Smad3/Akt/mTOR/S6K/S6RP signaling plays a critical role in follistatin-mediated muscle growth that operates independently of myostatin-driven mechanisms.
Abstract
Follistatin is essential for skeletal muscle development and growth, but the intracellular signaling networks that regulate follistatin-mediated effects are not well defined. We show here that the administration of an adeno-associated viral vector expressing follistatin-288aa (rAAV6:Fst-288) markedly increased muscle mass and force-producing capacity concomitant with increased protein synthesis and mammalian target of rapamycin (mTOR) activation. These effects were attenuated by inhibition of mTOR or deletion of S6K1/2. Furthermore, we identify Smad3 as the critical intracellular link that mediates the effects of follistatin on mTOR signaling. Expression of constitutively active Smad3 not only markedly prevented skeletal muscle growth induced by follistatin but also potently suppressed follistatin-induced Akt/mTOR/S6K signaling. Importantly, the regulation of Smad3- and mTOR-dependent events by follistatin occurred independently of overexpression or knockout of myostatin, a key repressor of muscle development that can regulate Smad3 and mTOR signaling and that is itself inhibited by follistatin. These findings identify a critical role of Smad3/Akt/mTOR/S6K/S6RP signaling in follistatin-mediated muscle growth that operates independently of myostatin-driven mechanisms.
Introduction
Follistatin (Fst) is essential for muscle fiber formation and growth (Lee, 2007; Medeiros et al., 2009), as its depletion leads to perinatal lethality associated with impaired muscle development (Matzuk et al., 1995; Lee et al., 2010). Fst was originally thought to promote muscle fiber hypertrophy by preventing the repressive effects of myostatin on myogenic precursor differentiation and growth of developing muscle fibers (Nakamura et al., 1990; Lee and McPherron, 2001), which have been demonstrated in many species, including humans (Grobet et al., 1997; Kambadur et al., 1997; McPherron et al., 1997; Zimmers et al., 2002; Schuelke et al., 2004; Clop et al., 2006; Shelton and Engvall, 2007). However, the effects of Fst knockdown or transgenic overexpression upon muscle development can be recapitulated in myostatin-null mice (Lee, 2007; Lee et al., 2010). Consequently, the ability of Fst to act as an inhibitory binding partner to other members of the TGF-β family with similar growth-repressing attributes to myostatin has become increasingly scrutinized (Nakamura et al., 1990; Lee and McPherron, 2001; Li et al., 2007; Zhou et al., 2010). Importantly, although attention devoted to Fst as a prospective therapeutic for loss of muscle mass and strength has focused on this role as an extracellular inhibitor of TGF-β family ligands (Miller et al., 2006; Haidet et al., 2008; Nakatani et al., 2008; Rodino-Klapac et al., 2009), little is known about the intracellular mechanisms that regulate muscle growth and adaptation in response to Fst.
Like several other TGF-β family ligands, myostatin engages a heterodimeric receptor complex with serine/threonine kinase activity that in turn mediates phosphorylation and nuclear retention of Smad2 and Smad3 regulatory proteins via a process that is facilitated by the common Smad, Smad4 (Massagué et al., 2005). Within the nucleus, activated Smad complexes interact with a variety of transcriptional coregulators to achieve activation or repression of a cell type– and context-specific subset of TGF-β pathway target genes (Massagué et al., 2005). In skeletal muscle, the activity of Smad3 contributes to the inhibition of myogenic transcription factors (Liu et al., 2001) and the activation of ubiquitin ligases that mediate proteasomal degradation of contractile proteins (Sartori et al., 2009; Lokireddy et al., 2011). Importantly, although the activity of Smad2/3 is increased in models of muscle pathology associated with increased TGF-β pathway signaling (Trendelenburg et al., 2009; Zhou et al., 2010), attenuation of Smad3 activity can promote muscle anabolism, and inhibit the deleterious effects of elevated TGF-β signaling on muscle regeneration (Carlson et al., 2008; Sartori et al., 2009; Lokireddy et al., 2011). Given that myostatin promotes the nuclear retention of Smad proteins to facilitate transcriptional activation/repression of specific genes, it is logical to propose that the expression of Fst in muscle may promote growth by inhibiting myostatin-regulated Smads. However, studies also suggest that the TGF-β signaling cascade can influence the development and postnatal adaptation of skeletal muscle via interaction with other signaling axes that operate in skeletal muscle, including the Akt/mammalian target of rapamycin (mTOR) pathway (Sartori et al., 2009; Trendelenburg et al., 2009).
The synthesis of proteins in muscle is heavily influenced by signaling that activates the serine/threonine kinases Akt (also known as protein kinase B, PKB) and mTOR (Bodine et al., 2001; Rommel et al., 2001; Lai et al., 2004; Ruvinsky et al., 2009). As well as interacting with each other, downstream targets of Akt and mTOR that promote protein synthesis include the eukaryotic initiation factor 4 complex (eIF4) and the S6 protein kinases (S6K; Sarbassov et al., 2004, 2005). Inhibition of mTOR activity by rapamycin administration (Harding et al., 1989; Siekierka et al., 1989) can prevent hypertrophy of skeletal muscle after administration of insulin-like growth factor I (IGF1) or β-adrenergic agonists, or expression of constitutively active Akt (Bodine et al., 2001; Rommel et al., 2001; Lai et al., 2004; Kline et al., 2007). Ablation of S6K isoforms or of the S6 ribosomal proteins (S6RPs) that they target also compromises skeletal muscle development (Mieulet et al., 2007; Ruvinsky et al., 2009). The repressive effects of myostatin on muscle growth appear to attenuate Akt and mTOR signaling (Morissette et al., 2006; Trendelenburg et al., 2009), which suggests that interventions that block myostatin may stimulate Akt and/or mTOR to promote muscle hypertrophy. As Fst also promotes muscle hypertrophy in myostatin-null mice, it is important to determine the cellular processes that promote skeletal muscle growth in response to Fst, and to distinguish them from interventions that only inhibit myostatin. On the basis of these reported observations, we sought to test the hypothesis that increased expression of Fst promotes the hypertrophy of skeletal muscle by augmenting mTOR/S6K-dependent protein anabolism via a Smad-dependent mechanism.
Here, we use recombinant adeno-associated viral vectors (rAAV6 vectors) that efficiently transduce skeletal muscles in vivo (Gregorevic et al., 2004, 2006) to show, for the first time, that an acute postnatal intervention designed to increase expression of the tissue-restricted 288aa Fst isoform promotes dramatic increases in skeletal muscle mass and contractile capacity. These effects are mTOR- and S6K-dependent and influenced by Smad3 activity, but occur independently of myostatin-mediated signaling. Our data demonstrate a critical role for Smad3 and mTOR in the regulation of Fst-mediated muscle growth in vivo. These findings have important implications for our understanding of the mechanisms regulating skeletal muscle adaptation in health and disease.
Results
Postnatal expression of Fst-288 promotes profound skeletal muscle hypertrophy
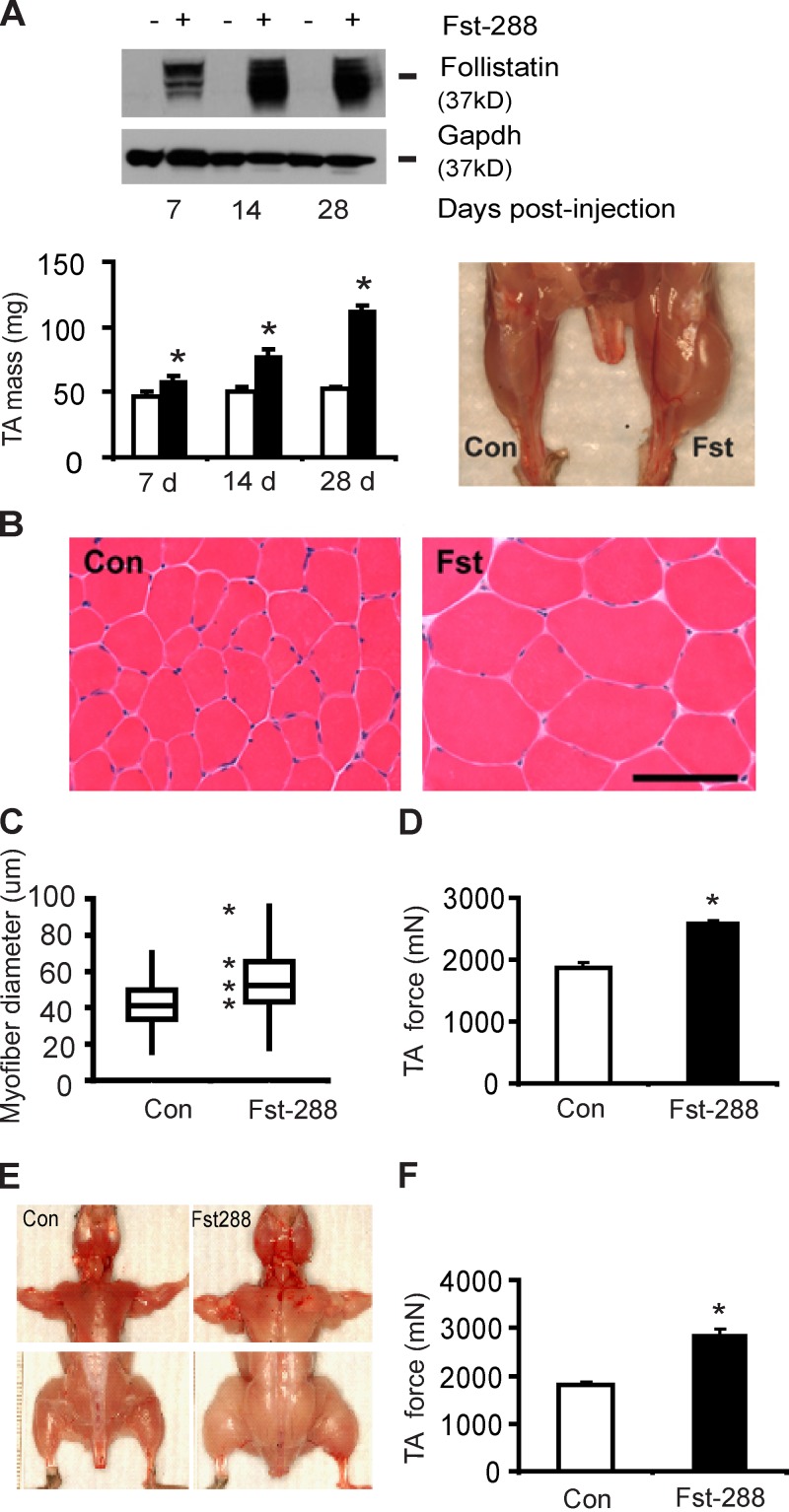
A single injection of a recombinant adeno-associated viral vector expressing the 288-aa Fst isoform (rAAV6:Fst-288) into the tibialis anterior (TA) muscles of 8-wk-old mice increased local expression of Fst-288, and more than doubled the mass of treated muscles by 28 d after treatment (Fig. 1 A). Histological examination revealed that the increase in muscle mass after rAAV6:Fst-288 administration was a product of muscle fiber hypertrophy (Fig. 1, B and C), but not a change in total muscle fiber number (Fig. S1 A). Muscles directly injected with rAAV6:Fst-288 demonstrated an ∼40% increase in maximal force-producing capacity within 28 d of treatment, compared with control-injected muscles (Fig. 1 D).
Figure 1.
Post-natal expression of Fst-288 promotes profound skeletal muscle hypertrophy. (A) Injection of the anterior musculature of the mouse hind limb with a viral vector designed to express Fst-288 (rAAV6:Fst-288) increased the expression of Fst in treated TA muscles, and increased muscle mass by >100% (*, P < 0.05 vs. control) within 28 d. (B and C) Muscle hypertrophy was a product of increased muscle fiber size (reported here as representative hematoxylin and eosin–stained cryosections, and as box and whisker plots comprising minimum, lower quartile, median, upper quartile, and maximum values for myofiber diameter). Bar, 100 µm. (D) Increased TA muscle isometric force producing capacity in C57BL/6 mice 4 wk after intramuscular injection of rAAV6:Fst-288 (*, P < 0.05 vs. control). (E) Intravenous administration of rAAV6:Fst-288 to mice facilitates systemic dissemination of the vector, resulting in considerable hypertrophy of muscles throughout the body. (F) Increased TA muscle isometric force producing capacity in C57BL/6 mice 8 wk after intravenous injection of rAAV6:Fst-288 (*, P < 0.05 vs. control). Graphs show data from at least four independent experiments. Error bars indicate ± SEM.
Unlike the predominant 315-aa Fst isoform commonly found in the circulation, the less-abundant 288aa Fst variant typically remains localized in the immediate vicinity of the cell from which it is secreted, owing to its affinity for heparin sulfate motifs that feature in the extracellular matrix near the cell surface (Patel, 1998). In our hands, the morphological effects observed in injected muscles were comparable when using vectors expressing the tissue-restricted Fst-288 or the circulating Fst-315 isoform (Fig. S1, B and C). However, intramuscular levels of Fst-315 were reduced compared with Fst-288 (Fig. S1 D), likely because the longer isoform can disseminate from the site of expression (Haidet et al., 2008).
As Fst-288 typically remains confined to the tissue in which it is expressed, we used the ability of rAAV6 vectors to systemically transduce skeletal muscles after intravascular administration (Gregorevic et al., 2004, 2006) to determine whether delivery of rAAV6:Fst-288 to mice as a single tail vein injection could promote hypertrophy of muscles throughout the body. Systemic administration of rAAV6:Fst-288 to wild-type mice increased the size of muscle fibers and the mass of individual muscles throughout the body (Fig. 1 E). Similarly to individually injected muscles, the hypertrophy caused by systemic rAAV6:Fst-288 administration was associated with a significant (∼60%) increase in the force producing capacity of limb muscles (Fig. 1 F).
Fst-288 stimulates protein synthesis and mTOR signaling in skeletal muscle
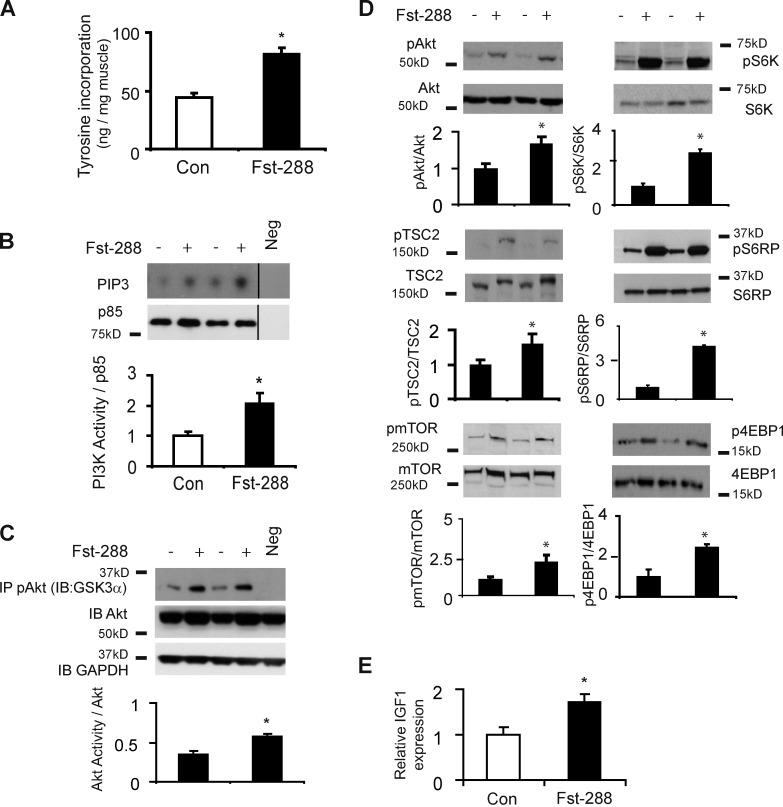
TA muscles examined 14 d after local injection of rAAV6:Fst-288 demonstrated an almost doubled fractional rate of protein synthesis compared with control-injected muscles (Fig. 2 A). As phosphatidylinositol 3-kinase (PI3K) and mTOR signaling can be inhibited by TGF-β family members including myostatin (Amirouche et al., 2009; Trendelenburg et al., 2009), and the targets of these proteins contribute to the regulation of protein synthesis in muscle, we subsequently assessed the effect of Fst-288 expression on PI3K activity. As shown in Fig. 2 B, PI3K activity was significantly elevated in response to the administration of rAAV6:Fst-288 at this time point. This finding was further corroborated by increased levels of Akt kinase activity (as measured by GSK3α fusion protein phosphorylation) in response to Fst-288–mediated muscle growth (Fig. 2 C). Treated muscles also exhibited increased phosphorylation of AktS473, TSC2S939 mTORS2448, S6KT389, S6RPS235/236, and 4EBP1T37/46 (Fig. 2 D), thereby demonstrating activation of this established signaling cascade. Because IGF1 is an established regulator of striated muscle growth that can potentiate PI3K/Akt/mTOR signaling (Musarò et al., 1999; Rommel et al., 2001), we also investigated whether rAAV6:Fst288 administration promotes IGF expression, and found that IGF1 expression was increased 14 d after injection of rAAV6:Fst288 (Fig. 2 E). Our data demonstrate that skeletal muscle hypertrophy induced by Fst is associated with activation of the Akt/mTOR/S6K signaling cascade. However, it is interesting to note that at 3 d after rAAV6:Fst-288 administration, a time point where growth had not yet occurred, we observed increased mTOR/S6K phosphorylation but no significant change to PI3K activity or the phosphorylation of Akt and its substrate TSC2 (Fig. S2, A–C). Moreover, we did not observe evidence of increased phosphorylation of the known mTOR activators extracellular-signal-regulated kinase (ERK) and ribosomal S6 kinase (RSK) at this early time point (Fig. S2 D).
Figure 2.
Fst-288 stimulates protein synthesis and mTOR signaling in skeletal muscles. (A) Muscles examined 14 d after injection of rAAV6:Fst-288 demonstrated significantly up-regulated fractional rates of protein synthesis (as determined by tyrosine incorporation; *, P < 0.05 vs. control). (B) Fst-288 significantly increased PI3K activity (measured here as conversion of PIP2 to PIP3, and normalized over P85 levels; *, P < 0.05 versus control; Neg, negative control with no antibody). Black lines indicate that intervening lanes have been spliced out. (C) Fst-288 increased Akt kinase activity as measured by GSK3α fusion protein phosphorylation (*, P < 0.05 versus control; Neg, negative control with no ATP). (D) Fst-288 expression promoted the phosphorylation of AktS473, TSC2S939, mTORS2448, S6KT389, S6RPS235/236, and 4EBP1T37/46 (*, P < 0.05 vs. respective controls). (E) IGF1 mRNA expression was elevated in treated muscles (*, P < 0.05 versus control). Error bars indicate ± SEM.
Fst-288 promotes skeletal muscle hypertrophy and mTOR signaling independently of myostatin
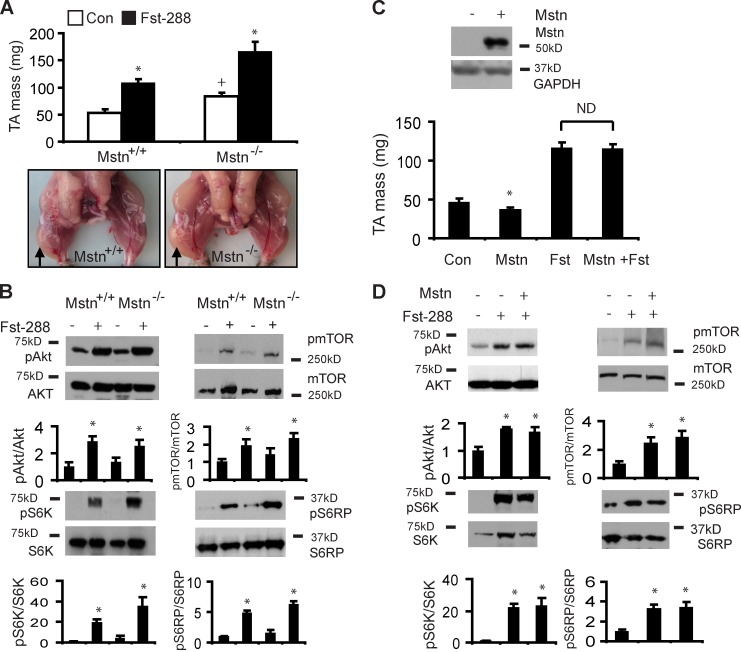
As Fst can regulate muscle growth independent of its well-characterized binding partner myostatin, we examined the regulation of mTOR-related signaling by Fst-288 in myostatin-null mice (Lee, 2007) and in wild-type mice, where an rAAV6 vector designed to overexpress myostatin (rAAV6:Mstn) was co-delivered. Although myostatin-null mice exhibit increased muscle mass compared with wild-type littermates, direct injection of TA muscles with rAAV6:Fst-288 produced a proportionally similar doubling of muscle mass in myostatin-null mice as observed after treatment of wild-type littermates (Fig. 3 A). Accordingly, we found that rAAV6:Fst-288 administration increased the phosphorylation of Akt, mTOR, S6K, and S6RP in the muscles of myostatin-null mice by a similar ratio as that observed in wild-type littermates after treatment (Fig. 3 B).
Figure 3.
Fst-288 promotes skeletal muscle hypertrophy and mTOR signaling independently of myostatin expression. (A) Although 8-wk-old myostatin-null mice exhibit increased muscle size and mass compared with wild-type littermates (+, P = 0.006 for TA mass Mstn−/− vs. Mstn+/+ mice), a proportionally comparable hypertrophic response is observed 4 wk after the administration of rAAV6:Fst-288 to the TA muscles of each strain (*, P < 0.001 for TA mass between treated and respective control muscles). Arrows identify muscles injected with rAAV6:Fst-288 in representative control littermates (Mstn+/+) and Mstn−/− mice. (B) Increased phosphorylation of Akt, mTOR, S6K, and S6RP is evident 28 d after administration of rAAV6:Fst-288 to the muscles of Mstn+/+ and Mstn−/− mice (*, P < 0.05 between treated and respective control muscles). (C) Local injection of a rAAV6 vector expressing myostatin reduced TA muscle mass by ∼20% by 28 d after injection (*, P < 0.05 vs. control muscles). There was no significant effect of myostatin overexpression on Fst-induced muscle growth when muscles were examined 28 d after injection with rAAV6:Fst-288 or coinjection of rAAV6:Fst-288 with rAAV6:Mstn. (D) Co-delivery of rAAV6:Mstn with rAAV6:Fst-288 did not attenuate the phosphorylation of Akt, mTOR, S6K, or S6RP otherwise observed after injection of rAAV6:Fst-288 alone (*, P ≤ 0.05 vs. control muscles). Graphs show data from at least four independent experiments. Error bars indicate ± SEM.
In agreement with the reported muscle wasting effects of elevated myostatin expression (Zimmers et al., 2002), we found that the administration of rAAV6:Mstn to the muscles of wild-type mice increased local expression of myostatin and caused atrophy of the injected muscles (Fig. 3 C). The muscle wasting observed after rAAV6:Mstn injection was associated with reduced phosphorylation of Akt, mTOR, and S6RP (Fig. S3). However, the co-administration of rAAV6:Mstn with rAAV6:Fst-288 did not attenuate the Fst-mediated hypertrophic response compared with that observed in contralateral muscles receiving rAAV6:Fst-288 only (Fig. 3 C). Consistent with these observations, similar increases in the phosphorylation of Akt, mTOR, S6K, and S6RP were observed in the muscles of wild-type mice after local coinjection of rAAV6:Fst-288 and rAAV6:Mstn, compared with muscles receiving rAAV6:Fst-288 only (Fig. 3 D).
Inhibition of mTOR or deletion of S6K1/2 attenuates skeletal muscle hypertrophy and protein synthesis induced by Fst-288
Having established that injection of rAAV6:Fst-288 causes increased activity and phosphorylation of Akt, mTOR, S6K, and S6RP, we next investigated whether the administration of rapamycin, an inhibitor of mTOR activation (Harding et al., 1989; Siekierka et al., 1989; Shioi et al., 2003), could prevent Fst-induced muscle growth. In mice receiving an intramuscular injection of rAAV6:Fst-288 and daily administration of rapamycin, the hypertrophic response of treated muscles was attenuated (relative to the response in vehicle-treated mice) by 42% and 35% at 14 d and 28 d after injection, respectively (Fig. 4 A). The mean myofiber diameter in muscles injected with rAAV6:Fst-288 that were collected from mice receiving rapamycin was also reduced compared with the myofibers in the treated muscles from animals receiving vehicle (Fig. 4 B), and, at the signaling level, rapamycin ablated Fst-288–induced (as well as basal) phosphorylation of mTOR, S6K, S6RP, and 4EBP1 (Fig. 4 C). The administration of rapamycin to mice was also associated with ∼50% attenuation of the anabolic effect of rAAV6:Fst-288 on the fractional protein synthesis rate (Fig. 4 C). On the basis of these findings, we next investigated whether the key mTOR substrate S6K (Shima et al., 1998; Pende et al., 2004; Mieulet et al., 2007) is critical in stimulating muscle growth in response to rAAV6:Fst-288 administration. Although initial experiments in S6K1-null mice demonstrated a lack of measurable S6K1 protein and smaller muscles than those of age-matched wild-type littermates (Fig. S4 A), the muscles of S6K1-null mice exhibited a similar relative increase in muscle mass (Fig. S4 B), phosphorylation of S6RPS235/236 (Fig. S4 C), and myofiber diameter (Fig. S4 D) after injection of rAAV6:Fst-288. We then tested the effect of Fst-288 in mice lacking both S6K1 and -2, which have similar reductions in muscle mass to the S6K1-null mouse (Fig. S4 E). In S6K1/2-null mice, the relative gain in muscle mass observed 28 d after local injection of rAAV6:Fst-288 was modestly (∼20%) attenuated (Fig. 4 E), and the induction (and absolute abundance) of phosphorylated S6RP was considerably reduced (Fig. 4 F). Given that RAS/ERK signaling can also regulate S6RP activity (Wang et al., 2001; Pende et al., 2004), we assessed ERK phosphorylation in muscles harvested 28 d after administration of rAAV6:Fst-288 but found that Fst-288 did not affect ERK phosphorylation (Fig. S4 F). Collectively, our data demonstrate that the postnatal hypertrophic response of muscles to increased expression of Fst-288 involves the regulation of protein synthesis by mTOR, and activation of the S6 protein kinases.
Figure 4.
Inhibition of mTOR or deletion of S6K1/2 attenuates skeletal muscle hypertrophy and protein synthesis induced by Fst-288. (A) Daily administration of rapamycin to mice 3 h before hind limb injection of rAAV6:Fst-288 attenuated the Fst-mediated increase in TA muscle mass by 42% after 14 d, and by 35% after 28 d (*, P < 0.05 for rapamycin vs. vehicle at the respective time points). Data are expressed as the magnitude of hypertrophic response observed relative to the magnitude of hypertrophic response caused by rAAV6:Fst-288 alone (normalized to 100% effect here). (B) As illustrated by hematoxylin and eosin staining, rapamycin attenuated the increase in myofiber diameter observed 14 d after injection of rAAV6:Fst-288, but did not affect the myofiber size in control muscles. Bar, 100 µm. (C) Daily administration of rapamycin for 14 d ablated basal and Fst-induced phosphorylation of mTOR, S6K, and S6RP (V, vehicle; R, rapamycin; *, P < 0.05 vs. control muscles). (D) The potentiation of protein synthesis rates typically observed in muscles 14 d after injection of rAAV6:Fst-288 (expressed as the percentage increase relative to control leg) was diminished in animals receiving rapamycin, compared with vehicle (*, P < 0.05 for response in rapamycin treated vs. vehicle treated mice). (E) Compared with the equivalently treated muscles of age-matched wild-type mice, the muscles of S6K1/2-null mice demonstrated a 20% reduction in the magnitude of hypertrophic response (as measured by TA muscle mass) observed 28 d after administration of rAAV6:Fst-288 (*, P < 0.05 vs. wild-type mice). (F) The phosphorylation of S6RP was markedly reduced, but not entirely abolished, in the treated muscles of S6K1/2-null mice (+, P < 0.05 for Fst-288 vs. control muscles in S6K1/2-null mice; *, P < 0.05 for treated muscles in S6K1/2-null vs. wild-type mice). Graphs shows data from at least four independent experiments. Error bars indicate ± SEM.
Smad3 regulates Fst-induced hypertrophy and is an essential intermediary that regulates mTOR/S6K signaling
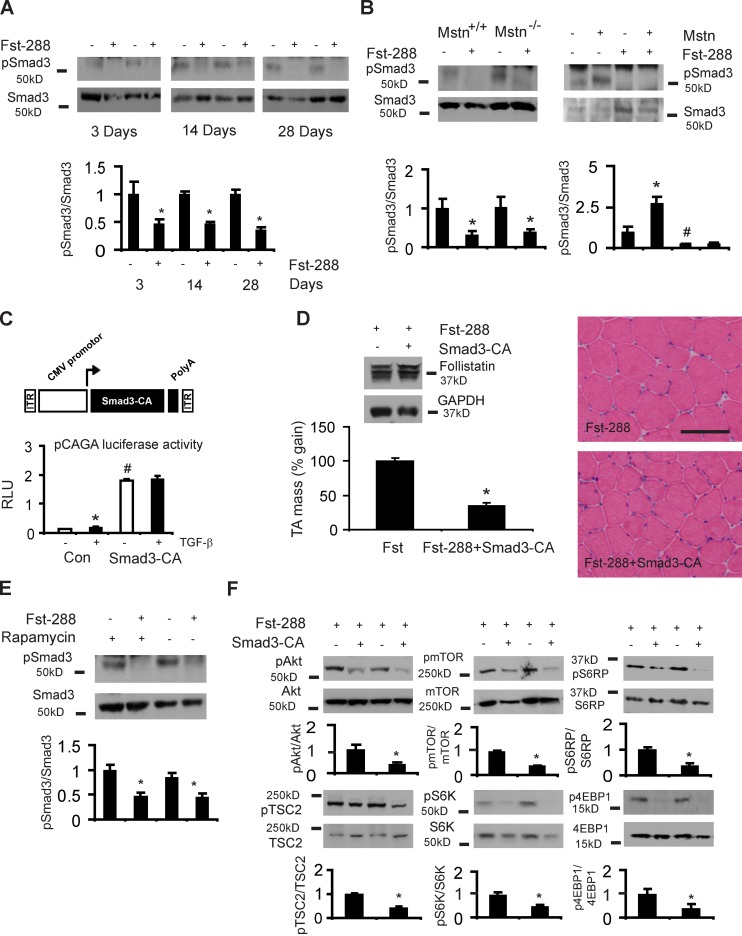
Having established that the growth-promoting effect of Fst in skeletal muscle is dependent on mTOR signaling, and because Fst inhibits the actions of extracellular TGF-β ligands that promote Smad signaling, we sought to test the hypothesis that Fst-mediated hypertrophic effects (including activation of mTOR) are dependent on attenuation of Smad signaling. As shown in Fig. 5 A, we found that phosphorylation of Smad3S423/425 is markedly reduced in muscles after rAAV6:Fst-288 administration. Importantly, the inhibitory effect of Fst-288 on Smad3 phosphorylation was entirely conserved despite the genetic ablation or vector-mediated overexpression of myostatin (Fig. 5 B). To determine the role of Smad3 in mediating the hypertrophic effects of Fst, we designed a constitutively active Smad3 vector (rAAV6:Smad3-CA) by substituting aspartic acid for the three C-terminal serines in Smad3, as shown previously (Fig. 5 C; Liu et al., 1997). We confirmed the functionality of Smad3-CA by assessing its effect using a pCAGA luciferase reporter assay in vitro (Fig. 5 C). Although TGF-β treatment significantly increased pCAGA luciferase activity in HEK293T cells, the overexpression of Smad3-CA markedly increased luciferase activity to such an extent that that the reporter construct was no longer additionally responsive to TGF-β stimulation (Fig. 5 C). These data confirm that Smad3-CA markedly up-regulates TGF-β signaling and serves as an appropriate tool to test the effect of Fst-288 when challenged by forced activation of Smad3. As shown in Fig. 5 D, the overexpression of Smad3-CA in mouse muscles attenuated the magnitude of the Fst-induced hypertrophic response by ∼65%, and significantly repressed muscle fiber hypertrophy in spite of continued Fst expression (Fig. 5 D). Interestingly, we found that the inhibitory effect of Fst on Smad3 phosphorylation was conserved in the presence of rapamycin (Fig. 5 E). Based on these data, we hypothesized that Fst may activate mTOR signaling downstream of its effect on Smad3 signaling. We therefore assessed the effect of Smad3-CA on mTOR signaling when co-administered with Fst, and found that the expression of Smad3-CA in the presence of Fst-288 markedly down-regulated the phosphorylation of AktS473, TSC2S939, mTORS2448, S6KT389, S6RPS235/236, and 4EBP1T37/46 (Fig. 5 F). These data demonstrate that the attenuation of Smad3 phosphorylation is required for Fst-mediated muscle hypertrophy, as well as for the potentiation of mTOR/S6K signaling and protein synthesis that contributes to this hypertrophic process.
Figure 5.
Smad3 regulates Fst-induced hypertrophy and is a key intermediary that regulates PI3K/mTOR signaling. (A) Smad3S423/425 phosphorylation is reduced at 3, 14, and 28 d after Fst-288 administration (*, P < 0.05 vs. control muscles). (B) Smad3 phosphorylation is reduced 28 d after rAAV6:Fst-288 administration to the muscles of myostatin-null mice (Mstn−/−), similarly compared with the treated muscles of littermate controls (Mstn+/+; *, P < 0.05 vs. control muscles). Injection of the muscles of wild-type mice with an rAAV6 vector expressing myostatin (Mstn) increases Smad3 phosphorylation, but coinjection of muscles with rAAV6:Mstn and rAAV6:Fst-288 still results in diminished Smad3 phosphorylation (shown here after 28 d; *, P < 0.05 vs. control muscles; #, P < 0.05 vs. control muscles). (C) Smad3-CA was validated in 293T cells using a pCAGA luciferase assay normalized to β-gal expression. (D) 8-wk-old mice were injected with either rAAV6:Fst-288, or rAAV6:Fst-288 and rAAV6:Smad3-CA, and examined after 28 d (*, P < 0.001 vs. control). Data are expressed as the percentage increase in muscle mass relative to the control leg, where the effect of Fst-288 is normalized to 100%. Hematoxylin and eosin–stained cryosections demonstrate attenuated muscle fiber hypertrophy in muscles examined 28 d after receiving rAAV6:Fst-288 and rAAV6:Smad3-CA compared with rAAV6:Fst-288 alone. Bar, 100 µm. (E) Smad3 phosphorylation in response to rAAV6:Fst-288 injection was assessed in mice additionally treated with vehicle or rapamycin for 14 d (*, P < 0.05 vs. control muscles). (F) Akt/mTOR signaling was assessed 28 d after the co-administration of Fst with Smad3-CA (*, P < 0.01 vs. control muscles). Graphs show data from at least four independent experiments. Error bars indicate ± SEM.
Discussion
This is the first demonstration that a single-dose, postnatal administration of an rAAV vector designed to express Fst-288 promotes dramatic and sustained increases in skeletal muscle mass and strength. Importantly, we show that although the hypertrophic response to Fst-288 utilizes, but does not entirely depend upon, activation of mTOR and S6 protein kinases to promote protein anabolism, Smad3 is the critical intermediary that regulates mTOR signaling in response to Fst. In addition, importantly, its forced expression dramatically reduces growth induced by Fst. We also show that the control of these signaling mechanisms and resultant muscle growth caused by Fst occurs independent of myostatin expression, thus identifying key mechanisms by which Fst regulates skeletal muscle growth in vivo.
The administration of rAAV6:Fst-288 to mice increased muscle mass and force-producing capacity by a magnitude that equals (and in most cases exceeds) other postnatal interventions designed to promote skeletal muscle hypertrophy. These outcomes are likely attributable to the capacity of Fst to interact with (and thus inhibit the growth-repressive effects) of not only myostatin but also Activin A, which has been linked to conditions associated with loss of muscle mass and strength, including cancer cachexia, sepsis, and sarcopenia (Harada et al., 1996; Baccarelli et al., 2001; Michel et al., 2003; Zhou et al., 2010). The use of Fst-288 as a potential therapeutic (to bind and counteract effects of myostatin or Activin A) is therefore particularly attractive, especially given the capacity for Fst-288 to remain restricted to the tissue in which it is expressed/introduced (in this case, constraining the effect of the intervention to skeletal muscle). Tissue-directed expression or administration of Fst-288 could also circumvent issues associated with systemic “off-target” effects of TGF-β signaling, particularly in tissues where TGF-β networks can regulate cancer progression (Massagué, 2008).
We found that expression of Fst-288 in muscles stimulated protein synthesis, and was associated with robust phosphorylation of mTOR, S6K, and S6RP. Accordingly, we also found that the hypertrophic response of muscles to Fst-288 expression, including the associated stimulation of protein synthesis, was attenuated ∼40% by the inhibition of mTOR, and more modestly by the simultaneous deletion of the key mTOR substrates S6K1 and S6K2. We observed significant increases in PI3K and Akt activity associated with Fst-mediated hypertrophy. Interestingly, our findings also demonstrate that before muscle mass increases become evident, the expression of Fst promotes mTOR signaling in a manner that is initially independent of Akt and PI3K activity, and of ERK or RSK, which have also been shown to regulate mTOR (Pende et al., 2004; Goodman et al., 2011). As another mechanism of mTOR activation that is independent of Akt, it is possible that Fst-288 may promote increased amino acid uptake, which in turn can stimulate mTOR signaling, via a process that employs translocation of mTORC1 to lysosomal membranes (Sancak et al., 2010). Previous studies that used soluble type II Activin receptors (ActRIIB.Fc) as a means to sequester myostatin away from muscle fibers have shown that Akt 1 and 2 were dispensable in regulating ActRIIB.Fc-mediated muscle hypertrophy (Goncalves et al., 2010). Unlike our studies, Goncalves et al. (2010) did not detect changes in Akt phosphorylation, which suggests that different modes of signaling are associated with muscle hypertrophy mediated by increased expression of Fst, and the administration of molecules based on the type II Activin receptor. This is an important distinction to make between the biological properties of Fst and soluble type II Activin receptors, as the two molecules are thought to exert positive effects upon muscle growth by binding with, and inhibiting, extra cellular myostatin.
Our observations demonstrate that Fst-mediated inhibition of Smad3 activity is critical for the activation of Akt and mTOR signaling, and ultimately, the control of protein synthesis in skeletal muscle. Accordingly, expression of a constitutively active Smad3 mutant not only prevented the Fst-induced phosphorylation of mTOR/S6K/S6RP, but attenuated the Fst-induced hypertrophic response by ∼65%. Because the inhibition of mTOR did not completely prevent Fst-induced hypertrophy, it is likely that other mTOR-independent mechanisms promote anabolism in this model of muscle growth. We propose that the reduced phosphorylation of Smad3 that we observed after rAAV6:Fst-288 administration (owing to Fst inhibiting the actions of TGF-β family members that otherwise promote phosphorylation of Smad3) will directly impact upon the transcription of TGF-β target genes that influence protein synthesis and degradation in skeletal muscle. We are presently undertaking studies to identify the specific gene targets of Smads that are relevant to this mode of muscle growth.
The effects of Fst on Smad3/Akt/mTOR signaling that we observed were not dependent on the inhibition of myostatin, as they were entirely preserved (along with similar relative increases in muscle mass) regardless of whether we administered rAAV6:Fst-288 to the muscles of wild-type mice alone, in conjunction with a vector designed to overexpress myostatin, or to the muscles of myostatin-null mice. Although myostatin is a potent negative regulator of these networks in skeletal muscle, it is clear from our studies and the work of others that other TGF-β family members likely contribute to the repression of muscle growth via regulation of Smad3 phosphorylation (Lee et al., 2005) and mTOR signaling (Trendelenburg et al., 2009), and that Fst could serve as an inhibitory binding partner for these other members as well (Lee, 2007). Collectively, our studies demonstrate for the first time that at a signaling level, Fst exerts its effects through myostatin-independent mechanisms.
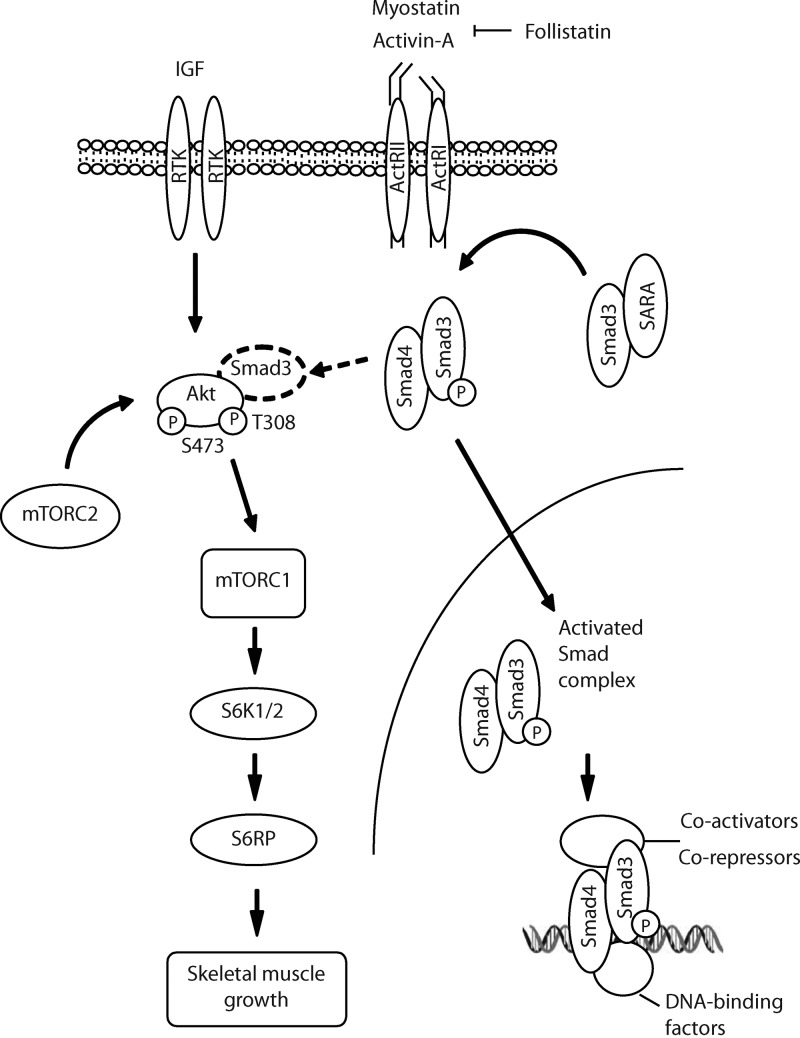
Our findings raise the question as to how the inhibition of specific TGF-β family ligands and a resultant diminution of Smad3 activity can potentiate the Akt/mTOR/S6K/S6RP signaling cascade in skeletal muscle. Others have shown that the transcription of genes that are targets of TGF-β signaling in muscle utilizes activation of PI3K via autocrine-regulatory processes (Song et al., 2003; Gardner et al., 2011). As we have shown that a constitutively active Smad3 can inhibit the phosphorylation of Akt and mTOR signaling, we therefore propose a model whereby the initial suppression of TGF-β signaling cascades by Fst potentiates the activation of the IGF/PI3K/Akt/mTOR/S6K axis via Smad3, which in turn can further repress TGF-β–related signaling (Fig. 6). As Akt can sequester Smad3 away from the TGF-β type I receptor, thereby preventing its phosphorylation and regulation of transcription (Conery et al., 2004; Remy et al., 2004), the potentiation of the Akt/mTOR/S6K axis could enable Akt-mediated inhibition of Smad3-dependent signals that otherwise repress muscle growth. Given the considerable increase in skeletal muscle mass we observed in response to expression of Fst-288, it is likely that these events work in concert to maximize commands to enhance protein synthesis. The specific transcriptional targets of these signaling events are a focus of our ongoing research.
Figure 6.
Proposed model of signaling associated with Fst-mediated skeletal muscle hypertrophy. Fst inhibits the activation of the TGF-β signaling pathway by binding to multiple extracellular ligands including myostatin and Activin A. Inhibiting the actions of myostatin and Activin A diminishes phosphorylation of Smad3, which alters the transcription of Smad target genes, and potentiates Akt/mTOR signaling that stimulates protein synthesis. Increased phosphorylation of Akt contributes to the inhibition of Smad3-dependent signals that otherwise repress muscle growth (arrow with broken line), thereby maximizing protein synthesis networks regulated by Fst.
Combined, these findings demonstrate that the expression of Fst-288 in skeletal muscles promotes significant hypertrophy via the suppression of Smad3 phosphorylation, which leads to increased protein synthesis driven in part by potentiation of the mTOR/S6K/S6RP signaling cascade, an established regulator of protein synthesis and cell size (Shima et al., 1998; Shioi et al., 2003; Ruvinsky et al., 2009). We propose that other Smad-based and non-Smad signaling events that are regulated by myostatin and related TGF-β family members most likely contribute to the transcriptional regulation of key genes associated with muscle anabolism. Our novel findings provide important insight into the intracellular mechanisms associated with Fst-mediated growth of mammalian skeletal muscle. These observations also support further investigation of Fst-288 as a possible intervention to increase skeletal muscle mass and functional capacity during development, as well as in the context of a variety of serious clinical conditions associated with muscle wasting and dysfunction.
Materials and methods
Antibodies
All antibodies used for Western blotting were obtained from Cell Signaling Technology, except for antibodies against Fst (R&D Systems), pSmad3 (Epitomics, Inc.), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology, Inc.).
Production of AAV vectors
cDNA constructs encoding for Fst-288, Fst-315, myostatin (Thermo Fisher Scientific), and Smad3-CA (GenScript) were cloned into an AAV expression plasmid consisting of a cytomegalovirus (CMV) promoter/enhancer and a SV40 poly-A region flanked by AAV2 terminal repeats (Blankinship et al., 2004), using standard cloning techniques. Transfection of these plasmids with the pDGM6 packaging plasmid into HEK293 cells generated type-6 pseudotyped viral vectors that were harvested and purified as described previously (Blankinship et al., 2004). In brief, HEK293 cells were plated at a density of 3.2–3.8 × 106 cells on a 10-cm culture dish, 8–16 h before transfection with 10 µg of a vector-genome-containing plasmid and 20 µg of the packaging/helper plasmid pDGM6, by means of the calcium phosphate precipitate method to generate pseudotype 6 vectors. 72 h after transfection, the media and cells were collected and homogenized through a microfluidizer (Microfluidics) before 0.22 µm clarification (Millipore). The vector was purified from the clarified lysate by affinity chromatography over a HiTrap heparin column (GE Healthcare), and ultracentrifuged overnight before resuspension in sterile physiological Ringer’s solution. The purified vector preparations were titered with a customized sequence-specific quantitative PCR-based reaction (Applied Biosystems).
Animal experiments
All experiments were conducted in accordance with the relevant codes of practice for the care and use of animals for scientific purposes (National Institutes of Health, 1985; and the National Health and Medical Council of Australia, 2004). C57BL/6 mice were bred in-house at the Baker IDI Heart and Diabetes Institute. Myostatin-null mice were provided by S.-J. Lee (Johns Hopkins University, Baltimore, MD). S6K1- and S6K1/2-null mice were a gift of G. Thomas (University of Cincinnati, Cincinnati, OH), the latter strain being supplied from the colony maintained by K. Hannan, R. Hannan, and R. Thompson (Peter MacCallum Cancer Centre, Victoria, Australia). For local vector delivery, mice were deeply anesthetized with isoflurane, and ∼4 × 109 vector genomes of a given vector were injected in 30 µl of HBSS directly into the anterior compartment of the hind limb, which is occupied by the TA and extensor digitorum longus (EDL) muscles. Control injections of the contralateral limb used a vector lacking a functional gene (referred to as rAAV6:MCS). For systemic delivery studies, ∼4 × 1012 vector genomes were administered in a 200-µl volume of HBSS via the tail vein. For rapamycin experiments, rapamycin (EMD) was dissolved overnight in a solution containing 0.2% carboxymethylcellulose sodium salt (Sigma-Aldrich) and 0.25% polysorbate-80 (Sigma-Aldrich) in water, as described previously (Shioi et al., 2003). In these experiments, mice received 2 mg/kg/d of rapamycin or vehicle as a daily intraperitoneal injection commencing 3 h before rAAV6:Fst injection and continuing for 14 or 28 d, inclusive. For tissue harvest, mice were humanely killed via a cervical dislocation, and the muscles were rapidly excised and weighed before subsequent processing.
Luciferase assays
pCAGA luciferase activity was measured to validate the constitutive activation of Smad3. 293T cells were transfected in 12-well plates with either 300 ng per well of GFP or Smad3-CA plasmids, as well as 300 ng per well of the pCAGA luciferase reporter (a gift from Z. Chai, Baker IDI Heart and Diabetes Institute, Melbourne, Victoria, Australia), and 200 ng per well of β-galactosidase (β-gal). Media was changed 5 h later and supplemented with either vehicle or 5 ng/ml TGF-β (PeproTech) for 24 h. Cells were then lysed with cell lysis buffer (Promega), and luciferase activity was measured using a luminometer (Berthold). β-gal was detected using a β-gal detection assay (Promega). In brief, lysate was frozen and thawed at −80°C twice, followed by centrifugation at 13,000 rpm and the addition of 2× β-gal buffer for 1 h at 37°C. β-gal expression was then measured at 420 nm. Luciferase activity is presented as the ratio of pCAGA luciferase activity to β-gal reporter activity, and data are representative of three independent experiments.
Quantitative RT-PCR
Total RNA was collected from TA muscles cells using TRIZOL (Invitrogen). 500–1,000 ng of RNA was reverse transcribed using the High Capacity RNA-to-cDNA kit (Applied Biosystems). cDNA for IGF1 was then analyzed by quantitative RT-PCR using Taqman assay on demand kits (ABI) and ABI detection software. 18S was used to standardize cDNA concentrations. Data were analyzed using the ΔΔCT method of analysis and are normalized over the 18S housekeeping gene.
Histology
Harvested muscles were placed in Tissue-Tek OCT Compound (Sakura) cryoprotectant and frozen in liquid nitrogen-cooled isopentane. The frozen samples were subsequently cryosectioned at 10 µm thickness and stained with hematoxylin and eosin to examine morphology (Rafael et al., 1996). Sections were mounted using DePeX mounting medium (BDH), and images of stained sections were captured at room temperature using a U-TV1X-2 camera mounted to a microscope (IX71), and a PlanC 10×/0.25 NA objective lens (all from Olympus). DP2-BSW acquisition software (Olympus) was used to acquire images. To determine myofiber size, cryosections were immunolabeled for laminin (B2, used at 1:600 [Millipore] with Alexa Fluor 594–labeled goat anti–rat secondary antibody used at 1:1,200; Invitrogen), washed, and coverslipped with Vectashield mounting medium (Vector Laboratories). Digital dark-field images of the immunolabeled sections were obtained at room temperature using a Spot-2 camera (controlled by Spot acquisition software; both from Diagnostic Instruments) mounted to a microscope (E1000; Nikon) equipped with a 20×/0.75 NA objective lens. Cell diameter was measured from the digital images by tracing the perimeter of individual muscle fibers through on-screen image assessment, and calculating the minimum diameter passing through the centroid of each object of interest (ImagePro Plus software suite; Media Cybernetics). On average, >500 fibers were counted for each muscle section examined.
Western blotting
TA muscles were homogenized in RIPA-based lysis buffer (Millipore) with Complete EDTA-free protease and phosphatase inhibitor cocktails (Roche). Lysis was followed by centrifugation at 13,000 g for 10 min at 4°C, and samples were denatured for 5 min at 95°C. Protein concentration was determined using a Pierce micro protein assay kit (Thermo Fisher Scientific). Protein fractions were subsequently separated by SDS-PAGE using precast 4–12% Bis-Tris gels (Invitrogen), blotted onto nitrocellulose membranes (Bio-Rad Laboratories), and incubated with the appropriate antibody overnight and detected as described previously (Winbanks et al., 2011). Quantification of labeled Western blots was performed using ImageJ pixel analysis (National Institutes of Health Image software), and data are normalized to a control value of 1. Western blots were quantified by normalizing the band density of phosphorylated proteins to their total protein level, whilst all other proteins were quantified by normalizing the band to GAPDH. Western blots are representative of at least three independent experiments.
PI3K assay
TA muscles injected with rAAV6:Fst-288 or rAAV6:MCS were collected 3 or 14 d after injection to measure PI3K activity as described previously (Serunian et al., 1991). In brief, samples containing 1 mg protein in lysis buffer (10% vol/vol glycerol, 137 mM NaCl, 20 mM Tris-HCl, pH 7.4, 1% Igepal [Sigma-Aldrich], 4 µg/ml aprotinin, 4 µg/ml leupeptin, 10 mM EDTA, 1 mM EGTA, 20 mM NaF, 1 mM sodium pyrophosphate, 1 mM vanadate, 1 mM PMSF, and 4 µg/ml pepstatin A) were immunoprecipitated using an antibody against the p85 subunit of PI3K (06–497; Millipore,) and protein A Sepharose CL-4B beads (GE Healthcare). Half of the washed bead suspension was reserved for Western blotting. The remaining washed beads were pelleted, resuspended in Hepes buffer, and incubated with a 1:1 phosphatidylinositol:phosphatidylserine substrate (Sigma-Aldrich) in the presence of [γ-32P]ATP (PerkinElmer) for 10 min. The reaction was stopped and the radiolabeled phospholipids were resolved on a silicon-based thin layer chromatography plate (Merck). The plate was exposed using autoradiography film (GE Healthcare) for up to 5 d. PIP3 signals were standardized against total p85 protein.
Akt kinase assay
A nonradioactive Akt kinase assay (Cell Signaling Technology) was performed according to the manufacturer’s instructions. TA muscles injected with rAAV6:Fst-288 or rAAV6:MCS were collected, and 500 µg of protein extracted in lysis buffer (see “PI3K assay”) was subjected to overnight immunoprecipitation using an antibody against the S473 site of Akt. A portion of the protein lysate was set aside as the total cell lysate. Immunoprecipitated beads were then pelleted, washed, and incubated with kinase buffer, ATP, and a GSK3α fusion protein for 30 min at 30°C. The reaction was then inactivated and samples were analyzed by Western blotting. Akt kinase activity was standardized against total Akt levels.
Ex vivo protein synthesis
EDL muscles were removed with intact tendons and preincubated for 30 min in 2.0 ml of warmed (30°C) modified Krebs-Henseleit buffer (KHB), as described previously (Dyck et al., 1997). KHB consisted of 4.5% NaCl, 5.75% KCl, 6.1% CaCl2, 10.55% KH2PO4, 19.1% MgSO4 × 7H2O, and 16% vol/vol NaHCO3, pH 7.4, supplemented with 4% bovine serum albumin (Bovostar; Bovogen Biologicals), and 5 mM glucose before gassing with 95:5% O2/CO2. The intracellular protein pool was labeled as described previously (Clark and Mitch, 1983). In brief, EDL muscles were transferred to a vial containing 2.0 ml of pulse (radioactive) buffer comprising 5 µCi/ml of [3H]Tyrosine (GE Healthcare) and 500 mM l-Tyrosine in KHB for 1 h and supplemented with 200 nM Rapamycin (Sigma-Aldrich), or vehicle. After labeling, the muscles were rinsed in nonradioactive KHB, dry blotted, weighed, snap frozen in liquid nitrogen, and stored at −80°C. The frozen muscles were homogenized in 500 µl of 10% trichloroacetic acid and centrifuged at 10,000 g for 15 min (4°C). The pellets corresponding to the insoluble protein fractions were resuspended in 500 µl of 1 M NaOH and allowed to dissolve overnight at room temperature. To estimate tyrosine incorporation, 100 µl of each resuspended sample was added to 5 mL of scintillation fluid, and 3H radioactivity was measured for triplicates of each sample in a Beckman scintillation counter.
Assessment of muscle function
As described previously (Gregorevic et al., 2006), the contractile properties of the mice TA muscles were assessed in situ by delivering a series of electrical stimuli to the tibial motor nerve via percutaneous electrodes, and recording tension generated during contraction via a force transducer (305C-LR; Aurora Scientific) attached to the distal tendon with surgical silk suture. The mice were placed under tribromoethanol (Sigma-Aldrich) anesthesia before testing, and were humanely killed via cervical dislocation immediately at the conclusion of evaluation, while still deeply anesthetized. At the conclusion of the protocol, muscles were rapidly excised, dissected free of tendon and connective tissue, and weighed. Total muscle cross-sectional area (CSA) was calculated by dividing muscle mass by the product of fiber length and 1.06 mg/mm3, the density of mammalian skeletal muscle. Specific force was determined by normalizing maximum isometric tetanic force to CSA.
Statistical analysis
One-way and two-way analysis of variance (ANOVA) tests were used to assess statistical differences across multiple conditions, with the Student-Newman-Keuls post hoc test used for comparisons between the specific group means. Comparisons between two conditions used the Student’s t test. All differences reported are P < 0.05 unless otherwise stated. Data are presented as the mean ± SEM unless indicated, and are representative of at least four independent experiments.
Online supplemental material
Fig. S1 shows that Fst-288 and Fst-315 promote comparable degrees of hypertrophy when administered to the TA muscle in vivo. Fig. S2 shows that muscles examined 3 d after administration of rAAV6:Fst-288 demonstrate increased phosphorylation of S6K and S6RP, without altered activity of the upstream signaling molecules PI3K, Akt, and TSC2. Fig. S3 shows that administration of rAAV6:Mstn to mouse TA muscles results in reduced phosphorylation of Akt, mTOR, and S6RP. Fig. S4 shows that deletion of S6K1 does not inhibit Fst-mediated muscle growth or inhibit S6RP phosphorylation in response to Fst-288. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201109091/DC1.
Supplementary Material
Acknowledgments
We thank Se-Jin Lee (Johns Hopkins University) and George Thomas (University of Cincinnati) for kindly providing access to the myostatin-null mice and S6K-null mice, respectively. We also thank Ross Hannan, Rick Thompson, and Kate Hannan (Peter MacCallum Cancer Centre) for providing S6K1/2-null mice. We also thank Clinton Bruce (Baker IDI Heart and Diabetes Institute) for advice on ex vivo measurement of protein synthesis.
This work was supported by funding from the National Health and Medical Research Council (NHMRC; Australia) awarded to P. Gregorevic (526648), and C.A. Harrison (1006488), from the Muscular Dystrophy Association (USA) awarded to P. Gregorevic (69684), and funding from the National Institutes of Health (AG033610) awarded to J.S. Chamberlain. M.A. Febbraio is supported by an NHMRC Senior Principal Research Fellowship. C.A. Harrison is supported by a NHMRC Career Development Fellowship (1013533). J.R. McMullen is supported by an Australian Research Council Future Fellowship (FT0001657) and Honorary NHMRC Senior Research Fellowship (586604). P. Gregorevic is supported by a Senior Research Fellowship sponsored by Pfizer Australia. The Baker IDI Heart and Diabetes Institute is supported in part by the Operational Infrastructure Support Program of the Victorian Government.
Author contributions: C.E. Winbanks and P. Gregorevic designed the research. C.E. Winbanks, K.L. Weeks, R.E. Thomson, P.V. Sepulveda, C. Beyer, H. Qian, J.L. Chen, J.M. Allen, G.I. Lancaster, and P. Gregorevic performed the experimental work. C.A. Harrison, J.R. McMullen, J.S. Chamberlain, and M.A. Febbraio contributed reagents and analytical tools and technical advice. C.E. Winbanks, K.L. Weeks, P.V. Sepulveda, and P. Gregorevic analyzed the data. C.E. Winbanks and P. Gregorevic wrote the manuscript.
The authors declare they have no conflicts of interest.
Footnotes
Abbreviations used in this paper:
- β-gal
- β-galactosidase
- EDL
- extensor digitorum longus
- ERK
- extracellular-signal-regulated kinase
- Fst
- follistatin
- IGF
- insulin-like growth factor
- KHB
- Krebs-Henseleit buffer
- mTOR
- mammalian target of rapamycin
- PI3K
- phosphatidylinositol 3-kinase
- RSK
- ribosomal S6 kinase
- S6RP
- S6 ribosomal protein
- TA
- tibialis anterior
References
- Amirouche A., Durieux A.C., Banzet S., Koulmann N., Bonnefoy R., Mouret C., Bigard X., Peinnequin A., Freyssenet D. 2009. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 150:286–294 10.1210/en.2008-0959 [DOI] [PubMed] [Google Scholar]
- Baccarelli A., Morpurgo P.S., Corsi A., Vaghi I., Fanelli M., Cremonesi G., Vaninetti S., Beck-Peccoz P., Spada A. 2001. Activin A serum levels and aging of the pituitary-gonadal axis: a cross-sectional study in middle-aged and elderly healthy subjects. Exp. Gerontol. 36:1403–1412 10.1016/S0531-5565(01)00117-6 [DOI] [PubMed] [Google Scholar]
- Blankinship M.J., Gregorevic P., Allen J.M., Harper S.Q., Harper H., Halbert C.L., Miller A.D., Chamberlain J.S. 2004. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol. Ther. 10:671–678 (published erratum appears in Mol. Ther. 2009. 17:1482) 10.1016/j.ymthe.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., Yancopoulos G.D. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3:1014–1019 10.1038/ncb1101-1014 [DOI] [PubMed] [Google Scholar]
- Carlson M.E., Hsu M., Conboy I.M. 2008. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 454:528–532 10.1038/nature07034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.S., Mitch W.E. 1983. Muscle protein turnover and glucose uptake in acutely uremic rats. Effects of insulin and the duration of renal insufficiency. J. Clin. Invest. 72:836–845 10.1172/JCI111054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clop A., Marcq F., Takeda H., Pirottin D., Tordoir X., Bibé B., Bouix J., Caiment F., Elsen J.M., Eychenne F., et al. 2006. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 38:813–818 10.1038/ng1810 [DOI] [PubMed] [Google Scholar]
- Conery A.R., Cao Y., Thompson E.A., Townsend C.M., Jr, Ko T.C., Luo K. 2004. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat. Cell Biol. 6:366–372 10.1038/ncb1117 [DOI] [PubMed] [Google Scholar]
- Dyck D.J., Peters S.J., Glatz J., Gorski J., Keizer H., Kiens B., Liu S., Richter E.A., Spriet L.L., van der Vusse G.J., Bonen A. 1997. Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am. J. Physiol. 272:E340–E351 [DOI] [PubMed] [Google Scholar]
- Gardner S., Alzhanov D., Knollman P., Kuninger D., Rotwein P. 2011. TGF-β inhibits muscle differentiation by blocking autocrine signaling pathways initiated by IGF-II. Mol. Endocrinol. 25:128–137 10.1210/me.2010-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves M.D., Pistilli E.E., Balduzzi A., Birnbaum M.J., Lachey J., Khurana T.S., Ahima R.S. 2010. Akt deficiency attenuates muscle size and function but not the response to ActRIIB inhibition. PLoS ONE. 5:e12707 10.1371/journal.pone.0012707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C.A., Frey J.W., Mabrey D.M., Jacobs B.L., Lincoln H.C., You J.S., Hornberger T.A. 2011. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J. Physiol. 589:5485–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Meuse L., Miller D.G., Russell D.W., Chamberlain J.S. 2004. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10:828–834 10.1038/nm1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P., Allen J.M., Minami E., Blankinship M.J., Haraguchi M., Meuse L., Finn E., Adams M.E., Froehner S.C., Murry C.E., Chamberlain J.S. 2006. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 12:787–789 10.1038/nm1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobet L., Martin L.J., Poncelet D., Pirottin D., Brouwers B., Riquet J., Schoeberlein A., Dunner S., Ménissier F., Massabanda J., et al. 1997. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 17:71–74 10.1038/ng0997-71 [DOI] [PubMed] [Google Scholar]
- Haidet A.M., Rizo L., Handy C., Umapathi P., Eagle A., Shilling C., Boue D., Martin P.T., Sahenk Z., Mendell J.R., Kaspar B.K. 2008. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc. Natl. Acad. Sci. USA. 105:4318–4322 10.1073/pnas.0709144105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Shintani Y., Sakamoto Y., Wakatsuki M., Shitsukawa K., Saito S. 1996. Serum immunoreactive activin A levels in normal subjects and patients with various diseases. J. Clin. Endocrinol. Metab. 81:2125–2130 10.1210/jc.81.6.2125 [DOI] [PubMed] [Google Scholar]
- Harding M.W., Galat A., Uehling D.E., Schreiber S.L. 1989. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 341:758–760 10.1038/341758a0 [DOI] [PubMed] [Google Scholar]
- Kambadur R., Sharma M., Smith T.P., Bass J.J. 1997. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 7:910–916 [DOI] [PubMed] [Google Scholar]
- Kline W.O., Panaro F.J., Yang H., Bodine S.C. 2007. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J. Appl. Physiol. 102:740–747 10.1152/japplphysiol.00873.2006 [DOI] [PubMed] [Google Scholar]
- Lai K.M., Gonzalez M., Poueymirou W.T., Kline W.O., Na E., Zlotchenko E., Stitt T.N., Economides A.N., Yancopoulos G.D., Glass D.J. 2004. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol. Cell. Biol. 24:9295–9304 10.1128/MCB.24.21.9295-9304.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J. 2007. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2:e789 10.1371/journal.pone.0000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., McPherron A.C. 2001. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA. 98:9306–9311 10.1073/pnas.151270098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Reed L.A., Davies M.V., Girgenrath S., Goad M.E., Tomkinson K.N., Wright J.F., Barker C., Ehrmantraut G., Holmstrom J., et al. 2005. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc. Natl. Acad. Sci. USA. 102:18117–18122 10.1073/pnas.0505996102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Lee Y.S., Zimmers T.A., Soleimani A., Matzuk M.M., Tsuchida K., Cohn R.D., Barton E.R. 2010. Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. 24:1998–2008 10.1210/me.2010-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Kumar R., Underwood K., O’Connor A.E., Loveland K.L., Seehra J.S., Matzuk M.M. 2007. Prevention of cachexia-like syndrome development and reduction of tumor progression in inhibin-deficient mice following administration of a chimeric activin receptor type II-murine Fc protein. Mol. Hum. Reprod. 13:675–683 10.1093/molehr/gam055 [DOI] [PubMed] [Google Scholar]
- Liu X., Sun Y., Constantinescu S.N., Karam E., Weinberg R.A., Lodish H.F. 1997. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl. Acad. Sci. USA. 94:10669–10674 10.1073/pnas.94.20.10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Black B.L., Derynck R. 2001. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 15:2950–2966 10.1101/gad.925901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokireddy S., McFarlane C., Ge X., Zhang H., Sze S.K., Sharma M., Kambadur R. 2011. Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol. Endocrinol. 25:1936–1949 10.1210/me.2011-1124 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Massagué J. 2008. TGFbeta in Cancer. Cell. 134:215–230 10.1016/j.cell.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Seoane J., Wotton D. 2005. Smad transcription factors. Genes Dev. 19:2783–2810 10.1101/gad.1350705 [DOI] [PubMed] [Google Scholar]
- Matzuk M.M., Lu N., Vogel H., Sellheyer K., Roop D.R., Bradley A. 1995. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 374:360–363 10.1038/374360a0 [DOI] [PubMed] [Google Scholar]
- McPherron A.C., Lawler A.M., Lee S.J. 1997. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 387:83–90 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- Medeiros E.F., Phelps M.P., Fuentes F.D., Bradley T.M. 2009. Overexpression of follistatin in trout stimulates increased muscling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297:R235–R242 10.1152/ajpregu.91020.2008 [DOI] [PubMed] [Google Scholar]
- Michel U., Ebert S., Phillips D., Nau R. 2003. Serum concentrations of activin and follistatin are elevated and run in parallel in patients with septicemia. Eur. J. Endocrinol. 148:559–564 10.1530/eje.0.1480559 [DOI] [PubMed] [Google Scholar]
- Mieulet V., Roceri M., Espeillac C., Sotiropoulos A., Ohanna M., Oorschot V., Klumperman J., Sandri M., Pende M. 2007. S6 kinase inactivation impairs growth and translational target phosphorylation in muscle cells maintaining proper regulation of protein turnover. Am. J. Physiol. Cell Physiol. 293:C712–C722 10.1152/ajpcell.00499.2006 [DOI] [PubMed] [Google Scholar]
- Miller T.M., Kim S.H., Yamanaka K., Hester M., Umapathi P., Arnson H., Rizo L., Mendell J.R., Gage F.H., Cleveland D.W., Kaspar B.K. 2006. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 103:19546–19551 10.1073/pnas.0609411103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M.R., Cook S.A., Foo S., McKoy G., Ashida N., Novikov M., Scherrer-Crosbie M., Li L., Matsui T., Brooks G., Rosenzweig A. 2006. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ. Res. 99:15–24 10.1161/01.RES.0000231290.45676.d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musarò A., McCullagh K.J., Naya F.J., Olson E.N., Rosenthal N. 1999. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 400:581–585 10.1038/23060 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Takio K., Eto Y., Shibai H., Titani K., Sugino H. 1990. Activin-binding protein from rat ovary is follistatin. Science. 247:836–838 10.1126/science.2106159 [DOI] [PubMed] [Google Scholar]
- Nakatani M., Takehara Y., Sugino H., Matsumoto M., Hashimoto O., Hasegawa Y., Murakami T., Uezumi A., Takeda S., Noji S., et al. 2008. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 22:477–487 10.1096/fj.07-8673com [DOI] [PubMed] [Google Scholar]
- Patel K. 1998. Follistatin. Int. J. Biochem. Cell Biol. 30:1087–1093 10.1016/S1357-2725(98)00064-8 [DOI] [PubMed] [Google Scholar]
- Pende M., Um S.H., Mieulet V., Sticker M., Goss V.L., Mestan J., Mueller M., Fumagalli S., Kozma S.C., Thomas G. 2004. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 24:3112–3124 10.1128/MCB.24.8.3112-3124.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafael J.A., Cox G.A., Corrado K., Jung D., Campbell K.P., Chamberlain J.S. 1996. Forced expression of dystrophin deletion constructs reveals structure-function correlations. J. Cell Biol. 134:93–102 10.1083/jcb.134.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I., Montmarquette A., Michnick S.W. 2004. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat. Cell Biol. 6:358–365 10.1038/ncb1113 [DOI] [PubMed] [Google Scholar]
- Rodino-Klapac L.R., Haidet A.M., Kota J., Handy C., Kaspar B.K., Mendell J.R. 2009. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 39:283–296 10.1002/mus.21244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009–1013 10.1038/ncb1101-1009 [DOI] [PubMed] [Google Scholar]
- Ruvinsky I., Katz M., Dreazen A., Gielchinsky Y., Saada A., Freedman N., Mishani E., Zimmerman G., Kasir J., Meyuhas O. 2009. Mice deficient in ribosomal protein S6 phosphorylation suffer from muscle weakness that reflects a growth defect and energy deficit. PLoS ONE. 4:e5618 10.1371/journal.pone.0005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y., Bar-Peled L., Zoncu R., Markhard A.L., Nada S., Sabatini D.M. 2010. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 141:290–303 10.1016/j.cell.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296–1302 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 307:1098–1101 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Sartori R., Milan G., Patron M., Mammucari C., Blaauw B., Abraham R., Sandri M. 2009. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Cell Physiol. 296:C1248–C1257 10.1152/ajpcell.00104.2009 [DOI] [PubMed] [Google Scholar]
- Schuelke M., Wagner K.R., Stolz L.E., Hübner C., Riebel T., Kömen W., Braun T., Tobin J.F., Lee S.J. 2004. Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 350:2682–2688 10.1056/NEJMoa040933 [DOI] [PubMed] [Google Scholar]
- Serunian L.A., Auger K.R., Cantley L.C. 1991. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 198:78–87 10.1016/0076-6879(91)98010-4 [DOI] [PubMed] [Google Scholar]
- Shelton G.D., Engvall E. 2007. Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul. Disord. 17:721–722 10.1016/j.nmd.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Shima H., Pende M., Chen Y., Fumagalli S., Thomas G., Kozma S.C. 1998. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 17:6649–6659 10.1093/emboj/17.22.6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T., McMullen J.R., Tarnavski O., Converso K., Sherwood M.C., Manning W.J., Izumo S. 2003. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 107:1664–1670 10.1161/01.CIR.0000057979.36322.88 [DOI] [PubMed] [Google Scholar]
- Siekierka J.J., Hung S.H., Poe M., Lin C.S., Sigal N.H. 1989. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 341:755–757 10.1038/341755a0 [DOI] [PubMed] [Google Scholar]
- Song K., Cornelius S.C., Reiss M., Danielpour D. 2003. Insulin-like growth factor-I inhibits transcriptional responses of transforming growth factor-beta by phosphatidylinositol 3-kinase/Akt-dependent suppression of the activation of Smad3 but not Smad2. J. Biol. Chem. 278:38342–38351 10.1074/jbc.M304583200 [DOI] [PubMed] [Google Scholar]
- Trendelenburg A.U., Meyer A., Rohner D., Boyle J., Hatakeyama S., Glass D.J. 2009. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 296:C1258–C1270 10.1152/ajpcell.00105.2009 [DOI] [PubMed] [Google Scholar]
- Wang L., Gout I., Proud C.G. 2001. Cross-talk between the ERK and p70 S6 kinase (S6K) signaling pathways. MEK-dependent activation of S6K2 in cardiomyocytes. J. Biol. Chem. 276:32670–32677 10.1074/jbc.M102776200 [DOI] [PubMed] [Google Scholar]
- Winbanks C.E., Wang B., Beyer C., Koh P., White L., Kantharidis P., Gregorevic P. 2011. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 286:13805–13814 10.1074/jbc.M110.192625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wang J.L., Lu J., Song Y., Kwak K.S., Jiao Q., Rosenfeld R., Chen Q., Boone T., Simonet W.S., et al. 2010. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 142:531–543 10.1016/j.cell.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Zimmers T.A., Davies M.V., Koniaris L.G., Haynes P., Esquela A.F., Tomkinson K.N., McPherron A.C., Wolfman N.M., Lee S.J. 2002. Induction of cachexia in mice by systemically administered myostatin. Science. 296:1486–1488 10.1126/science.1069525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.