Abstract
Background
In patients with myeloproliferative neoplasia (MPN) the development of fibrosis and increased vessel density correlate with poor prognosis. The JAK2V617F mutation constitutively activates JAK2, which phosphorylates signal transducer activator of transcription (STAT), up-regulating vascular endothelial growth factor (VEGF), which might be responsible for angiogenesis in MPN. Galectins are involved in the development of fibrosis and angiogenesis and might also be involved in activation of the JAK/STAT pathway in MPN.
Methods
106 MPN patients, 36 essential thrombocythemia (ET), 25 polycythemia vera (PV) and 45 primary myelofibrosis (PMF), were analyzed for the expression pattern of galectin-1, galectin-3, pSTAT3, pSTAT5 and MVD by immunostaining of bone marrow biopsy sections followed by automated image analysis. The JAK2 mutational status was analysed through real time PCR in blood samples.
Results
The expression of galectin-1 was significantly higher in all MPN patients compared to normal controls. Galectin-3 was expressed more in PV patients. MVD was significantly higher in all MPN patients and correlated with galectin-1 and pSTAT5 expression. pSTAT5 expression showed a trend of higher expression in patients carrying the JAK2V617F mutation as well as in PV patients. PMF patients and all JAK2V617F positive patients showed a significantly higher pSTAT3 expression compared to control and ET patients.
Conclusion
The findings suggest the involvement of galectin-1 in MPN development, regardless of the subtype. Furthermore involvement of galectin-3 in PV development, pSTAT5 in that of PV and JAK2V617F positive patients and angiogenesis, as well as pSTAT3 is involved in the pathogenesis of PMF.
Keywords: MPN, myeloproliferative neoplasia, galectin, JAK, STAT, angiogenesis, MVD
Introduction
Myeloproliferative neoplasia (MPNs) are clonal bone marrow stem cell disorders, characterized by proliferation of the myeloid, erythroid and/or megakaryocytic cell lineages resulting in increased numbers of granulocytes, erythrocytes and/or platelets in the peripheral blood. The three classical Philadelphia chromosome-negative (Ph-) MPNs are polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF) [1,2].
In patients with a MPN, fibrosis and increased vessel density correlate with poor prognosis [3,4]. Galectins are involved in the development of both fibrosis [5,6] and angiogenesis [7] in other organs, and therefore might be involved in MPN development.
Galectins mediate cell adhesion and stimulate cell migration, proliferation and apoptosis, through β-galactoside moieties on the cell surface interacting with integrins, laminin and fibronectin. Galectin-1 (gal-1) is involved in tumour angiogenesis and since increased microvessel density (MVD) has been reported in MPNs [8-10], gal-1 might be involved in the regulation of angiogenesis in MPN. Increased galectin-3 (gal-3) expression has been shown to be involved in liver fibrosis [5,11]. Therefore, we studied the gal-1 and gal-3 expression in bone marrow trephines of Ph- MPNs.
The signal transducer and activator of transcription (STAT) proteins are activated via the JAK/STAT pathway, by Janus Kinases (JAKs). A somatic mutation in the JAK2 gene, JAK2V617F, has been shown to be present in >95% of PV patients and in approximately 50% of ET and PMF patients [12,13]. The JAK2V617F mutation disrupts the inhibitory function of the pseudokinase domain in the JAK2 gene, resulting in constitutively activation of JAK2 and phosphorylation of STAT5 [8-10,14-16]. Phosphorylated STAT5 (pSTAT5) is known to be increased in PV patients [17,18] and it was shown that activation of STAT3 induces up-regulation of vascular endothelial growth factor (VEGF) [19]. Therefore, we studied the JAK2 mutational status, pSTAT3 and pSTAT5 expression along with MVD in bone marrow trephines of patients with Ph-MPNs.
Materials and methods
Study population
The study was carried out on bone marrow trephines obtained from patients recorded at the Maastricht University Medical Centre, Maastricht, between January 1992 and December 2009, recorded at the Haga Hospital, The Hague, between January 2006 and December 2009 and recorded at the VieCuri Medical Centre, Venlo, between January 2005 and July 2010. The study was approved by the local institutional ethics committee. The study population consisted of 106 patients with a myeloproliferative neoplasm, with a mean age of 63.6 years at time of diagnosis (SD±14.7) ranging from 17 to 86 years. The patient population included in the study consisted of 36 ET (33.9%), 25 PV (23.6%), and 45 PMF (42.5%) patients. None of the patients received therapy when the biopsy was taken. All patients were clinically and histological diagnosed according to the World Health Organization (WHO) 2008 classification [20] and independently reviewed by two pathologists. Of the patients 45 (42.5%) were men and 61 (57.5%) were women. Fifty-six patients were carriers of the JAK2V617F mutation (19 ET, 17 PV and 20 PMF patients), 24 patients were carriers of the JAK2 wild type (15 ET, 2 PV and 7 PMF patients) and of 26 patients the JAK2 mutational status was unknown, because of insufficient DNA to detect the JAK2 status by PCR or because the patients died prior to the availability of the JAK2V617F test (see Table 1). The patients were subdivided for the grading of myelofibrosis (mf) into mf 0/1 and mf 2/3; 43 patients belonged to the mf 0/1 group (19 ET, 12 PV, 12 PMF) of which 24 were JAK2V617F positive and 11 carried the JAK2 wild type gene and 61 belonged to the mf 2/3 group (17 ET, 12 PV, 32 PMF) of which 31 were JAK2V617F positive and 13 carried the JAK2 wild type gene.
Table 1.
Clinical and laboratory findings of patients with ET, PV, PMF and the control group.
| Essential thrombocythemia n=36 | Polycythemia vera n=25 | Primary myelofibrosis n=45 | Control bone marrow n=36 | |
|---|---|---|---|---|
| Males/females | 11/25 | 8/17 | 26/19 | 23/13 |
| Age, y, mean (SD) | 59 (17.70) | 65 (13.56) | 67 (10.73) | 56 (14.33) |
| JAK2 wild type/ JAK2 mutation/ JAK2 unknown | 15/19/2 | 2/17/6 | 7/20/18 | 36/0/0 |
| White blood cell count*109/L, mean (SD) | 9.37 (2.89) | 16.08 (12.22) | 11.89 (11.38) | 8.16 (4.44) |
| Minimum-maximum | 4.4-18.30 | 5.70-62.00 | 0.90-70.60 | 2.80-23.80 |
| Haemoglobin, mmol/L, mean (SD) | 8.53 (1.26) | 9.89 (1.81) | 7.09 (1.58) | 8.16 (1.10) |
| Minimum-maximum | 6.10-12.00 | 6.70-13.50 | 3.30-10.60 | 6.10-11.10 |
| Haematocrit L/L, mean (SD) | 0.43 (0.06) | 0.52 (0.09) | 0.35 (0.08) | 0.39 (0.05) |
| Minimum-maximum | 0.29-0.61 | 0.37-0.68 | 0.15-0.50 | 0.30-0.52 |
| Thrombocytes*109/L, mean (SD) | 929 (346) | 662 (316) | 564 (532) | 263 (137) |
| Minimum-maximum | 327-1862 | 112-1371 | 15-2644 | 49-585 |
The control group consisted of 36 morphologically normal negative staging biopsies from patients with non-Hodgkin lymphoma and Hodgkin lymphoma with a mean age of 55.8 years.
Immunohistochemistry
The bone marrow biopsy specimens were decalcified using the EDTA decalcification for four hours, followed by standard tissue processing and paraffin embedding. From the paraffin-embedded blocks 3µm sections were cut for immunohistochemical staining and mounted on starfrost slides (Knittel Gläser, Germany). All the antibodies were tested for specificity on positive and negative tumour control slides and also individually tested on decalcified control bone marrow biopsies, resulting in a variation of immunohistochemical techniques, optimised for all individual antibodies.
Antihuman galectin-1 (R&D systems, Minneapolis, MN) was used at a dilution of 1:500 and antihuman galectin-3 (R&D systems, Minneapolis, MN) at a dilution of 1:50. After deparaffinization and blocking of endogenous peroxidase activity (0.3% H2O2 in methanol) antigen retrieval was performed by boiling in citric acid (pH 6.0) for 10 minutes in a water bath of 100°C. After blocking with 5% bovine serum albumin/phosphate buffered saline (BSA/PBS), primary antibody was applied in 0.5% BSA/PBS. Slides were then incubated with a biotin-labelled secondary antibody (gal-1: polyclonal swine anti-rabbit, Dako (Glostrup, Denmark) and gal-3: rabbit anti-goat, Dako (Glostrup, Denmark) at a dilution of 1:200 and 1:500 respectively for 30 minutes. Staining was performed with the StrepABComplex/HRP kit (Dako, Glostrup, Denmark) according to the manufacturer’s instructions. After developing the colour with freshly made diaminobenzidine solution (Dako, Glostrup, Denmark), slides were counterstained with haematoxylin (Merck, Whitehouse Station, NJ), dehydrated and mounted in Entellan (Merck).
Immunohistochemical staining of pSTAT3 and pSTAT5 was carried out using the antihuman rabbit monoclonal antibody pSTAT3 (Tyr705) and pSTAT5 (Tyr694) at a dilution of 1:50 and 1:200 respectively (Cell signaling Technology, Danvers, MA). After deparaffinization and antigen retrieval by boiling for 20 minutes in 1mM Tris EDTA pH 8.0 in a warm water bath, endogenous peroxidase activity was blocked in 3% H2O2 in methanol. After blocking with blocking solution (Tris Buffered Saline Tween (TBST) with 5% horse serum), primary antibody was applied in TBST with 5% horse serum (pSTAT3) and TBST with 1% BSA (pSTAT5) overnight. The slides were then incubated with powervision (Immunologic, Duiven, The Netherlands) for 40 minutes. Development of the colour and counterstaining as described above.
The 142 trephines (MPN patients plus control patients) were immunohistochemically analysed using an automated immunostainer (Dako autostainer Link 48) with CD34 (clone QBend 10, Dako). CD34 was incubated for 20 minutes at room temperature. The reaction was revealed by means of the Dako Envision Flex Kit (Dako) according to the manufacturer’s instructions.
Quantification of staining
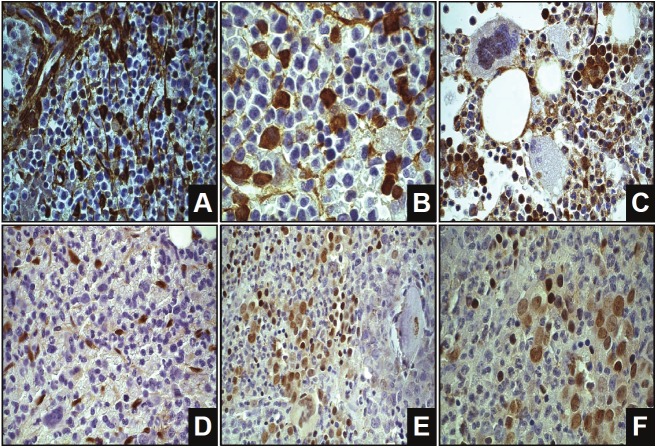
Gal-1, gal-3, pSTAT3 and pSTAT5 staining (see Figure 1) was quantified using an image processing and analysis system (Leica, Cambridge, UK) linked to a Leica DML3000 light microscope (Leica Quantimet, Germany). The program used in this system was QWin (Leica’s Windows-based image analysis tool-kit-Leica, Cambridge, UK). The surface area of galectin present was measured separately in cell nuclei and in stroma. All measurements were conducted at 40x magnification, in minimal three to maximal five complete hot spot bone marrow fields per slide, to measure total tissue, total cytoplasmic area positive and negative staining (gal-1 and gal-3), total nuclei positive (pSTAT3 or pSTAT5) and total nuclei count. The amount of positivity was calculated as the percentage of the total tissue area (gal-1 and gal-3) or the percentage of positive nuclear pixels related to the total number of nuclear pixels (pSTAT3 and pSTAT5).
Figure 1.
Examples of galectin-1, galectin-3, pSTAT3 and pSTAT5 staining. The brown colour represents the gal-1 staining in A and B, gal-3 staining in C, pSTAT3 staining in D and pSTAT5 staining in E and F. A. Galectin-1 (630x) B. Galectin-1 (1000x) C. Galectin-3 (630x) D. pSTAT3 (630x) E. pSTAT5 (630x) F. pSTAT5 (1000x).
MVD was assessed by counting the number of CD34 positive capillary-, arteriolar- or sinuslumen in five 1 mm2 fields at 100x magnification, calculating the mean over these five fields.
The grading of fibrosis was done according to the European consensus on grading of bone marrow fibrosis [21].
To validate the data obtained at the molecular level, we tried to isolate DNA from bone marrow biopsies. However, the quality of the DNA was very poor and the DNA was too fragmented to be used.
Statistical analysis
The data were statistically evaluated using the SPSS 15 statistical package, analyzed descriptively (descriptives, explore and crosstabs). Statistical comparison was performed by Mann-Whitney U-test when comparing medians. Differences were considered significant when p-value was less then 0.05. Pearson’s test was performed for correlating the expression of gal-1 with MVD, gal-3 with MVD, pSTAT3 with MVD and pSTAT5 with MVD.
For the analysis of pSTAT5, bone marrow of the Haga hospital, The Hague, was withdrawn, due to inappropriate staining of the bone marrow. Only 30 ET patients, 16 PV and 34 PMF patients and a total of 20 control bone marrows were available for pSTAT5 analysis.
In some cases bone marrow tissue was lost during the pre-treatment of the slides; for gal-1 we report 1 missing value, for pSTAT5 6, and for MVD 5 missing values. For the grading of myelofibrosis we report 2 missing values.
Results
The results of all staining percentages are summarized in Table 2 and 3. Qualitative microscopic evaluation of gal-1 staining showed its expression mainly in the immature myeloid cell component. A weak expression of gal-1 was seen in the cytoplasm of the megakaryocytes, no expression of gal-1 was seen in the erythroid cell line. Gal-1 was expressed significantly more in bone marrow of PMF patients compared to the control slides (p=0.036). The mean percentage of gal-1 for all MPN patients together was 7.8% and 6.3% for the control patients (p=0.027). The expression between gal-1 and MVD was significantly correlated (p=0.007).
Table 2.
Percentage of gal-1, gal-3, pSTAT3 and pSTAT5 in ET, PV, PMF, all MPN patients and control patients.
| Essential thrombocythemia n=36 | Polycythemia vera n=25 | Primary myelofibrosis n=45 | All MPN patients n=106 | Control patients n=36 | |
|---|---|---|---|---|---|
| Galectin-1, %*, mean (SD) | 7.80 (4.37) | 8.15 (4.50) | 7.70 (3.35) | 7.84 (3.97) | 6.25 (2.65) |
| Minimum-maximum (CI) | 1.12-20.32 (5.90-9.49) | 0.49-12.79 (5.69-10.09) | 3.65-14.56 (6.27-8.95) | 0.49-20.32 (6.76-8.65) | 3.22-15.60 (4.23-8.57) |
| Galectin-3, %*, mean (SD) | 7.24 (4.82) | 10.23 (5.01) | 7.72 (5.90) | 8.15 (5.43) | 8.58 (4.51) |
| Minimum-maximum (CI) | 0.64-18.69 (5.76-9.52) | 0.20-19.51 (6.53-12.75) | 0.56-23.50 (6.28-11.43) | 0.20-23.50 (7.19-9.89) | 2.01-18.80 (5.85-12.99) |
| pSTAT3, %#, mean (SD) | 4.18 (1.96) | 5.19 (4.21) | 5.52 (3.29) | 4.99 (3.20) | 4.21 (2.28) |
| Minimum-maximum (CI) | 1.12-8.91 (3.17-4.94) | 1.02-16.53 (2.77-7.63) | 0.84-13.68 (3.98-6.42) | 0.84-16.53 (3.99-5.49) | 0.96-7.92 (2.67-5.17) |
| pSTAT5, %#, mean (SD) | 2.91 (2.18) | 4.72 (3.58) | 3.31 (2.60) | 3.46 (2.74) | 3.62 (2.46) |
| Minimum-maximum (CI) | 0.26-7.40 (2.06-3.75) | 0.40-11.77 (2.82-6.63) | 0.00-13.71 (2.41-4.22) | 0.00-13.71 (2.84-4.07) | 1.18-9.29 (2.08-4.65) |
| MVD, 1 mm2, mean (SD) | 37.72 (22.18) | 47.55 (27.45) | 58.47 (31.56) | 48.79 (28.92) | 27.95 (11.25) |
| Minimum-maximum (CI) | 3.40-89.60 (29.70-49.07) | 5.80-111.20 (35.77-70.80) | 12.80-122.40 (50.30-76.39) | 3.40-122.40 (44.16-59.03) | 5.60-57.80 (17.94-34.27) |
calculated as percentage positive area of total tissue area.
calculated as percentage positive nuclei of total nuclei count.
Table 3.
Percentage of gal-1, gal-3, pSTAT3 and pSTAT5 in JAK2 positive and JAK2 negative patients.
| JAK2 positive n=56 | JAK2 negative n=24 | |
|---|---|---|
| Galectin-1, %*, mean (SD) | 8.50 (4.04) | 7.36 (3.58) |
| Minimum-maximum (CI) | 1.12-20.32 (7.41-9.59) | 1.48-14.56 (5.82-8.91) |
| Galectin-3. %*. mean (SD) | 8.93 (5.34) | 7.04 (5.18) |
| Minimum-maximum (CI) | 0.56-23.50 (7.49-10.37) | 0.55-18.69 (4.80-9.28) |
| pSTAT3. %#. mean (SD) | 5.53 (2.80) | 4.18 (1.97) |
| Minimum-maximum (CI) | 1.02-14.67 (4.77-6.29) | 1.12-8.91 (3.33-5.03) |
| pSTAT5. %#. mean (SD) | 4.22 (3.48) | 3.04 (1.60) |
| Minimum-maximum (CI) | 0.26-13.71 (3.02-5.41) | 0.90-6.47 (2.27-3.81) |
| MVD. 1 mm2. mean (SD) | 52.77 (30.58) | 49.01 (25.98) |
| Minimum-maximum (CI) | 3.60-122.40 (44.72-63.18) | 17.20-111.20 (40.64-65.24) |
calculated as percentage positive area of total tissue area.
calculated as percentage positive nuclei of total nuclei count.
Gal-3 was present in immature and mature myeloid cells and was only weakly expressed in megakaryocytes, endothelial cells and erythropoietic cells. Statistical analysis of gal-3 revealed a significant difference between PV and ET patients (p=0.019) and between PV and PMF (p=0.044) patients, with higher gal-3 expression in PV patients. There was no significant correlation between gal-3 and MVD and no significant difference between patients with different JAK2 mutational status.
pSTAT3 was localized in immature and mature myeloid cells and in endothelial cells. In the evaluated bone marrow biopsy trephines, the percentage of pSTAT3 was higher in JAK2V617F positive patients compared to patients with wild type JAK2 (p=0.018). There was also a significant correlation between pSTAT3 and MVD (p=0.000).
pSTAT5 was expressed in immature myeloid cells, the nuclei of adipocytes, some endothelial cells and in the nuclei of megakaryocytes and partly a weak expression in the cytoplasm of megakaryocytes. pSTAT5 was significantly correlated with the MVD (p=0.020). No statistically significant difference but a trend was reached between patients carrying the JAK2V617F mutation and patients without the mutation as well as in PV patients compared to ET and PMF patients.
In the total MPN group the mean MVD was significantly higher compared to the control group (p=0.000) and the MVD was significantly higher expressed in PV (p=0.006) and PMF (p=0.000) patients compared to the control group. ET patients compared to PMF patients showed also a statistically significant difference with a higher MVD expression in PMF patients (p=0.003). PMF patients showed higher MVD (58.5 vessels/mm2) than ET (37.7 vessels/mm2) and PV patients (47.6 vessels/mm2). Comparing the JAK2V617F positive patients to the JAK2V617F negative patients the MVD was not significantly different.
Concerning the myelofibrosis grading and the stainings we report a statistically significant higher gal-1 (p=0.013) and gal-3 (p=0.012) expression in the mf 0/1 group compared to the mf 2/3 group. For MVD there was a higher expression of MVD in the mf 2/3 group (p=0.001) compared to the mf 0/1 group and also the Pearson correlation showed a significant correlation of MVD with the grading of myelofibrosis (p=0.000).
Discussion
In this study, the expression of gal-1, gal-3, pSTAT3 and pSTAT5 along with the MVD in bone marrow cells was immunohistochemically measured in ET, PV, PMF and control bone marrows.
Gal-1 is known to be involved in tumour angiogenesis [7]. The higher expression of gal-1 and MVD in the total group of MPN patients in our study together with a significant correlation between gal-1 and MVD, suggests a role of gal-1 in the increased angiogenesis in MPN patients. These results assign a possible target for the angiogenesis inhibitor anginex, as gal-1 was identified as receptor for anginex. Anginex blocks the adhesion and migration of angiogenically activated endothelial cells, leading to apoptosis and inhibition of angiogenesis [22]. In gal-1-null mice treatment with anginex did not inhibit tumour growth in contrast to the wild type mice where tumour growth and vessel density was significantly inhibited with anginex treatment [7].
Increased expression of gal-3 has been associated with liver fibrosis secondary to diverse types of injury [11]. However, in the mf 0/1 group we saw a higher gal-3 expression compared to the mf 2/3 group. Also we saw no significant correlation between gal-3 and MVD. These findings contradict the relation between increasing fibrosis, MVD and gal-3 expression in MPN trephines. On the other hand we were able to show higher gal-3 expression in PV patients. Recently, it was also demonstrated that gal-3 is predominantly expressed in Chronic Myeloid Leukemia (CML) cells, where gal-3 expression support the molecular signalling pathways for maintaining CML in the bone marrow and resistance to therapy [23,24]. Therefore there are indications that gal-3 might play a role in MPN pathogenesis.
Constitutive activation of STAT proteins is present in a variety of haematological disorders [25-29]. STAT3 activation has been reported in PV and ET and low pSTAT3 levels in PMF patients [17,30]. However, our study does not confirm these results, possibly due to a relative high amount of PMF patients and lower amounts of PV and ET patients.
Activated STAT3 has an important role in the regulation of megakaryopoiesis and thrombopoiesis in vivo, via activation of Bcl-xL inhibiting apoptosis of megakaryocytes [31]. The bone marrow of PMF patients is characterized by a proliferation of the megakaryocytic cell line. The megakaryocytes often demonstrate dense clustering with cloud like nucleus [20]. The increased megakaryocytes with deviated forms in the bone marrow of PMF patients might be due to the decreased megakaryocyte apoptosis as result of increased STAT3 activation in PMF patients. The higher pSTAT3 expression in JAK2V617F positive patients indicates an increased STAT3 activation generated by the presence of the JAK2V617F mutation. In diverse cancer types it was shown that constitutive activation of STAT3 induces vascular endothelial growth factor (VEGF) expression [19]. In our study we show a correlation between pSTAT3 and MVD, indicating that the increased MVD seen in MPN patients, especially in PMF patients, might be induced by the constitutive activation of STAT3 resulting in increased expression of VEGF.
Our finding of higher pSTAT5 expression in PV and JAK2V617F positive patients is in line with earlier published data [14,17,32,33]. This indicates that the presence of the JAK2V617F mutation generates increased levels of pSTAT5. However, in our study the pSTAT5 expression did not reach statistical significant difference but only showed a trend between patients carrying the JAK2V617F mutation and patients without the mutation as well as in PV patients compared to ET and PMF patients. This might be due to the high number of patients with an unknown JAK2 status and also to the small PV patient population. The correlation between pSTAT5 and MVD might suggest other pathways involved in the increased MVD seen in MPN patients. pSTAT5 can interact with p85, a regulatory subunit of PI3K/Akt pathway, and might increase VEGF via the PI3K/Akt and mammalian target of rapamycine (mTor) pathway as was already shown in chronic myeloid leukaemia (CML) [34-36].
In line with other studies[37,38], we found the bone marrow MVD in the total MPN group and in PV and PMF patients to be significantly higher compared to the control group. The increased MVD reflects increased angiogenic activity which might be induced by hypoxia, via hypoxia-inducible factor (Hif) and VEGF, or by normoxia, directly via VEGF.
Regarding the MVD and fibrosis in MPN patients, Boveri et al. [39] found a higher MVD along with a higher grading of fibrosis, which is line with our study. Other studies showed higher MVD in PMF, post-ET myelofibrosis and post-PV myelofibrosis patients compared to ET and PV patients indicating that angiogenesis is primarily involved in later stages of the disease [38-41].
In conclusion, the characteristic megakaryopoietic abnormalities and also the higher MVD expression in PMF trephines can be explained by a higher pSTAT3 expression in PMF patients. Also gal-1 expression is correlated with the MVD with anginex as potential new therapy for MPN patients. pSTAT5 expression showed a trend of higher expression in PV and JAK2V617F positive patients, possible induced by the JAK2V617F mutation and also gal-3 expression seems correlated with PV. Further, the increased MVD expression in MPN patients with higher myelofibrosis grading suggests the important role of angiogenesis in the development of myelofibrosis.
Based upon these data we support the concept that the microenvironment plays an important role in haematological malignancies [42,43]. Interactions between stroma and haematopoietic cells in MPNs constitute possible targets for therapy.
References
- 1.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 2.Murray J. Myeloproliferative disorders. Clin Med. 2005;5:328–332. doi: 10.7861/clinmedicine.5-4-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponzoni M, Savage DG, Ferreri AJ, Pruneri G, Viale G, Servida P, Bertolini F, Orazi A. Chronic idiopathic myelofibrosis: independent prognostic importance of bone marrow microvascular density evaluated by CD105 (endoglin) immunostaining. Mod Pathol. 2004;17:1513–1520. doi: 10.1038/modpathol.3800224. [DOI] [PubMed] [Google Scholar]
- 4.Vener C, Fracchiolla NS, Gianelli U, Calori R, Radaelli F, Iurlo A, Caberlon S, Gerli G, Boiocchi L, Deliliers GL. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood. 2008;111:1862–1865. doi: 10.1182/blood-2007-09-112953. [DOI] [PubMed] [Google Scholar]
- 5.Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. doi: 10.1002/(sici)1097-0215(19990517)81:4<519::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, Shirai K. Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56:57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 7.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 9.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 10.Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 11.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, Skoda RC. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 13.Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22:1299–1307. doi: 10.1038/leu.2008.113. [DOI] [PubMed] [Google Scholar]
- 14.James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 15.Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, Berger R, Clark JJ, Willis SG, Nguyen KT, Flores NJ, Estey E, Gattermann N, Armstrong S, Look AT, Griffin JD, Bernard OA, Heinrich MC, Gilliland DG, Druker B, Deininger MW. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 16.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 17.Teofili L, Martini M, Cenci T, Petrucci G, Torti L, Storti S, Guidi F, Leone G, Larocca LM. Different STAT-3 and STAT-5 phosphorylation discriminates among Ph-negative chronic myeloproliferative diseases and is independent of the V617F JAK-2 mutation. Blood. 2007;110:354–359. doi: 10.1182/blood-2007-01-069237. [DOI] [PubMed] [Google Scholar]
- 18.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, Zhao ZJ. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Fourth Edition. 2008. WHO Classification of Tumours, Volume 2. IARC WHO Classification of Tumours, No 2. [Google Scholar]
- 21.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90:1128–1132. [PubMed] [Google Scholar]
- 22.Griffioen AW, van der Schaft DW, Barendsz-Janson AF, Cox A, Struijker Boudier HA, Hillen HF, Mayo KH. Anginex, a designed peptide that inhibits angiogenesis. Biochem J. 2001;354:233–242. doi: 10.1042/0264-6021:3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto-Sugitani M, Kuroda J, Ashihara E, Nagoshi H, Kobayashi T, Matsumoto Y, Sasaki N, Shimura Y, Kiyota M, Nakayama R, Akaji K, Taki T, Uoshima N, Kobayashi Y, Horiike S, Maekawa T, Taniwaki M. Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia. Proc Natl Acad Sci USA. 2011;108:17468–17473. doi: 10.1073/pnas.1111138108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng YL, Huang WC, Chen CL, Tsai CC, Wang CY, Chiu WH, Chen YL, Lin YS, Chang CF, Lin CF. Increased galectin-3 facilitates leukemia cell survival from apoptotic stimuli. Biochem Biophys Res Commun. 2011;412:334–340. doi: 10.1016/j.bbrc.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 25.Carlesso N, Frank DA, Griffin JD. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med. 1996;183:811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouilleux-Gruart V, Gouilleux F, Desaint C, Claisse JF, Capiod JC, Delobel J, Weber-Nordt R, Dusanter-Fourt I, Dreyfus F, Groner B, Prin L. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87:1692–1697. [PubMed] [Google Scholar]
- 27.Weber-Nordt RM, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart V, Mertelsmann R, Finke J. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- 28.Xia Z, Baer MR, Block AW, Baumann H, Wetzler M. Expression of signal transducers and activators of transcription proteins in acute myeloid leukemia blasts. Cancer Res. 1998;58:3173–3180. [PubMed] [Google Scholar]
- 29.Frank DA. STAT signaling in the pathogenesis and treatment of cancer. Mol Med. 1999;5:432–456. [PMC free article] [PubMed] [Google Scholar]
- 30.Roder S, Steimle C, Meinhardt G, Pahl HL. STAT3 is constitutively active in some patients with Polycythemia rubra vera. Exp Hematol. 2001;29:694–702. doi: 10.1016/s0301-472x(01)00637-3. [DOI] [PubMed] [Google Scholar]
- 31.Kirito K, Osawa M, Morita H, Shimizu R, Yamamoto M, Oda A, Fujita H, Tanaka M, Nakajima K, Miura Y, Ozawa K, Komatsu N. A functional role of Stat3 in in vivo megakaryopoiesis. Blood. 2002;99:3220–3227. doi: 10.1182/blood.v99.9.3220. [DOI] [PubMed] [Google Scholar]
- 32.Grimwade LF, Happerfield L, Tristram C, McIntosh G, Rees M, Bench AJ, Boyd EM, Hall M, Quinn A, Piggott N, Scorer P, Scott MA, Erber WN. Phospho-STAT5 and phospho-Akt expression in chronic myeloproliferative neoplasms. Br J Haematol. 2009;147:495–506. doi: 10.1111/j.1365-2141.2009.07870.x. [DOI] [PubMed] [Google Scholar]
- 33.Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, Oku S, Abe H, Katayose KS, Kubuki Y, Kusumoto K, Hasuike S, Tahara Y, Nagata K, Matsuda T, Ohshima K, Harada M, Shimoda K. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 34.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 35.Nyga R, Pecquet C, Harir N, Gu H, Dhennin-Duthille I, Régnier A, Gouilleux-Gruart V, Lassoued K, Gouilleux F. Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J. 2005;390:359–366. doi: 10.1042/BJ20041523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 37.Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R, Iurlo A, Radaelli F, Fermo E, Bucciarelli P, Bori S, Coggi G, Deliliers GL. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol. 2007;128:966–973. doi: 10.1309/FP0N3LC8MBJUFFA6. [DOI] [PubMed] [Google Scholar]
- 38.Panteli K, Zagorianakou N, Bai M, Katsaraki A, Agnantis NJ, Bourantas K. Angiogenesis in chronic myeloproliferative diseases detected by CD34 expression. Eur J Haematol. 2004;72:410–415. doi: 10.1111/j.1600-0609.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- 39.Boveri E, Passamonti F, Rumi E, Pietra D, Elena C, Arcaini L, Pascutto C, Castello A, Cazzola M, Magrini U, Lazzarino M. Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular correlations. Br J Haematol. 2008;140:162–168. doi: 10.1111/j.1365-2141.2007.06885.x. [DOI] [PubMed] [Google Scholar]
- 40.Arora B, Ho CL, Hoyer JD, Mesa RA, Tefferi A. Bone marrow angiogenesis and its clinical correlates in myelofibrosis with myeloid metaplasia. Haematologica. 2004;89:1454–1458. [PubMed] [Google Scholar]
- 41.Steurer M, Zoller H, Augustin F, Fong D, Heiss S, Strasser-Weippl K, Gastl G, Tzankov A. Increased angiogenesis in chronic idiopathic myelofibrosis: vascular endothelial growth factor as a prominent angiogenic factor. Hum Pathol. 2007;38:1057–1064. doi: 10.1016/j.humpath.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Duhrsen U, Hossfeld DK. Stromal abnormalities in neoplastic bone marrow diseases. Ann Hematol. 1996;73:53–70. doi: 10.1007/s002770050203. [DOI] [PubMed] [Google Scholar]
- 43.Scadden DT. The stem cell niche in health and leukemic disease. Best Pract Res Clin Haematol. 2007;20:19–27. doi: 10.1016/j.beha.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]