Fausto V., Aloe L., Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatry. 2006;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Cutuli D., Rossi S., Burello L., Laricchiuta D., De Chiara V., Foti F., De Bartolo P., Musella A., Gelfo F., Centonze D., et al. Before or after does it matter? Different protocols of environmental enrichment differently influence motor, synaptic and structural deficits of cerebellar origin. Neurobiol. Dis. 2011;42:9–20. doi: 10.1016/j.nbd.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Galani R., Berthel M.C., Lazarus C., Majchrzak M., Barbelivien A., Kelche C., Cassel J.C. The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol. Learn. Mem. 2007;88:1–10. doi: 10.1016/j.nlm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Kazlauckas V., Pagnussat N., Mioranzza S., Kalinine E., Nunes F., Pettenuzzo L., Souza D.O., Portela L.V., Porciuncula L.O., Lara D.R. Enriched environment effects on behavior, memory and BDNF in low and high exploratory mice. Physiol. Behav. 2011;102:475–480. doi: 10.1016/j.physbeh.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 56.Rasmuson S., Olsson T., Henriksson B.G., Kelly P.A., Holmes M.C., Seckl J.R., Mohammed A.H. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression and binding in the rat hippocampus. Brain Res. Mol. Brain Res. 1998;53:285–290. doi: 10.1016/s0169-328x(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 57.Wood D.A., Rebec G.V. Environmental enrichment alters neuronal processing in the nucleus accumbens core during appetitive conditioning. Brain Res. 2009;1259:59–67. doi: 10.1016/j.brainres.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moy S.S., Nadler J.J., Young N.B., Perez A., Holloway L.P., Barbaro R.P., Barbaro J.R., Wilson L.M., Threadgill D.W., Lauder J.M., et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spencer C.M., Graham D.F., Yuva-Paylor L.A., Nelson D.L., Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav. Neurosci. 2008;122:710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- 60.Moy S.S., Nadler J.J., Poe M.D., Nonneman R.J., Young N.B., Koller B.H., Crawley J.N., Duncan G.E., Bodfish J.W. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav. Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.