Abstract
The lymphatic vasculature plays a pivotal role in maintaining tissue fluid homeostasis, immune surveillance, and lipid uptake in the gastrointestinal organs. Therefore, impaired function of the lymphatic vessels caused by genetic defects, infection, trauma, or surgery leads to the abnormal accrual of lymph fluid in the tissue and culminates in the swelling of affected tissues, known as lymphedema. Although millions of people suffer from lymphedema worldwide which causes impaired wound healing, compromised immune defense and, in rare case, lymphangiosarcoma, no effective therapy is currently available. In addition, recent advances in cancer biology have disclosed an indispensable function of the lymphatic vessel in tumor growth and metastasis. Therefore, understanding the detailed mechanisms governing lymphatic vessel formation and function in pathophysiologic conditions is essential to prevent or treat these diseases. Here, we will review the developmental processes of the lymphatic vessels and postnatal lymphatic neovascularization, focusing on the role of recently identified bone-marrow (BM) derived PODOPLANIN expressing (PODOPLANIN+) cells as lymphatic endothelial progenitor cells (LEPCs).
Introduction
The vasculature, which includes the blood vessels and the lymphatic vessels, is indispensable for the development and the survival of mammals. Extensive efforts have been made to study the biology of blood vessels; however, recent investigations on the molecular mechanisms controlling the development of the lymphatic vessels during embryogenesis and the diseases related to lymphatic dysfunctions have gained much attention. In the adult, the lymphatic vasculature is composed of three distinct yet interconnected parts: lymphatic capillaries, precollectors, and lymphatic collecting vessels. The lymphatic capillaries are single-layered vessels consisting of staggered LECs, which lack mural cells and are surrounded by few basement membranes. The LECs of the lymphatic capillaries are anchored to extracellular matrix by anchoring filaments. These features allow the lymphatic capillaries to have a high permeability to macromolecules as well as interstitial fluids. In contrast to the LECs in the capillaries, precollectors and collecting vessels are surrounded by smooth muscle cells and contain valves that maintain the unidirectional transport of the lymphatic fluids toward the blood vessels. Eventually, the collecting lymphatic vessels carry the lymphatic fluid to the blood system through the jugular and subclavian veins, where blood vessels and lymphatic vessels, in the adult, are connected.
The primary function of the lymphatic vessels is to maintain tissue fluid homeostasis and lipid uptake. In addition, the lymphatic vessels play a critical role in immune surveillance by transporting foreign substances to the lymph nodes, where T or B lymphocytes reside. Recent studies suggest that the lymphatic vessel regeneration can also be an integral part of wound healing process (Cho et al., 2006; Niessen et al., 2011). Furthermore, defects in the lymphatic vessels can lead to diseases, such as lymphedema, accompanied by several complications, including dermal fibrosis, impaired wound healing, and enhanced susceptibility to infection. Additionally, lymphatic vessels facilitate tumorigenesis by providing a route for tumor metastasis. Here, we will review the development of lymphatic vessels and discuss the potential usage of BM-derived cells for disease-related lymphatic dysfunctions.
Development of lymphatic vasculature
In developing mouse embryos, specification and differentiation of LECs begin with the expressions of SOX-18, a member of the SOX (Sry-related HMG box) transcription factor family, and PROX-1, a prospero homeobox transcription factor. Sox18 is expressed as early as embryonic day (E) 9 in a subset of cardinal veins. Then, shortly after, cells co-expressing Sox18 and Prox1 appear and dorsolaterally migrate out to form the lymphatic sacs (Francois et al., 2008; Wigle and Oliver, 1999). While overexpressing SOX-18 significantly induces the expression of LEC markers, such as Prox1, ephrinB2, and Vegfr3, Sox18 deficient mice display embryonic lethality with abnormal patterning of the lymphatic vessels (Francois et al., 2008). In fact, SOX-18 can directly bind to the promoter and induce the expression of Prox1, which is the master switch for LEC development. A Prox1−/− mice study shows that PROX-1 appears to be dispensable for the initiation of LEC generation, but essential for budding and sprouting of the LECs (Wigle et al., 2002; Wigle and Oliver, 1999). PROX1 can also provide the identity of LECs to blood endothelial cells (BECs), and is continuously required for the maintenance of LEC identity by expressing LEC markers and blocking the expression of BEC genes (Hong et al., 2002; Johnson et al., 2008; Petrova et al., 2002). Recent studies show that COUP-TFII, an orphan nuclear receptor, can also interact with SOX18 to activate and maintain the expression of PROX1; it can also regulate PROX1 downstream targets (Lee et al., 2009; Yamazaki et al., 2009). This SOX18-COUP-TFII-PROX1 complex plays a critical role in the initiation and maintenance of the lymphatic vasculature throughout life.
The VEGFC and VEGFR3 pathway is a critical regulator for a proper migration/sprouting of LECs to form a lymphatic sac. Around E10.5, VEGFC is expressed in the mesenchymal cells around the jugular veins, from which the first arising LECs sprout and migrate (Karkkainen et al., 2004). Consistent with the expression pattern of VEGFC and VEGFR3, genetic knockout mice studies reveal an indispensable role of this pathway in the development of lymphatic vasculature. In Vegfc−/− mice, PROX1+ LECs develop normally; however, they are unable to migrate out of the cardinal veins and cause a failure in the formation of lymphatic sacs. This suggests that VEGFC plays a critical role in the migration of LECs (Karkkainen et al., 2004). Due to a severe cardiovascular defect, Vegfr3−/− mice die prior to LEC development (Dumont et al., 1998), precluding the investigation of VEGFR3 function in the process. More recently, a study showed that a kinase activity is required for the sprouting of LECs, and ligand binding domain is critical for the proliferation of already formed lymphatic sacs (Zhang et al., 2010).
PODOPLANIN is a 38 kD integral membrane mucoprotein and is predominantly expressed in the endothelium of lymphatic capillaries. Podoplanin deficient mice die at birth due to respiratory failure, and manifest malfunctioning lymphatic vessels and congenital lymphedema (Schacht et al., 2003). Recently, PODOPLANIN has gained much attention as a critical regulator of the separation between blood vessels and lymphatic vessels. PODOPLANIN can induce aggregation and activation of platelets by binding to CLEC-2 receptor on platelets (Bertozzi et al., 2010; Kato et al., 2003; Suzuki-Inoue et al., 2010; Uhrin et al., 2010). The failure of platelet aggregation, in the absence of Podoplanin, causes the development of a mixed lympho-blood vessels (Bertozzi et al., 2010; Uhrin et al., 2010), which phenocopies the defects seen in Syk−/−, Slp76−/− or PLCg2−/− mice (Abtahian et al., 2003; Ichise et al., 2009). The inactivation of Slp76 in platelets and megakaryocytes results in the generation of mixed lympho-blood vessels (Bertozzi et al., 2010), suggesting that, at least, SPL76 is needed in platelets to signal the PODOPALNIN-CLEC2 interaction, which induces the separation of blood and lymphatic vessels. Further maturation and remodeling of the lymphatic sacs into functional lymphatic vessels, consisting of collecting lymphatic vessels and lymphatic capillaries, are regulated by several key molecules, such as FOXC2, EphB2 and Tie1-Ang2. Out of these key molecules, FOXC2, a fork head transcription factor, notably plays a critical role in the formation of functional collecting lymphatic vessels, as demonstrated by defective valve formation and aberrantly increased recruitment of pericytes in Foxc2−/− mice (Petrova et al., 2004). Mechanistically, NFATc is one of direct downstream targets of FOXC2 for the lymphatic vessel maturation (Norrmen et al., 2009). Connexins 37 and 43 appear to be other targets of FOXC2 to regulate the valve formation in the collecting lymphatic vessels (Kanady et al., 2011). For further discussion about lymphatic vessel development, please read previously published literatures (Karpanen and Alitalo, 2008; Tammela and Alitalo, 2010).
BM as a potential source of lymphatic endothelial cells
BM cells participate in many different pathophysiologic processes, including hematopoiesis, angiogenesis, and inflammation. The contribution of BM-derived cells to non-hematopoietic tissues, such as neuroectodermal cells, skeletal myoblasts, and cardiomyocytes, has been reported (Krause, 2002), which was later highly debated (Spyridonidis et al., 2005). On the other hand, the role of hematopoietic stem cells (HSCs) and endothelial progenitor cells (EPCs), derived from BM for blood vessel formation, is largely accepted (Asahara et al., 2011). This has raised a question as to whether LEPCs can also be derived from BM as well.
Similar to blood vessels, the formation of lymphatic vessels (lymphatic neovascularization) is achieved by two interdependent ways: lymphangiogenesis (the formation of new lymphatic vessels from preexisting lymphatic vasculature) and lymphvasculogenesis (de novo generation of lymphatic vessel through stem or progenitor cells). It had been believed that lymphatic vessels in the adult were generated exclusively by lymphangiogenesis (Cao et al., 2006; He et al., 2004; Karkkainen et al., 2004; Karpanen et al., 2001; Nagy et al., 2002); however, recent studies have provided compelling evidence that lymphvasculogenesis may also contribute to new lymphatic vessel formation (Kerjaschki et al., 2006; Maruyama et al., 2005; Religa et al., 2005; Salven et al., 2003). First potential evidence of LEPCs in hematopoietic organs was reported by Salven et al, that a subpopulation of human fetal liver co-expresses CD34, VEGFR3 and CD133 (AC133) (Salven et al., 2003). In culture, this specific cell population displays a robust proliferative potential, becomes adherent, and expresses other lymphatic markers, such as LYVE-1 and PODOPLANIN, and blood endothelial markers, such as VE-cadherin and CD105, suggesting the dual potential of these cells as LEPC and EPC. This initial finding was further corroborated by subsequent studies. Two groups showed that, using BM transplantation, donor-derived GFP+ cells were found closely localized or incorporated in the newly formed lymphatic vessels under inflammatory conditions (Maruyama et al., 2005; Religa et al., 2005). A more compelling evidence was provided by Kerjaschki et al (2006), in which the presence of male donor-derived LECs in the lymphatic vessels was clearly revealed in the transplanted kidneys of female recipients (Kerjaschki et al., 2006). Together, these studies strongly support the notion that lymphvasculogenesis, via precursor cells of lymphatic vessels derived from either BM or other organs, can play crucial roles in new lymphatic vessel formation during postnatal period. Nevertheless, these studies failed to address the identity and characteristics of the cells that contributed to the postnatal lymphvasculogenesis, namely lymphatic endothelial progenitor cells (LEPCs).
Some tissue macrophages express lymphatic markers, such as LYVE-1 and PROX-1, and secrete VEGFC (Cursiefen et al., 2004; Maruyama et al., 2005). A recent report showed that CD11b+ cells were found in the sites of lymphangiogenesis in cornea undergoing inflammation (Maruyama et al., 2005). The depletion of macrophages by clodronate liposomes resulted in a suppression of lymphangiogenesis with concomitant decrease in recruited CD11b+ cells, suggesting that CD11b+ cells can play an important role in inflammation-induced lymphangiogenesis. However, this study was not able to show direct evidence that macrophages are physically incorporated into the lymphatic vessels. By taking advantage of genetic lineage tracing strategy, Zumsteg et al (2009) have provided compelling evidence that BM-derived myelomonocytic lineage cells can contribute to new lymphatic vessels through lymphvasculogenesis (Zumsteg et al., 2009). In Rip1Tag2 mice undergoing pancreatic cell carcinogenesis, peritumoral lymphatic vessels contained GFP+ cells derived from transplanted donor BM cells, some of which coexpressed PROX-1 and either PODOPLANIN or LYVE-1. Furthermore, the incorporated BM-derived cells in the lymphatic vessels were shown to be myeloid lineage cells. For this analysis, Rip1Tag2 mice were reconstituted with BM from either CXCR1+/GFP mice, in which GFP was preferentially expressed in monocytes under the control of CXCR1, or CD11bCre;Z/EZ mice, in which cells that have expressed CD11b are permanently marked by GFP expression via a loxP-Cre recombination. In addition, BM-derived macrophages exhibited significantly augmented expression of lymphatic markers with concomitant decrease in hematopoietic CD45 and macrophage marker F4/80 in tube formation assay, supporting the notion that myelomonocytic lineage cells can transdifferentiate into LECs in newly formed lymphatic vessels. Collectively, these studies clearly showed the contribution of BM cells to lymphatic vessels as structural components.
BM-derived PODOPLANIN+ cells can function as LEPCs
Recently, we reported that, a subpopulation of BM cells, PODOPLANIN+ cells function as LEPCs by transdifferentiating into lymphatic endothelial cells and physically incorporating into lymphatic vessels under inflammatory and tumorigenic conditions (Lee et al., 2010). Around 1% of freshly isolated BM mononuclear cells (BM-MNCs) expressed PODOPLANIN. In culture of BM-MNCs in the presence of lymphatic cytokines (VEGFA and VEGFC) and EGF, PODOPLANIN+ cells reached ~20%, most of which also expressed CD11b. Interestingly, the expressions of stem/progenitor markers, such as c-KIT and Sca-1, were exclusively limited to the PODOPLANIN+ cells, i.e. PODOPLANIN+CD11b+ cells. Other lymphatic markers, such as VEGFR-3 and LYVE-1, showed the same expression pattern. When further cultured in LEC medium, the PODOPLANIN+CD11b+ cells exhibited enhanced LEC marker expression with concomitant loss of CD45, while PODOPLANIN−CD11b+ cells continued to express CD45 with low expression of LEC markers, suggesting that progenitor function is almost exclusively restricted to PODOPLANIN+CD11b+ cells. Indeed, these cells were able to integrate into newly formed lymphatic vessels while losing the expression of CD45 in several independent mouse models, in which lymphatic vessel growth occur, confirming that the PODOPLANIN+CD11b+ cells include LEPCs. Therefore, it is tempting to speculate that the expression of PODOPLANIN may confer stem/progenitor potential to certain populations of BM-MNC-derived CD11b+ cells. A report by Blin-Wakkach (Blin-Wakkach et al., 2006) showed that the BM-derived B220+CD11b+ cells were induced to differentiate into B lymphocyte by acquiring B lymphocyte specific genes and losing myeloid specific genes in response to IL-7 treatment. Another study also demonstrated that the Flt3+CD11b+ cells generated mature dendritic cells (DCs) (Hieronymus et al., 2005). Interestingly, the Flt3+CD11b+ cells express stem/progenitor cell markers including CD117 (c-KIT), CD184 (CXCR4), CD93 (Ly68/AA4.1), and CD133 (AC133). When induced to differentiate into DCs, the expression of those surface antigens significantly decreased, although the expression of CD11b remained relatively unchanged. These results suggest that CD11b+ cells possess certain plasticity in their function that ranges from stem/progenitor cells to differentiated cells. Therefore, we envision that CD11b+ myelomonocytes attain expression of PODOPLANIN upon influence from environmental cues, including lymphangiogenic cytokines, and become LEPCs, which can subsequently differentiate into lymphatic endothelial cells. Whether the expression of c-Kit and Sca1 can be also induced by enhanced PODOPLANIN expression remains to be determined. Alternatively, some c-KIT+CD11b+ progenitor cells in BM can attain the expression of PODOPLANIN upon influence from environmental cues. The possibility that a rare population of PODOPLANIN+CD11b+c-KIT+ cells is selectively expanded in response to cytokines can also be possible. Considering that the axis of SOX18-COUPTFII-PROX1 is critical for lymphatic specification and maintenance throughout embryogenesis and adult life as discussed above, it would also be interesting to see if such embryonic program is reactivated in response to environmental cues and induce a certain fraction of BM cells to adopt lymphatic identity.
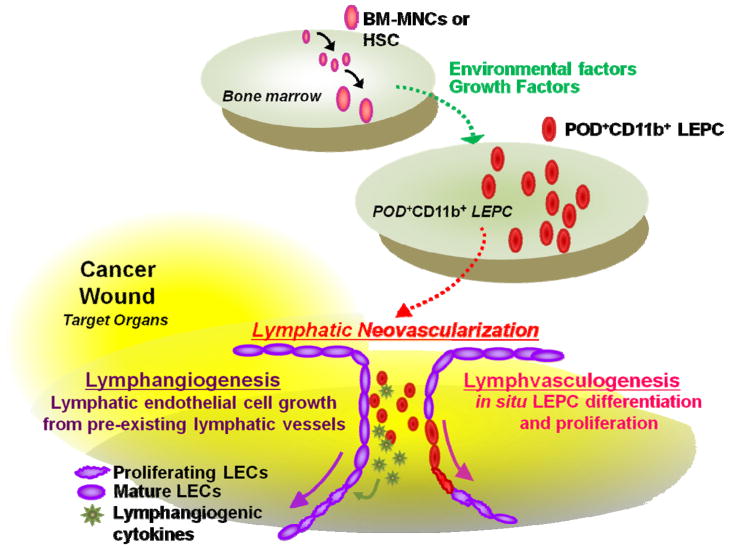
Our in vivo cell injection studies demonstrated that culture-isolated PODOPLANIN+ cells are not only incorporated into lymphatic vessels but also localized perivascularly and augmented peritumoral lymphatic vascular density (Lee et al., 2010). PODOPLANIN+ cells showed high pro-proliferative potential as well as high levels of lymphangiogenic cytokines. Furthermore, PODOPLANIN+ cells could facilitate fusion among newly formed lymphatic vessels by bridging the ends of the vessels (i.e. lymphatic vascular anastomosis), which lead to lymphatic neovascularization (Fantin et al., 2010). Together, this evidence implies that PODOPLANIN+ cells induce new lymphatic vessel growth through both lymphvasculogenesis and lymphangiogenesis (Figure 1).
Figure 1.
Lymphatic neovascularization mediated by BM-derived POD+CD11b+ cells. Pathophysiologic conditions such as tumors or wounds can induce generation and mobilization of POD+CD11b+ (POD+) LEPCs from BM to circulation, via growth factors or cytokines, including VEGFA and VEGFC. Although the mechanisms by which BM-MNCs or HSCs become POD+CD11b+ LEPCs need further investigation, the selective expression of POD in CD11b+ myelomonocytes in response to environmental cues can confer CD11b+ cells to express stem/progenitor cell characteristics. Alternatively, POD+CD11b+ cells in BM can be exclusively expanded in response to the above stimuli. The mobilized POD+CD11b+ LEPCs migrate to peripheral tissues and contribute to new lymphatic vessel formation through lymphvasculogenesis (i.e. LEPCs become LECs) and lymphangiogenesis (i.e. LEPCs provide lymphangiogenic factors to enhance proliferation of pre-existing LECs). POD; PODOPLANIN, HSCs; hematopoietic stem cells, BM-MNCs; bone marrow mononuclear cells
Clinical application of BM-derived cells for lymphatic disorders
Lymphedema is a disease caused by insufficiency of functional lymphatic vessels, leading to a failure of fluid uptake from surrounding tissues. Lymphedema can result from idiopathic causes or genetic defects, such as genetic mutations in FOXC2 and VEGFR3 (i.e. primary lymphedema), or from known causes, such as infection, trauma, and surgery (i.e. secondary lymphedema). Unfortunately, there is no known cure or effective treatment available at the moment. A fundamental approach to treat lymphedema would target new lymphatic vessel growth by providing lymphangiogenic signals that can grow lymphatic vessels and/or functional cells that can constitute lymphatic vessels. For example, VEGFC has been one of the most extensively studied lymphangiogenic factors. Using mouse models for secondary lymphedema and for wounds, the treatment of VEGFC either as a form of recombinant protein or via several vehicles, such as viral vectors and naked plasmids, showed increased lymphatic capillary formation, accompanied by reduced lymphedema (Saaristo et al., 2006; Saaristo et al., 2004; Szuba et al., 2002; Yoon et al., 2003). Recent evidence also hinted such therapeutic potential by BM-derived cells. Conrad et al (2009) reported that human and mouse MSCs express PROX-1 and enhance the expression of PODOPLANIN and VEGFR-3 in culture with VEGFC or LEC-conditioned medium (Conrad et al., 2009). These MSCs were shown to enhance lymphatic vessel growth and promote recovery from lymphedema. Given the mounting evidence that BM-derived PODOPLANIN+ cells can robustly induce lymphatic vessel growth through lymphangiogenesis and lymphvasculogenesis, future studies should address whether PODOPLANIN+ cells can attenuate or reverse lymphedema and intractable wound. One recent study reported that 9-cis retinoic acid, which is involved in a variety of cellular events, was able to promote recovery from lymphedema, at least, by activating the fibroblast growth factor (FGF) pathway (Choi et al., 2012). It may also be interesting to investigate whether 9-cis retinoic acid may augment LEPC action or mobilization on the foci of lymphatic vessel formation. One concern with using LEPCs in patients with post-cancer lymphedema to promote new lymphatic vessel formation is that dormant tumors may potentially grow or metastasize via lymphatic vasculature (Sleeman and Thiele, 2009). Another concern is that LEPCs may have potentially weak lymphvasculogenic effects, similar to the vasculogenic effects of other bone marrow cells in blood vessel formation (Gothert et al., 2004; Larrivee et al., 2005; Purhonen et al., 2008). However, we believe that the lymphatic endothelial cells lack basement membrane and have simpler structures; so, the LEPC contribution to the increased lymphatic vessel formation through lymphvasculogenesis may be more robust than EPC or other BM cell contribution to blood vessel formation.
PODOPLANIN expressed in tumor cells has been used for detecting various types of tumors and for evaluating their invasiveness, including kaposi sarcoma, hemangioendothelioma, epithelioid methothelioma, and esophageal squamous cell carcinoma (Kalof and Cooper, 2009). Our study further showed that the number of BM and circulating PODOPLANIN+ cells in the mononuclear cell fraction can reflect the tumor burden in mice subcutaneously implanted with melanoma cells or breast cancer cells. These data suggest that circulating PODOPLANIN+ cells may be used as a biomarker to gauge the development and the progression of certain types of tumors.
Acknowledgments
We appreciate Woan-Sang Kim for preparing and critical reading of the manuscript. This work was supported in part by Idea Grant Award from Department of Defense (W81XWH-09-1-0278); NIH grants DP3DK094346; and NIH contract, HHSN268201000043C (Program of Excellence in Nanotechnology Award); NSF-EBICS (Emergent Behaviors of Integrated Cellular Systems) grant, CBET-0939511.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Changwon Park, Department of Pharmacology, University of Illinois College of Medicine, Chicago, IL 60612, USA.
Ji Yoon Lee, Department of Medicine, Emory University School of Medicine, Atlanta, GA 30322, USA.
Young-sup Yoon, Department of Medicine, Emory University School of Medicine, Atlanta, GA 30322, USA.
References
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin-Wakkach C, Wakkach A, Quincey D, Carle GF. Interleukin-7 partially rescues B-lymphopoiesis in osteopetrotic oc/oc mice through the engagement of B220+ CD11b+ progenitors. Experimental hematology. 2006;34:851–859. doi: 10.1016/j.exphem.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4946–4951. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Lee S, Kyoung Chung H, Suk Lee Y, Eui Kim K, Choi D, Park EK, Yang D, Ecoiffier T, Monahan J, et al. 9-cis retinoic Acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic Acid for secondary lymphedema. Circulation. 2012;125:872–882. doi: 10.1161/CIRCULATIONAHA.111.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ, Ott HC, Jauch KW, Bruns CJ. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2009;119:281–289. doi: 10.1161/CIRCULATIONAHA.108.793208. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. The Journal of clinical investigation. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer research. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- Hieronymus T, Gust TC, Kirsch RD, Jorgas T, Blendinger G, Goncharenko M, Supplitt K, Rose-John S, Muller AM, Zenke M. Progressive and controlled development of mouse dendritic cells from Flt3+CD11b+ progenitors in vitro. J Immunol. 2005;174:2552–2562. doi: 10.4049/jimmunol.174.5.2552. [DOI] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development. 2009;136:191–195. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes & development. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalof AN, Cooper K. D2-40 immunohistochemistry--so far! Advances in anatomic pathology. 2009;16:62–64. doi: 10.1097/PAP.0b013e3181915e94. [DOI] [PubMed] [Google Scholar]
- Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Developmental biology. 2011;354:253–266. doi: 10.1016/j.ydbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature immunology. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annual review of pathology. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer research. 2001;61:1786–1790. [PubMed] [Google Scholar]
- Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, Tsuruo T. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. The Journal of biological chemistry. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nature medicine. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- Krause DS. Plasticity of marrow-derived stem cells. Gene therapy. 2002;9:754–758. doi: 10.1038/sj.gt.3301760. [DOI] [PubMed] [Google Scholar]
- Larrivee B, Niessen K, Pollet I, Corbel SY, Long M, Rossi FM, Olive PL, Karsan A. Minimal contribution of marrow-derived endothelial precursors to tumor vasculature. J Immunol. 2005;175:2890–2899. doi: 10.4049/jimmunol.175.5.2890. [DOI] [PubMed] [Google Scholar]
- Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, Yun SH, Yoon YS. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–1859. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. The Journal of clinical investigation. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. The Journal of experimental medicine. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen K, Zhang G, Ridgway JB, Chen H, Kolumam G, Siebel CW, Yan M. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood. 2011;118:1989–1997. doi: 10.1182/blood-2010-11-319129. [DOI] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. The Journal of cell biology. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nature medicine. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. The EMBO journal. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- Saaristo A, Tammela T, Farkkila A, Karkkainen M, Suominen E, Yla-Herttuala S, Alitalo K. Vascular endothelial growth factor-C accelerates diabetic wound healing. The American journal of pathology. 2006;169:1080–1087. doi: 10.2353/ajpath.2006.051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo A, Tammela T, Timonen J, Yla-Herttuala S, Tukiainen E, Asko-Seljavaara S, Alitalo K. Vascular endothelial growth factor-C gene therapy restores lymphatic flow across incision wounds. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1707–1709. doi: 10.1096/fj.04-1592fje. [DOI] [PubMed] [Google Scholar]
- Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. The EMBO journal. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. International journal of cancer Journal international du cancer. 2009;125:2747–2756. doi: 10.1002/ijc.24702. [DOI] [PubMed] [Google Scholar]
- Spyridonidis A, Zeiser R, Follo M, Metaxas Y, Finke J. Stem cell plasticity: the debate begins to clarify. Stem cell reviews. 2005;1:37–43. doi: 10.1385/scr:1:1:037. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. The Journal of biological chemistry. 2010;285:24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. The EMBO journal. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes to cells : devoted to molecular & cellular mechanisms. 2009;14:425–434. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. The Journal of clinical investigation. 2003;111:717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Han W, Shen B, Luo J, Shibuya M, He Y. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell research. 2010;20:1319–1331. doi: 10.1038/cr.2010.116. [DOI] [PubMed] [Google Scholar]
- Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PloS one. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]