Abstract
As a prerequisite for studying the intracellular metabolome of mycobacteria, several methods were evaluated for efficient breakage of the cell using Mycobacterium bovis (BCG) as a model microorganism. Several pulping methods, treating with an Ultra-Turax®, deep-freezing in liquid nitrogen followed by mechanical grinding, sonicating with probe head or cup horn, and bead beating prior to solvent extraction were applied and compared. Gravimetry, electron microscopy, and nuclear magnetic resonance spectrometry were used to analyze the extracts.
All analytical methods prove that sonicating is superior to mechanical grinding of deep-frozen cells. Two methods indicated that sonicating with a probe head enhances the efficiency of cell disruption compared to sonicating with a cup horn. The highest extract yield and chemical diversity were achieved by a combination of mechanical grinding and sonicating.
Within the scope of a metabolomic analysis, the method of choice to treat mycobacterial cells is a combination of deep-freezing in liquid nitrogen and mechanical grinding followed by sonicating with a probe head.
Keywords: Metabolome analysis, Extraction methods, mycobacteria, NMR, qNMR, breakage, cell wall, metabolites
Introduction
Metabolome analysis - in the generic sense of elucidating the full complement of the low molecular weight compounds of an organism, and as such including current definitions of metabolomics and metabonomics [1,2] - is becoming recognized as a complementary and perhaps most traceable approach (including the insight necessary for rational drug discovery) to a comprehensive understanding of any pathogen.
Since exhaustive organic extraction of the cell material is the first step of any metabolome analytical method, finding a way to open the cell wall is an essential prerequisite. Due to differences in the architecture of bacterial cell surfaces, the initial choice of the cell breakage method is of great importance. The mycobacteria, with their thick complex cell walls, are perhaps the most challenging from this perspective.
Without disputing the proven usefulness of available mechanical breakage techniques, there are intrinsic limitations for the extraction of mycobacterial cells and for the use in metabolome analysis. Each method must carefully be assessed in light of the physiochemical and physiological properties of the genus, in this case Mycobacteria, and for the purpose of the cell fractionation. If only certain cell fractions are to be isolated, a different method might be useful as if a complete breakage of the cell wall is desired. The French press cell, a method applying hydraulic pressure and pressure shearing (hydrodynamic shear) and widely used for gram-negative and some gram-positive bacteria as well as mycobacteria, normally breaks the cell wall without damaging subcellular particles [3]. This may be an advantage, if certain organelles are to be isolated, but is disadvantageous in a chemical investigation aiming at small intracellular compartments and their chemical components, which can be extracted only after a complete degradation of the corresponding compartmental substructure.
Cell breakage methods can be classified into two main groups: mechanical and chemical. One mechanical method by which degradation into smaller fragments can be achieved is sonication. Rapid vibration of a resonating probe produces high-intensity sound waves, which generate microscopic air bubbles. These transient cavities are thought to create high-shear gradients by microstreaming [4]. Nevertheless, the reproducibility of breakage is limited, since the result depends on several factors, like treatment time and sample viscosity. Additionally, it is very difficult to accommodate the French press cell and the ultrasonic disintegration method with biosafety requirements. Another approach is ballistic disintegration, which comes into operation by the use of a Bead Beater [4]. Here, shear forces develop when a suspension of cells together with small glass or plastic beads is shaken or agitated, and will violently break the bacterial cells [4,5]. A major disadvantage of this method is the abrasion of chamber material (see results below), and its impracticality when using organic solvents. The classical approach of shearing or mechanical grinding is a simple method, where frozen lyophilized cells are broken by grinding cell paste or by using an agate mortar and pestle [4,6,7]. The efficiency of this process depends on the organism and the skills of the operator, as well as time spent. This procedure has been efficiently used for the breakage of archaebacteria [7]. Finally, some microorganisms have been successfully lysed by microwave disruption [8]. However, since this lysis method has been attributed primarily to thermal effects, it appears unsuitable for a chemical investigation, because the secondary metabolites, which are the center of attention of a metabolomic investigation, might be heat labile.
Chemical [9–13], osmotic [14], and alkali [3] lysis, are alternatives to mechanical breakage methods. However, their major disadvantage for metabolome investigation is the contamination of the cell extract with chemicals and the unpredictable occurrence of artifacts or reaction products during downstream analysis. Several detergents, for example, contain aromatic groups, which disturb UV measurements or show unfavorable elution properties in chromatography due to their soapy nature.
Within the scope of an isolation project to comprehend the metabolome of mycobacteria falls the development of a reliable method to break the mycobacterial cell walls. Mycobacteria are known for their extremely resilient cell wall, a massive “core”, comprised of peptidoglycan which is covalently bond, via a linker (L-Rha-D-GlcNAc-P), to a linear galactofuran, attached to several strands of a highly branched arabinofuran, which are in turn bound to mycolic acids [15–17]. Thus, a regime of several pulping methods, treating with Ultra-Turax®, deep-freezing in liquid nitrogen followed by mechanical grinding, sonicating with probe head or cup horn, and bead beating was compared. The first step, i.e., freezing the cells with liquid N2, leads to actual “freezing” of all metabolic processes in the cells. The mechanical cell disruption by means of sonication has the advantage that the solvents used for extraction become part of the disruption process themselves. Therefore, the first two steps of a metabolome analysis, i.e. quenching and extraction, overlap with the cell disruption process. An adequate choice of the solvents is vital to separate the extracted material according to polarity, which leads to the next steps, fractionation and analysis of the cell material.
The vaccine strain of Mycobacterium bovis (BCG) was chosen as a test microorganism because of reduced biosafety requirements and high anatomical similarity to M. tuberculosis. To evaluate the pulping effectiveness, the cells were subsequently extracted by exhaustive maceration with CHCl3 and MeOH. Electron micrographs of the cells were taken in all stages of the procedure in order to visualize the cell wall and the degree of destruction. In addition, 1H-NMR spectra of the extracts were acquired and the extract weights were recorded. Thus, the determination of the efficiency of the extraction methods is based on three parameters: optical inspection, chemical diversity, and quantity/gravimetry.
Material and Methods
Preparation of cell material
Mycobacterium bovis (BCG), Romanian substrain I.C was obtained from the National Institute of Research and Development for Microbiology and Immunology Cantacuzino, a vaccine production facility in Bucharest, Romania. The log-phase culture was grown in Sauton’s medium, washed in phosphate buffer, and lyophilized. It shall be noted that lyophilisation is not an essential part of the presented extraction concept. The whole procedure is independent of prior lyophilization of mycobacterial cells. The dried cell material (200 g) was pre-extracted by using an Ultra-Turax® with CHCl3 followed by MeOH as solvents. From the residual cell mass, six batches of 4 g dry weight each were deep-frozen in liquid nitrogen and mechanically ground with a pistil in a mortar for 5 minutes. The resulting samples of each batch were divided into three equal aliquots, which were weighed accurately. One aliquot remained as ground (g) sample, the second was further sonicated with a cup-holder resulting in sample gsc(=ground and sonicated with cup), the third aliquot was sonicated with a probe head resulting in sample gsp(=ground and sonicated with probe),. Six batches of all samples were used for further analysis.
Twelve more batches of 2 g dry weight each, from the Ultra-Turax® treated cell-mass were sonicated with both methods resulting in samples sc(=sonicated with cup) and sp(=sonicated with probe), six batches each, while six batches of 2 g-samples dry weight were processed with a bead-beater (0.1 mm diameter zirconia beads, 3 minutes) to yield 6 batches of sample b(=bead beaten), (Table 1). All samples, except for sample b, which contained massive chamber and/or rotor abrasion material, were exhaustively extracted by maceration with CHCl3, followed by MeOH to give 60 extracts.
Table 1.
Abbreviations and extract treatments
| Abbreviation | Treatment |
|---|---|
| g | ground |
| gsc | ground and sonicated with cup |
| gsp | ground and sonicated with probe |
| sc | sonicated with cup |
| sp | sonicated with probe |
| bb | bead beaten |
Electron microscopy
Electron micrographs of samples g, gsc, gsp, sc, sp as well as from the untreated (= u) cells were taken with a Hitachi S-3000N Variable Pressure Scanning Electron Microscope (SEM).
Sonicating
Sonicating was carried out with an ultrasonic liquid processor (Misonix XL-2020 Sonicator®, 600 W). Each sonicated sample (10 mL CHCl3 as solvent, ice jacket) was treated 3 times for 60 s at a fixed frequency of 20 kHz using either a cup horn or probe head. Cup horns are high intensity ultrasonic water baths that allow samples to be processed in completely closed containers. The ultrasonic probe never comes in contact with the sample so that sample loss, escape and/or cross contamination cannot occur. High intensity probe heads are tipped horns, furnished with replaceable tips, such as the microtip horns used in this study.
Gravimetry
All weight measurements were carried out with an AND® analytical balance at a precision of 0.01 mg.
Nuclear Magnetic Resonance Spectroscopy
For NMR spectroscopy, 30.0 mg samples of the recombined CHCl3 and MeOH extracts were partitioned in CHCl3-MeOH-H2O two phase systems, separated, evaporated, and subsequently dissolved in CHCl3 and CD3OD-D2O (2:1, v/v), respectively, with an isotopic purity of 99.8 % D, to give a final volume of 1 mL corresponding to a filling height of 50 mm in 5 mm tubes. The spectra were recorded on a Bruker Avance 500 spectrometer. Chemical shifts (δ in ppm) were referenced to the CD3OD multiplet at 3.300 ppm or to the CDCl3 singlet at 7.240 ppm, respectively. For all NMR experiments, off-line data analysis was performed using the NUTS software package, Acorn NMR Inc., U.S.A.
Bead-Beating
Zirkonia beads of 0.1 mm in diameter were mixed with 1 g of cell material in 200 mL (total volume 350 mL) of methanol in a Biospec® Bead Beater with a stainless steel chamber. The external jacket was filled with ice water and the mixture was blended 3 times for 2 min.
Results
Gravimetric analysis
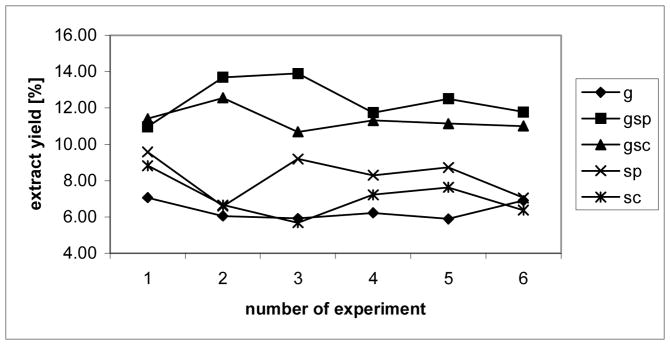
Gravimetric comparison of the extract yields shows that (i) sonicating of the cell material (gsp: 12.43%, st.dev. = 1.17; gsc: 11.35%, st.dev. = 0.65; sp: 8.24%, st. dev. = 1.19; and sc: 7.07%, st. dev. = 1.09; n=6) give higher yields than mechanical grinding (g: 6.35%, st. dev. = 0.52; n=6), and that (ii) sonicating with probe head (gsp: 12.43%; sp: 8.24%) in comparison with sonicating with cup horn (gsc: 11.35%; sc: 7.07%) gives superior results (Fig. 1).
Figure 1. Gravimetric analysis of the extracts.
Gravimetric analysis of the extracts. g = ground cells, gsp = ground and sonicated with probe head, gsc = ground and sonicated with cup horn, sc = sonicated with cup holder, sp = sonicated with probe head
Electron microscopic analysis
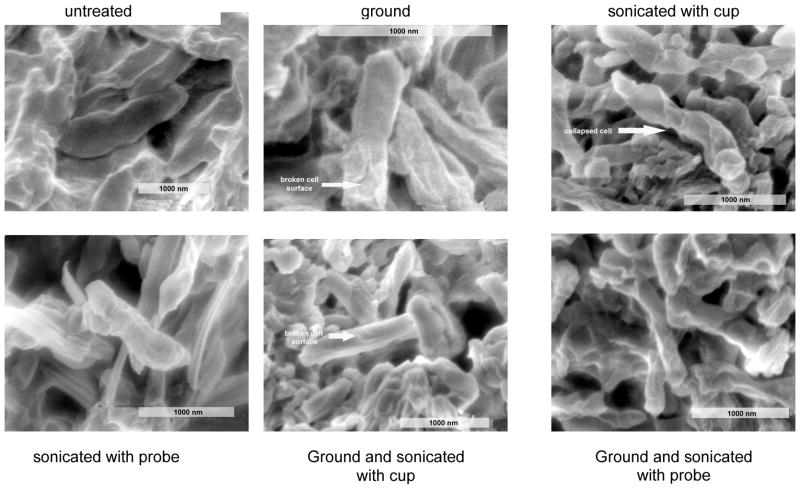
Comparison of the electron microscopic pictures shows that all samples, which have been deep-frozen with liquid nitrogen and mechanically ground, display disruptions of the mycobacterial cell surface. Furthermore they prove that sonicating enhances cell destruction, whereas no remarkable differences are detectable between sonicating using a probe-head and sonicating using a cup-holder (Fig. 2).
Figure 2. Electron micrographs of the cell material in all stages of the treatment.
Electron micrographs of the cell material in all stages of the treatment. Deep freezing and grinding lead s to broken cell surfaces. Sonicating enhances the disruption of the cell wall.
Nuclear magnetic resonance analysis
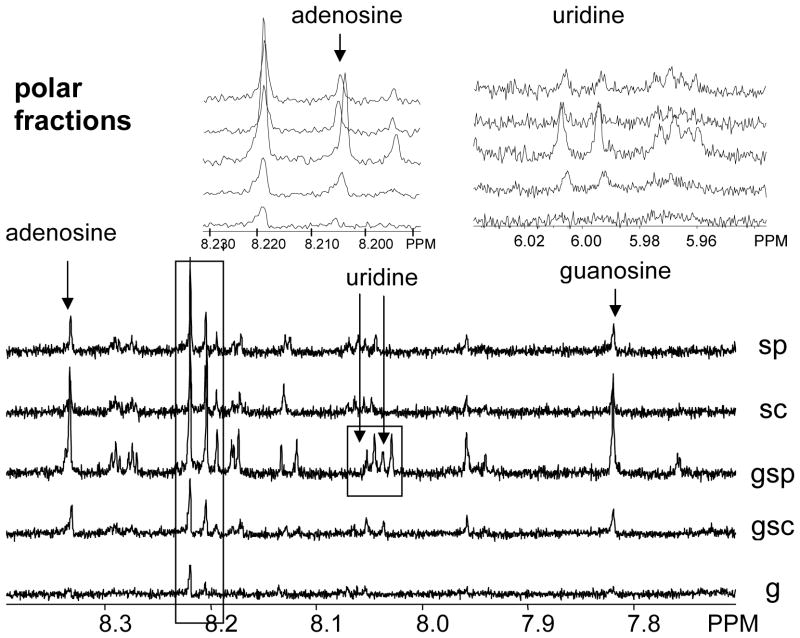
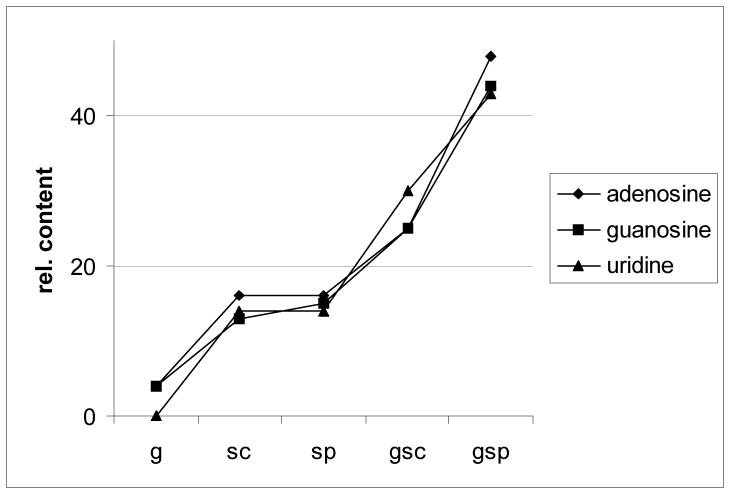
NMR as a method that allows comprehending the chemical shifts and J patterns of the extracted compounds provides structural information and therefore gives rise to the chemical diversity and nature of the extracts. In contrast to the widespread HPLC analysis, this method enables detection and assignment of compound classes, without depending on UV chromophores or elution properties. All the polar extracts of these samples, that were sonicated, show prominent signals in the (hetero-)aromatic region at 6.5 to 9.0 ppm. Sonicating with probe head improves the signal integral intensity, a qualitative measure of the yield, in those cases where the cells have been ground. These signal groups can be assigned to intracellular material, namely nucleosides (Fig. 3 and 4; adenosine: d = 8.205 ppm, (H-2), 8.345 ppm, H-8; guanosine: d = 7.830 ppm (H-8); uridine: d = 6.065 ppm (H-4), 8.050 ppm (H-5); [18]), which in turn provides proof that the cell walls are at least permeable, and more likely been broken. Interestingly, these results show that chemical diversity in the extracts can be improved by sonicating with a probe head but not with a cup horn. Signals of sugar components are visible between 3.0–4.5 ppm, but since severe overlapping does not allow functional determination without chromatography, it cannot be concluded whether they originate from intracellular material or from the cell wall. Quantification of the nucleosides by means of quantitative 1H NMR (qHNMR), performed according to the general experimental procedure described in Pauli et. al. [19], provides a selective quantitative measure for the deliberation of intracellular metabolites and confirms that the amount of extracted intracellular compounds as a function of the extraction method increases in the following order: g < sc < sp < gsc < gsp (Fig. 5).
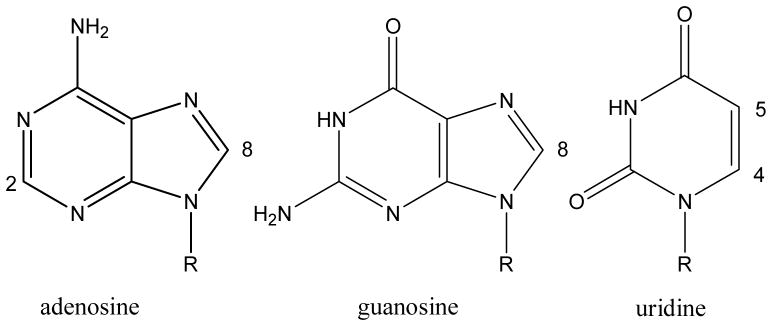
Figure 3. Chemical structures of nucleosides.
Chemical structures of the analyzed nucleosides. Sonication with probe head does not represent an advantage compared to sonication with cup horn in terms of cell disruption (R = ribose)
Figure 4. NMR spectra of polar fractions.
1H-NMR spectra of the polar fractions: diagnostic signals of the nucleosides adenosine and guanosine are contained in the sonicated fractions, while uridine can only be detected in the fraction that was ground and sonicated with probe head. It shall be noted that, even at 500 MHz, a minimum of 128 scans had to be accumulated in order to achieve sufficient signal/noise for identification of the highly coupled signals of the extract components and for reliable qNMR quantification.
Figure 5. Relative content of nucleosides.
Relative content of nucleosides in equal amounts of the extracts prepared by the different extraction methods, as determined by qHNMR. Signals specific to the three nucleosides were quantitated (NMR integral) relative to the solvent as an internal reference.
Non-polar fractions do not show significant differences in signal diversity as detected by NMR. This is expected since the majority of the extract is derived from cell wall and cell wall associated lipophilic material. The concentration of possible cytosolic constituents is most likely too low to provide significant NMR peak pattern in crude extracts and, thus, would need further separation of the non-polar extracts.
Bead beating
Bead beating was found to be unsuitable for the purpose of cracking mycobacterial cell walls for metabolomic analysis, because massive abrasion of the chamber material and/or the rotor was observed. The method was therefore abandoned.
Discussion
All three methods, gravimetry, scanning electron microscopy, and nuclear magnetic resonance spectroscopy confirm that (i) sonicating optimizes the mycobacterial extraction process with respect to extract mass, destructive modifications of cell surfaces, and chemical diversity; (ii) mechanical grinding of the deep-frozen cells before extraction results in better extract yields and improved cell cracking as evident from the optical appearance of the extracted cells as well as increased NMR signal diversity (chemical shift as an indicator of chemical diversity) and integrals (qNMR quantitation). Furthermore, the NMR results indicate that mycobacterial cells can be cracked by sonicating, giving access to cytosolic metabolites. An increase in chemical shift diversity is clearly achieved for polar extractibles (intracellular/cytosolic material), whereas the non-polar extracts (cell-wall material) result in almost identical proton NMR spectra of the crude extracts. Finally, it is noteworthy that the regimen of (organic) extraction solvents can be adjusted to the polarity of the target analytes of the metabolome. In order to extract ionic/ionizable compounds, much more polar solvents can be used, such as methanol/water mixtures, also under pH-defined conditions. Since sonication only requires the presence of a liquid phase for cell disruption, polar aqueous solvents are equally suitable for combined extraction and mechanical disruption of mycobacteria using the protocol described above.
Acknowledgments
Source of support: NIAID/NIH under grant R21-AI052847-01
The authors gratefully acknowledge the research support from NIAID/NIH under grant R21-AI052847-01. Furthermore, we thank the Research Resources Center at the University of Illinois at Chicago for performing the SEM analysis.
References
- 1.Reo NV. NMR-based metabolomics. Drug Chem Toxicol. 2002;25:375–382. doi: 10.1081/dct-120014789. [DOI] [PubMed] [Google Scholar]
- 2.Lindon JC, Holmes E, Nicholson JK. So what's the deal with metabonomics? Anal Chem. 2003;75:385A–391A. doi: 10.1021/ac031386+. [DOI] [PubMed] [Google Scholar]
- 3.Sprott GD, Koval SF, Schnaitman CA. Cell Fractionation. In: Gerhardt P, Murray PRGE, Wood WA, Krieg NR, editors. Methods for General and Molecular Bacteriology. ACM; Washington: 1994. pp. 72–103. [Google Scholar]
- 4.Hughes DE, Wimpenny JWT, Lloyd D. The disintegration of microoganisms. In: Norris DWRJR, editor. Methods in Microbiology. Academic Press, Inc; New York: 1971. pp. 1–54. [Google Scholar]
- 5.Salton MRJ. Isolation of cell walls from gram-positive bacteria. Meth Enzymol. 1974;31:653–667. doi: 10.1016/0076-6879(74)31071-3. [DOI] [PubMed] [Google Scholar]
- 6.Jarrel KF, Fafuy D, Herbert AM, Kalmokoff ML. A general method of isolating high molecular weight DNA from methanogenic archaea (archaebacteria) Can J of Microbiol. 1992;38:65–68. doi: 10.1139/m92-010. [DOI] [PubMed] [Google Scholar]
- 7.Weil CF, Cram DS, Sherf BA, Reeve JN. Structure and comparative analysis of the genes encoding component C of methyl coenzyme M reductase in the extremely thermophylic archaebacterium Methanothermus fervidus. J Bacteriol. 1988;170:4718–4726. doi: 10.1128/jb.170.10.4718-4726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujikawa H, Ushioda H, Kudo Y. Kinetics of Escherichia coli destruction by microwave irradiation. Appl Environ Microbiol. 1992;58:920–924. doi: 10.1128/aem.58.3.920-924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helenius A, McCaslin DR, Fries E, Tanford C. Properties of detergents. Meth Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- 10.Zulauf MU, Fuerstenberger M, Grabo P, Jaeggi P, Regenass M, et al. Critical micellar concentrations of detergents. Meth Enzymol. 1989;172:528–538. doi: 10.1016/s0076-6879(89)72032-2. [DOI] [PubMed] [Google Scholar]
- 11.Schnaitman CA. Protein composition of the cell wall and cytoplasmatic membrane of Echerichia coli. J Bacteriol. 1970;104:890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filip C, Fletcher G, Wulff JL, Earhart CF. Solubilization of the cytoplasmatic membrane of Escherichia coli by the ionic detergent sodium lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamekura M, Oesterhelt D, Wallace R, Anderson P, Kushner DJ. Lysis of halobacteria in Bacto-Peptone by bile acids. Appl Environ Microbiol. 1988;54:990–995. doi: 10.1128/aem.54.4.990-995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laddaga RA, MacLeod RA. Effects of wash treatment on the ultrastructure and lysozyme penetration of the outer membrane of various marine and two terrestrial gram-negative bacteria. Can J Microbiol. 1982;28:318–324. [Google Scholar]
- 15.Besra GS, Chatterjee D. Lipids and carbohydrates of Mycobacterium tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection, and control. ASM Press; Washington: 1994. pp. 285–302. [Google Scholar]
- 16.Brennan P, Draper P. Ultrastructure of Mycobacterium tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection, and control. ASM Press; Washington: 1994. pp. 285–302. [Google Scholar]
- 17.Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis. 2003;83:91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 18.Gosselin G, Bergogne MC, Imbach JL. Synthesis and antiviral evaluation of b-L-Xylo-furanosyl nucleosides of the five naturaly occuring nucleic acids. Journal of Heterocyclic Chemistry. 1993;30:1229–1233. [Google Scholar]
- 19.Pauli GF, Jaki BU, Lankin DC. Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod. 2005;68:133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]