Abstract
Tuberous sclerosis complex (TSC) is caused by heterozygous mutations in either the TSC1 (hamartin) or the TSC2 (tuberin) gene. Among the multisystemic manifestations of TSC, the neurodevelopmental features cause the most morbidity and mortality, presenting a considerable clinical challenge. Hamartin and tuberin form a heterodimer that inhibits the mammalian target of rapamycin complex 1 (mTORC1) kinase, a major cellular regulator of protein translation, cell growth and proliferation. Hyperactivated mTORC1 signaling, an important feature of TSC, has prompted a number of preclinical and clinical studies with the mTORC1 inhibitor rapamycin. Equally exciting is the prospect of treating TSC in the perinatal period to block the progression of brain pathologies and allow normal brain development to proceed. We hypothesized that low-dose rapamycin given prenatally and/or postnatally in a well-established neuroglial (Tsc2-hGFAP) model of TSC would rescue brain developmental defects. We developed three treatment regimens with low-dose intraperitoneal rapamycin (0.1 mg/kg): prenatal, postnatal and pre/postnatal (combined). Combined rapamycin treatment resulted in almost complete histologic rescue, with a well-organized cortex and hippocampus almost identical to control animals. Other treatment regimens yielded less complete, but significant improvements in brain histology. To assess how treatment regimens affected cognitive function, we continued rapamycin treatment after weaning and performed behavioral testing. Surprisingly, the animals treated with the combined therapy did not perform as well as postnatally-treated animals in learning and memory tasks. These results have important translational implications in the optimization of the timing and dosage of rapamycin treatment in TSC affected children.
INTRODUCTION
Tuberous sclerosis complex (TSC) is a tumor suppressor disorder caused by heterozygous mutations in either the TSC1 (hamartin) or the TSC2 (tuberin) genes (1,2). Loss of heterozygosity (LOH) of either gene (3,4) and activation of the mTORC1 kinase (3,5) are important molecular features associated with TSC pathology. Although TSC affects multiple organs, neurodevelopmental defects result in the most substantial morbidity and mortality. Tubers, subependymal nodules and subependymal giant cell astrocytomas (SEGAs) represent common TSC neurodevelopmental abnormalities (6,7) and are associated with intellectual disability, autism and epilepsy. The prevention and management of these developmental brain lesions are major challenges in caring for patients.
The hamartin and tuberin heterodimer inhibit the rapamycin-sensitive mTORC1 signal transduction pathway that controls translation, proliferation and cell growth (8–10). Preclinical trials have demonstrated significant rescue of many neurologic defects using rapamycin (11–15), a macrolide that, upon binding to the intracellular binding protein FKBP12, inhibits the ability of the mTORC1 kinase to signal to its downstream effectors. These studies have paved the way for several human clinical trials that have recently resulted in the approval of everolimus, a rapamycin derivative, for the treatment of SEGAs (16,17). Nonetheless, the ability of rapamycin to rescue perinatal defects remains largely untested. A recent report demonstrated a promising effect of one dose of prenatally-administered rapamycin on the longevity of a Tsc1-Nestin mouse model of TSC (13). Here, we substantially extend those studies by comparing and contrasting the histological and behavioral effects of different perinatal rapamycin treatment regimens in the previously reported Tsc2-hGFAP neuroglial mouse model of TSC (18).
Using the Tsc2-hGFAP mouse model, we have shown that the loss of Tsc2 in radial glial progenitor cells recapitulates many brain manifestations of TSC. Tsc2-hGFAP animals have cortical and cellular hypertrophy, heterotopias, defects in lamination and myelination, astrogliosis and die at about one month of age (18). These defects are a combination of prenatal and postnatal neurodevelopmental abnormalities and thus provide a good model for the study of perinatal treatment of TSC. Using the Tsc2-hGFAP model, we sought to systematically determine the most effective treatment regimens to rescue neurodevelopmental defects. We show that combined rapamycin treatment resulted in almost complete rescue of neuronal and glial pathologies, while prenatal or postnatal treatment alone yielded less complete but significant improvements in brain histology. Surprisingly, the animals treated with combined therapy did not perform as well as postnatally-treated animals on learning and memory tasks. These results show that rapamycin can rescue perinatal neurodevelopmental defects and support the cautious design of trials that will assess early rapamycin treatment of TSC-affected children.
RESULTS
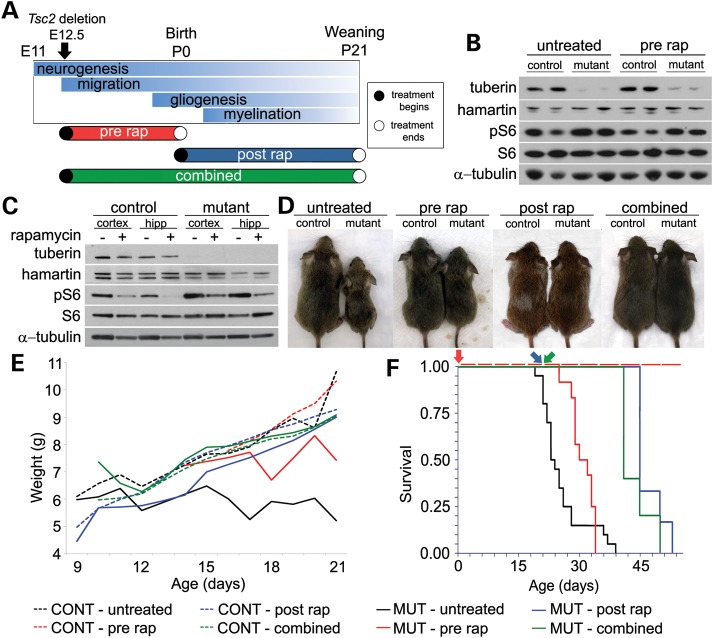
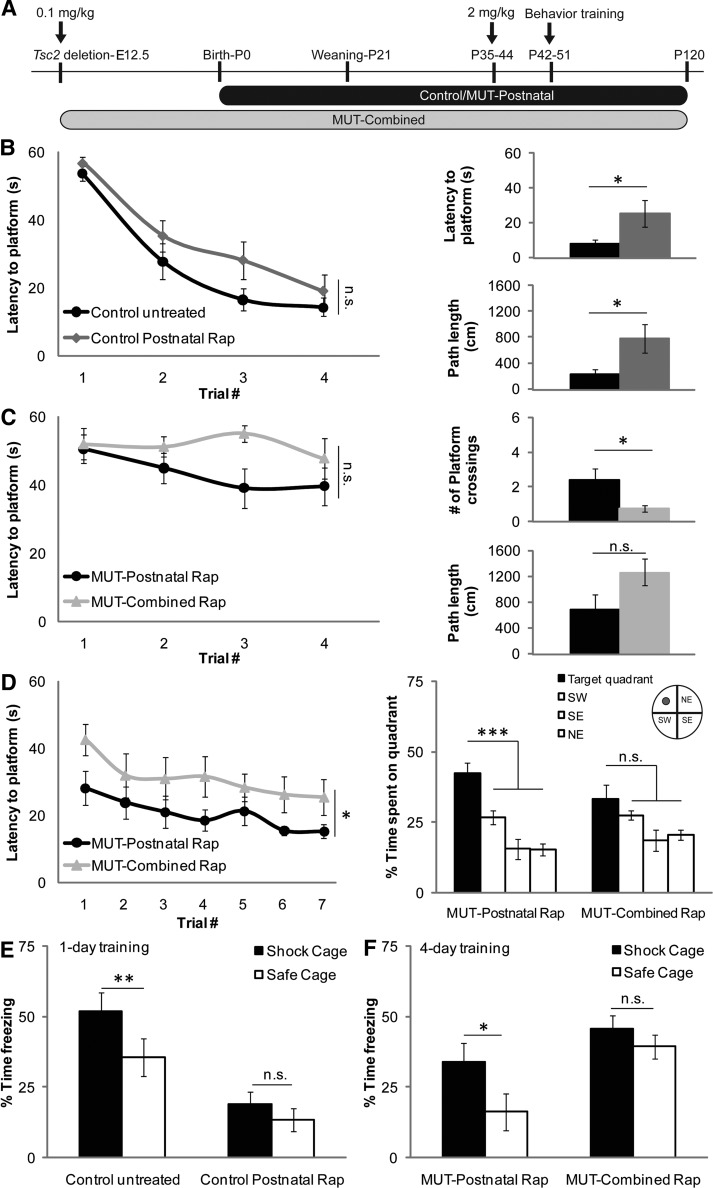
To examine whether rapamycin could rescue some or all defects in Tsc2-hGFAP mice, we designed three rapamycin treatment regimens to target prenatal and/or postnatal developmental abnormalities (Fig. 1A). We optimized a rapamycin dosage (0.1 mg/kg i.p. daily) that suppressed mTORC1 activity as measured by levels of phosphorylated ribosomal protein S6 (Fig. 1B and C), but did not kill embryos or retard postnatal growth. This dose is 10–50-fold lower than that used in other studies involving TSC mouse models (11–14). Rapamycin treatment was stopped at birth in the prenatal group or at postnatal day 21 (P21) in the combined and postnatal groups. Animals were either sacrificed for brain histology or observed for longevity.
Figure 1.
Prenatal, postnatal and combined rapamycin treatments improved health, weight gain and longevity. (A) Rapamycin treatment regimens and their relationship to brain development. Prenatal treatment was from E12.5-birth (P0); postnatal treatment from P0 to weaning (P21); combined treatment from E12.5 to P21. In the cohorts used for histology and longevity studies, prenatal treatment stopped at birth (open circle), postnatal and combined treatments were stopped at P21 (open circle). (B) Brain lysates from newborn animals after 0.1 mg/kg prenatal rapamycin treatment from E12.5 to birth were analyzed by immunoblotting. The levels of phosphorylated S6 (pS6), an indicator of mTORC1 activation, were decreased in the lysates of the rapamycin-treated Tsc2-hGFAP (mutant) animals, although not to control levels. Total S6 was unaffected. (C) Immunoblots of cortical and hippocampal lysates from P21 mice treated with 0.1 mg/kg i.p. rapamycin from P0 to P21. Levels of pS6 in the untreated Tsc2-hGFAP cortex and hippocampus were increased. Treatment reduced mutant pS6 levels to approximately untreated control levels. Total levels of S6 remained unchanged. Based on these results, daily 0.1 mg/kg i.p. was used for further experiments. (D) Control and Tsc2-hGFAP mutant mice at P21 after the different rapamycin treatment regimens. (E) Graph demonstrating an improvement in weight gain for all rapamycin regimens compared with untreated Tsc2-hGFAP mice (solid black line). Rapamycin treatment regimens had no effect on weight gain in control animals. (F) The Kaplan–Meier survival plots of untreated and all treatment regimens after the cessation of rapamycin. Colored arrows correspond to day of rapamycin cessation for specific groups. Median age of death: MUT-untreated P23 (n = 20); MUT-prenatal P31 (n = 12; P= 0.08); MUT-postnatal P45 (n = 6; P < 0.001); MUT-combined P41 (n = 5; P = 0.0001).
In all treatment groups, rapamycin improved the health of Tsc2-hGFAP mice (Fig. 1D, E, F). The postnatal and combined treatment groups were healthy and appeared indistinguishable from controls at P21, but began to die from seizures at about P40 (Fig. 1F). These results further demonstrated that rapamycin treatment must be continued to prevent death in mutant animals as shown in other models (11,12). Nonetheless, the median age of survival of both postnatal and combined groups was significantly longer than untreated mutants. Prenatal treatment had a modest effect on health, and did not significantly alter the median survival. These attenuated effects of prenatal treatment are likely due to the reactivation of mTORC1 after the cessation of treatment at P0 as demonstrated by increased phosphorylated S6 (pS6) immunostaining (Fig. 2A).
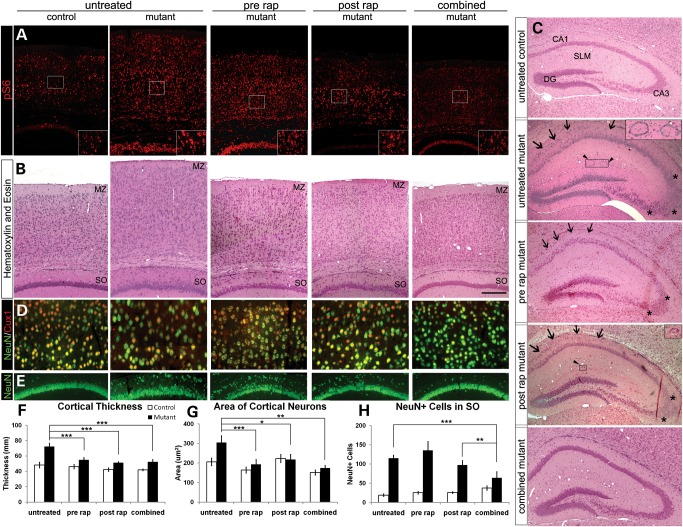
Figure 2.
Effects of daily 0.1 mg/kg rapamycin treatment regimens on cortical histology and organization. (A) Cortical and hippocampal phosphorylated S6 (pS6) immunohistochemistry. Untreated Tsc2-hGFAP brains at postnatal day 21 demonstrated an increased pS6 signal compared with control. Postnatal and combined treatments decreased the intensity of pS6 staining in Tsc2-hGFAP brain consistent with suppression of mTORC1. In the prenatally-treated brains, pS6 expression was comparable with untreated Tsc2-hGFAP levels, suggesting reactivation of mTORC1 during the period of no treatment from P0 to P21. (B) H&E staining of the cerebral cortex. Note the well-formed MZ in the brains from prenatal and combined treated Tsc2-hGFAP mice. (C) H&E staining of hippocampus. Combined treatment resulted in a mutant hippocampus that was almost identical in organization to the control. The CA1 and CA3 regions were indistinguishable between control and combined treatment groups, but remain somewhat split and disorganized (arrows and asterisks) in the prenatal and postnatal groups. The ring heterotopia in the SLM of the mutant mice (arrowheads and insets) were absent in prenatal and combined treatments, and were occasionally seen in the postnatal group (two out of seven animals examined; 15/15 untreated Tsc2-hGFAP mice have ring heterotopias). (D) Higher magnification of cortical layers II/III showing NeuN immunohistochemistry of enlarged neurons of the untreated mutant and the rescue of cell size by the various treatments. (E) NeuN immunohistochemistry of the CA1 region of the hippocampus. Note the ectopic cells in the SO of untreated Tsc2-hGFAP mice that are decreased in number in postnatal treatment, and almost disappear in combined treatment brains. (F) Bar graph showing the rescue of cortical thickness in all treatment regimens (***P< 0.001). The thickness of the control cortex was unaffected by the different rapamycin regimens. (G) Bar graph showing the rapamycin rescue of neuronal hypertrophy (*P< 0.05; **P< 0.01; ***P< 0.001). Control neuronal size was not significantly affected by rapamycin. (H) Bar graph showing the number of ectopic neurons in the SO of the hippocampus in different treatment groups. Combined treatment had the greatest effect on reducing the number of ectopic neurons in the SO (**P< 0.005; ***P< 0.0005). Data represent mean ±SEM. MZ, marginal zone; SLM, stratum lacunosum-moleculare; SO, stratum oriens; DG, dentate gyrus.
Phosphorylation of S6 in the brains of the postnatal and combined treatment groups at P21 (Fig. 2A) was decreased compared with untreated mutants, consistent with the immunoblot analysis used to optimize the dose of 0.1 mg/kg (Fig. 1C). Further histologic analysis demonstrated significant reduction in cortical enlargement and cellular hypertrophy in all three treatment groups (Fig. 2B, D, F, G). The effects of combined treatment on cortical and hippocampal organization were most striking (Fig. 2B, C, E). Prenatal and combined treatment restored the cell sparse marginal zone (MZ) that was thinner and less defined in the untreated and postnatal-treated brains (Fig. 2B). The hippocampal pyramidal layer of the combined treatment group was well organized and almost indistinguishable from control (Fig. 2C and E). Prenatal and postnatal treatment also improved hippocampal organization, although the CA1 and CA3 regions remained somewhat disorganized. All untreated Tsc2-hGFAP animals have distinctive ring heterotopia in the stratum lacunosum moleculare (SLM) (Fig. 2C). No heterotopia were seen in the prenatal or combined treatment samples. Small heterotopia were seen in two out of seven postnatal-treated animals. We then examined ectopic NeuN-positive cells in the stratum oriens (SO) to assess how rapamycin affected the abnormal position of these cells. Postnatal and combined treatments reduced the number of ectopic cells in the SO, but combined treatment reduced the number of ectopic cells to almost control levels (Fig. 2E and H). These results demonstrate that rapamycin can correct the abnormal migration of Tsc2-deficient neurons. The additive effect of pre- and postnatal rapamycin treatment on hippocampal organization is consistent with the in utero and postnatal development of this structure (19).
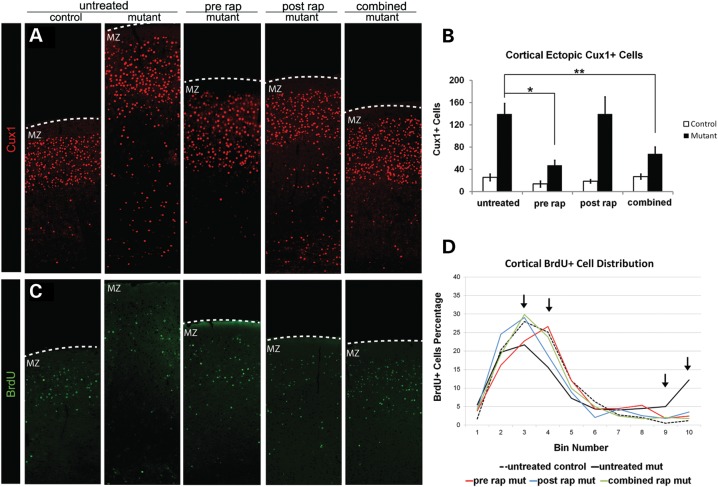
To examine the effects of rapamycin on cortical lamination, we performed immunohistochemistry to detect the layer II–IV-specific transcription factor Cux1 (20) (Fig. 3A and B). Both prenatal and combined rapamycin decreased the number of ectopic Cux1-positive cells in cortical layers V and VI of Tsc2-hGFAP mice. Postnatal treatment had little effect on the distribution of Cux1-positive cells. These results suggest that in utero administration of rapamycin can rescue the abnormal migration of cortical neurons to appropriate layers of the cortex. To further assess the effect of rapamycin on neuronal migration, we analyzed the fate of cortical neurons born at E15.5 using BrdU birthdating (Fig. 3C, D and Supplementary Material, Fig. S1). In control mice, the majority of BrdU-labeled neurons at E15.5 appear in the upper cortical layers. More BrdU-labeled neurons are present in the lower cortical layers of the untreated mutant, suggesting that the loss of Tsc2 affects the migration of some late-born neurons. Prenatal treatment increased the distribution of BrdU-labeled cells toward more superficial layers, whereas postnatal and combined treatments resulted in a BrdU distribution that was most similar to controls. These demonstrate that rapamycin can alter the abnormal migration of Tsc2-deficient neurons during both prenatal and postnatal cortical development.
Figure 3.
Effects of daily 0.1 mg/kg rapamycin treatment regimens on cortical lamination. (A) Cux1 immunohistochemistry. Note the ectopic Cux1-positive cells in the deep cortical layers of the untreated Tsc2-hGFAP brain. (B) Bar graph of ectopic Cux1-positive cells. Prenatal and combined rapamycin treatment significantly reduced the number of ectopic Cux1 cells in layers V and VI (*P< 0.05; **P< 0.005). (C) BrdU immunohistochemistry at P21 showing cortical cells BrdU-labeled at E15.5. (D) Histogram showing distribution of E15.5 BrdU-labeled cells in different treatment groups. Bin 1 is at the pial surface and bin 10 is at the ventricular zone. Untreated Tsc2-hGFAP mice have more BrdU-labeled neurons in the lower bins 9 and 10. All treatments increased the proportion of BrdU-labeled cells in the more superficial bins. Arrows indicate significant differences between control and untreated, and among treatment regimens (See Supplementary Material, Fig. S2 for a bar graph representation). Data represent mean ±SEM. MZ, marginal zone.
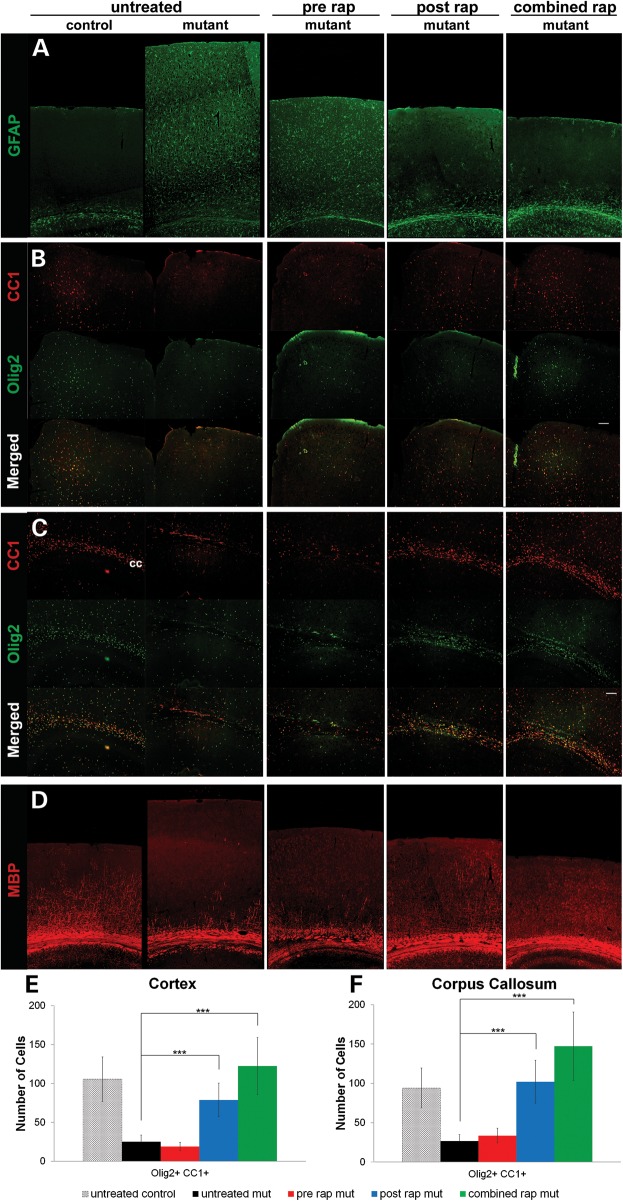
Loss of Tsc1 or Tsc2 in astrocytes causes reactive astrogliosis characterized by increased expression of glial fibrillary acidic protein (GFAP) (Fig. 4A) (18,21). Only postnatal and combined treatments prevented the astrogliosis. Prenatal rapamycin treatment had no effect on the overexpression of GFAP at P21 likely due to the reactivation of mTORC1 by P21 (Fig. 2A). The effects of rapamycin on oligodendrocytes and myelination were examined. Untreated Tsc2-hGFAP mice at P21 showed a decreased number of mature cortical and callosal oligodendrocytes (Fig. 4B, C, E, F). Prenatal rapamycin treatment had little effect on mature oligodendrocyte number. Postnatal and combined treatments significantly restored the number of mature oligodendrocytes in both the cortex and corpus callosum of mutant animals. These results are consistent with the late embryonic and postnatal maturation of oligodendroglia (22). Cortical myelination is also a postnatal event. Accordingly, we observed substantial rescue of myelination defects in only the postnatal and combined treatment groups (Fig. 4D).
Figure 4.
Effects of daily 0.1 mg/kg rapamycin treatment regimens on astrocytes, oligodendroglia and myelination. (A) Glial fibrillary acid protein (GFAP) immunohistochemistry. Prominent astrogliosis in untreated mutant was defined by increased cortical GFAP expression. Postnatal and combined rapamycin treatment normalized GFAP expression. Prenatal treatment had little effect on the upregulation of GFAP. (B and C) CC1 and Olig2 immunohistochemistry to identify mature oligodendroglia in the midline cortex (B) and lateral corpus callosum (C). Many mature oligodendroglia were found in the control midline cortex and corpus callosum. In the same structures of the untreated Tsc2-hGFAP, there was a marked decrease in mature oligodendrocytes. Postnatal and combined treatments restored the cortical oligodendrocyte distribution to control levels (***P< 0.001), whereas prenatal treatment had little effect. (D) Myelin basic protein (MBP) immunohistochemistry. MBP distribution in the cortex is almost absent from untreated Tsc2-hGFAP mice. Postnatal and combined treatment demonstrated significant restoration of the normal MBP pattern. Data represent means ±SEM.
While haploinsufficiency for Tsc1 and Tsc2 is associated with modest behavioral deficits (14,23,24), the consequences of LOH on behavior have been more difficult to assess due to the lethality associated with many of these mouse models (18,21,25). We found that 2 mg/kg rapamycin i.p. three times a week beginning at P10 was able to maintain the health and extend the lifespan of Tsc2-hGFAP mice as long as treatment continued (Supplementary Material, Fig. S2). Therefore, to asses if histologic rescue in the combined and postnatal groups led to improved brain function, postnatal and combined treatment groups were switched from 0.1 mg/kg daily to 2 mg/kg rapamycin three times a week between postnatal days 35 and 44 to maintain their health and permit behavioral analysis (Fig. 5A). Behavior testing was initiated after the first four doses of rapamycin and spanned P42–P120 (1.5–4 months). After the completion of behavioral testing, brains were isolated for histologic analysis.
Figure 5.
Effects of rapamycin treatments on learning and memory. (A) Rapamycin treatment regimens and timing for behavior testing. Animals were treated with 0.1 mg/kg rapamycin daily starting at E12.5 (combined group) or birth (postnatal group). Between P35 and P44, rapamycin dosing was changed to 2 mg/kg three times a week to maintain the health of the animals. Treatment continued throughout behavior testing and ended at P120. (B) Left panel: latency to platform during a 1-day MWM training protocol (n = 10 control untreated group, n = 10 control postnatal rapamycin group; two-way repeated-measures of ANOVA with treatment and trial number as between-subjects factors: F(1,18) = 2.95, P = 0.103). Right panels: latency to platform and path length traveled during probe trial 24 h after completion of training (two-tailed, unpaired student's t-test; latency to platform P = 0.04; path length P = 0.03) (C) Left panel: latency to platform during a 1-day MWM training protocol (n = 8 both groups; two-way repeated-measures of ANOVA with treatment and trial number as between-subjects factors: F(1,14) = 3.01, P = 0.105). Right panels: platform crossings and path length traveled during probe trial 24 h after completion of training (two-tailed, unpaired Student's t-test; platform crossings P = 0.036; path length P = 0.09) (D) Left panel: latency to platform during a 7-day MWM training protocol (n = 8 MUT-postnatal rapamycin group, n = 9 MUT-combined rapamycin group, two-way repeated-measures of ANOVA: group main effect F(1,15) = 5.271, P = 0.037). Right panel: quadrant preference during a probe trial 48h after training (one-way repeated-measures of ANOVA (MUT-postnatal group) and ANOVA on ranks (MUT-combined group) with quadrant as between-subjects factor: F(3,21) = 12.831, P< 0.001; Chi-square = 4.600, 3 d.f., P = 0.204). (E) Percent time spent freezing in shock cage or safe cage after 1 day of training in a context discrimination protocol (n = 10 both groups; two-tailed, paired Student's t-test P = 0.009 (control untreated); P = 0.085 (control postnatal rapamycin). (F) Percent time spent freezing in shock cage or safe cage after 4 days of training in a context discrimination protocol [n = 7 MUT-postnatal rapamycin group, n = 9 MUT-combined rapamycin group; two-tailed, paired student's t-test P = 0.014 (MUT-postnatal rapamycin group); P = 0.37 (MUT-combined rapamycin group)]. *P< 0.05, **P< 0.01, ***P< 0.001. Data represent means ± SEM.
Spatial memory, which is sensitive to the manipulation of the TSC2-mTOR cascade, was tested using a modified 1-day version of the Morris water maze (MWM) task (26–28). Consistent with this, control mice treated with 2 mg/kg rapamycin had significantly impaired long-term memory as indicated by increased latency (P = 0.04) and path length (P = 0.03) to cross the previous location of the hidden platform during a probe trial administered 24 h after training (Fig. 5B). When the performance of the postnatal and the combined Tsc2-hGFAP treatment groups were compared, no significant difference in acquisition was observed (Fig. 5B versus C, left panels). Probe trials showed a decreased path length and significantly increased number (P = 0.036) of platform crossings for the postnatal group when compared with those observed for the combined treatment group, suggesting enhanced memory. As the 1-day paradigm does not typically give rise to strong spatial localization, these animals were given daily training (four trials) for an additional 7 days. Consistent with our probe trial results, the postnatal group showed improved memory for the platform location on day 1 compared with the combined group. This difference was maintained throughout the training (P = 0.037), with both groups acquiring the location of the hidden platform at similar rates (Fig. 5D). When spatial localization was assessed in a probe trial given 48 h after the last day of training, a significant preference for the target quadrant was observed for the postnatal (P < 0.001), but not the combined treatment groups (P = 0.204). No significant differences in either swimming speed or performance in a visible platform task were observed between the two treatment groups (Supplementary Material, Fig. S3).
Heterozygous Tsc2+/− mice have an impaired ability to discriminate between two similar contexts, a deficit that could be lessened by pre-training rapamycin treatment (14). Untreated control mice can distinguish between two similar contexts after 1 day of training as indicated by significantly more freezing in the context in which a mild foot shock was delivered (Fig. 5E). In agreement with the previous results, control animals treated with rapamycin were unable to perform this discrimination. When both rapamycin-treated Tsc2-hGFAP groups were tested after 3 days of training, only the postnatal-treated group was able to distinguish between the two contexts (Fig. 5F). Altogether, these results show that postnatal rapamycin can enable Tsc2-hGFAP animals to learn and remember. While combined rapamycin treatment produced a most complete histologic rescue, these animals had impaired memory when compared with the postnatal group.
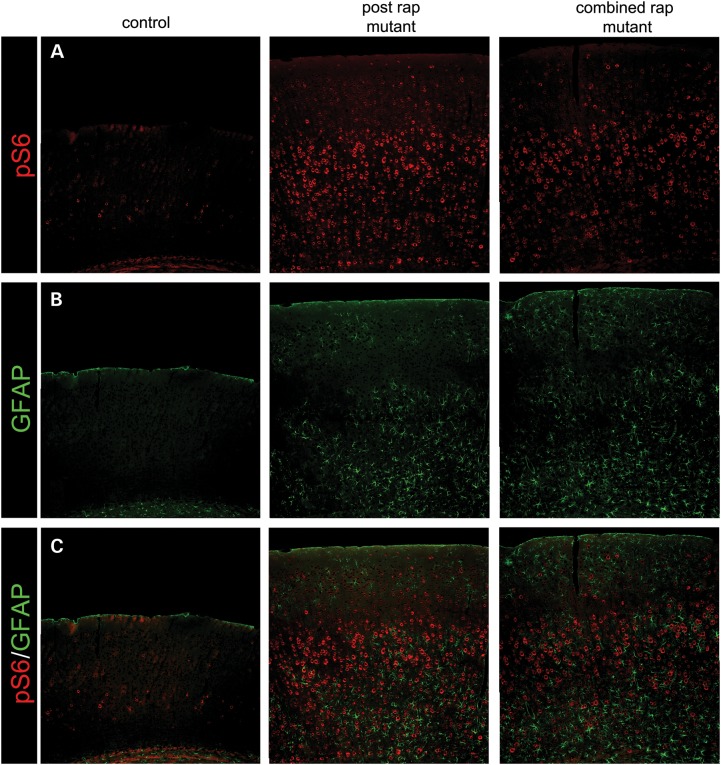
After the completion of behavioral testing, we analyzed the brains of the postnatal and combined groups to assess if 2.5 months of 2 mg/kg rapamycin treatment changed the brain histology that was observed at P21 after daily 0.1 mg/kg rapamycin. We found increased expression of phosphorylated S6 (240/244) above control levels (Fig. 6A). While 2 mg/kg rapamycin three times a week was enough to maintain the health of the animals, it was not sufficient to suppress mTORC1 to control levels as we observed in the P21 brains. We also noted an increase in cortical thickness in the mutant animals compared with controls that would also be consistent with increased mTORC1 activity. The increased pS6 was accompanied by an increase in GFAP (Fig. 6B), indicative of astrogliosis associated with mTORC1 hyperactivity as has been reported previously (18,21,29). Hippocampal and cortical organization, lamination and myelination were unchanged compared with the histologic analysis observed at P21 after daily 0.1 mg/kg rapamycin in the postnatal and combined groups (Supplementary Material, Fig. S4).
Figure 6.
Effects of 2 mg/kg rapamycin on cortical phosphorylated S6 levels and GFAP expression after behavioral testing. (A) There is increased expression of pS6 levels in the cortex of both the postnatal and combined groups, indicating that 2 mg/kg of rapamycin was not sufficient to maintain the mTORC1 inhibition observed at P21 after 0.1 mg/kg (Fig. 2). (B) The increased pS6 expression is accompanied by cortical astrogliosis. (C) Merge of A and B.
DISCUSSION
In this study we demonstrate the prevention of Tsc2-associated neurodevelopmental abnormalities in a neuroglial mouse model of TSC using different perinatal rapamycin treatment regimens. Prenatal, postnatal and combined rapamycin treatment regimens were well tolerated and improved the health of Tsc2-hGFAP mice. However, discontinuation of rapamycin at P0 or P21 ultimately led to death, a finding consistent with previous reports (11,12). Of the three regimens tested, the combined treatment was most effective in restoring neurodevelopment. However, the observed reductions in cortical and hippocampal developmental pathologies did not correlate with memory function. The postnatal-treated animals performed better than the combined treatment group in two hippocampus-dependent memory tasks.
The combined treatment, which targeted both in utero and postnatal neurodevelopment, remarkably achieved a histologic rescue that was almost indistinguishable from untreated control animals. The results of combined treatment were an additive effect of rapamycin on prenatal and postnatal developmental events. For example, the migration of cortical neurons to their appropriate layer is predominantly a prenatal event. Only prenatal and combined treatments were able to rescue the MZ and the distribution of Cux1-positive cells. Postnatal treatment had little effect on antenatal neuronal migration defects. Likewise, postnatal and combined treatments mainly affected developmental defects that are predominantly postnatal or continue through the postnatal period such as oligodendrogenesis, myelination and the later stages of hippocampal development (19,22). These results suggest developmental windows of opportunity for the perinatal rescue of TSC pathology with rapamycin.
The Tsc2-hGFAP mutant mice used in this study died postnatally, even if rapamycin treatment was maintained at 0.1 mg/kg given daily (Fig. 1F). In order to test the consequences of rapamycin treatment on learning and memory, 2 mg/kg rapamycin was administered three times a week during the behavioral testing. This dosing was not adequate to suppress mTORC1 activation to control levels. This finding has clinical relevance and suggests that partial mTORC1 inhibition in human TSC patients may be adequate to improve neurologic function.
Although both combined and postnatal groups did appear to learn the MWM 7-day task, the performance of the postnatal group was found to be significantly improved when compared with the combined treatment group (Fig. 5C). This improved performance was also observed when long-term memory was assessed, as indicated by increased search times in the immediate vicinity where the platform was previously located. Consistent with this, combined treatment animals were incapable of performing a context discrimination task, suggesting that prenatal rapamycin exposure has subtle effects that significantly affect memory consolidation in this group. As the mTORC1 pathway participates in a number of processes critical for neurodevelopment such as neuronal growth, axon guidance, synapse formation and myelination, disruption of these processes could contribute to the observed memory dysfunction in the combined treatment group (30–33). Additional ultrastructural and dose–response studies would be needed to assess these possibilities.
The results of these and a previous study raise the feasibility of administering rapamycin to women carrying a TSC-affected fetus and/or treating TSC-affected children within the first few years of life (13). Moreover, many TSC-associated functional deficits such as epilepsy and autism spectrum disorders often manifest within the first few years of life (7). In utero medical treatment has precedence. Periconceptual folic acid prevents neural tube defects (34). Rapamycin is classified as a class C teratogen. Animal studies suggest adverse effects on the fetus, but there are no well-controlled human studies. There have been a few reports of pregnant mothers having received rapamycin in the transplant literature (35). Most of the infants were phenotypically normal; however, no formal follow-up cognitive testing has been done. In spite of these observations, the negative effects of rapamycin on the behavior of the combined treatment group suggests that more preclinical studies need to be done to better assess the neuroanatomic and physiologic consequence of mTORC1 inhibition on the developing mammalian brain. Nonetheless, the postnatal studies are perhaps more encouraging and raise the possibility of clinical trial in infants or young children. Further studies are needed to assess dosages that would be beneficial, but not induce a failure to thrive phenotype as we and others have observed with high doses of rapamycin.
In summary, we demonstrate several novel developmental effects of rapamycin on a neuroglial-LOH model of TSC. These results suggest that rapamycin treatment during the early perinatal period might be an opportune interval to treat developmental disorders caused by mTORC1 dysregulation. Partial mTORC1 rescue in human TSC patients may provide significant therapeutic benefit. Our results will help better design future preclinical and clinical trials for TSC and other mTORopathies.
MATERIALS AND METHODS
Mice
Tsc2-hGFAP mice (Tsc2ko/flox;hGFAP-Cre) were generated and genotyped as previously described (18). All protocols were approved by the University of Texas Health Science Center at Houston Animal Welfare Committee.
Rapamycin
Rapamycin (MP Biomedicals) was dissolved in 100% methanol at 1.0 mg/ml for storage at −20°C. Before use, rapamycin was diluted with PBS and administered i.p. at 0.1 mg/kg daily. Prenatal treatment was conducted by administering rapamycin to pregnant dams from embryonic day 12.5 (E12.5), the approximate day of Cre expression in radial glial progenitors, until delivery (prenatal group). Postnatal treatment started at birth (P0) and ended at weaning (P21) (postnatal group). A third group was treated prenatally from E12.5 and then after birth until weaning (pre/post = combined group). One group of treated animals was used for histologic analysis, and another group was observed for longevity. For behavior testing, animals were switched from 0.1 mg/kg rapamycin daily to 2 mg/kg rapamycin three times a week between P35 and P44.
Western analysis, histology and immunohistochemistry
Brain lysates were made from P21 animals and analyzed as previously described (18). Briefly, P21 mice were anesthetized with 2.5% avertin and transcardially perfused with cold PBS followed by 4% paraformaldehyde. Hematoxylin and eosin staining and immunohistochemistry were performed as previously reported (18). Antibodies used were: phosphorylated (Ser 240/244) S6 (1:100, Cell Signaling), Cux1 (1:50, Santa Cruz), BrdU (1:50, Becton Dickinson), GFAP (1:400, Sigma), NeuN (1:100), MBP (1:200) (Millipore), Olig2 (1:200) (Millipore) and CC1 (1:100, Calbiochem). Antibodies used for western analysis were: tuberin (1:500), hamartin (1:500), α-tubulin (1:2000), S6 (1:500), pS6 (S240/244) (1:500), pS6 (S235/236) (1:500) from Cell Signaling.
Quantitative analysis
Three sections from three mice of each group were used for all quantitation. Sections were matched and a standard area was used for counting. ANOVA was used for analysis of data. The log-rank test was used to compare survival curves. For BrdU analysis, the cortex was divided equally into 10 bins, and a standardized width was used for counts.
Learning and memory testing
Experimenters were blind to the treatment groups. Animals were trained in two variations of the hidden platform version of the MWM task: 1-day version and a 7-day version (26,27,36,37). For 1-day training protocol, animals were given 12 consecutive trials (4 min inter-trial interval or ITI) in 1 day and a probe test 24 h later. For the 7-day protocol, animals were given four training trials a day (4 min ITI) for six consecutive days. A probe trial was given 48 h later. Movement within the maze was monitored by a video camera linked to tracking software. Analysis of data was done with unpaired, two-tailed Student's t-test (number of platform crossings and path length) and repeated-measure ANOVAs (learning curves and quadrant preference).
Context discrimination testing was done as previously described (38). Briefly, animals were pre-exposed (no shock) to two contexts that shared certain features (horizontal grid floor, background noise, animal handling to and from the room) while differing in others (differently spaced grids, scent, distal cues, floor shape and color). Animals were given one trial a day of 3 min in each chamber with a minimum of 3.5 h between trials. In the shock chamber, animals were given a 2 s, 0.75 mA shock given at 148 s, while no shock was given in the safe chamber. Discrimination of the two contexts was assessed by comparing the time spent freezing (monitored in 2 s intervals) in each chamber during a test trial done on day 4 during which no shock was given. Analysis of the data was done with paired, two-tailed Student's t-test. Data were considered significant at P < 0.05.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by National Institutes of Health grants RO1-NS060804 to M.J.G., RO1 NS053588 to P.D., National Institutes of Health award TL1RR024147 to R.M.R. and the generous support of the Schissler Foundation to S.W.W.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Seonhee Kim and Anthony Moore for helpful discussions and suggestions. N.S.R. and H.C.W. made equal contributions and their order is arbitrary.
Conflict of Interest statement. None declared.
REFERENCES
- 1.European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 2.van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S., Lindhout D., van den Ouweland A., Halley D., Young J., et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 3.Chan J.A., Zhang H., Roberts P.S., Jozwiak S., Wieslawa G., Lewin-Kowalik J., Kotulska K., Kwiatkowski D.J. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J. Neuropathol. Exp. Neurol. 2004;63:1236–1242. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 4.Crino P., Aronica E., Baltuch G., Nathanson K. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology. 2010;74:1716–1723. doi: 10.1212/WNL.0b013e3181e04325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crino P. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J. Child Neurol. 2004;19:716–725. doi: 10.1177/08830738040190091301. [DOI] [PubMed] [Google Scholar]
- 6.DiMario F.J., Jr Brain abnormalities in tuberous sclerosis complex. J. Child Neurol. 2004;19:650–657. doi: 10.1177/08830738040190090401. [DOI] [PubMed] [Google Scholar]
- 7.Crino P., Nathanson K., Henske E. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 8.Tapon N., Ito N., Dickson B.J., Treisman J.E., Hariharan I.K. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov D.D., Ali S.M., Sabatini D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Gao X., Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng L., Xu L., Gutman D., Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meikle L., Pollizzi K., Egnor A., Kramvis I., Lane H., Sahin M., Kwiatkowski D. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: Effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderl S., Freeland M., Kwiatkowski D.J., Goto J. Therapeutic value of prenatal rapamycin treatment in a mouse brain model of Tuberous Sclerosis Complex. Hum. Mol. Genet. 2011;20:4597–4604. doi: 10.1093/hmg/ddr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehninger D., Han S., Shilyansky C., Zhou Y., Weidong L., Kwiatkowski D., Ramesh V., Silva A. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat. Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto J., Talos D., Klein P., Qin W., Chekaluk Y., Anderl S., Malinowska I., DiNardo A., Bronson R., Chan J., et al. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc. Natl Acad. Sci. USA. 2011;108:E1070–E1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campen C., Porter B. Subependymal giant cell astrocytoma (SEGA) treatment update. Curr. Treat Options Neurol. 2011;13:380–385. doi: 10.1007/s11940-011-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreuger D., Care M., Holland K., Agricola K., Tudor C., Mangeshkar P., Wilson K., Byars A., Sahmoud T., Franz D. Everolimus for subependymal giant-cell astrocytoma in tuberous sclerosis. N. Engl. J. Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 18.Way S., McKenna III J., Mietzsch U., Reith R., Wu H., Gambello M. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danglot L., Triller A., Marty S. the development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- 20.Nieto M., Monuki E.T., Tang H., Imitola J., Haubst N., Khoury S., Cunningham J., Gotz M., Walsh C. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J. Comp. Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 21.Uhlmann E.J., Wong M., Baldwin R.L., Bajenaru M.L., Onda H., Kwiatkowski D.J., Yamada K., Gutmann D.H. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann. Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 22.Rowich D., Kriegstein A. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 23.Goorden S., van Woerden G., van der Weerd L., Cheadle J., Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann. Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- 24.Young D., Schenk A., Yang S., Jan Y., Jan L. Altered ultrasonic vocalizations in a tuberous sclerosis mouse modle of autism. PNAS. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meikle L., Talos D.M., Onda H., Pollizzi K., Rotenberg A., Sahin M., Jensen F.E., Kwiatkowski D.J. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J. Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzowski J., McGaugh J. Antisense oligodeoxynucleotide mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. PNAS. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Dash P., Orsi S., Moore A. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J. Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosunov A., Wu X., Weiner H., Mikell C., Goodman R., Crino P., McKann G. Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia. 2008;49:53–62. doi: 10.1111/j.1528-1167.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y., DiNardo A., Kramvis I., Meikle L., Kwiatkowski D.J., Sahin M., He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie D., DiNardo A., Han J., Baharanyi H., Kramvis I., Huynh T., Dabora S., Codeluppi S., Pandolfi P., Pasquale E., et al. Tsc2-Rheb signaling reguilates EphA-mediated axon guidance. Nat. Neurosci. 2010;13:163–173. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavazoie S., Alvarez V., Ridenour D., Kwiatkowski D.J., Sabatini B. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 33.Tsai P., Sahin M. Mechanisms of neurocognitive dysfunction and therapeutic considerations in tuberous sclerosis complex. Curr. Opin. Neurol. 2011;24:106–113. doi: 10.1097/WCO.0b013e32834451c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry R., Li Z., Erickson J., Moore C., Wang H., Mulinare J., Zhao P., Wong L., Gindler J., Hong S., et al. Prevention of neural-tube defects with folic acid in China. China-U.S. collaborative project for neural tube prevention. N. Engl. J. Med. 1999;341:1864. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 35.Briggs G., Freeman R., Yaffe S. A Reference Guide to Fetal and Neonatal Risk: Drugs in Pregnancy and Lactation. Philadelphia: Lippincott Williams and Wilkins; 2011. Sirolimus; pp. 1336–1337. [Google Scholar]
- 36.Schenk F., Morris R. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp. Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- 37.Morris R., Garrud P., Rawlins J., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 38.Frankland P., Castari V., Filipkowski R., McDonald R., Silva A. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.