Abstract
Computational electrophysiology has proven useful to investigate the mechanisms of cardiac arrhythmias at various spatial scales, from isolated myocytes to the whole heart. Here we review how mathematical modeling has aided our understanding of human atrial myocyte electrophysiology to study the contribution of structural and electrical remodeling to human atrial fibrillation. We suggest potential new avenues of investigation and model development.
Models of the human atrial cardiomyocyte
It is well established that computational electrophysiology allows for an integrated understanding of cardiac (patho)physiological processes that may complement and inform experimental work with new testable predictions. In recent years, mathematical models of heart cell electrophysiology have become increasingly complicated and biophysically detailed due to increased availability of experimental data. Increased complexity is justified, for example, by the need to capture the subcellular nature of ion channel gating or compartmentation of signaling proteins. Specialized models for atrial and ventricular cells of several species have been made available, as well as for Purkinje, atrio-ventricular, and sino-atrial node cells, characterized by specific amalgams of ion channel expression and function. Limited availability of human samples for experiments has made perhaps even more crucial the development of human elecrophysiological models.
The Courtemanche et al. (1998) and Nygren et al. (1998) models have been the first attempts to establish a quantitative framework for elucidating the ionic mechanisms of human atrial myocyte electrophysiology and atrial fibrillation (AF) (Courtemanche et al. 1999). These models focused primarily on the description of transmembrane ion channels generating the atrial action potential (AP), whereas little emphasis was placed on intracellular Ca2+ dynamics. The Nygren and Courtemanche models have vastly different properties, especially in their rate-dependent behavior, due to marked differences in Ca2+ handling (Cherry et al. 2008). Maleckar et al. (2009) refined the K+ current description in the Nygren model, based on newly available data, to improve the representation of repolarization processes and reproduce AP rate-dependence. However, because of limited appropriate experimental data the formulation of Ca2+ handling was not improved.
Recently, Koivumaki et al. (2011) extended the Nygren model to describe the heterogeneous subcellular Ca2+ dynamics of human atrial cells accounting for a delay between peripheral and central sarcoplasmic reticulum (SR) Ca2+ release, which is expected in cells lacking t-tubules. However, atrial myocytes from human tissue exhibit an extensive t-tubular network (Richards et al. 2011), like atrial myocytes from large mammals (Dibb et al. 2009; Lenaerts et al. 2009). The role of Ca2+ in chronic AF (cAF), where indeed remodeling of the t-tubular network may occur, was not investigated.
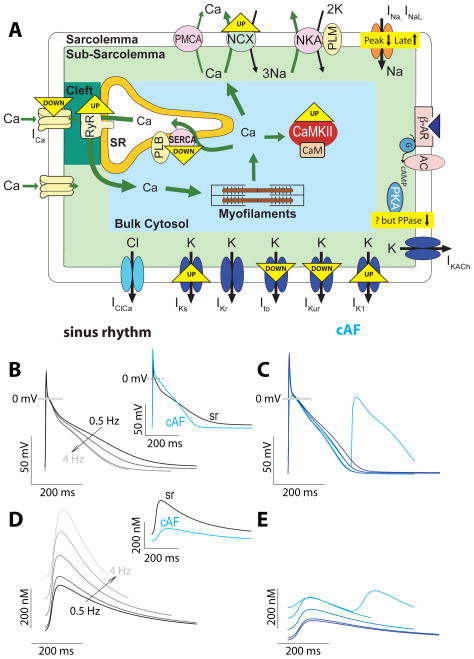
Understanding AF requires an integrated quantitative understanding of ionic currents and Ca2+ transport in healthy and remodeled human atrium. We have recently developed a new human atrial model (Fig. 1A) that provides an accurate representation of electrophysiology and Ca2+ homeostasis in human atrial myocytes in sinus rhythm and chronic AF conditions ((Grandi et al. 2011), Fig. 1B–E), built upon recent Ca2+ handling data at physiological temperature in atrial myocytes from patients in sinus rhythm and with cAF.
Figure 1.
A) Schematic representation of the electrophysiological and Ca2+ handling processes formulated in human atrial myocyte models. Alterations associated to AF are highlighted. Simulated APs (B and C) and Ca2+ transients (D and E) at various pacing rates in sinus rhythm and cAF respectively [Modified from (Grandi et al. 2011)]. Sinus rhythm (black) and cAF (blue) APs and Ca2+ transients at 1-Hz pacing frequency are shown in insets for comparison. AC: adenylate cyclase, ATP: adenosine triphosphate, β-AR: β-adrenergic receptor, CaM: calmodulin, cAMP: cyclic adenosine monophosphate, G: guanine nucleotide-binding protein, PKA: protein kinase A, PLB: phospholamban, PLM: phospholemman, PMCA: plasmalemmal Ca2+ pump, PPase: protein phosphatase.
While all models have been shown to replicate many fundamental aspects of atrial electrophysiology, the latter two have highlighted the strong impact of Ca2+ and Na+ dynamics on the AP and its rate-dependent characteristics.
Remodeling in human AF
AF is characterized by severe structural, electrical and contractile remodeling that is believed to contribute to the arrhythmogenic substrate and favor AF maintenance (see (Grandi et al. 2012) and key molecular/ionic bases of AF-induced remodeling in Fig. 1A). We (Grandi et al. 2011) and others (Courtemanche et al. 1999; Zhang et al. 2005) have accounted for the marked reduction in ICaL (Christ et al. 2004; Grandi et al. 2011; Van Wagoner et al. 1999; Voigt et al. 2009), Ito and IKur densities (Caballero et al. 2010), as well as IK1 enhancement (Van Wagoner et al. 1997) in cAF vs. sinus rhythm. For the first time, the reported enhancement in IKs (Caballero et al. 2010) and late INa (Sossalla et al. 2010) were incorporated in our cAF model (Grandi et al. 2011). cAF-induced alterations result in shortening of AP duration (APD, Fig. 1B, inset) and effective refractory period (ERP), and loss of rate-adaptation of both atrial repolarization (Fig. 1C vs. 1B, see below for proposed mechanism) and refractoriness compared to sinus rhythm, in agreement with experimental data (Van Wagoner et al. 1999; Workman et al. 2001).
Perturbations in intracellular Ca2+ handling (Fig. 1A) are important players in AF-induced atrial remodeling. Na+/Ca2+ exchange (NCX) is upregulated in cAF, (El-Armouche et al. 2006; Grandi et al. 2011; Neef et al. 2010; Voigt et al. 2009), whereas SR Ca2+ pump (SERCA) expression is decreased, causing slower Ca2+ transient decay vs. sinus rhythm (El-Armouche et al. 2006; Grandi et al. 2011; Voigt et al. 2009). SR Ca2+ leak is increased in AF, due to enhanced ryanodine receptor (RyR) activity (Neef et al. 2010; Voigt et al. 2009). Intracellular Ca2+ transient amplitude is diminished (Fig. 1D, inset), mostly due to reduced Ca2+ influx via ICaL. The positive dependency of Ca2+ transient amplitude on pacing frequency is markedly attenuated in our cAF model compared to sinus rhythm (Fig. 1E vs. Fig. 1D).
Ca2+ abnormalities (and enhanced Ca2+ loading expected at high atrial rates during AF) are in turn likely to influence atrial electrophysiology, via effects on Ca2+-dependent currents, as INCX, IKs, IClCa. It has been proposed that Ca2+-activated K+ (SK2) channels play a role in AF, although the functional impact of these channels is still controversial (Nagy et al. 2009), as knockout of SK2 channels causes AP prolongation and AF in mouse (Li et al. 2009). Incorporation into our model may well be warranted once more data on SK2 channel density, gating, and Ca2+-sensitivity are available. Stretch-activated Cl− channels may also be involved in AF (e.g., secondary to structural remodeling).
Rate-dependent APD adaptation
Typically, the human atrial APD shortens when paced at faster rates (Fig. 1B), but the mechanisms underlying this rate-dependent APD adaptation are not fully understood. Our human atrial myocyte model (and (Koivumaki et al. 2011)) predicted this is due to the increase of [Na+]i at fast pacing rates, which causes outward (repolarizing) shifts in Na+/K+ pump (NKA) and NCX currents that shorten the APD. An effect of [Na+]i accumulation to shorten APD has been shown previously in guinea pig (Faber and Rudy 2000), canine (Decker et al. 2009) and human (Grandi et al. 2010; Iyer et al. 2004) ventricle. Notably, we confirmed experimentally in human atrial myocytes the prediction of our model that acute block of (outward) INKA causes AP prolongation, followed by APD shortening as INKA increases secondary to [Na+]i increase, supporting the involvement of [Na+]i in APD and rate-dependent APD adaptation in human atrial cells (Grandi et al. 2011). The diminished Ca2+ transients and shorter APs in the remodeled human atrial myocyte cause less Ca2+ extrusion and less Na+ entry via Na+/Ca2+ exchange, and the blunted positive inotropy limits predicted Na+ accumulation, thus causing smaller outward shifts in NCX and NKA and contributing to the predicted (and measured) impairment of APD rate-adaptation in cAF (Fig. 1C).
Cell Signaling Pathways
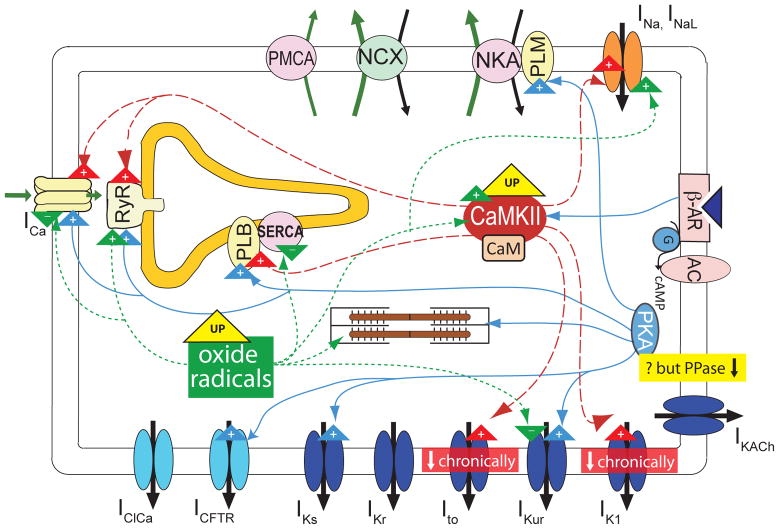
The (mal)adaptive alterations associated with human chronic AF may be accounted for, or complicated by, activation and/or crosstalk between cellular signaling pathways (Fig. 2).
Figure 2.
Schematic representation of adrenergic (blue), CaMKII (red) and oxidative (green) signaling (all enhanced in cAF) and effects on targets. CFTR: cystic fibrosis transmembrane conductance regulator.
Ca2+/calmodulin-dependent protein kinase II (CaMKII)
CaMKII phosphorylates several key Ca2+ handling and regulatory proteins (Maier and Bers 2007), myofilament proteins, and various sarcolemmal ion channels (Bers and Grandi 2009) (Fig. 2). CaMKII is more expressed and more phosphorylated in human cAF (Neef et al. 2010; Tessier et al. 1999), and may be responsible for the reported increase in RyR activity (Neef et al. 2010). Conversely, a blunted effect of CaMKII inhibition on ICaL in human cAF has been reported (Neef et al. 2010). Reduced inhibition of SERCA by hyperphosphorylated phospholamban (El-Armouche et al. 2006) in cAF could help maintaining a normal SR Ca2+ load despite increased RyR activity. CaMKII acutely enhances Ito in human atrial myocytes (Tessier et al. 1999), and this may reduce Ca2+ influx and minimize Ca2+ overload during AF by shortening the AP. On the other hand, a larger Ito is expected to accentuate the AP early repolarization, thus increasing the driving force for ICaL. The effects of Ito changes on Ca2+ influx are potentially complex and modeling may help identifying the net impact of these two opposite mechanisms. Increased CaMKII activity may also explain the AF-induced increase in late Na+ current (Sossalla et al. 2010).
Autonomic regulation
Both sympathetic and vagal activation have been shown capable of producing proarrhythmic changes in atrial APD and refractoriness and contributing to induction and/or perpetuation of AF.
β-adrenergic stimulation (Fig. 2) increases human atrial ICaL (Christ et al. 2004; Van Wagoner et al. 1999) and IKur (Li et al. 1996), which are predicted to result in no net effect on atrial APD (Grandi et al. 2011), consistent with experiments (Workman 2010). The increased ICaL elevates the AP plateau and, along with enhanced phospholamban phosphorylation, increases [Ca2+]i and favors afterdepolarizations (Workman 2010). Chronic AF potentiates the effect of β-adrenergic stimulation to increase human atrial ICaL (Christ et al. 2004; Van Wagoner et al. 1999), although basal ICaL is markedly reduced in cAF, possibly due to increased phosphatase activity (Christ et al. 2004).
Vagal stimulation activates the muscarinic-receptor activated K+ channel IKACh, which shortens APD (Koumi et al. 1994) and ERP, thus promoting reentry. Our model demonstrated an acetylcholine dose-dependent reduction in human atrial APD, consistent with previous modeling studies (Maleckar et al. 2008), and Ca2+ transient amplitude (Grandi et al. 2011). Chronic AF appears to induce constitutively active IKACh in human atrium, but attenuates the acetylcholine-mediated increase in atrial IKACh (Dobrev et al. 2005).
Inflammation and oxidative stress
Increased myocardial oxidative stress (Mihm et al. 2001; Van Wagoner 2008) and inflammation (Van Wagoner 2008) have been associated with AF, and might be involved in AF pathogenesis. Several ion channels (Zima and Blatter 2006) (Fig. 2), myofilament proteins (Mihm et al. 2001), and protein kinases (e.g., CaMKII (Howe et al. 2004)) and phosphatases are subject to redox modulation. S-nitrosylation of the L-type Ca2+ channel alpha subunit is increased in AF, and exogenously applied glutathione partially restores the AF-related ICaL reduction (Carnes et al. 2007), suggesting that oxidative stress may play a role in ICaL downregulation. Oxidation increases RyR open probability and may contribute to AF-associated RyR hyperactivity. K+ channels (e.g., Ito and IKATP) are also sensitive to redox states. Kv1.5 current is inhibited by S-nitrosylation (Nunez et al. 2006), which may contribute to IKur suppression in AF. Redox-dependent modulation of INa has also been reported (Fearon and Brown 2004) and may account for the increased late component in cAF (both directly and via CaMKII activation).
Crosstalk and/or synergy between β-adrenergic and acetylcholine or CaMKII pathways, or the effects of oxidative stress (and oxidation vs. nitrosylation) deserve further investigation and would be important extensions for future modeling studies, as done in ventricular models (Soltis and Saucerman 2010),(Heijman et al. 2011). In fact, while the effects on targets in Figure 2 have been shown, a direct causal link to AF remodeling has not yet been established. In addition, Ca2+ loading and subsequently altered Ca2+ signaling may be involved in AF-associated remodeling of ion-channel expression and/or function (Makary et al. 2011; Qi et al. 2008). The incorporation of such processes would be a challenging next step to take in modeling AF.
Multiscale simulations
Animal and clinical studies have suggested that AF is a reentrant arrhythmia sustained by reentrant circuits propagating in a remodeled atrial tissue substrate (Jalife 2011). Rapid ectopic activity (possibly generated in the pulmonary veins) may also contribute to AF maintenance. These mechanisms are thoroughly reviewed in a recent article by (Wakili et al. 2011). Thus, understanding the mechanisms underlying initiation of reentry requires the integration of multiscale data (at ionic channel, cellular, tissue and whole atria levels) into multidimensional computational frameworks.
Numerous studies have used tissue-level simulations to determine the mechanisms that help maintain, or terminate, the small reentrant sources (rotors) during AF. A recent paper analyzed systematically the relative importance of ionic currents and transporters in modulating excitability, refractoriness and rotor dynamics in the human atrium (Sanchez et al. 2011). The study highlighted the fundamental role of NKA in modulating APD, APD restitution, ERP, and reentrant dominant frequency (DF) both in sinus rhythm and AF. The importance of IK1, which was previously indicated as crucial in stabilizing rotors during cAF (Pandit et al. 2005), was confirmed. Also, INa was shown to alter atrial rotor dynamics by affecting conduction velocity and ERP. Specific blockade of IKur or Ito can also terminate rotor activity (Pandit et al. 2005), although the impact of these currents may be limited due to their downregulation in cAF (Sanchez et al. 2011). Emphasis has also been placed recently on the effects of structural remodeling (e.g., dilation, fibrosis) associated with AF (Krogh-Madsen et al. 2012). Ashihara et al. (2012) have also coupled fibroblasts to atrial myocytes in human atrial tissue simulations and predicted reduced APDs, conduction velocity and excitability, as previously shown (MacCannell et al. 2007). They concluded that fibroblast proliferation in atria might cause complex fractionated atrial electrograms, which are normally the target of cathether ablation, during persistent AF.
While a homogeneous 2-D tissue geometry is often chosen to avoid confounding interpretation of the underlying mechanisms due to added complexities of structural and electrophysiological heterogeneities (Pandit et al. 2005; Sanchez et al. 2011), atrial anatomical properties and heterogeneity may play a role in maintaining reentry (Kuo and Trayanova 2006) and should be considered. Human atria are characterized by significant regional electrophysiological differences (Wang et al. 1993), due to intrinsic variations in the ionic currents through the atria (Caballero et al. 2010; Voigt et al. 2010; Wang et al. 1993). We (Grandi et al. 2011) and others (Nygren et al. 1998) accounted for variability in AP morphology within and between atria. Clinical electroanatomic mapping showed localized sites of high-frequency activity during AF in humans (Sanders et al. 2005). In most experiments DF left-to right gradients are observed (Jalife 2011), and electrical isolation of the pulmonary vein from the left atrium is an effective strategy for preventing AF in many patients with paroxysmal AF (Medi et al. 2010). We simulated left-to-right gradients in repolarizing currents, and showed that the APD left-to-right gradient observed in sinus rhythm cells was reduced in cAF (Grandi et al. 2011), supporting the hypothesis that continuous fibrillatory activity may induce substantial remodeling and increase the likelihood of the appearance of new sources in either atrium (Sanders et al. 2005).
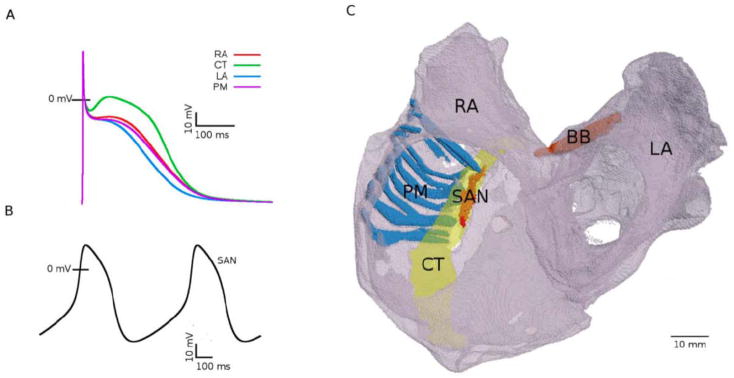
3-D human atria models have been developed (Harrild and Henriquez 2000; Jacquemet et al. 2006; Seemann et al. 2006) but somewhat limited by the lack of a complete set of experimental human data at the various scales. Recently, Aslanidi et al. (2011) developed a multiscale computational framework combining an integrated human atrial anatomical model with heterogeneous AP models for various cell types (Fig. 3) and coupled with a human torso model simulating body surface ECGs. This may constitute a useful platform that takes into account both spatial heterogeneity and anisotropy to study propagation in the normal and fibrillating atria and address some of the aforementioned issues. We hope that more recent and biophysically detailed models that include accurate descriptions of Ca2+ handling (Grandi et al. 2011; Koivumaki et al. 2011) will be incorporated in this rich platform.
Figure 3.
Heterogeneous model of the human atria [From (Aslanidi et al. 2011) with permission]. A) AP profiles in the right atrium, left atrium, pectinate muscle and crista terminalis cells. B) Spontaneous APs in the SAN. C) 3-D anatomical model of the human atria showing the main conductive bundles.
Therapeutics
Computational models have proven useful in understanding and developing novel antiarrhythmic drug therapy for AF. Modeling has been used to investigate the electrophysiological effects (Tsujimae et al. 2007) and mechanisms of AF termination (Comtois et al. 2008) by available drugs. Recently, this approach has been taken a step forward to determine the relationship between drug dynamic properties and atrial-selectivity (vs. ventricles), AF-selectivity (vs. sinus rhythm), and AF-termination effectiveness (Aguilar-Shardonofsky et al. 2012), and suggested the use of simulations in developing therapeutic agents with optimized pharmacodynamic properties for AF treatment.
Conclusions
It is now clear that cardiac electrophysiology, Ca2+ (and Na+) dynamics are intimately associated with respect to atrial arrhythmias. Integrating our present understanding of how they interact with cellular signaling networks may help linking mechanistically maladaptive atrial remodeling and AF. Incorporation of these detailed models into a multiscale computational framework accounting for atrial structural and electrophysiological heterogeneity will have important predictive value in understanding complex aspects of AF initiation and/or maintenance and aiding future therapeutic strategies.
Acknowledgments
Sources of support: NHLBI Grants P01-HL080101, R37-HL30077-29 and the Leducq Foundation (to DMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Shardonofsky M, Vigmond EJ, Nattel S, Comtois P. In silico optimization of atrial fibrillation-selective sodium channel blocker pharmacodynamics. Biophys J. 2012;102:951–60. doi: 10.1016/j.bpj.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashihara T, Haraguchi R, Nakazawa K, Namba T, Ikeda T, Nakazawa Y, Ozawa T, Ito M, Horie M, Trayanova NA. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: implications for electrogram-based catheter ablation. Circ Res. 2012;110:275–84. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidi OV, Colman MA, Stott J, Dobrzynski H, Boyett MR, Holden AV, Zhang H. 3D virtual human atria: A computational platform for studying clinical atrial fibrillation. Prog Biophys Mol Biol. 2011;107:156–68. doi: 10.1016/j.pbiomolbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:180–7. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero R, de la Fuente MG, Gómez R, Barana A, Amorós I, Dolz-Gaitón P, Osuna L, Almendral J, Atienza F, Fernández-Avilés F, et al. In Humans, Chronic Atrial Fibrillation Decreases the Transient Outward Current and Ultrarapid Component of the Delayed Rectifier Current Differentially on Each Atria and Increases the Slow Component of the Delayed Rectifier Current in Both. Journal of the American College of Cardiology. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Carnes CA, Janssen PM, Ruehr ML, Nakayama H, Nakayama T, Haase H, Bauer JA, Chung MK, Fearon IM, Gillinov AM, et al. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–73. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- Cherry EM, Hastings HM, Evans SJ. Dynamics of human atrial cell models: Restitution, memory, and intracellular calcium dynamics in single cells. Progress in Biophysics and Molecular Biology. 2008;98:24–37. doi: 10.1016/j.pbiomolbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ T, Boknik P, Wohrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-Type Ca2+ Current Downregulation in Chronic Human Atrial Fibrillation Is Associated With Increased Activity of Protein Phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- Comtois P, Sakabe M, Vigmond EJ, Munoz M, Texier A, Shiroshita-Takeshita A, Nattel S. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2008;295:H1489–504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol Heart Circ Physiol. 1998;275:H301–321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovascular Research. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- Decker KF, Heijman J, Silva JR, Hund TJ, Rudy Y. Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium. Am J Physiol Heart Circ Physiol. 2009;296:H1017–26. doi: 10.1152/ajpheart.01216.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb K, Clarke J, Horn M, Richards M, Graham H, Eisner D, Trafford A. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail. 2009;2:482–9. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–80. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78:2392–404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon IM, Brown ST. Acute and chronic hypoxic regulation of recombinant hNa(v)1.5 alpha subunits. Biochem Biophys Res Commun. 2004;324:1289–95. doi: 10.1016/j.bbrc.2004.09.188. [DOI] [PubMed] [Google Scholar]
- Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–66. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. Journal of Molecular and Cellular Cardiology. 2010;48:112–121. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi E, Workman AJ, Pandit SV. Altered Excitation-Contraction Coupling in Human Chronic Atrial Fibrillation. J Atr Fibrillation. 2012;2 doi: 10.4022/jafib.495. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrild D, Henriquez C. A computer model of normal conduction in the human atria. Circ Res. 2000;87:E25–36. doi: 10.1161/01.res.87.7.e25. [DOI] [PubMed] [Google Scholar]
- Heijman J, Volders PG, Westra RL, Rudy Y. Local control of beta-adrenergic stimulation: Effects on ventricular myocyte electrophysiology and Ca2+-transient. J Mol Cell Cardiol. 2011;50:863–71. doi: 10.1016/j.yjmcc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279:44573–81. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J. 2004;87:1507–25. doi: 10.1529/biophysj.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet V, van Oosterom A, Vesin JM, Kappenberger L. Analysis of electrocardiograms during atrial fibrillation. A biophysical model approach. IEEE Eng Med Biol Mag. 2006;25:79–88. doi: 10.1109/emb-m.2006.250511. [DOI] [PubMed] [Google Scholar]
- Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovasc Res. 2011;89:766–75. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivumaki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study. PLoS Comput Biol. 2011;7:e1001067. doi: 10.1371/journal.pcbi.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumi S, Arentzen CE, Backer CL, Wasserstrom JA. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation. 1994;90:2213–24. doi: 10.1161/01.cir.90.5.2213. [DOI] [PubMed] [Google Scholar]
- Krogh-Madsen T, Abbott GW, Christini DJ. Effects of electrical and structural remodeling on atrial fibrillation maintenance: a simulation study. PLoS Comput Biol. 2012;8:e1002390. doi: 10.1371/journal.pcbi.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SR, Trayanova NA. Action potential morphology heterogeneity in the atrium and its effect on atrial reentry: a two-dimensional and quasi-three-dimensional study. Philos Transact A Math Phys Eng Sci. 2006;364:1349–66. doi: 10.1098/rsta.2006.1776. [DOI] [PubMed] [Google Scholar]
- Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'Hooge J, Heidbuchel H, Sipido KR, Willems R. Ultrastructural and Functional Remodeling of the Coupling Between Ca2+ Influx and Sarcoplasmic Reticulum Ca2+ Release in Right Atrial Myocytes From Experimental Persistent Atrial Fibrillation. Circ Res. 2009;105:876–885. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]
- Li G-R, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic Modulation of Ultrarapid Delayed Rectifier K+ Current in Human Atrial Myocytes. Circ Res. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. The Journal of Physiology. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–32. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–40. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D, Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ Res. 2011;109:1031–43. doi: 10.1161/CIRCRESAHA.111.253120. [DOI] [PubMed] [Google Scholar]
- Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am J Physiol Heart Circ Physiol. 2009;297:H1398–1410. doi: 10.1152/ajpheart.00411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleckar MM, Greenstein JL, Trayanova NA, Giles WR. Mathematical simulations of ligand-gated and cell-type specific effects on the action potential of human atrium. Prog Biophys Mol Biol. 2008;98:161–70. doi: 10.1016/j.pbiomolbio.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medi C, Sparks PB, Morton JB, Kistler PM, Halloran K, Rosso R, Vohra JK, Kumar S, Kalman JM. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol. 2010;22:137–41. doi: 10.1111/j.1540-8167.2010.01885.x. [DOI] [PubMed] [Google Scholar]
- Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- Nagy N, Szuts V, Horvath Z, Seprenyi G, Farkas AS, Acsai K, Prorok J, Bitay M, Kun A, Pataricza J, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009;47:656–63. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients With Atrial Fibrillation. Circ Res. 2010;106:1134–44. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- Nunez L, Vaquero M, Gomez R, Caballero R, Mateos-Caceres P, Macaya C, Iriepa I, Galvez E, Lopez-Farre A, Tamargo J, et al. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res. 2006;72:80–9. doi: 10.1016/j.cardiores.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical Model of an Adult Human Atrial Cell : The Role of K+ Currents in Repolarization. Circ Res. 1998;82:63–81. doi: 10.1161/01.res.82.1.63. [DOI] [PubMed] [Google Scholar]
- Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–21. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–54. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- Richards MA, Clarke JD, Saravanan P, Voigt N, Dobrev D, Eisner DA, Trafford AW, Dibb KM. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am J Physiol Heart Circ Physiol. 2011;301:H1996–2005. doi: 10.1152/ajpheart.00284.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Corrias A, Bueno-Orovio A, Davies MR, Swinton J, Jacobson I, Laguna P, Pueyo E, Rodriguez B. The Na+/K+ pump is an important modulator of refractoriness and rotor dynamics in human atrial tissue. Am J Physiol Heart Circ Physiol. 2011;302:H1146–59. doi: 10.1152/ajpheart.00668.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- Seemann G, Hoper C, Sachse FB, Dossel O, Holden AV, Zhang H. Heterogeneous three-dimensional anatomical and electrophysiological model of human atria. Philos Transact A Math Phys Eng Sci. 2006;364:1465–81. doi: 10.1098/rsta.2006.1781. [DOI] [PubMed] [Google Scholar]
- Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and beta-adrenergic signaling in regulation of cardiac myocyte Ca2+ handling. Biophys J. 2010;99:2038–47. doi: 10.1016/j.bpj.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, et al. Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–42. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85:810–9. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- Tsujimae K, Suzuki S, Murakami S, Kurachi Y. Frequency-dependent effects of various IKr blockers on cardiac action potential duration in a human atrial model. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H660–H669. doi: 10.1152/ajpheart.01083.2006. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–13. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-Type Ca2+ Currents and Human Atrial Fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–81. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Voigt N, Trafford AW, Ravens U, Dobrev D. Abstract 2630: Cellular and Molecular Determinants of Altered Atrial Ca2+ Signaling in Patients With Chronic Atrial Fibrillation. Circulation. 2009;120:S667-d-668. [Google Scholar]
- Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–80. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–68. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993;73:276–85. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- Workman AJ. Cardiac adrenergic control and atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:235–49. doi: 10.1007/s00210-009-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovascular Research. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- Zhang H, Garratt CJ, Zhu J, Holden AV. Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovascular Research. 2005;66:493–502. doi: 10.1016/j.cardiores.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–21. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]