Abstract
The death of a loved one is recognized as one of life's greatest stresses, with reports of increased mortality and morbidity for the surviving spouse or parent, especially in the early months of bereavement. The aim of this paper is to review the evidence to date to identify physiological changes in the early bereaved period, and evaluate the impact of bereavement interventions on such physiological responses, where they exist. Research to date suggests that bereavement is associated with neuroendocrine activation (cortisol response), altered sleep (electroencephalography changes), immune imbalance (reduced T-lymphocyte proliferation), inflammatory cell mobilization (neutrophils), and prothrombotic response (platelet activation and increased vWF-ag) as well as hemodynamic changes (heart rate and blood pressure), especially in the early months following loss. Additional evidence suggests that bereavement interventions have the potential to be of value in instances where sleep disturbance becomes a prolonged feature of complicated grief, but have limited efficacy in maintaining immune function in the normal course of bereavement.
Keywords: bereavement, complicated grief, mortality, intervention, cortisol, immune, prothrombotic, heart rate, sleep, blood pressure
Abstract
La muerte de un ser querido es reconocida como uno de los mayores estresores de la vida, especialmente en los primeros meses del duelo, lo que se basa en reportes del aumento de la morbi-mortalidad de la esposa o del padre sobreviviente. El objetivo de este artículo es revisar la evidencia disponible a la fecha para identificar los cambios fisiológicos en el primer período del duelo y evaluar el impacto de las intervenciones sobre éste en las respuestas fisiológicas cuando ellas se presentan. La investigación actual sugiere que el duelo se asocia especialmente en los primeros meses que siguen a la pérdida con: activación endocrina (respuesta de cortisol), sueño alterado (cambios electroencefalográficos), desbalance inmune ((proliferación reducida de linfocitos T), movilización de células inflamatorias (neutrófilos), respuesta protrombótica (activación plaquetaria y aumento del antígeno de Factor von Willebrand) y también hemodínámícos (frecuencia cardíaca y presión sanguínea). La evidencia adicional sugiere que las intervenciones sobre el duelo son potencialmente valiosas en situaciones donde el trastorno del sueño se constituye en una característica prolongada del duelo complicado, pero tienen una eficacia limitada en el mantenimiento de la función inmune durante el curso normal del duelo.
Abstract
La perte d'un être aimé est reconnue comme étant l'un des stress les plus importants de la vie, avec une morbidité et une mortalité augmentées chez l'époux ou le parent survivant, surtout dans les mois qui suivent la perte, Le but de cet article est d'analyser les preuves actuelles des modifications physiologiques de la période précoce qui suit cette perte et d'évaluer l'impact des interventions sur ces réponses physiologiques, là où elles existent. Jusqu'à présent, la recherche suggère qu'une perte affective est associée à une activation neuroendocrinienne (réponse cortisolique), un sommeil altéré (modifications électroencéphalographiques), un déséquilibre immunitaire (prolifération diminuée des lymphocytes T), une mobilisation des cellules de l'inflammation (neutrophiles), une réponse pro-thrombotique (activation plaquettaire et augmentation des antigènes du facteur von Willebrand soit vWF-ag) ainsi que des modifications hémodynamiques (fréquence cardiaque et pression artérielle), surtout dans les mois qui suivent la perte. Des preuves supplémentaires suggèrent que les interventions après une perte affective peuvent avoir un effet dans les cas où les perturbations du sommeil prolongent un deuil compliqué mais elles sont peu efficaces sur le maintien de la fonction immunitaire au cours de l'évolution normale du deuil.
Introduction
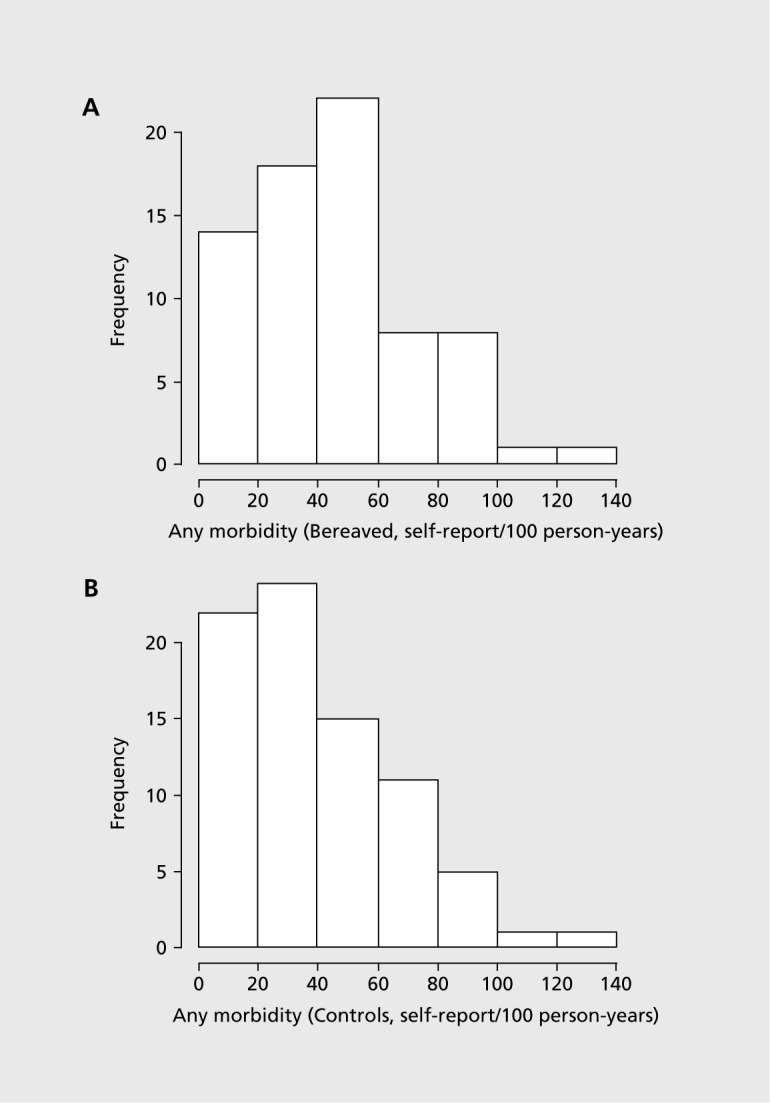
The death of a loved one is recognized as one of life's greatest stresses and has long been associated with increased health risk, especially for the surviving spouse or parent, although this is sometimes considered to be incidental rather than bereavement-related. In 1963, a follow-up of 4486 widowers, comparing their mortality to that of married men,1 reported a 40% increased mortality rate in the first 6 months of bereavement, with little differential thereafter. This finding, demonstrating a relationship between spousal bereavement and adverse health, has been confirmed.2-4 In a recent study2 bereaved participants had a higher risk than nonbereaved participants of dying from any cause (RR 1.27; 95% CI 1.2 to 1.35) including cardiovascular disease, coronary heart disease, stroke, all cancer, smoking-related cancer, and accidents or violence. In one 10-year follow-up study, it was shown that increased health risk may continue for many years after bereavement, especially in surviving spouses (Figure 1).5
Figure 1. Distribution of total morbidity rates per 1 00 person/ years in bereaved and control cohorts in a 10-year follow-up of bereaved spouses: A bereaved cohort and B control cohort. The difference between groups in morbidity rates arose from a general elevation in the distribution of morbidity incidence among the bereaved, relative to the controls with morbidity levels due to mental health (61 % elevation, P=0.05) and circulatory system disorders (66% elevation, P=0.01) compared with controls).5 Figure reproduced from ref 5: Jones MP, Bartrop RW, Forcier L, Penny R. The long-term impact of bereavement upon spouse health: a 10-year follow-up. Acta Neuropsychiatry. 2010;22:212-217. Copyright @Wiley-Blackwell 2010.
While the increased health risk in bereavement is well documented, the mechanism remains largely unexplained, possibly due to the perceived difficulties in conducting research at a time of great distress. Proposed explanations for the increased risk in bereaved individuals include the tendency of unfit people to marry similarly unfit spouses, and the possibility that the spouses may share with the bereaved the same pathogenic environment and dietary and social factors.6,7 However, the increased risk among the bereaved persists after adjustment for spousal covariates,8 bias from common socioeconomic environmental and common lifestyles, accidents shared with spouses,7 age, ethnicity, and education.3 It is therefore plausible that much of the increased health risk in bereavement stems from the impact of psychological grief reactions on, or in conjunction with, physiological responses, resulting in the early phases of bereavement becoming a vulnerable period for the bereaved person.
The aim of this review is to document the evidence to date, identify physiological changes in the early bereaved period, and evaluate the impact of bereavement interventions on such physiological responses, where they exist.
Neuroendocrine response
Neuroendocrine response during early bereavement has been evaluated in several studies.9-13 In one early study, morning blood cortisol levels measured three times over a 1- to 2-month period were not significantly higher in anticipatory bereavement compared with nonbereaved subjects, but were approximately 3% higher in the bereaved group following the death of their husbands, suggesting that bereavement, but not anticipatory bereavement, is associated with increased adrenocortical activity.10
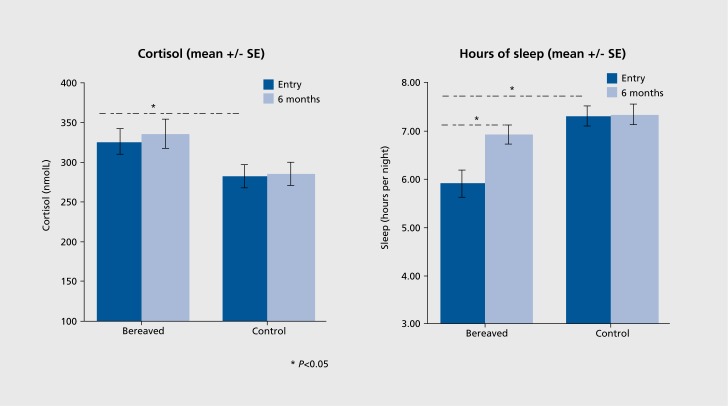
This finding of elevated cortisol in the early period of bereavement has been confirmed in several studies since, at 10 days after unanticipated loss in one study,12 and likewise 11 days after loss in a sample of bereaved spouses and parents in another.13 In this study13 (Figure 2), men had even higher cortisol levels than women, which was accounted for by their self-reported increased alcohol intake, possibly an indicator of vulnerability to stress.
Figure 2. Morning blood cortisol levels and self-reported hours of sleep in bereaved participants at 2 weeks (entry) and 6 months compared with nonbereaved controls in the Cardiovascular Health in Bereavement Study.13 *P<0.05.
It appears that cortisol remains elevated for at least the first 6 months of bereavement.12,13 For some, cortisol elevation may become chronic, as observed in one study that found increased afternoon saliva cortisol levels in adults several years following parental loss in early childhood, with higher levels inversely associated with quality of life.14
Cortisol, often referred to as a stress hormone, has been previously associated with increased cardiac risk,15 reduced immune function,16 and reduced quality of life.14,17 Hypercortisolemia in bereavement may help explain why some groups, mainly the elderly, are at higher health risk compared with younger individuals. This potential impact is highlighted in a recent study of 24 older bereaved adults that reported elevated blood cortisol: dehydroepiandrosterone-sulphate (DHEAS) levels compared with a matched nonbereaved control group.9 Both cortisol and DHEAS are outputs of the hypothalamic-pituitary-adrenal (HPA) axis and a higher ratio of cortisol to DHEAS is observed in older age, as production of DHEAS, an immune enhancer, decreases naturally whereas cortisol levels do not. As such, elevated cortisol in a group with reduced ability to produce DHEAS is likely to have greater impact in elderly bereaved, with greater potential for immune alteration.
Disturbed sleep
Evidence of sleep disturbance in bereavement stems from three main study approaches: community-based studies,18,19 self-reporting questionnaires following bereavement, and quantification of sleep patterns using electroencephalography (EEG). In one study conducted in Sweden, the relative risk of sleep disturbances was 1.95 (CI = 1.5-3.4) in 509 widows whose husbands had died from cancer 3 years prior compared with women whose husbands were still alive.18 In another study of 2800 randomly selected Japanese residents, bereavement was associated with an increased risk of not maintaining uninterrupted sleep and a higher incidence of using hypnotic medications (odds ratio of 1.65 and 2.12 respectively).19 Similarly, another study of 105 bereaved individuals, examined at least 6 months after their loss, reported significantly lower sleep quality and efficiency, with worse self-reported sleep measures associated with higher levels of depression.20
These reports of an association between bereavement and altered sleep have been confirmed by studies using electroencephalography (EEG) monitoring, although studies have mainly focused on elderly samples. In one study of 31 elderly bereaved spouses, stratified by the presence or the absence of major depression 3.5 years after loss, subjects with major depression had significantly lower sleep efficiency, more early-morning awakening, shorter rapid eye movement (REM) latency, higher REM sleep percentage, and lower rates of delta wave generation in the first non-REM (NREM) period, compared with bereaved subjects without depression. Interestingly in this study, sleep in bereavement without depression was similar to that of nonbereaved control subjects.21 These findings have been confirmed in another evaluation of 14 elderly bereaved subjects who were experiencing subsyndromal depressive symptoms, with evidence of diminished REM sleep latency, prolonged first REM sleep period, and impaired sleep efficiency at 5.5 months following loss.22
Cognitive arousal has been associated with disrupted sleep in individuals with insomnia and may be one mechanism underlying sleep disturbances in bereavement. After controlling for the effects of age, time since loss, and depression severity, greater frequency of bereavement-related intrusive thoughts and avoidance behaviors were associated with longer sleep latency and lower deep sleep phases on EEG measurements in a study of 40 men and women with major bereavementrelated depression 7.4 months after loss.23 It is not surprising that disturbed sleep patterns are a prominent feature of bereavement, as sleep disturbance is a prominent feature of depressive symptomatology, affecting more than 80% of people experiencing depression.24,25 In bereavement, reduced sleep time, likely a result of an increased hypothalamic-pituitary-adrenal axis activation, may exacerbate depressive symptoms since a strong bidirectional relationship between sleep and depression has been previously suggested.26
While sleep disturbance can become persistent and debilitating in some bereaved individuals, for most uncomplicated bereavements, sleep returns to prebereavement levels.13,27 Preservation of normal sleep after spousal bereavement has been previously associated with fewer depressive symptoms in the first 2 years after loss, with bereaved individuals who reported no depressive symptoms recording normal sleep EEG patterns.24 However, for those who develop complicated grief (CG), a situation associated with negative health outcomes28 and increased risk of mortality in elderly,29 sleep disturbance has been suggested as an important therapeutic target in bereavement.30
Immune/inflammatory changes
Immune function is one of the physiological changes most studied to date in bereavement. Enumerative measures include quantifying the number of cells in various subpopulations, usually using monoclonal antibodies that bind to unique surface markers on cell types such as T-lymphocyte cells or natural killer (NK) cells16,31 and functional measures usually conducted in vitro by measuring antibody response to a specific antigen.
One of the first studies to report immune changes in early bereavement identified reduced T-lymphocyte responses to autogenic stimulation. In this work, 26 bereaved spouses were assessed at 2 and 8 weeks following loss and compared with a sample of nonbereaved controls. Response to the mitogenic stimulant phytohemagglutinin (PHA) was significantly depressed in the bereaved group at 6 weeks after bereavement, but not at the 2-week assessment.32 Since this study, altered T-cell responses have been reported following the death of a loved one at 1 month following loss of a spouse,33 at approximately 12 months, but not at 6 months, following loss or a close friend or lover in a sample of homosexual men participating in a longitudinal study of HIV-1 infection34 and at 40 days, although not at 10 days or 6 months, following sudden death of a relative.12
While research groups report reduced T-lymphocyte proliferation in bereavement, it appears that the absolute number of lymphocytes do not consistently alter,10-12 as only one study of bereaved parents showed small changes in lymphocyte subpopulations,35 suggesting that parental response to the death of a child may be different in some aspects of physiological response to other bereaved groups.
In addition to reduced T-lymphocyte responses, an association between reduced NK cell activity and bereavement has been reported.10,12 Natural killer cells, an important defense against tumours and viral infections, were higher in bereaved subjects with greater depression scores and also with those reporting insomnia in one study.10 Additionally, a higher depression score was associated with an absolute loss of suppressor/cytotoxic cells, and an increase in the ratio of T helper to T suppressor/cytotoxic cells in bereaved women,36 lower immunoglobulin-M levels at 4 to 6 weeks following loss37 and reduced lymphocyte response in other bereaved populations.36,38
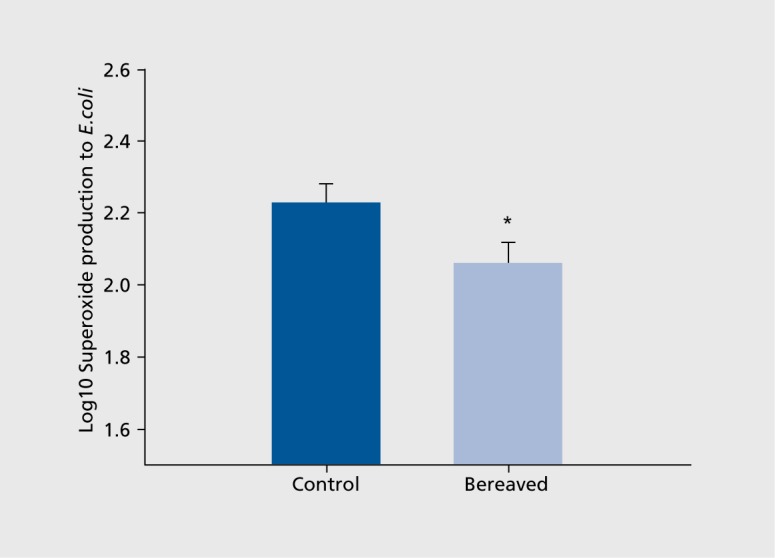
While evidence to date suggests that lymphocyte function may decrease in bereavement, more recent evidence suggest neutrophils (nonspecific inflammatory cells) may increase in number39 and decrease in function in elderly subjects during the early grieving period.9 In a prospective evaluation of 80 bereaved spouses at 2 weeks and 6 months following loss of a spouse or parent, neutrophil count was significantly higher in bereaved participants compared with a matched nonbereaved sample at 2 weeks with reduction to nonbereaved levels at 6 months.39 Additionally, in this sample, smoking was independently associated with this higher neutrophil count, highlighting the complex potential interactions between altered health behaviors and physiological response. A recent evaluation of neutrophil function in 24 elderly bereaved subjects at 2 months following loss found reduced neutrophil superoxide production in response to a challenge with Escherichia coli (E. coli), suggesting altered early ability to respond to an antigen during the early months of bereavement in this elderly population (Figure 3).9 While the significance of increased leukocytes in bereavement is unclear to date, inflammation plays a significant role in atherosclerosis, and inflammatory markers, including leukocytes, correlate with cardiovascular mortality.40,41
Figure 3. Neutrophil superoxide production on stimulation with Escherichia coli between bereaved and nonbereaved groups; error bars are standard error of the mean. Reproduced from ref 9: Khanfer R, Lord J, Phillips A. Neutrophil function and cortisol: DHEAS ratio in bereaved older adults. Brain Behav Immun. 2011;25:1182-1186. Copyright @Academic Press 2011.
In the longer term, an unresolving grief response may be a risk factor for altered immune response, as in one study bereaved participants, who were characterized by harm-avoidant temperament and long-lasting dysphoric mood at 6 months following the unexpected death of their spouse, had greater reduced immune responsiveness compared with participants whose grief levels were significantly lower.12 Coping style in bereavement may also be a determinant of immune function in bereavement,34 and be associated with perceptions of better health status 12 months following loss.37
As identified earlier, timing of assessment appears important, suggesting that immune imbalance is not an immediate response in bereavement. Assessments in the first few weeks of bereavement reported increased circulation of inflammatory cells (neutrophils and macrophages) but not changes to lymphocyte and NK cells. However, assessments conducted 1 to 2 months after loss have found altered immune response (decreased lymphocyte and NK cell function) and in assessments conducted after 6 months since loss normal immune and inflammatory function was reported, except for the bereaved who continued to demonstrate unresolved or sustained high levels of grief response.
Hemodynamic response to bereavement
Heart rate
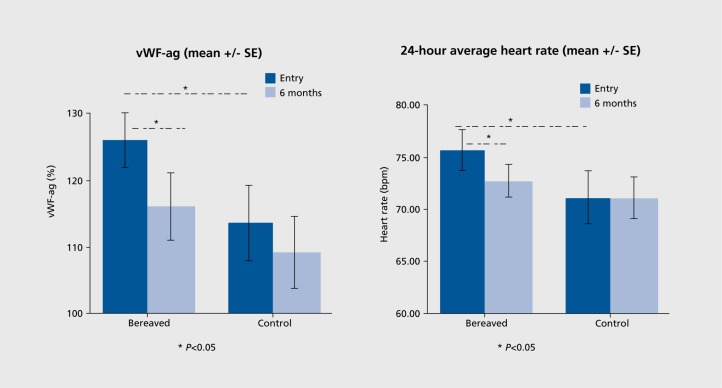
To date only two studies have reported on heart rate (HR) during bereavement although increased HR has been reported to be associated with psychological stress in other life circumstances.42-44 In the first of these studies 10 bereaved participants showed significantly higher HR (approximately 5 beat differences) than either depressed or control participants 2 months after loss. This finding was confirmed in the Cardiovascular Health in Bereavement (CARBER) study42 in which hourly measurements revealed significantly higher HR in the acutely bereaved compared with the reference group, whereas at 6 months HR in the bereaved had fallen to nonbereaved levels (Figure 4). In CARBER, higher HR was associated with higher levels of anxiety and cortisol, suggesting that elevated HR in bereavement is mediated by hypothalamic-piluitary-adrenal axis activation.
Figure 4. vWF-ag levels and 24-hour average HR in bereaved participants at 2 weeks (entry) and 6 months compared with nonbereaved controls in the Cardiovascular Health in Bereavement Study.42 vWF, von Willebrand factor.
Elevated HR in bereavement may be a significant contributor to health risk in early bereavement as higher HR has been linked to greater cardiovascular risk and mortality45,46 and coronary artery plaque rupture.47 In one study of patients with existing heart disease, an increase of five beats per minute in a 24-hour assessment, as seen in the acutely bereaved participants in the CAREER study,42 increased the risk of new coronary events by 14%, after controlling for the other risk factors.48 Lower HR found in those taking HR-lowering medications in the CARBER study,42 while not surprising, would suggest that these medications could be cardioprotective during early bereavement,49 especially in those who are at significant cardiovascular risk.
Blood pressure
Traumatic grief symptoms 6 months after the death of a spouse predicted higher self-reported blood pressure (BP) at 13- and 25-month follow-up in a prospective survey of 150 widows and widowers.28 Higher clinic systolic BP was reported in a sample of bereaved individuals, compared with a control group, in a longitudinal study of surviving spouses from deceased Alzheimer patients, studied at 6-month intervals for 18 months.50 Longerterm raised BP was reported in family members of deceased soldiers51 where the stress of mourning was associated with higher prevalence of hypertension after controlling for other cardiac risk factors.51 Over time, on average 4 years, the proportion of hypertensive participants decreased suggesting that BP takes considerable time to resolve after bereavement.51
More recently, data from the CAREER study42 suggests that raised BP is a prominent physiological feature of bereavement in the early grieving months, as 24-hour ambulatory monitoring revealed a significantly higher blood pressure load (percentage of day BP above 140 mm Hg) compared with nonbereaved matched controls (39% vs 29%) at both 2 weeks and at 6 months following loss, with older age independently associated with higher BP levels.42
While short-term hemodynamic changes, as reported above, may have limited clinical significance for healthy younger individuals, small changes could increase risk for older individuals or those with known cardiovascular disease (CVD). For example, a 2-mm Hg reduction in mean systolic BP has been associated with 7% lower CVD and 10% lower risk of stroke and death,52 making BP a potential target for preventative strategies in bereavement.
Platelet activation and coagulation factors
Increased levels of circulating Von Willebrand factor (vWF) and increased platelet activation have been recently observed in the early weeks of bereavement, with both changes resolved 6 months later (Figure 4).39 Von Willebrand factor, a major hemostatic regulatory molecule synthesised by endothelium and involved in platelet aggregation, has previously been associated with post-traumatic stress53 and clinical depression54,55 and is an independent risk factor for myocardial infarction.56 The finding of increased platelet activation may partially contribute to increased CV risk in those already with pre-existing risk factors. Circulating activated platelets play an important role in thrombosis57 and most, but not all, acute coronary occlusions occur as the result of rupture of an unstable atherosclerotic plaque and superimposed thrombus formation58 As such, one approach to cardiovascular prevention for those at increased risk in bereavement could be short-term use of antithrombotic medications, such as aspirin, in the early weeks of bereavement, as has been previously proposed for other transient periods of increased risk.49
The effect of bereavement interventions on physiological correlates
Neuroendocrine
Specific interventions designed to reduce cortisol response in bereavement have not been reported, although a randomized controlled clinical trial that examined the effect of support group sessions on immune response reported significantly lower plasma cortisol levels in the intervention group compared with the control group following 10 weekly 90-minute support group sessions.59 In this study, a reduction in physician visits was also reported in the intervention group,59 although it is unclear which aspect of the intervention contributed to these findings.
Sleep
To date two intervention approaches to improve sleep in CG have been reported; one a nonpharmacological approach and the other using a tricyclic antidepressant medication. Findings from one study suggest that a 16-week Complicated Grief Treatment (CGT) intervention has the potential to improve sleep, albeit modestly, in individuals suffering CG.60 In this study of 67 bereaved individuals with elevated scores greater than or equal to 30 on the Inventory of Complicated Grief,61 suggestive of intense grief reactions, subjects who were randomized to receive the CGT intervention reported lowered grief scores although scores remained elevated in participants after treatment, and they continued to experience clinically significant sleep problems.61
The potential effectiveness of cognitive behavioral therapy was highlighted in another study of 11 recently bereaved family members.62 In this study, the intervention consisted of cognitive behavioral therapy-insomnia (CBT-I) which included educational information about cognitive restructuring, stimulus control, sleep hygiene, relaxation techniques and goal setting, and monitoring. Self-reported sleep measures and depression scores decreased over the 5-week intervention period, although sleep actigraphy data (that provide limited measures of sleep patterns and circadian rhythms) showed no significant changes over the study period. However, this study was limited by not including a control group to help determine whether the improvement was related to the treatment or the natural course of bereavement and the limitations of using actigraphy as a sleep measure.
Use of nortriptyline appears to improve sleep quality in elderly bereaved, although removal of the treatment appeared to result in loss of some effect.63,64 In one study 10 elderly bereaved subjects, compared with matched healthy controls, were monitored using EEG study techniques while on and after discontinuation of nortriptyline, remission of depressive symptoms while still on treatment was associated with significant improvements in sleep EEG measures and sleep efficiency. In this study sleep quality continued to show improvement coincident with sustained clinical remission after ceasing treatment, suggesting that nortriptyline may be clinically useful in treating sleep disturbances in older people with bereavement-related depression.22
Taylor and colleagues64 built on the above studies by conducting a double-blind, randomized controlled trial to examine the effect of nortriptyline on depressive symptoms and sleep quality, employing EEG sleep study measures in 27 elderly bereaved participants, all diagnosed with depression within 7 weeks of their loss. The 16-week intervention was associated with better EEG measures while on treatment at 4 months compared with a placebo group, but not at 6 months, which was 2 months after discontinuation of treatment, suggesting that EEG sleep characteristics in bereavement-related depression persist into remission.
Immunity
Four studies have reported the outcome of interventions to enhance immune function in bereavement, two demonstrating no intervention effect65,66 while two studies found potential benefit for individuals with HIV.59,67 In one randomized controlled trial of 18 middle-aged Dutch widows, recruited 3 months after loss, no differences were found between groups in psychological or immune measures following a 4-month group grief counselling program.65 Similarly, another study testing the effect of relaxation sessions on grief, stress symptoms, and immune response functioning in a sample of 27 bereaved widows reported no intervention effect despite a reduction in psychological grief symptoms. However, in a randomized controlled clinical trial, the potential for behavioral interventions to have beneficial immunological and clinical health effects following bereavement among HIV-1-infected individuals was highlighted.59 In this study, support group sessions were associated with reduced blood cortisol levels and fewer physician visits, and a stable CD4+ cell count for the intervention group over the 6-month study period, whereas the CD4+ cell count decreased in HIV-positive participants in the control group.59 This same research group suggested that the bereavement support group intervention may prove to be not only a primary therapy for psychological distress but also an adjunctive therapy for sustained control of plasma viral load in conjunction with highly active antiretroviral therapy in this population with pre-existing immune depression.67
Discussion
Despite the difficulties in conducting physiologically based studies in the early bereavement period, current evidence suggests that such a severely distressing life event is associated with increased cortisol secretion that potentially contributes to increased cognitive arousal resulting in sleep disturbance, especially in those with intense or prolonged grief reactions. It is likely that cortisol secretion and disruptions to sleep partially contribute towards or exacerbate immune, hemodynamic, and prothrombotic responses, especially in the early months following loss and in those where grief intensity is particularly high (Figure 5).
Figure 5. Representation of the complex interactions between psychological and physiological correlates of bereavement.
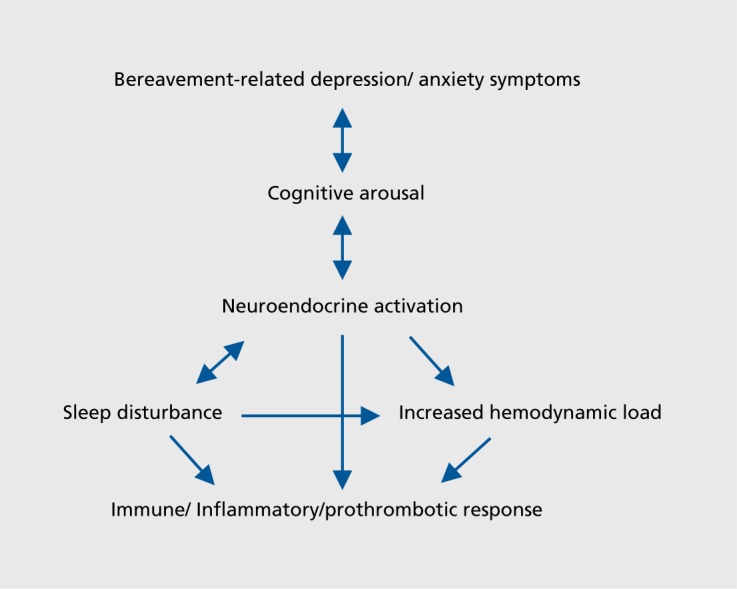
The impact of bereavement interventions on physiological correlates is difficult to ascertain due to the limited number of controlled intervention studies to date and the limitation of studies conducted in predominantly elderly populations. Both use of CGT and norytriptyline therapies show potential promise in instances where sleep disturbance becomes a prolonged feature of CG, especially in older people, although further randomized controlled studies with adequately powered samples and longer term follow-up data are required before such therapies could be recommended broadly.
While bereavement is associated with increased mobilization of inflammatory cells and changes in immune function, it is unclear if the temporary changes are causally related to the increased health risk. Evidence to date suggests that bereavement interventions to promote immune function have limited use in the normal course of bereavement, except in populations with pre-existing immunosuppression, where evidence suggests a role preventing decline in immune function.
Recent prospectively gathered evidence of hemodynamic and prothrombotic changes in the early weeks of bereavement provide insight into the impact of early bereavement on known cardiac risk factors and inform potential preventative approaches to reduce cardiovascular risk during this heightened vulnerable period, especially in those already at increased risk.
One noninvasive potential preventative approach in bereavement may be to focus on modifying or avoiding behaviors, such as tobacco smoking, alcohol consumption, and changes to diet, that, in the presence of altered physiology, could further increase health risk. Additionally, reducing the risk of acquiring infections by implementing simple preventative strategies such as frequent handwashing may also be useful, since immune imbalance appears prevalent during early bereavement. While being prepared for a loved one's death can result in decreased psychological stress symptoms,13 there is a lack of studies on the effects of anticipatory bereavement on physiological correlates, a potential area of future research. In samples where mortality risk is reported in surviving spouses, no difference between expected deaths and unexpected deaths has been reported.68 However, the anticipatory bereavement period may provide opportunity for potential preventative strategies targeting health outcome. Indeed, it is worth noting that in one matched retrospective cohort study69 that compared mortality risk among 30 838 couples where the deceased used hospice care and an equal number of couples where the deceased did not, analysis of spousal mortality revealed that bereaved spouses whose deceased partners had used hospice services, compared with “control bereaved” subjects who did not, were less likely to die themselves in the first 18 months of bereavement, with an adjusted odds ratio of 0.92 for widows and 0.95 for widowers. This study highlights the possible protective influence of social support during the anticipatory bereavement period on spousal outcome. In this study, hospice care was described as including nursing services, physician visits, homemaker assistance, social assistance, and bereavement counseling.
The focus at the time of bereavement is naturally directed to the deceased person; the health and welfare of bereaved survivors is of concern to both surviving family members and their health care practitioners. Further research is warranted, building on the body of evidence to date, to continue to prospectively evaluate physiological correlates in bereavement and also to test preventive interventions targeted at reducing health risk during this universal and inevitable life stressor.
Contributor Information
Thomas Buckley, University of Sydney, NSW, Australia.
Dalia Sunari, University of Sydney, NSW, Australia.
Andrea Marshall, University of Sydney, NSW, Australia.
Roger Bartrop, University of Sydney, NSW, Australia; University of Western Sydney, NSW, Australia.
Sharon McKinley, University of Technology, Sydney, NSW, Australia.
Geoffrey Tofler, University of Sydney, NSW, Australia; Department of Cardiology, Royal North Shore Hospital, St Leonards, NSW, Australia.
REFERENCES
- 1.Young M., Benjamin B., Wallis C. The mortality of widowers. Lancet. 1963;13:454–456. doi: 10.1016/s0140-6736(63)92193-7. [DOI] [PubMed] [Google Scholar]
- 2.Hart C., Hole D., Lawlor D., Smith G., Lever T. Effect of conjugal bereavement on mortality of the bereaved spouse in participants of the Renfrew/Paisley Study. J Epidemiol Community Health. 2007;61:455–460. doi: 10.1136/jech.2006.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manor O., Eisenbach Z. Mortality after spousal loss: are there sociodemographic differences? SocSciMed. 2003;56:405–413. doi: 10.1016/s0277-9536(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein P., Gatz M., Berg S. A twin study of mortality after spousal bereavement. Psychol Med. 1998;28:635–643. doi: 10.1017/s0033291798006692. [DOI] [PubMed] [Google Scholar]
- 5.Jones MP., Bartrop RW., Forcier L., Penny R. The long-term impact of bereavement upon spouse health: a 10-year follow-up. Acta Neuropsychiatries. 2010;22:212–217. doi: 10.1111/j.1601-5215.2010.00482.x. [DOI] [PubMed] [Google Scholar]
- 6.Genevro JL., Marshall T., Miller T. Report on bereavement and grief research. Death Stud. 2004;28:491–575. doi: 10.1080/07481180490461188. [DOI] [PubMed] [Google Scholar]
- 7.Martikainen P., Valkonen T. Mortality after the death of a spouse: rates and causes of death in a large finnish cohort. Am J Public Health. 1996;86:1087–1093. doi: 10.2105/ajph.86.8_pt_1.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer C., Quesenberry CP Jr., Wi S. Mortality following conjugal bereavement and the effects of a shared environment. Am J Epidemiol. 1995;141:1142–1152. doi: 10.1093/oxfordjournals.aje.a117387. [DOI] [PubMed] [Google Scholar]
- 9.Khanfer R., Lord J., Phillips A. Neutrophil function and cortisol: DHEAS ratio in bereaved older adults. Brain Behav Immun. 2011;25:1182–1186. doi: 10.1016/j.bbi.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Irwin M., Daniels M., Risch S., Bloom E., Weiner H. Plasma cortisol and natural killer cell activity during bereavement. Biol Psychiatry. 1988;24:173–178. doi: 10.1016/0006-3223(88)90272-7. [DOI] [PubMed] [Google Scholar]
- 11.Spratt M., Denney D. Immune variables, depression, and plasma cortisol over time in suddenly bereaved parents. J Neuropsychiatry Clin Neurosci. 1991;3:299–306. doi: 10.1176/jnp.3.3.299. [DOI] [PubMed] [Google Scholar]
- 12.Gerra G., Monti D., Panerai A., et al. Long-term immune-endocrine effects of bereavement: relationships with anxiety levels and mood. Psychiatry Res. 2003;121:145–158. doi: 10.1016/s0165-1781(03)00255-5. [DOI] [PubMed] [Google Scholar]
- 13.Buckley T., Bartrop R., McKinley S., et al. Prospective study of early bereavement on psychological and behavioural cardiac risk factors. Intern Med J. 2009;39:370–378. doi: 10.1111/j.1445-5994.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Fraser R., Ingram MC., Anderson NH., Morrison C., Davies E., Connell JM. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension. 1999;33:1364–1368. doi: 10.1161/01.hyp.33.6.1364. [DOI] [PubMed] [Google Scholar]
- 16.Segerstrom S., Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breier A. A.E. Bennett award paper. Experimental approaches to human stress research: assessment of neurobiological mechanisms of stress in volunteers and psychiatric patients. Biol Psychiatry. 1989;26:438–462. doi: 10.1016/0006-3223(89)90066-8. [DOI] [PubMed] [Google Scholar]
- 18.Valdimarsdottir U., Helgason AR., Furst CJ., Adolfsson J., Steineck G. Longterm effects of widowhood after terminal cancer: a Swedish nationwide follow-up. Scand J Public Health. 2003;31:31–36. doi: 10.1080/14034940210165109. [DOI] [PubMed] [Google Scholar]
- 19.Doi Y., Minowa M., Okawa M., Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol. 2000;10:79–86. doi: 10.2188/jea.10.79. [DOI] [PubMed] [Google Scholar]
- 20.Germain A., Caroff K., Buysse DJ., Shear MK. Sleep quality in complicated grief. J Trauma Stress. 2005;18:343–346. doi: 10.1002/jts.20035. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds CF 3rd., Hoch CC., Buysse DJ., et al. Electroencephalographic sleep in spousal bereavement and bereavement-related depression of late life. Biol Psychiatry. 1992;31:69–82. doi: 10.1016/0006-3223(92)90007-m. [DOI] [PubMed] [Google Scholar]
- 22.Pasternak RE., Reynolds CF 3rd., Hoch CC., et al. Sleep in spousally bereaved elders with subsyndromal depressive symptoms. Psychiatry Res. 1992;43:43–53. doi: 10.1016/0165-1781(92)90140-x. [DOI] [PubMed] [Google Scholar]
- 23.Hall M., Buysse DJ., Dew MA., Prigerson HG., Kupfer DJ., Reynolds CF 3rd. Intrusive thoughts and avoidance behaviors are associated with sleep disturbances in bereavement-related depression. Depress Anxiety. 1997;6:106–112. [PubMed] [Google Scholar]
- 24.Armitage R., Hoffmann R. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–246. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds C., Kupfer D. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10:199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- 26.Riemann D., Berger M., Voderholzer U. Sleep and depression-results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 27.Richardson S., Lund D., Caserta M., Dudley W., Obray S. Sleep patterns in older bereaved spouses. Omega. 2003;47:361–383. [Google Scholar]
- 28.Prigerson H., Bierhals A., Kasl S., et al. Traumatic grief as a risk factor for mental and physical morbidity. Am J Psychiatry. 1997;154:616–623. doi: 10.1176/ajp.154.5.616. [DOI] [PubMed] [Google Scholar]
- 29.Dew MA., Hoch CC., Buysse DJ., et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 30.Monk TH., Germain A., Reynolds CF. Sleep disturbance in bereavement. PsychiatrAnn. 2008;38:671–675. doi: 10.3928/00485713-20081001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller GE., Cohen S. Psychological interventions and immune system: a meta-analytic review and critique. Health Psychol. 2001;20:47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Bartrop RW., Luckhurst E., Lazarus L., Kiloh LG., Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977;1:834–836. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- 33.Schleifer SJ., Keller SE., Camerino M., Thornton JC., Stein M. Suppression of lymphocyte stimulation following bereavement. JAMA. 1983;250:374–377. [PubMed] [Google Scholar]
- 34.Goodkin K., Feaster DJ., Tuttle R., et al. Bereavement is associated with time-dependent decrements in cellular immune function in asymptomatic human immunodeficiency virus type 1-seropositive homosexual men. Clin Diagn Lab Immunol. 1996;3:109–118. doi: 10.1128/cdli.3.1.109-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spratt ML., Denney DR. Immune variables, depression, and plasma cortisol over time in suddenly bereaved parents. J Neuropsychiatry Clin Neurosci. 1991;3:299–306. doi: 10.1176/jnp.3.3.299. [DOI] [PubMed] [Google Scholar]
- 36.Irwin M., Daniels M., Bloom ET., Smith TL., Weiner H. Life events, depressive symptoms, and immune function. Am J Psychiatry. 1987;144:437–441. doi: 10.1176/ajp.144.4.437. [DOI] [PubMed] [Google Scholar]
- 37.Lindstrom TC. Immunity and health after bereavement in relation to coping. Scand J Psychol. 1997;38:253–259. doi: 10.1111/1467-9450.00034. [DOI] [PubMed] [Google Scholar]
- 38.Linn MW., Linn BS., Jensen J. Stressful events, dysphoric mood, and immune responsiveness. Psychol Rep. 1984;54:219–222. doi: 10.2466/pr0.1984.54.1.219. [DOI] [PubMed] [Google Scholar]
- 39.Buckley T., Morel-Kopp MC., Ward C., et al. Inflammatory and thrombotic changes in early bereavement: a prospective evaluation. Eur J Cardiovasc Prev Rehabil. In press. doi: 10.1177/1741826711421686. [DOI] [PubMed] [Google Scholar]
- 40.Phillips AN., Neaton JD., Cook DG., Grimm RH., Shaper AG. Leukocyte count and risk of major coronary heart disease events. Am J Epidemiol. 1992;136:59–70. doi: 10.1093/oxfordjournals.aje.a116421. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM., Cushman M., Stampfer MJ., Tracy RP., Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 42.Buckley T., Mihailidou A., Bartrop R., et al. Haemodynamic changes during early bereavement: potential contribution to increased cardiovascular risk. Heart Lung Circulation. 2011;20:91–98. doi: 10.1016/j.hlc.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor M., Allen J., Kaszniak A. Autonomic and emotion regulation in bereavement and depression. J Psychosom Res. 2002;52:183–185. doi: 10.1016/s0022-3999(02)00292-1. [DOI] [PubMed] [Google Scholar]
- 44.Gumming J., Olphin T., Law M. Self-reported psychological states and physiological responses to different types of motivational general imagery. J Sport Exerc Psychol. 2007;29:629–644. doi: 10.1123/jsep.29.5.629. [DOI] [PubMed] [Google Scholar]
- 45.Kizilbash MA., Daviglus ML., Dyer AR., et al. Relation of heart rate with cardiovascular disease in normal-weight individuals: the Chicago Heart Association Detection Project in Industry. Prev Cardiol. 2008;11:141–147. doi: 10.1111/j.1751-7141.2008.08004.x. [DOI] [PubMed] [Google Scholar]
- 46.Palatini P., Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004;26:637–644. doi: 10.1081/ceh-200031959. [DOI] [PubMed] [Google Scholar]
- 47.Heidland UE., Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104:1477–1482. doi: 10.1161/hc3801.096325. [DOI] [PubMed] [Google Scholar]
- 48.Aronow WS., Ahn C., Mercando AD., Epstein S. Association of average heart rate on 24-hour ambulatory electrocardiograms with incidence of new coronary events at 48-month follow-up in 1,311 patients (mean age 81 years) with heart disease and sinus rhythm. Am J Cardiol. 1996;78:1175–1176. doi: 10.1016/s0002-9149(96)90077-6. [DOI] [PubMed] [Google Scholar]
- 49.Tofler GH., Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 50.Grant I., Adler KA., Patterson TL., Dimsdale JE., Ziegler MG., Irwin MR. Health consequences of Alzheimer's caregiving transitions: effects of placement and bereavement. Psychosom Med. 2002;64:477–486. doi: 10.1097/00006842-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Santic Z., Lukic A., Sesar D., Milicevic S., Ilakovac V. Long-term follow-up of blood pressure in family members of soldiers killed during the war in Bosnia and Herzegovina. Croat Med J. 2006;47:416–423. [PMC free article] [PubMed] [Google Scholar]
- 52.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 53.von Kanel R., Hepp U., Traber R., et al. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008;158:363–373. doi: 10.1016/j.psychres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Morel-Kopp MC., McLean L., Chen Q., et al. The association of depression with platelet activation: evidence for a treatment effect. J Thromb Haemost. 2009;7:573–581. doi: 10.1111/j.1538-7836.2009.03278.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Zhang S., Jin Y., Qin G., Yu L., Zhang J. Elevated levels of plateletmonocyte aggregates and related circulating biomarkers in patients with acute coronary syndrome. IntJ Cardiol. 2007;115:361–365. doi: 10.1016/j.ijcard.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Kucharska-Newton AM., Couper DJ., et al. Hemostasis, inflammation, and fatal and nonfatal coronary heart disease: long-term follow-up of the atherosclerosis risk in communities (ARIC) cohort. Arterioscier Thromb Vase Biol. 2009;29:2182–2190. doi: 10.1161/ATVBAHA.109.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markovitz JH., Shuster JL., Chitwood WS., May RS., Tolbert LC. Platelet activation in depression and effects of sertraline treatment: an open-label study. Am J Psychiatry. 2000;1 57:1006–1008. doi: 10.1176/appi.ajp.157.6.1006. [DOI] [PubMed] [Google Scholar]
- 58.Servoss SJ., Januzzi JL., Muller JE. Triggers of acute coronary syndromes. Prog Cardiovasc Dis. 2002;44:369–380. doi: 10.1053/pcad.2002.123470. [DOI] [PubMed] [Google Scholar]
- 59.Goodkin K., Feaster DJ., Asthana D., et al. A bereavement support group intervention is longitudinally associated with salutary effects on the CD4 cell count and number of physician visits. Clin Diagn Lab immunol. 1998;5:382–391. doi: 10.1128/cdli.5.3.382-391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Germain A., Shear K., Monk TH., et al. Treating complicated grief: effects on sleep quality. Behav Sleep Med. 2006;4:152–163. doi: 10.1207/s15402010bsm0403_2. [DOI] [PubMed] [Google Scholar]
- 61.Prigerson HG., Maciejewski PK., Reynolds CF 3rd., et al. Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59:65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- 62.Carter PA., Mikan SQ., Simpson C. A feasibility study of a two-session home-based cognitive behavioral therapy-insomnia intervention for bereaved family caregivers. Palliat Support Care. 2009;7:197–206. doi: 10.1017/S147895150900025X. [DOI] [PubMed] [Google Scholar]
- 63.Pasternak RE., Reynolds CF 3rd., Houck PR., et al. Sleep in bereavementrelated depression during and after pharmacotherapy with nortriptyline. J Geriatr Psychiatry Neurol. 1994;7:69–73. doi: 10.1177/089198879400700201. [DOI] [PubMed] [Google Scholar]
- 64.Taylor MP., Reynolds CF 3rd., Frank E., et al. EEG sleep measures in laterlife bereavement depression, a randomized, double-blind, placebo-controlled evaluation of nortriptyline. Am J Geriatr Psychiatry. 1999;7:41–47. [PubMed] [Google Scholar]
- 65.Beem EE., Hooijkaas H., Cleiren MH., et al. The immunological and psychological effects of bereavement: does grief counseling really make a difference? A pilot study. Psychiatry Res. 1999;85:81–93. doi: 10.1016/s0165-1781(98)00135-8. [DOI] [PubMed] [Google Scholar]
- 66.Kang HY., Yoo YS. Effects of a bereavement intervention program in middle-aged widows in Korea. Arch Psychiatr Nurs. 2007;21:132–140. doi: 10.1016/j.apnu.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Goodkin K., Baldewicz TT., Asthana D., et al. A bereavement support group intervention affects plasma burden of human immunodeficiency virus type 1. Report of a randomized controlled trial. J Hum Virol. 2001;4:44–54. [PubMed] [Google Scholar]
- 68.Ward AW. Mortality of bereavement. BMJ. 1976;1:700–702. doi: 10.1136/bmj.1.6011.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christakis NA., Iwashyna TJ. The health impact of health care on families: a matched cohort study of hospice use by decedents and mortality outcomes in surviving, widowed spouses. Soc Sci Med. 2003;57:465–475. doi: 10.1016/s0277-9536(02)00370-2. [DOI] [PubMed] [Google Scholar]