Abstract
Background
Local drug delivery from polymer-coated stents has demonstrated efficacy for preventing in-stent restenosis; however, both the inflammatory effects of polymer coatings and concerns about late outcomes of drug-eluting stent use indicate the need to investigate innovative approaches, such as combining localized gene therapy with stent angioplasty. Thus, we investigated the hypothesis that adenoviral vectors (Ad) could be delivered from the bare-metal surfaces of stents with a synthetic complex for reversible vector binding.
Methods and Results
We synthesized the 3 components of a gene vector binding complex: (1) A polyallylamine bisphosphonate with latent thiol groups (PABT), (2) a polyethyleneimine (PEI) with pyridyldithio groups for amplification of attachment sites [PEI(PDT)], and (3) a bifunctional (amine- and thiol-reactive) cross-linker with a labile ester bond (HL). HL-modified Ad attached to PABT/PEI(PDT)-treated steel surfaces demonstrated both sustained release in vitro over 30 days and localized green fluorescent protein expression in rat arterial smooth muscle cell cultures, which were not sensitive to either inhibition by neutralizing anti-Ad antibodies or inactivation after storage at 37°C. In rat carotid studies, deployment of steel stents configured with PABT/PEI(PDT)/HL-tethered adenoviral vectors demonstrated both site-specific arterial AdGFP expression and adenovirus-luciferase transgene activity per optical imaging. Rat carotid stent delivery of adenovirus encoding inducible nitric oxide synthase resulted in significant inhibition of restenosis.
Conclusions
Reversible immobilization of adenovirus vectors on the bare-metal surfaces of endovascular stents via a synthetic complex represents an efficient, tunable method for sustained release of gene vectors to the vasculature.
Keywords: restenosis, stents, gene therapy
Stent angioplasty has resulted in a paradigm shift in the treatment of occlusive vascular disease; however, neither bare-metal stents (BMS) nor polymer-coated drug-eluting stents (DES) represent ideal therapies at this time.1–3 Therefore, the present research focused on a molecular therapy approach to improved stent angioplasty with investigations of gene-delivery stents. Gene delivery from stents was first reported by our group using a poly(lactide-co-glycolide) polymer-coated stent,4 and investigations by others5–7 have also established the efficacy of this approach with several different therapeutic genes. Local delivery of gene therapy vectors from stents represents an advantageous strategy from several standpoints. Higher local arterial concentrations of gene vectors can be achieved at sites of disease activity with stent immobilization than with nonimmobilized vectors administered as an injectable suspension, while avoiding the distal spread of vectors. In addition, because cell activation and proliferation in the setting of in-stent restenosis are observed primarily in the vicinity of stent struts,8 the tethering of gene vectors to stent wires approximates vectors to the anticipated site of action.
A paramount consideration in the design of gene-delivery stents is the method of gene vector attachments to stents. Bulk polymer coatings on stents result in an inflammatory response that has been observed regardless of the type of polymer used or the therapeutic agent delivered.9 Thus, our group previously investigated gene vector delivery from the bare-metal surfaces of stents using a surface pretreatment with a custom-synthesized, water-soluble polybisphosphonate that provided a molecular monolayer for the affinity binding of adenovirus (Ad) vectors to the stent surfaces via anti-Ad antibody or a recombinant protein, D1, which consisted of the adenovirus-binding domain of the coxsackie adenovirus receptor.10 However, protein-based local delivery poses a number of important challenges for translational directions, including structural stability issues, species specificity, and vector-affinity optimization factors.
Therefore, in the present studies, we investigated an entirely synthetic strategy for tethering viral vectors to the bare-metal surfaces of stents. We sought to create a 3-component synthetic complex that includes a bifunctional (amine- and thiol-reactive) cross-linking agent (HL) for reactive binding to both the gene vector and the stent-surface–associated polybisphosphonates and incorporates a central hydrolyzable ester bond that enables vector release. The other components of the complex are a water-soluble polybisphosphonate that binds to the metallic surface of the stent, with engrafted latent thiol groups (PABT) for thioconjugation reactions, and a thiol amplifier, PEI(PDT), to enable increased levels of HL-Ad complexes to be attached to stent surfaces with thiol-based reactions.
Methods
Complete details of the research methods used in the present study are available in the online Data Supplement.
PABT/PEI(PDT) Pretreatment of Steel Surfaces and Subsequent Adenovirus Immobilization
Adenovirus immobilization on steel surface was achieved by the subsequent exposure of stainless steel stents and meshes to aqueous solutions of PABT and PEI(PDT), followed by covalent attachment of HL-modified viral vectors (see Data Supplement for details of chemical synthesis [supplemental Figures I through III] and vector immobilization). Adenoviral vectors that contain the genes for green fluorescent protein (GFP), firefly luciferase (Luc), and human inducible nitric oxide synthase (iNOS) are fully described in the Data Supplement.
Cell Culture Experiments
Animal experiments were approved by the IACUC of the Children’s Hospital of Philadelphia in accordance with National Institutes of Health Guidelines. Rat aortic smooth muscle cells (A10 cells; ATCC, Gaithersburg, Md) were cultured to 90% confluence. To determine any potential detrimental effects of HL modification on adenovirus infectivity, A10 cells were transduced with AdGFP modified with HL concentrations of 0, 25, 75, or 225 μmol/L at an adenovirus multiplicity of infection (MOI) of 1000. In the experiments that compared the relative effectiveness of nonimmobilized and mesh-tethered adenovirus vectors, equal amounts of free and mesh-immobilized AdGFP (1.6×108 particles) were added in triplicate into wells of 96-well plates either immediately on formulation or after a lag period of 20 hours’ storage in PBS at 37°C. In separate experiments, 1.6×108 mesh-immobilized or 2×109 unmodified AdGFP particles (a dose shown to achieve comparable expression to that of the immobilized counterpart) were exposed to escalating titers of neutralizing anti-knob antibody for 15 minutes before and then for the duration of transduction of A10 cells. In all in vitro studies, GFP expression was assessed after 48 hours by fluorescence microscopy and by live cell fluorometry (485 to 535 nm). To determine iNOS activity after transduction of A10 cells with unmodified (MOI 1 to 500; virus particle number range 2.74×106 to 1.37×109) or meshtethered (1.6×108 particles) AdiNOS, nitrite concentrations in the conditioned media were assayed by the Griess method.11
Rat Carotid Stent Angioplasty
Carotid stent angioplasties were performed in 450- to 500-g male Sprague-Dawley rats (Taconic Farms, Hudson, NY). Either BMS NIR stents (8 mm length; Boston Scientific, Natick, Mass) or the same stents formulated with 109 particles of AdGFP, AdLuc, or AdiNOS immobilized via HL tethering were used throughout the in vivo experiments. Animals implanted with AdGFP-configured stents (n=5) and respective BMS controls (n=5) were euthanized 2 and 7 days after stenting for reverse-transcription polymerase chain reaction and reporter-expression studies. Rats treated with AdLuc (n=4) and control BMS (n=4) underwent bioluminescence imaging (IVIS 100, Xenogen, Alameda, Calif) 5 minutes after local perivascular application of 3 mg of luciferin dissolved in 300 μL of 25% Pluronic gel (BASF Corp, Florham Park, NJ). Rats treated with either deployed AdiNOS (n=10) stents or control (n=10) BMS were euthanized 14 days after stenting. The stented carotid arteries were perfusion-fixed with formalin and embedded in methyl methacrylate (Technovit 9100, Heraeus, Kulzer, Germany).
Statistical Methods
Data are expressed as mean±SE. The significance of differences between means of experimental groups was determined with Student’s t tests.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Surface Immobilization of Adenovirus Vectors
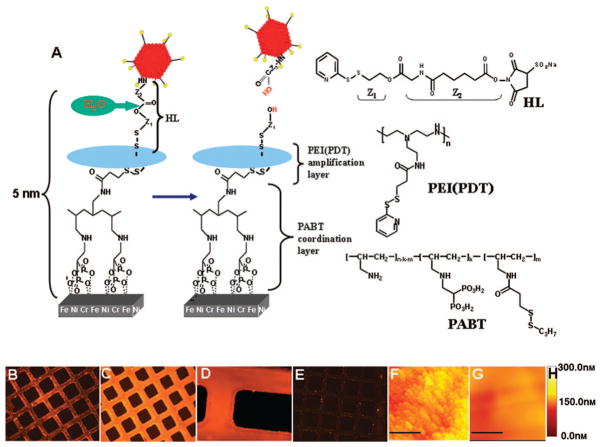
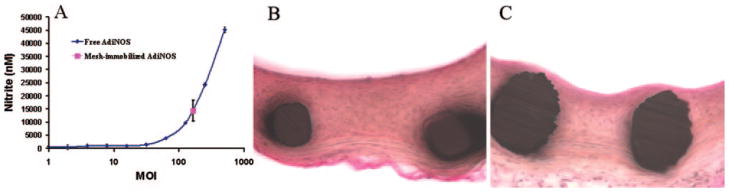
The surfaces of the stainless steel stents and meshes were rendered thiolated by a buildup of a PABT molecular monolayer and subsequent deprotection of thiol groups with a reducing agent, TCEP [tris-(2-carboxyethyl) phosphine; Figure 1A]. This strategy by itself was sufficient to achieve a significant binding of thiol-reactive adenovirus particles on the surface of model steel samples (Figure 1B); however, we chose to expand the amount of available thiol groups on the metal surfaces using an additional exposure of thiol-activated metal samples to an aqueous solution of PEI(PDT). Importantly, only a small fraction of the PDT groups in PEI(PDT) are consumed in the reaction with surface thiols (from PABT), which leaves the bulk of the PDT groups intact to effectively amplify the vector binding capacity. An additional reduction step with a reducing agent, DTT, was used to selectively reduce PDT disulfides into thiols while preserving disulfide bridges between PABT and PEI(PDT).12 Using a novel thiol quantification assay based on the reversible binding of the thiol-reactive dye AMCA-HPDP, we demonstrated that this amplification protocol resulted in a 4.5-fold increase of reactive thiol groups on model steel surfaces (739.3±45.9 versus 164.5±9.7 pmol/cm2; supplemental Figure IV) and led to more effective adenovirus tethering (compare Figure 1B and 1C).
Figure 1.
Adenoviral vector tethering on steel surfaces. A, Scheme illustrating specific chemical interactions to enable adenovirus binding. Type 5, E1, E3-deleted, replication defective adeno-viruses were modified via reacting lysine residues of capsid proteins with a bifunctional amine/thiol-reactive hydro-lyzable cross-linker (HL; see supplemental Figure I) that possessed a hydrolyzable ester bond separating fragments Z1 and Z2. Stainless steel meshes or stents were consecutively exposed to a solution of polyallylamine bisphosphonate comprising latent thiol groups (PABT) and a reducing agent, TCEP, to activate thiol groups on the surface. To expand the amount of available thiol functions, a subsequent treatment with polyethylene-amine modified with pyridyldithio groups, PEI(PDT), and DTT was used. Finally, HL-modified adenoviral vectors reacted with thiolated metal surfaces, which led to covalent tethering of adenovirus. The subsequent release of covalently immobilized adenovirus is dependent on the hydrolysis of the ester bond in the HL backbone. B through E, Fluorescence microscopy demonstrating Cy3-labeled adenovirus (red) tethered to steel surfaces (rhodamine filter set, 100× magnification for B, C, and E; 200× magnification for D). The absence of the PEI(PDT) amplification step (B) results in less vector binding than with the amplification protocol (C, D), whereas no PABT modification of the steel surface precludes adenovirus attachment (E). F through H, Atomic force microscopy of PABT/PEI(PDT)-modified and plain, nonmodified stainless steel surface after exposure to HL-modified adenovirus. Atomic force microscopy confirms the presence of adenovirus on the surface of steel mesh formulated according to the amplification protocol (F), whereas no vector binding was observed with the mesh that was not derivatized with PABT (G); the bar length in F and G is 1 μm. H, Atomic force microscopy depth scale.
In parallel with the steel surface modification, a fraction of the lysine residues in the capsid proteins of adenovirus vectors were covalently modified with a bifunctional (amine-and thiol-reactive) HL. Finally, HL-modified adenovirus vectors were tethered via disulfide bridges to the metal surfaces with a conjugation reaction between HL-derived PDT groups and thiol groups introduced onto the metal surfaces through the PABT/PEI (PDT) interaction (Figure 1A). Because the resultant disulfide bonds are stable under the nonreducing conditions of the extracellular microenviron-ment,13 the release of immobilized adenovirus and ensuing gene transfer in vitro and in vivo rely solely on the ester hydrolysis in the HL backbone (Figure 1A). The rate of hydrolysis is dependent on the specific chemical context in which the ester group is positioned and therefore potentially can be modulated by the use of HL possessing identical amino-reactive and thiol-reactive functionalities but differing in the backbone structure adjacent to the hydrolyzable ester link (not shown).
The binding of adenovirus vectors on the surfaces of steel meshes (Figure 1B and 1C) and stents (Figure 1D) was confirmed by fluorescence microscopy with adenovirus co-modified with both hydrolyzable HL and the amino-reactive fluorescent dye Cy3-NHS2. Cy3-labeled adenovirus was un-detectable on steel surfaces that were not modified with PABT/PEI(PDT) (Figure 1E). Furthermore, atomic force microscopy of the stainless steel mesh surface derivatized via PABT/PEI(PDT) and exposed to HL-modified adenovirus vector (Figure 1F), but not control mesh formulated without PABT and exposed to the same vector (Figure 1G), demonstrated copious surface coverage with morphologically intact adenovirus particles.
Previous research by our group had shown that the aqueous bisphosphonate exposure described above did not result in a macroscopic coating but instead formed a molecular monolayer several angstroms in thickness, as demonstrated by x-ray photoelectron spectroscopy.10 For the present formulations, scanning electron microscopy studies were performed that also demonstrated that PABT/PEI(PDT) aqueous pre-treatment of the steel surfaces did not result in a detectable coating (supplemental Figure V, panels A and B). Furthermore, viral vector surface attachment was also demonstrated by this technique (supplemental Figure V, panels C and D), in agreement with Figure 1G (atomic force microscopy results).
Release of Surface-Immobilized Adenovirus Vectors
To determine the release rate of HL-immobilized adenovirus from the PABT/PEI(PDT)-modified stainless steel surfaces, we prepared steel mesh disks with HL-treated, Cy3-labeled adenovirus and quantified the release of vector as the decrease of surface-associated fluorescence (Figure 2). As predicted by the postulated mechanism of release via HL hydrolysis, a sustained release of surface-tethered vector was observed, with 50% of the vector load detached after 2 weeks. More than 20% of adenovirus was still associated with the steel surface after 30 days (Figure 2).
Figure 2.
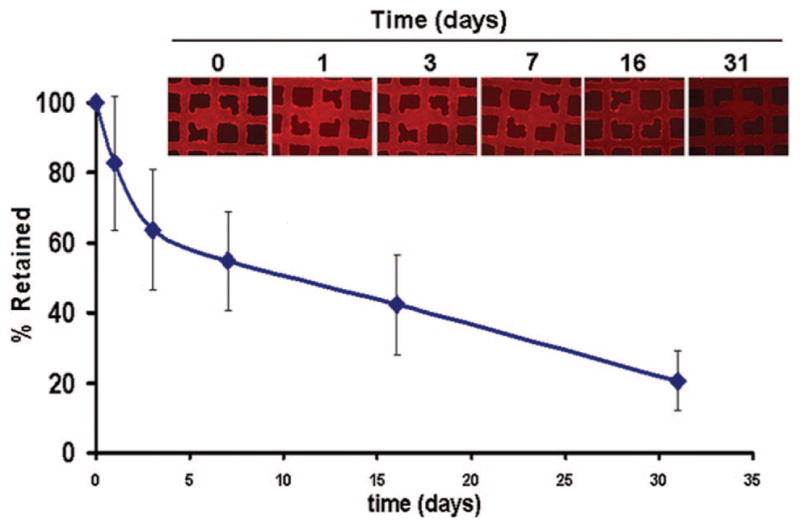
Controlled release of Cy3-labeled adenovirus vectors covalently attached to PABT/PEI(PDT)-modified steel meshes via hydrolyzable HL. The amount of vector retained on mesh surface over time was determined by fluorescence microscopy of steel mesh surfaces with bound Cy3-labeled adenovirus vectors, shown in the inserted fluorescent micrographs (original magnification ×200), as specified in Methods.
Impact of the Extent of Adenovirus Surface Modification on Vector Infectivity
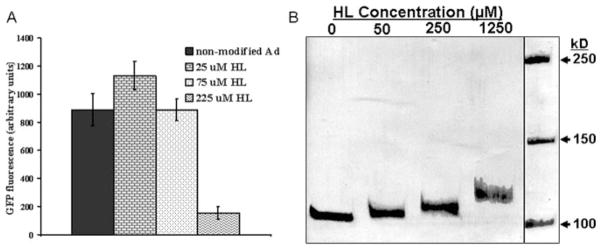
The infectivity of adenovirus was previously shown to depend on interactions between the fiber knob domain of adenovirus and the coxsackie adenovirus receptor on the surface of susceptible cells,14 as well as on the engagement of cell-surface αvβ5-integrins by the penton base proteins of the viral capsid.15 Random covalent modifications of capsid proteins with HL could potentially involve residues crucial for adenoviral vector receptor binding and internalization and therefore could hypothetically adversely affect the ability of modified vectors to transduce vascular cells. To elucidate potential deleterious effects of HL modification on virus infectivity, we transduced subconfluent A10 cells with an equal amount of either nonmodified AdGFP particles or AdGFP modified with 25, 75, and 225 μmol/L HL. As illustrated in Figure 3A, no decrease of adenovirus transduction capacity, as assessed by GFP fluorometry of transduced cells, was observed with HL concentrations up to 75 μmol/L. Higher concentrations of HL in the reaction mixture (225 μmol/L) resulted in an 82% drop of the infective adenovirus titer. Thus, the 75-μmol/L concentration of HL, which has no impact on the ability of adenovirus vector to transduce smooth muscle cells, was used for all subsequent in vivo experiments. In support of these observations, we also showed that the increase in apparent molecular weight of the most abundant capsid protein, hexon, due to covalent modification with a cross-linker was proportional to the HL concentration and was clearly observed with an HL concentration as low as 50 μmol/L in the reaction mixture (Figure 3B).
Figure 3.
The effects of HL on transduction effectiveness and apparent molecular weight of AdGFP vector. A, AdGFP activity after HL exposure is maintained at lower reactive levels of HL. A10 cells were transduced at an MOI of 1000 with either unmodified or HL-modified AdGFP as indicated. Transduction was determined fluorometrically and demonstrated no significant effect of HL on transgene expression in culture except at the highest concentration of HL. B, HL conjugation increases vector mass; polyacrylamide electrophoresis (PAGE) of native and HL-modified adenovirus. Unmodified or HL-conjugated AdGFP (2×1010 particles) was dissociated by boiling in Laemmli buffer before 5% PAGE and Coomassie blue staining, which demonstrated that increased levels of HL that have been reacted with AdGFP result in greater vector size.
In Vitro Transduction With HL-Immobilized Adenovirus
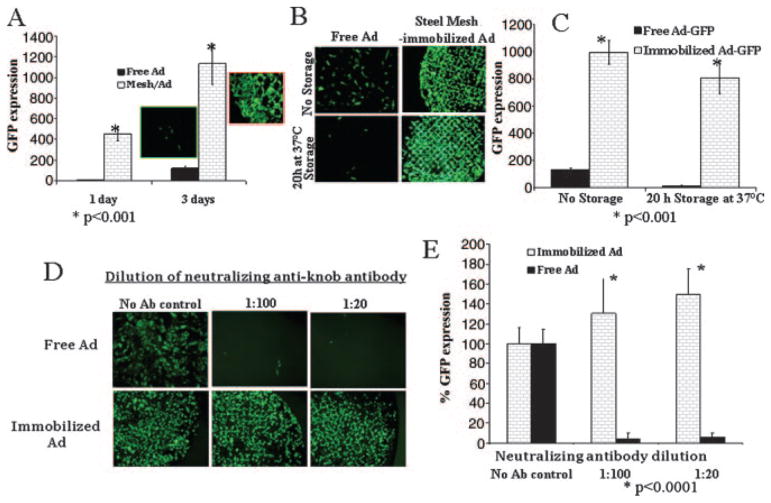
For the cell culture transduction experiments, 316L steel mesh disks were formulated with HL-tethered AdGFP. Placement of these disks on top of confluent A10 cells resulted in 98% transduction of cells underlying the disks with virtually no GFP expression in distal cells (Figure 4A). The same amount of AdGFP administered as a free vector suspension in cell culture media resulted in significantly lower transgene expression (Figure 4A).
Figure 4.
In vitro transduction with unmodified versus HL-modified mesh-immobilized AdGFP. A, Immobilized adenovirus results in greater levels of GFP activity than the equivalent amount of free adenovirus: PABT/PEI(PDT)-treated stainless steel meshes were configured with 1.6×108 particles of HL-modified AdGFP. A10 cells were transduced with the mesh-immobilized adenovirus or with an equal amount of unmodified vector. Transduction was determined by fluorometry in the well-scan mode. B and C, 37°C incubations do not diminish immobilized adenovirus transgene activity, unlike with free adenovirus. The formulations of mesh-tethered or free adenoviral vector were added to cells either immediately on preparation or after 20 hours’ storage in PBS at 37°C. Transduction was determined by fluorescence microscopy (B) with the FITC filter set, at 40×, and by fluorometry in the well-scan mode (C). D and E, Neutralizing anti-Ad antibodies do not diminish immobilized adenovirus transgene activity, unlike free adenovirus controls: PABT/PEI(PDT)-treated stainless steel meshes (1.6×108 AdGFP per mesh) or 2×109 free AdGFP particles were exposed to neutralizing anti-knob antibody before infection of the cells (see Methods for details). Transduction was determined by fluorescence microscopy (D) with the FITC filter set, 40×, and by fluorometry in the well-scan mode (E). Ab indicates antibody.
HL-Ad 37°C Stability Studies: Cell Culture Results
Adenovirus vectors are known to be temperature-labile, and their infectivity rapidly deteriorates at 37°C.16 The instability of adenovirus vector can hypothetically lead to circumstances in which adenovirus particles that are not released immediately from the surface of metal implants are infection/transduction-defective on their release at delayed time points. To address this potential stability problem, we stored AdGFP-configured mesh disks in PBS at 37°C for 20 hours before placement into A10 cell culture. No significant decrease of GFP expression was observed with HL-immobilized AdGFP as the result of this 37°C exposure. In contrast, exposure of nonimmobilized AdGFP to 37°C temperatures resulted in a profound decrease of infectivity (Figure 4B and 4C).
HL-Ad Resistance to Neutralizing Antibodies: Cell Culture Results
Preincubation of nonimmobilized AdGFP with 0.5 μmol/L neutralizing antibody before infection of A10 cells resulted in a 95% decrease of ensuing transgene expression. However, the exposure of mesh disks with HL-immobilized AdGFP to the same antibody at a 500-fold higher concentration did not affect focal GFP expression in the cells underlying the disks (Figure 4D and 4E). Thus, covalent tethering of adenovirus to the steel disks conferred protection from neutralizing anti-knob antibodies, which are prevalent in both laboratory animals17 and humans18 and represent a formidable problem for successful adenovirus-based gene therapy.
In Vivo Studies: HL-Ad Reporter Results
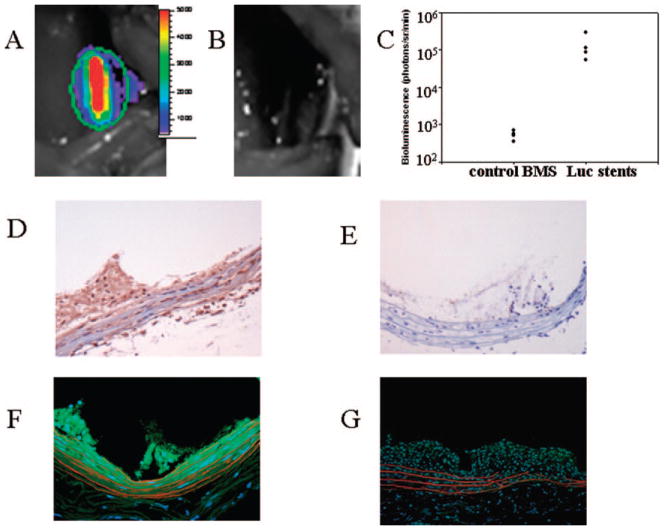
To characterize vascular gene delivery from the stent surfaces in vivo, AdLuc and AdGFP were separately reacted with HL, followed by tethering to the surface of PABT/PEI (PDT)-modified stainless steel stents with HL. Overall arterial transgene expression was determined by intravital bioluminescence performed 2 days after HL-modified AdLuc stent deployment in rat carotid arteries. A robust signal emitted from the stented segment of carotid arteries was present in all tested animals (Figure 5A and 5C). No spread of luminescent signal to the nonstented portion of arteries or to adjacent anatomic structures was noted. Furthermore, an absence of luciferase activity was noted in the rats implanted with control BMS (without PABT exposure) incubated with HL-modified AdLuc (Figure 5B and 5C).
Figure 5.
Reporter studies with a rat stent angioplasty mode demonstrating efficient delivery and transgene expression with vector immobilized on the surfaces of the stents. A–C, Luciferase optical imaging studies with luciferin bioluminescence. Stainless steel NIR stents were formulated with 109 particles of AdLuc tethered via PABT/PEI(PDT)/HL interactions. Two days after deployment of AdLuc stents (A) or control BMS (B), the animals underwent bioluminescence imaging after local periarterial delivery of 3 mg of luciferin in a Pluronic gel. The individual data from 8 animals are presented in a point scatterplot with a logarithmic scale y-axis (C). D and E, AdGFP immobilized on stent surfaces. Immunostaining studies documented efficient transgene expression. Stainless steel NIR stents were formulated with 109 particles of AdGFP immobilized by a PABT/PEI(PDT)/HL conjugation strategy. Animals stented with AdGFP stents (D) or control BMS (E) were euthanized 7 days after stent deployment. Cryoembedded arterial sections were immunostained with DAB/peroxidase (brown staining) with anti-GFP antibody and counterstained with hematoxylin. Original magnification ×200. F and G, AdGFP immobilized on stent surfaces: fluorescent microscopy studies. Stainless steel NIR stents were formulated with 109 particles of AdGFP immobilized by a PABT/PEI(PDT)/HL conjugation strategy. Animals stented with AdGFP stents (F) or control BMS (G) were euthanized 7 days after stent deployment. Sections were directly examined for GFP fluorescence after Evans blue/DAPI staining. F and G are merged images obtained with DAPI, FITC, and rhodamine filter sets; original magnification ×200.
To obtain a spatial assessment of the reporter-expression distribution after stent-based adenoviral vector delivery, the HL/AdGFP-modified stents were deployed in an additional series of rats. Arteries retrieved at 2 and 7 days after stenting were assessed for GFP fluorescence by 2 methods: quantitative DAB-peroxidase immunohistochemistry10 and confirmatory fluorescence microscopy.10 Per immunochemical analysis, GFP-positive cells were abundant in parietal thrombus, media, and adventitia of the arteries harvested 2 days after stenting (Table 1). Likewise, GFP-transduced cells were demonstrated in the media, adventitia, and nascent neointima of arteries retrieved 7 days after deployment of AdGFP-configured stents (Figure 5D; Table 1), whereas only background nonspecific staining was seen in arteries treated with control BMS (Figure 5E) at both time points. However, the distribution of GFP-immunopositive cells was comparable and not statistically different in both the 2- and 7-day explants (Table 1). Furthermore, we were able to confirm the presence of GFP-transduced cells by direct fluorescent microscopy (Figure 5F) after Evans blue staining, which shifts the autofluorescence of elastin into the red part of spectrum and unmasks the characteristic GFP-related signal.10
Table 1.
Efficient Arterial Transgene Expression After AdGFP-Stent Delivery: Immunohistochemistry Morphometry Results
| Group | No. of Rats | Labeling (% of GFP-Positive to Total Cells)
|
||
|---|---|---|---|---|
| Organized Thrombus/Neointima | Media | Adventitia | ||
| GFP stents (2 days) | 5 | 43.3±4.4 | 32.5±5.2 | 14.1±3.1 |
| GFP stents (7 days) | 5 | 46.4±5.3 | 25.6±2.4 | 28.8±6.8 |
Polymerase chain reaction studies confirmed the presence of AdGFP vector in the arterial segments underlying stents and demonstrated that no distal vector spread from the stented carotid segments to liver, spleen, blood, kidney, or myocardium (supplemental Figure VI). In addition, ongoing transcription of the GFP transgene at the 2- and 7-day time points was confirmed in stented but not in contralateral carotid segments by reverse-transcription polymerase chain reaction (supplemental Figure VII). Furthermore, with Cy3-labeled, HL-immobilized AdGFP, extensive surface coverage of stents harvested at day 7 after stenting with fluorescent vector particles was observed consistently (supplemental Figure VIII).
AdiNOS Delivery: Cell Culture Studies With HL-Immobilized AdiNOS
Nitric oxide is a key regulator of vascular homeostasis, with established antirestenotic properties.19 To investigate whether the production of nitric oxide in cultures of smooth muscle cells can be increased on delivery of reversibly immobilized AdiNOS, the conditioned media of A10 cells treated either with 1.6×108 particles of AdiNOS tethered to steel mesh disks via HL-PABT reactions as above or with incremental amounts of free AdiNOS were analyzed. Although transduction with free AdiNOS resulted in a dose-dependent increase in nitrites (as the stable end product of nitric oxide metabolism) in conditioned media, localized gene delivery with the AdiNOS-tethered mesh disks resulted in the accumulation of nitrites that corresponded to transduction with free AdiNOS at an MOI of 150 (Figure 6A).
Figure 6.
NO production and inhibition of in-stent restenosis with immobilized AdiNOS vectors. A, Stainless steel mesh-immobilized AdiNOS results in more efficient NO production than MOI equivalents of free adenovirus. Relative NO production (per Griess assay) after transduction of A10 cells with unmodified AdiNOS (MOI range 1 to 500) or with 1.6×108 AdiNOS particles (corresponding to MOI of 60) tethered via PABT/PEI(PDT)/HL complex formation. B and C, Significant inhibition of in-stent restenosis with stent delivery of immobilized AdiNOS. Representative photomicrographs of arterial sections treated with control BMS (B) and AdiNOS-tethered stents (C). Verhoeffvan Gieson elastic stain, original magnification ×200.
In Vivo Studies: HL-AdiNOS Results
In the therapeutic arm of the present studies, AdiNOS, which drives the synthesis of the inducible form of nitric oxide synthase, was used. This vector previously has been shown by us10 and others20–22 to provide antirestenosis efficacy when delivered in conjunction with balloon injury20 or stenting.22 In the present studies, an overexpression of iNOS in rat carotid arteries after deployment of stents configured with our synthetic complex that released an HL-modified vector resulted in a 56% reduction of neointimal formation as assessed by neointima/media ratio (Figure 6B and 6C; Table 2). The extent of cross-sectional stenosis was also reduced significantly as the result of AdiNOS delivery from stents (Table 2).
Table 2.
Inhibition of Restenosis Due to AdiNOS-Stent Delivery: Morphometric Data
| Group | No. of Rats | Neointima/Media Ratio | % of Stenosis | Neointima, mm2 |
|---|---|---|---|---|
| BMS | 10 | 2.26±0.21 | 30.87±3.0 | 0.456±0.043 |
| AdiNOS stents (Ad-tethered with the synthetic complex) | 10 | 1.06±0.10* | 17.54±1.8* | 0.248±0.028* |
P<0.001 for all 3 comparisons.
Discussion
These investigations addressed the challenge of arterial gene transfer with a novel stent-based gene-delivery system based on a synthetic complex for direct chemical coupling of gene vectors to the surface of BMS via labile HL that are capable of releasing vector on spontaneous hydrolysis. Prior reports23,24 have demonstrated that suppressed neointimal growth can resume if the inhibiting impact is withdrawn prematurely, which indicates the need for sustained release of gene vector over a 2- to 4-week vulnerable period; however, in studies by others, up to 90% of the gene therapy payload passively immobilized in stent coatings is typically released within 12 to 24 hours.5,7 In the present studies, the stents formulated with HL-tethered Cy3Ad and explanted at 7 days after deployment demonstrated significant surface coverage with labeled adenovirus vector (supplemental Figure VII). Conceptually, vector release rates could be modulated by the use of HLs with different chemical structures, with faster or slower hydrolysis rates, or perhaps by use of a mixture of HLs with different hydrolysis kinetics. This could enable the development of a truly disease-tailored gene-delivery platform with the peak of vector release adjusted to a specific, pathophysiology-based time course.
Although we used adenoviral vectors in the present study, the synthetic complex reported herein could be used to tether any type of viral gene therapy construct to metal surfaces. Furthermore, pharmaceuticals could be subjected to thiol derivatization and then, hypothetically, could also be linked to metal surfaces with the synthetic complexes, which raises the possibility of multiple local-delivery strategies that could incorporate synergistic therapeutic agents. It is also noteworthy that the synthetic complex used in the present study could be used to incorporate a peptide layer onto metal surfaces to enhance cellular interactions (for example, RGD-containing peptides could be used to promote reendothelialization).
Although the present studies focused on delivery mechanisms and demonstrated both efficient reporter transgene expression and anti–in-stent restenosis efficacy in a rat carotid stent angioplasty model, the results presented herein have certain limitations. The rat carotid stent angioplasty model system has been investigated and validated by others25–31 and reproduces key aspects of poststenting arterial injury that are comparable to observations in large-animal models and human clinical pathology investigations.25,27 Nevertheless, preclinical studies in general use large-animal models, such as pig coronary stenting; therefore, translational directions for the present approach should investigate large-animal stent angioplasty efficacy. Furthermore, there has been a paucity of studies in animal models assessing gene therapy for in-stent restenosis in a disease setting.6 Thus, the present formulations for gene-delivery stents ideally should be further investigated in large-animal disease models that come closest to human pathophysiology to elucidate both delivery mechanisms and efficacy.
It is concluded that a synthetic complex can mediate local, sustained release of adenovirus-gene vectors from metallic surfaces. The complex, which consisted of PABT/PEI(PDT)-pretreated metallic surfaces that in turn can tether HL-reacted adenovirus, results in the controlled release of active adeno-viral vectors, enhanced vector stability, and efficient trans-gene expression in vitro and in vivo and is effective for inhibiting in-stent restenosis via local delivery of AdiNOS.
Supplementary Material
CLINICAL PERSPECTIVE.
Stent angioplasty for coronary disease has made a major impact on improved therapeutic outcomes, and local delivery of highly potent drugs, such as paclitaxel or sirolimus, with polymer-coated drug-eluting stents is effective clinically for inhibiting the development of in-stent restenosis; however, it is also apparent that the current drug-eluting stents do not represent an ideal approach. Nevertheless, recent clinical and experimental results concerning drug-eluting stents indicate that local-delivery therapeutic strategies for cardiovascular disease will become increasingly important. The present study investigated local delivery of gene therapy from stent surfaces by use of a novel strategy of reversible chemical attachment of vectors to bare-metal stents via a 3-component, hydrolyzable synthetic complex. Adenoviral (Ad) vectors immobilized on the surfaces of stents with a packing density up to 5×109 adenovirus particles/cm2 exhibited prolonged (>1 month) release kinetics and demonstrated highly efficient site-specific transduction of target cell types in vitro and in vivo in rat carotid stent angioplasty studies. Moreover, the tethering of adenovirus vectors to steel surfaces allowed effective transduction in vitro in the presence of neutralizing anti-Ad knob antibodies that otherwise completely blocked activity of nonimmobilized vector. In rat carotid stent studies, the deployment of stents configured with 109 adenovirus vectors that were encoded for the inducible form of nitric oxide synthase (Ad-iNOS) resulted in a significant reduction of in-stent restenosis compared with bare-metal stents. These findings validate the concept of gene-eluting stents and warrant further translational investigations.
Acknowledgments
The authors thank Natalie Perry (The Children’s Hospital of Philadelphia) for her assistance in preparing this manuscript. We also thank Jeanne M. Connolly (The Children’s Hospital of Philadelphia) for her efforts with the illustrations. Thanks to Dee Breger, Drexel University, Philadelphia, for providing scanning electron microscopy services and expertise.
Sources of Funding
This research was supported by a grant from the National Institutes of Health, HL72108 (Dr Levy), and a Scientist Development Grant from the American Heart Association (Dr Fishbein). Support was also provided by the William J. Rashkind Endowment of The Children’s Hospital of Philadelphia.
Footnotes
The online-only Data Supplement, consisting of an expanded Methods section and figures, is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.746412/DC1.
Disclosures
None.
References
- 1.Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, Iida O, Sera F, Nanto S, Hori M, Nagata S. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116:910–916. doi: 10.1161/CIRCULATIONAHA.105.609057. [DOI] [PubMed] [Google Scholar]
- 2.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 3.Ellis SG, Colombo A, Grube E, Popma J, Koglin J, Dawkins KD, Stone GW. Incidence, timing, and correlates of stent thrombosis with the polymeric paclitaxel drug-eluting stent: a TAXUS II, IV, V, and VI meta-analysis of 3,445 patients followed for up to 3 years. J Am Coll Cardiol. 2007;49:1043–1051. doi: 10.1016/j.jacc.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi A, Palmer-Opolski M, Smith RC, Walsh K. Transgene delivery of plasmid DNA to smooth muscle cells and macrophages from a biostable polymer-coated stent. Gene Ther. 2003;10:1471–1478. doi: 10.1038/sj.gt.3302010. [DOI] [PubMed] [Google Scholar]
- 6.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, Yoon YS, Heidenreich R, Hanley A, Kearney M, Tio FO, Kuenzler P, Isner JM, Losordo DW. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, Oberhoff M. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol. 2005;25:754–759. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- 8.Yutani C, Ishibashi-Ueda H, Suzuki T, Kojima A. Histologic evidence of foreign body granulation tissue and de novo lesions in patients with coronary stent restenosis. Cardiology. 1999;92:171–177. doi: 10.1159/000006967. [DOI] [PubMed] [Google Scholar]
- 9.van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Jr, Ellis SG, Topol EJ. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94:1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 10.Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Vohs JM, Wong GS, Felderman H, Chen IW, Choi H, Wilensky RL, Levy RJ. Bisphosphonate-mediated gene vector delivery from the metal surfaces of stents. Proc Natl Acad Sci U S A. 2006;103:159–164. doi: 10.1073/pnas.0502945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooney R, Hynes SO, Duffy AM, Sharif F, O’Brien T. Adenoviral-mediated gene transfer of nitric oxide synthase isoforms and vascular cell proliferation. J Vasc Res. 2006;43:462–472. doi: 10.1159/000095163. [DOI] [PubMed] [Google Scholar]
- 12.Dent AH, Aslam M. The preparation of protein-protein conjugates. In: Aslam M, Dent AH, editors. Bioconjugation: Protein Coupling Techniques for the Biomedical Sciences. London, United Kingdom: Macmillan Reference Ltd; 1998. pp. 216–363. [Google Scholar]
- 13.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 14.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 15.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 16.Croyle MA, Yu QC, Wilson JM. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther. 2000;11:1713–1722. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- 17.Schulick AH, Vassalli G, Dunn PF, Dong G, Rade JJ, Zamarron C, Dichek DA. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries: potential for immunosuppression and vector engineering to overcome barriers of immunity. J Clin Invest. 1997;99:209–219. doi: 10.1172/JCI119149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AF, Ren J, Miao CY. Nitric oxide synthase gene therapy for cardiovascular disease. Jpn J Pharmacol. 2002;89:327–336. doi: 10.1254/jjp.89.327. [DOI] [PubMed] [Google Scholar]
- 20.Shears LL, II, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, Watkins SC, Tzeng E. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998;187:295–306. doi: 10.1016/s1072-7515(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 21.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, Makaroun MS, Billiar TR, Rhee RY. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 22.Wang K, Kessler PD, Zhou Z, Penn MS, Forudi F, Zhou X, Tarakji K, Kibbe M, Kovesdi I, Brough DE, Topol EJ, Lincoff AM. Local adenoviral-mediated inducible nitric oxide synthase gene transfer inhibits neointimal formation in the porcine coronary stented model. Mol Ther. 2003;7:597–603. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 23.Kishore R, Losordo DW. Gene therapy for restenosis: biological solution to a biological problem. J Mol Cell Cardiol. 2007;42:461–468. doi: 10.1016/j.yjmcc.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Sirois MG, Simons M, Kuter DJ, Rosenberg RD, Edelman ER. Rat arterial wall retains myointimal hyperplastic potential long after arterial injury. Circulation. 1997;96:1291–1298. doi: 10.1161/01.cir.96.4.1291. [DOI] [PubMed] [Google Scholar]
- 25.Finn AV, Gold HK, Tang A, Weber DK, Wight TN, Clermont A, Virmani R, Kolodgie FD. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J Vasc Res. 2002;39:414–425. doi: 10.1159/000064518. [DOI] [PubMed] [Google Scholar]
- 26.Indolfi C, Cioppa A, Stabile E, Di Lorenzo E, Esposito G, Pisani A, Leccia A, Cavuto L, Stingone AM, Chieffo A, Capozzolo C, Chiariello M. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury. J Am Coll Cardiol. 2000;35:214–221. doi: 10.1016/s0735-1097(99)00526-4. [DOI] [PubMed] [Google Scholar]
- 27.Indolfi C, Esposito G, Stabile E, Cavuto L, Pisani A, Coppola C, Torella D, Perrino C, Di Lorenzo E, Curcio A, Palombini L, Chiariello M. A new rat model of small vessel stenting. Basic Res Cardiol. 2000;95:179–185. doi: 10.1007/s003950050180. [DOI] [PubMed] [Google Scholar]
- 28.Indolfi C, Torella D, Coppola C, Curcio A, Rodriguez F, Bilancio A, Leccia A, Arcucci O, Falco M, Leosco D, Chiariello M. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ Res. 2002;91:1190–1197. doi: 10.1161/01.res.0000046233.94299.d6. [DOI] [PubMed] [Google Scholar]
- 29.Jaschke B, Milz S, Vogeser M, Michaelis C, Vorpahl M, Schomig A, Kastrati A, Wessely R. Local cyclin-dependent kinase inhibition by flavopiridol inhibits coronary artery smooth muscle cell proliferation and migration: implications for the applicability on drug-eluting stents to prevent neointima formation following vascular injury. FASEB J. 2004;18:1285–1287. doi: 10.1096/fj.04-1646fje. [DOI] [PubMed] [Google Scholar]
- 30.Jaschke B, Michaelis C, Milz S, Vogeser M, Mund T, Hengst L, Kastrati A, Schomig A, Wessely R. Local statin therapy differentially interferes with smooth muscle and endothelial cell proliferation and reduces neointima on a drug-eluting stent platform. Cardiovasc Res. 2005;68:483–492. doi: 10.1016/j.cardiores.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Vermeersch P, Nong Z, Stabile E, Varenne O, Gillijns H, Pellens M, Van Pelt N, Hoylaerts M, De Scheerder I, Collen D, Janssens S. L-Arginine administration reduces neointima formation after stent injury in rats by a nitric oxide-mediated mechanism. Arterioscler Thromb Vasc Biol. 2001;21:1604–1609. doi: 10.1161/hq1001.096645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.