Abstract
The cell signaling pathways of the mammalian target of rapamycin (mTOR) are broad in nature, but are tightly integrated through the protein complexes of mTORC1 and mTORC2. Although both complexes share some similar subcomponents, mTORC1 is primarily associated with the regulatory protein Raptor while mTORC2 relies upon Rictor. Pathways of mTOR that partner with Wnt as well as growth factor signaling are vital for endothelial and cardiomyocyte growth. In mature differentiated endothelial cells and cardiac cells, mTOR activation regulates both apoptotic and autophagic pathways during oxidative stress that can be dependent upon the activation of protein kinase B (Akt). These protective pathways of mTOR can promote angiogenesis and limit acute cell death to foster cardiac repair and tissue regeneration. However, under some conditions, blockade of mTOR pathways may be necessary to limit vasculopathy and promote microcirculatory flow. Future work that further elucidates the vital regulatory pathways of mTOR can offer new therapeutic insights for the treatment of cardiovascular diseases.
Keywords: Akt, cardiac, endothelial, erythropoietin, TORC1, TORC2, Wnt, wingless
Introduction
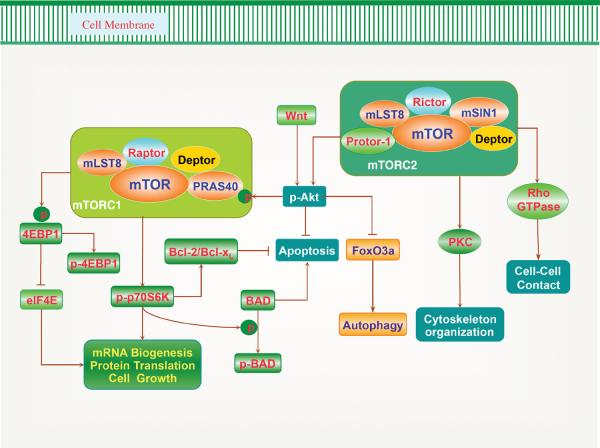
The signaling pathways of the mammalian target of rapamycin (mTOR) are closely linked to the formation of two different protein complexes (Chong et al. 2010; Hwang and Kim 2011) (Figure 1). The first complex, mTOR Complex 1 (mTORC1), relies upon the regulatory-associated protein of mTOR (Raptor) protein to enable mTORC1 to bind to its substrates. mTORC1 is also composed of the proline rich Akt substrate 40 kDa (PRAS40), the DEP domain-containing mTOR interacting protein (Deptor), and the mammalian lethal with Sec13 protein 8 (mLST8). mTORC1 controls the serine/threonine kinase ribosomal protein p70S6K and the eukaryotic initiation factor 4E-binding protein 1 (4EBP1). When 4EBP1 is hypophosphorylated, it can block protein translation by binding to eukaryotic translation initiation factor 4 epsilon (eIF4E) through the eukaryotic translation initiation factor 4 gamma (eIF4G), a protein that shepherds mRNA to the ribosome. Phosphorylation of 4EBP1 by mTORC1 leads to the dissociation of 4EBP1 from eIF4E to allow eIF4G to begin mRNA translation. mTORC1 phosphorylation also increases the kinase activity of p70S6K. Phosphorylation of the p70S6K serine/threonine kinase by mTORC1 results in mRNA biogenesis, translation of ribosomal proteins, and cell growth. In addition, PRAS40 can block the binding of the mTORC1 substrates p70S6K and 4EBP1 to Raptor (Chong et al. 2010; Hwang and Kim 2011).
Figure 1. Cellular signaling of mTOR.
The mammalian target of rapamycin (mTOR) functions through two complexes, mTORC1 and mTORC2. The components of mTORC1 include the catalytic multiple protein mTOR, regulatory-associated protein of mTOR (Raptor), proline rich Akt substrate 40 kDa (PRAS40), mammalian lethal with Sec13 protein 8 (mLST8), and DEP-domain-containing mTOR-interacting protein (Deptor). Once active, mTORC1 phosphorylates its two downstream targets, p70 ribosomal S6 kinase (p70S6K) and eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1), to regulate mRNA biogenesis, translation, and cell growth. p70S6K also promotes the phosphorylation of pro-apoptotic protein BAD and enhances the expression of anti-apoptotic protein Bcl-2/Bcl-xL. In addition to mTOR, mSLT8, and Deptor, mTORC2 also contains rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase interacting protein (mSIN1) and protein observed with Rictor-1 (Protor-1). mTORC2 primarily regulates cytoskeleton organization of the cell through its major downstream targets, protein kinase B (Akt) and protein kinase C (PKC). mTORC2 can also activate Rho GTPases and control cell to cell contact via Rho signaling pathways. Wnt can lead to Akt activation. Subsequently, Akt can directly phosphorylate PRAS40 to inhibit apoptosis and control autophagy through forkhead transcription factors, such as FoxO3a.
The second mTOR complex, mTORC2, is similar to mTORC1 in that it also is composed of mTOR, mLST8, and Deptor (Figure 1). However, mTORC2 has Rictor as a component rather than Raptor and associates with the mammalian stress-activated protein kinase interacting protein (mSIN1) and protein observed with Rictor-1 (Protor-1). Rictor is not sensitive to rapamycin and promotes the activity of mTORC2. mTORC2 controls actin cytoskeleton organization, cell size, endothelial cell survival and migration, and cell cycle progression. One target of mTORC2 is protein kinase B (Akt). Rictor allows mTORC2 to phosphorylate Akt at Ser473 to lead to its activation and to facilitate threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1). mTORC2 also regulates protein kinase C (PKC), P-Rex1, P-Rex2, Rho GTPases, and Rho signaling pathways that control cell to cell contact (Chong et al. 2010; Hwang and Kim 2011).
Stem cell proliferation
Expression of mTOR occurs in many systems of the body that include the cardiac, pulmonary, immune, reproductive, and gastrointestinal systems (Chong et al. 2010; Hwang and Kim 2011). As a result, mTOR can have a significant role in cell development with stem cell regulation (Table 1). In endothelial cells, mTOR may be necessary for endothelial progenitor cell development since inhibition of mTOR pathways with rapamycin lead to endothelial progenitor cell death that may result from inhibiting growth factor signaling (Miriuka et al. 2006). Growth factors, such as erythropoietin (EPO), rely upon mTOR pathway signaling. EPO controls angiogenesis, endothelial survival, and cardiomyocyte protection (Ammar et al. 2011; Maiese et al. 2005). EPO requires mTOR signaling for microglia survival during oxidative stress (Shang et al. 2011) and for osteoblastogenesis and osteoclastogenesis (Kim et al. 2012). However, in hematopoietic stem cells, mTOR may be associated with aging since mTOR activity is increased in the hematopoietic stem cells of older mice (Chen et al. 2009).
Table 1.
Regulatory Roles of mTOR in Cardiovascular System
| Targets | Biological function of mTOR |
|---|---|
| Stem Cells | Regulates proliferation/differentiation of embryonic stem cells Maintains the pluripotency of embryonic stem cells Promotes long-term renewal of embryonic stem cells Associates with aging of hematopoietic stem cells Mediates erythropoietin induced osteoblastogenesis and osteoclastogenesis Promotes endothelial progenitor cell development |
| Programmed Cell Death | Modulates apoptosis and autophagic cell death |
| Angiogenesis | Promotes the proliferation of endothelial progenitor cells and endothelial cells Prevents the induction of matrix metalloproteinases Promotes angiogenesis |
| Cardiovascular Disease | Protects cardiomyocytes against ischemia/reperfusion Inhibits autophagy in cardiomyocytes to prevent cardiac atrophy Prolonged activation leads to vasculopathy |
In relation to cardiac tissue, human embryonic stem cell-derived cardiomyocyte growth may be dependent upon mTOR, since pharmacological loss of mTOR can limit stem cell growth (Foldes et al. 2011). In general, it is believed that mTOR may have an important role for the proliferation and differentiation of embryonic stem cells. Deletion of the C-terminal six amino acids of mTOR that control kinase activity leads to a decrease in cell size and limits the proliferation of embryonic stem cells (Murakami et al. 2004). In addition, ablation of mouse mTOR gene results in the early lethality and arrest of embryonic stem cell proliferation (Gangloff et al. 2004). The mTOR pathway also has an important role in the maintenance of pluripotency and differentiation of cells. As a downstream target of mTOR, p70S6K is a critical factor for protein translational control. Expression of constitutively active p70S6K or siRNA-mediated knockdown of both tuberous sclerosis complex tuberin (TSC2) and Rictor to increase p70S6K activation leads to the differentiation of human embryonic stem cells (Easley et al. 2010). The activity of mTOR is also essential for the long-term renewal of human embryonic stem cells, since inhibition of mTOR impairs pluripotency, prevents cell proliferation, and enhances mesoderm and endoderm activities in embryonic stem cells (Zhou et al. 2009).
Stem cell proliferation and homeostasis through mTOR may require partnering with other pathways, such as the wingless pathway. Wnt proteins and their signaling pathways are vital to multiple cell process that involve stem cell proliferation, cell development, and cellular survival (Maiese et al. 2008). The Wnt pathway can increase the activity of mTOR through glycogen synthase kinase-3β (GSK-3β) (Chong et al. 2010). GSK-3β phosphorylates TSC2 on serine1337 and serine1341 in combination with the AMP activated protein kinase (AMPK) phosphorylation of TSC2 on serine1345 that results in the inhibition of mTOR activity. Wnt proteins counteract mTOR inhibition and foster mTOR activity by inhibiting GSK-3β through phosphorylation. In hematopoietic stem cells, the balance between Wnt and GSK-3β activation controls self renewal and lineage commitment (Huang et al. 2009).
Apoptosis, autophagy, and oxidative stress
Oxidative stress has a vital role in the onset of cardiovascular injury and can affect multiple related systems in the body that affect metabolic homeostasis, ischemic disease, and cardiopulmonary function (Maiese et al. 2010). The release of reactive oxygen species (ROS) leads to oxidative stress and promotes both cell injury as well as aging pathways. ROS can be generated in excessive quantities through different sources such as superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite. Under normal physiological conditions, ROS are scavenged by endogenous antioxidant systems that include superoxide dismutase, glutathione peroxidase, catalase, and vitamins that include C, D, E, and K.
Both apoptosis and autophagy may result in cardiac injury through oxidative stress (Figure 1). Apoptotic pathways can lead to cardiac failure, cardiomyocyte injury, cardiac metabolic disease, and reperfusion injury (Ammar et al. 2011; Das et al. 2011). Apoptosis has at least two phases that involve the early exposure of membrane phosphatidylserine (PS) residues and the subsequent destruction of genomic DNA (Chong et al. 2005). Externalization of membrane PS residues occur initially during cellular apoptosis and are a signal for the phagocytosis of cells. Apoptotic membrane PS exposure occurs in a broad array of cell that include cardiac, vascular, and inflammatory cells. The loss of membrane phospholipid asymmetry leads to the exposure of membrane PS residues on the cell surface and assists inflammatory cells to identify injured cells for phagocytosis. Exposure of membrane PS residues also affects the cardiovascular system since membrane PS externalization also occurs on platelets and has been associated with clot formation in the vascular system (Popescu et al. 2010).
Regulation of apoptosis by mTOR is mediated through 4EBP1 and p70S6K (Table 1). Activation of p70S6K by mTOR blocks apoptosis through pathways that can increase “anti-apoptotic” Bcl-2/Bcl-xL expression and inactivate the “pro-apoptotic” protein BAD (Pastor et al. 2009). In the absence of mTOR activity, 4EBP1 also binds to eIF4E to result in the translation of pro-apoptotic proteins and cardiac injury (Zhang et al. 2010). Blockade of apoptosis by mTOR also relies upon the serine-threonine kinase Akt. Akt activation leads to enhanced cell growth and cardiac protection during oxidative stress, endothelial cell injury, metabolic disease, and cardiac hypertrophy (Maiese et al. 2010). mTOR has been shown to require Akt activation to protect endothelial cells against apoptosis (Dormond et al. 2007) and mediate protection through the inactivation of forkhead transcription factors, such as FoxO3a (Chong et al. 2011; Dormond et al. 2007). Akt also functions to modulate apoptosis with mTOR through the inhibition of PRAS40 which can lead to the activation of apoptotic pathways (Thedieck et al. 2007). Phosphorylation of PRAS40 by Akt can inhibit the activity of this substrate and lead to its dissociation from mTORC1 and binding to cytoplasmic 14-3-3 proteins (Nascimento et al. 2010).
Autophagy is a process in which cells are able to recycle cytoplasmic components and dispose of defective organelles. This allows the early destruction of organelles and other cytoplasmic components but the preservation of cytoskeletal structures that is in contrast to processes that occur during apoptosis. Yet, autophagy can lead to cell death, such as during acute ischemic injury (Xin et al. 2011). In cardiomyocytes, cardiotoxicity by kinase inhibitor agents can be blocked during gene silencing of proteins responsible for autophagosome formation that involve Beclin 1 (autophagy-related gene 6 (Atg6)) (Zhao et al. 2010). However, autophagic degradation during normal physiology may be necessary with mTOR modulation to some degree since loss of Vps34, a known regulator of autophagy, can depress mTOR activation and result in cardiomegaly and decreased cardiac contractility (Jaber et al. 2012). In addition, benefits of exercise may require a brief inaction of mTOR for autophagic pathways to proceed (Ogura et al. 2011). In other circumstances, mTOR activation appears vital to block autophagy, possibly limit forkhead transcription FoxO3a activity, and prevent cardiac atrophy and dysfunction (Schips et al. 2011) (Table 1).
Angiogenesis, ischemic Injury, and cardioprotection
Angiogenesis, the process of new capillary formation, is an important component of cardiac tissue protection and regeneration that can be regulated by mTOR (Maiese et al. 2009). Inhibition of mTOR signaling, such as during the exposure to cigarette smoke, leads to a sequence of events with elevated matrix metalloproteinase-1, the blockade of tissue inhibitor of metalloproteases-3 that, and subsequent impaired angiogenesis (Lemaitre et al. 2011). Loss of mTOR activity also blocks endothelial proliferation and angiogenesis (Humar et al. 2002) as well as the proliferation of endothelial progenitor cells (Miriuka et al. 2006). Without effective angiogenesis following cardiac injury, tissue repair and regeneration may be limited or not occur at all (Table 1).
In regards to ischemic cardiac injury, activation of mTOR can be protective against oxidant ischemic/reperfusion injury in cardiomyocytes (Hernandez et al. 2011). Cardiac protection by mTOR may require the inhibition of GSK-3β through Wnt signaling (Vigneron et al. 2011), similar to other survival pathways that are both GSK-3β and Wnt dependent (Shang et al. 2012) (Figure 1). During periods of ischemic post-conditioning, mTOR is necessary to prevent apoptotic cardiac death (Wagner et al. 2010). In studies that block the phosphorylation and activation of p70S6K, cardiac infarct area and scar tissue are increased, further illustrating a cardioprotective role for the mTOR signaling pathway (Lajoie et al. 2009). It is important to note that prolonged activation of mTOR may have detrimental consequences to the cardiac system. Under these circumstances, inhibition of mTOR may be desirable. For example, chronic activation of mTOR can lead to vascular dysfunction. (Popescu et al. 2010) Inhibition of mTOR for prolonged treatment has been shown to reduce vasculopathy (Mancini et al. 2003) and to improve coronary flow through the microcirculation following cardiac transplantation (Sinha et al. 2008).
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest.
References
- Ammar HI, Saba S, Ammar RI, Elsayed LA, Ghaly WB, Dhingra S. Erythropoietin protects against doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2413–21. doi: 10.1152/ajpheart.01096.2010. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011;8:103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–91. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Ghosh J, Manna P, Sil PC. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem Pharmacol. 2011;81:891–909. doi: 10.1016/j.bcp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282:23679–86. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley CA, Ben-Yehudah A, Redinger CJ, Oliver SL, Varum ST, Eisinger VM, et al. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell Reprogram. 2010;12:263–73. doi: 10.1089/cell.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldes G, Mioulane M, Wright JS, Liu AQ, Novak P, Merkely B, et al. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy? J Mol Cell Cardiol. 2011;50:367–76. doi: 10.1016/j.yjmcc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–16. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Lal H, Fidalgo M, Guerrero A, Zalvide J, Force T, et al. A novel cardioprotective p38-MAPK/mTOR pathway. Exp Cell Res. 2011;317:2938–49. doi: 10.1016/j.yexcr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang Y, Bersenev A, O'Brien WT, Tong W, Emerson SG, et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin Invest. 2009;119:3519–29. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. Faseb J. 2002;16:771–80. doi: 10.1096/fj.01-0658com. [DOI] [PubMed] [Google Scholar]
- Hwang SK, Kim HH. The functions of mTOR in ischemic diseases. BMB Rep. 2011;44:506–11. doi: 10.5483/bmbrep.2011.44.8.506. [DOI] [PubMed] [Google Scholar]
- Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–8. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220–8. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie C, El-Helou V, Proulx C, Clement R, Gosselin H, Calderone A. Infarct size is increased in female post-MI rats treated with rapamycin. Can J Physiol Pharmacol. 2009;87:460–70. doi: 10.1139/y09-031. [DOI] [PubMed] [Google Scholar]
- Lemaitre V, Dabo AJ, D'Armiento J. Cigarette smoke components induce matrix metalloproteinase-1 in aortic endothelial cells through inhibition of mTOR signaling. Toxicol Sci. 2011;123:542–9. doi: 10.1093/toxsci/kfr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009;116:191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- Miriuka SG, Rao V, Peterson M, Tumiati L, Delgado DH, Mohan R, et al. mTOR inhibition induces endothelial progenitor cell death. Am J Transplant. 2006;6:2069–79. doi: 10.1111/j.1600-6143.2006.01433.x. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–8. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento EB, Snel M, Guigas B, van der Zon GC, Kriek J, Maassen JA, et al. Phosphorylation of PRAS40 on Thr246 by PKB/AKT facilitates efficient phosphorylation of Ser183 by mTORC1. Cell Signal. 2010;22:961–7. doi: 10.1016/j.cellsig.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Iemitsu M, Naito H, Kakigi R, Kakehashi C, Maeda S, et al. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun. 2011;414:756–60. doi: 10.1016/j.bbrc.2011.09.152. [DOI] [PubMed] [Google Scholar]
- Pastor MD, Garcia-Yebenes I, Fradejas N, Perez-Ortiz JM, Mora-Lee S, Tranque P, et al. mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem. 2009;284:22067–78. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010;116:993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schips TG, Wietelmann A, Hohn K, Schimanski S, Walther P, Braun T, et al. FoxO3 induces reversible cardiac atrophy and autophagy in a transgenic mouse model. Cardiovasc Res. 2011;91:587–97. doi: 10.1093/cvr/cvr144. [DOI] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011;8:270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4:187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SS, Pham MX, Vagelos RH, Perlroth MG, Hunt SA, Lee DP, et al. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. Am Heart J. 2008;155:889, e1–6. doi: 10.1016/j.ahj.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron F, Dos Santos P, Lemoine S, Bonnet M, Tariosse L, Couffinhal T, et al. GSK-3beta at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res. 2011;90:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- Wagner C, Tillack D, Simonis G, Strasser RH, Weinbrenner C. Ischemic postconditioning reduces infarct size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3beta, and apoptosis. Mol Cell Biochem. 2010;339:135–47. doi: 10.1007/s11010-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Xin XY, Pan J, Wang XQ, Ma JF, Ding JQ, Yang GY, et al. 2-methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci. 2011;38:631–8. doi: 10.1017/s031716710001218x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–16. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xue T, Yang X, Zhu H, Ding X, Lou L, et al. Autophagy plays an important role in Sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicol Appl Pharmacol. 2010;248:20–7. doi: 10.1016/j.taap.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:7840–5. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]