Abstract
Primary cultures obtained from embryonic nasal placodes can maintain olfactory neurons, olfactory ensheathing cells, and large numbers of gonadotropin releasing hormone-1 (GnRH) neurons. Depending on the age of the starting material, one can examine cell interactions important for placode formation or neuronal migration and axonal outgrowth. When generated at E11.5 in mouse, neuronal migration and axon outgrowth away from the main tissue mass occurs. This area of the explant, the periphery, is only a few cells thick. This characteristic offers the opportunity to image single cells and axons and allows for pharmacological and molecular manipulations as well as physiological recordings to be performed. Here we describe a system of culturing nasal explants used in our laboratory. This model system provides a method for obtaining physiological cellular responses with posthoc immunohistochemistry, or gene expression studies, on cells arising from the nasal placode.
Keywords: nasal explants, primary organ culture, GnRH neurons, olfactory neurons, olfactory ensheathing cells
Introduction
In mice, olfactory sensory axons and GnRH cells emerge from the nasal placode around E10 - E11. Due to difficulties in accessing embryos in utero, a nasal explant system was generated as a model to study migration of these cells. This model has been successfully used in a variety of species to study early development and physiology of the GnRH system and olfactory system (reviewed by Terasawa, 2001; Wray, 2010). Nasal explants generated at E11.5 thin to a few cells in thickness at the periphery and exhibit directed neuronal migration and axon outgrowth (Fueshko and Wray, 1994). These characteristics make this system suitable for imaging single cells and axons. In addition, the explants are exposed on the dorsal surface allowing pharmacological and molecular perturbations, as well as physiological recordings to be performed. Finally, landmarks remain after in situ recordings that allow posthoc immunohistochemical observations to be performed on recorded cells.
Many neuronal and non-neuronal cell types arise from the nasal placode. These include GnRH neurons, olfactory sensory neurons (OSNs), vomeronasal sensory neurons (VSNs), olfactory ensheathing cells, and the multipotent progenitor cells that give rise to subpopulations of these cell types (Murdoch et al., 2010; Wray, 2010; Forni et al., 2011). Nasal explants may be useful in studying the interactions of these cell types (e.g. OECs and olfactory axons) particularly for use with transgenic animals or gene targeting approaches.
GnRH cells, the focus of our protocols, originate in the nasal placode during early embryonic development and migrate along olfactory axons until reaching the developing hypothalamus. Within the brain, GnRH neurons are found scattered from the olfactory bulbs to the hypothalamus from where the majority of neurons project their axons to the median eminence (Wray, 2002). GnRH release at this site is pulsatile and affects the synthesis and secretion of gonadotropins from the pituitary and consequently, gonadal function (Belchetz et al., 1978). The small number of GnRH neurons (∼800 in mouse brain) and their widespread distribution (Hoffman and Finch, 1986) makes it difficult to study their physiology and cell signaling mechanisms. However, it has been shown that prenatal GnRH neurons in explants express many of the receptors found in GnRH neurons in the adult brain. They also exhibit spontaneous oscillations in intracellular calcium that correlates with electrical activity and pulsatile release (reviewed in Constantin, 2011). Therefore, prenatal GnRH cells maintained in nasal explants can also be used to identify the mechanisms underlying individual neuronal activity as well as neural network properties.
This unit describes a primary culture technique for GnRH neurons and other neurons and glia originating from the nasal placode at E11.5 (Basic Protocol). Further, we present two protocols utilizing the accessibility of GnRH neurons in explants, measuring their calcium activity and electrical activity.
NOTE: All procedures using live animals must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the use and care of laboratory animals.
Basic Protocol
Primary Culture of Nasal Explants Generated from Mouse Nasal Placodes
Introduction
This protocol describes the preparation of nasal explants from embryonic day (E) 11.5 mouse embryos (Fueshko and Wray, 1994). Nasal placodes are dissected from the developing nose and cut into butterfly shaped fragments. These fragments are explanted onto plasma/thrombin-coated coverslips in 35 mm culture dishes. The added culture medium consists of specific nutrients and substances to enable the survival and differentiation of neurons originating from the nasal placode. After 3 days in vitro (div) a proliferation inhibitor is added to the medium for 3 days to prevent dividing cell overgrowth. Olfactory sensory axons grow into the periphery and GnRH cells and olfactory ensheathing cells migrate out of the placodal area for the first 6 days of culture, forming a single layer on top of fibroblasts. Each nasal explant can be maintained, for a number of weeks, as a whole or divided in half (Kramer and Wray, 2000) for perturbation studies.
Materials
Cold PBS
70% ethanol
-
Dissection tools for removing uteri:
Blunt tipped operating scissors 6.5″
Scissors for removal of uteri 5.5″
Large grip forceps 5″
Small forceps
-
Dissection tools for embryos and nasal explants:
Dissecting microscope with at least a 2× magnification, and light source
Narrow spatula
#5 forceps (2)
4″ Iris Scissors
Plastic Pasteur pipettes
Scalpel and sterile scalpel micro blades (size 15°)
-
Culturing solutions (see Reagents and Solutions for details).
Gey's + glucose (G+G)
Chicken Plasma
Thrombin
Serum Free Media (SFM), filtered
-
Tissue Culture
Permanox Cell Culture Slides (Nalge Nunc International Cat. No. 160005) cut into ∼1 cm wide pieces with sterile scissors
Glass cover slips (12×24 mm) sterilized in 100% Ethanol for 24 hours and dried at 37 °C for 60 min
Tissue Culture Dishes (150×25 mm) (Becton Dickinson Labware Cat No. 35 3025)
Tissue Culture Dishes (35×10 mm) (Becton Dickinson Labware Cat No. 35 3001)
Vacuum Grease (111 Valve Lubricant and Sealant; Dow Corning 1864963-0807)
37 °C, 5% CO2 humidified incubator
Phosphate-buffered saline (PBS)
FUDR (5-Fluoro-2’-Deoxyuridine; Sigma, cat. no. F0503)
Tissue culture dishes (30 × 20 mm; Becton Dickinson Labware, cat. no. 35 3003)
Steps and Annotations
NOTE: To work with embryos at the proper age, timed matings are set up before the procedure. The embryonic dating scheme used here counts the morning that the males are removed from the females as E 0.5.
Collect and prepare the embryos
-
1
Terminate a time pregnant mouse (E11.5) with CO2 followed by cervical dislocation, as approved by NIH/IACUC. Use forceps to lift the skin over the abdomen, and cut the skin from above the vaginal opening towards the chest.
-
2
If you did not cut the muscle/peritoneum layer at the same time you need to repeat step number two with the lower skin layer.
-
3
Lift out the embryo-filled uterus, using forceps and detach the uterus with small scissors from the body cavity.
-
4
Transfer the uterus to PBS filled tissue culture dishes (100 mm) and rinse twice. Store uterus in cold PBS (4 °C) until use.
Embryo head preparation
-
5
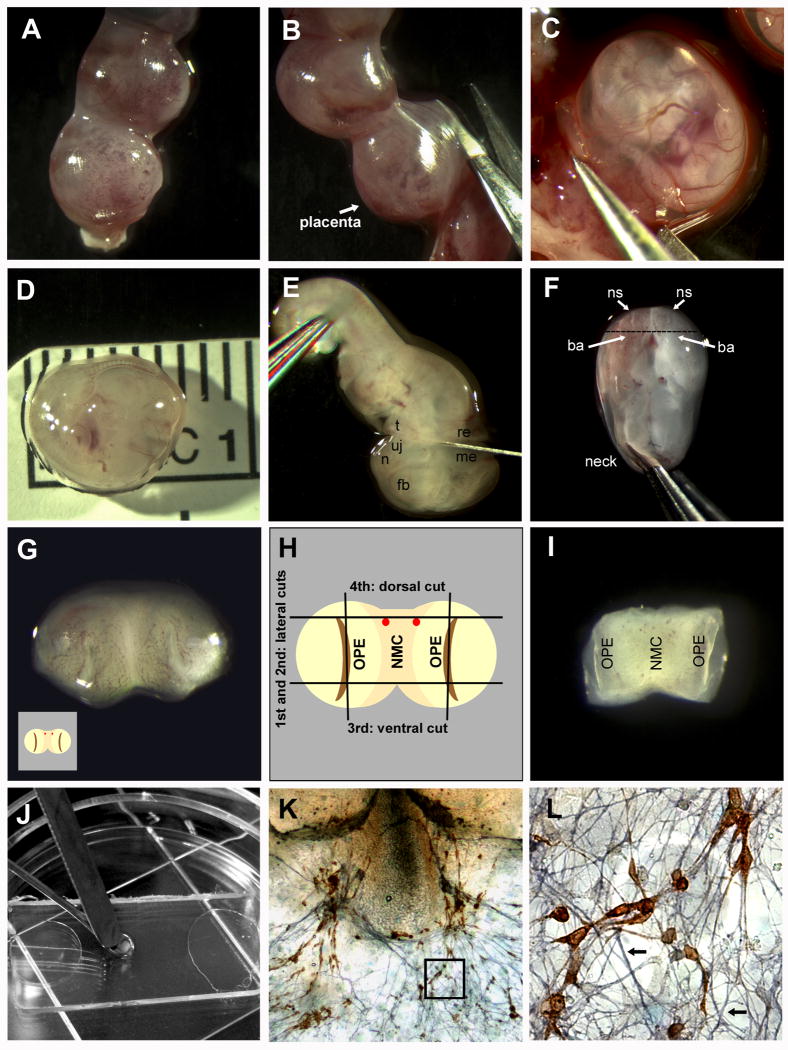
Align the uterus in a clean tissue culture dish under the dissecting scope to cut through the muscular side of the uterus with a pair of iris scissors (Fig 1A, B).
Cutting through the placental side of the uterus involves cutting through many blood vessels and placental tissue and increases risk of damaging the embryos before they have been removed.The amniotic sacks may peak out of the cut: leave the sacks intact, however if they are damaged during the cutting procedure it is not a problem. -
6
After opening the whole uterus, pull the amniotic sacks (with embryos inside) off of the placentas using #5 forceps (Fig 1C).
-
7
Discard the uterus.
-
8
Remove the embryo from the amniotic sack using two #5 forceps.
The amniotic sack consists of two layers, the thicker vascularized sack and a thinner inner layer, they may be removed simultaneously or one after the other. Discard the sacks (or use for genotyping).Avoid damaging the head and nasal region of the embryo. The rest of the body is not needed and may be used to hold the embryo in place when removing the amniotic sacs or cutting the head.Determine the size of the embryo by measuring the crown to rump distance using a plastic ruler under the tissue culture dish (Fig 1D). An E11.5 embryo should measure between 6.2 – 7.2 mm.Measure a few embryos out of one litter; they can be heterogeneous in size. 7.2 mm is the upper limit for obtaining consistent explants with large numbers of GnRH neurons. -
9
Cut off the head of the embryo with one clean cut all the way down to the plate using a scalpel (15° micro-blade) (Fig 1E), pull the body away and discard (or save for genotyping).
The angle of the head cut will influence the nasal cut performed later. The blade should reach across the head, cutting between the developing upper jaw (uj) and tongue (t) on one side, and between the mesencephalon (me) and rhombencephalon (re) on the other (Fig 1E). -
10
Move the heads (see Fig 2C) into a large drop of G+G solution in a fresh tissue culture dish by grabbing the back of the head with #5 forceps.
-
11
Transfer the tissue culture dish containing the heads into a refrigerator (4 °C) and move on to next litter, repeating steps 1 through 10. If you only prepare explants from one litter leave the heads at 4 °C for a minimum of 30 min to give the tissue time to absorb the G+G solution.
Figure 1.
Critical steps in nasal explant preparation. A One end of an isolated mouse uterus. B The uterus is cut through the muscular layer (opposite the placenta). C An embryo in its amniotic sac, still attached to the uterus, is removed with forceps. D The measured embryo is showing the ideal size of an E11.5 embryo (6.2 -7.2 mm from crown to tail). E A forceps is used to hold the embryo in place, while the head is removed with a scalpel. The cut is made between the tongue (t) and upper jaw (uj) at one side, and between the mesencephalon (me) and rhombencephalon (re) at the other side. The forebrain (fb), and nasal area (n) are also easily identifiable. F With the dorsal side of the head down, the two naris (ns) can be viewed from above. The branchial arch (ba) is also clearly visible. The head is grasped with forceps inserted into the mescencephalon where the base of the “neck” would be. G The nasal area, after the cut shown in F, with the outer surface viewed from above. Each olfactory pit (OPE in H) is visible as slight indentations on the right and left sides. H Schematic of G with the four cuts shown. The two lateral cuts going through the olfactory pit (OPE) on either side of the nasal mesenchyme (NMC) are illustrated. The two red dots shown are the blood vessels prominent in most embryos making a useful marker for the 3rd dorsal cut. I Explant, after the cuts are made in H, with the outside (exterior) face down (opposite view of H and I). This is the orientation the explant is plated. J Explant being transferred to permanox slip covered in plasma using two spatulas. K A 7 div explant stained for GnRH (SW 1:1000 stained in DAB; Fueshko and Wray, 1994) and peripherin (1:1000 stained in SG, blue; Fueshko and Wray, 1994) L Close-up of K (boxed area), with GnRH neurons migrating on peripherin positive fibers (arrows).
Figure 2.
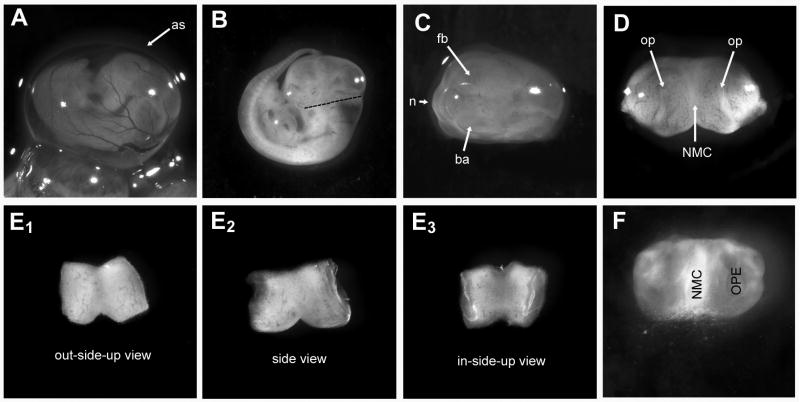
Stages of explant dissection. A E11.5 embryo in amniotic sac (as) attached to the uterus. B Embryo clear of both amniotic sacs. Dashed line indicates where the head should be cut. C Nasal area to be removed is clearly visible between the branchial arch (ba) and forebrain (fb). D After cutting the nasal area from the head, the cut side is against the surface of the plate, and the exterior of the nose is pointed towards the viewer. Olfactory pits (op) are visible as indentations on either side of the nasal mesenchyme (NMC). E1-E3 Views of the explant before and after plating. E1 The “out-side-up view” shows the explant in the same orientation as D. E2 The “side view” shows the explant with the exterior of the nose as an inverted V, and the each nasal pit is discernible as concaved areas on the right and left. The dorsal cut is now facing up. E3 The “in-side-up” view shows the exterior of the nose down on the plate (flipped over version of E1). This is the orientation that the explant should be plated. F A DIC image of the explant at 3 div: A halo around the explant is becoming visible, and the first neurons migrating out are radiating from around the bottom edge of the NMC (arrows). The OPEs are still recognizable.
Preparing nasal explants
-
12
Select embryo head, grasp back of head with #5 forceps and pull out of the droplet, set down on clean spot to remove some of excess fluid. Orient the head so that the top of the head is lying on the plate (Fig 1F).
This position offers a look at the inside of the “neck” and the outside of the nose, including the two nares (ns) and the branchial arches (ba). -
13
Grasp the back of the head with #5 forceps. Wet your blade with G+G solution, so the tissue does not stick. Use the scalpel to push back the branchial arches and cut at an angle in a single motion (Fig 1F) Take the head away with the forceps and angle the knife blade so that the nose lies flat, with the cut side down, on the tissue culture dish (Fig 1G).
-
14
To trim the explant, you will make four cuts: two lateral, a dorsal cut and a ventral cut (Fig 1H). Make the lateral cuts by cutting through each naris and use your forceps to move away the cut off tissue. Trim the dorsal cut by identifying two blood vessel clusters (Fig 1H) and cut through, or just above them. For the ventral cut, place the blade just above the inverted V where the two nasal areas fuse.
Do not trim too much from this side as the developing VNO, the origin site of GnRH neurons, is close to this region. -
15
Transfer the explant (see Fig 2E) to a new droplet of G+G solution using a plastic transfer pipette with a cut off tip and keep them in individual droplets on a fresh tissue culture dish at 4 °C for at least 30 min before plating.
Plating nasal explants
-
16
Orient the explant in the droplet using the dissecting scope. The explant needs to be inside up, meaning the nasal pits will have to face upwards, while the exterior of the explant will be laid down on the cover slip (Fig 1I, Fig 2E).
Avoid too much handling of the explant. Be careful and gentle with the tissue. Every time it is touched, damage occurs. -
17
Use either plastic or glass cover slips. Apply 10 μl of chicken plasma and spread it along the whole cover slip using a spatula.
The material of the cover slip depends on the future use of the explants. -
18
Using a wet spatula (G+G), carefully scoop up the explant onto the spatula and lift it out of the droplet keeping it in the proper orientation. Place the explant onto the cover slip Fig 1J).
Dropping the explant from the spatula may require a second spatula to slide it down from the initial spatula. -
19
Add 10 μl of Thrombin to the plasma/explant clot and gently mix the thrombin and plasma solutions by tipping the small tissue culture dish holding the cover slip.
-
20
Repeat the plating procedure until all explants have been plated. Place culture dishes with plated explants in large (150 × 25 mm) culture dishes for transport and storage (8/dish).
-
21
Keep the explants at room temperature for a minimum of 30 and a maximum of 45 min for the plasma/thrombin clot to solidify.
Feeding of the explants
-
22
Add 1.8 ml (or 1.5 ml if explants are on glass) of SFM to each culture dish containing a single explant. Use a 10 ml pipette to gently cover the explant with media. After applying media to all prepared explants, transfer the dishes into an incubator set at 37 °C and 5% CO2.
-
23
After two days, gently swirl the dishes containing the explants to assure proper coverage with media.
-
24
On day three, replace media with 1.5 ml (1.2 ml glass) fresh SFM media including FUDR [2 mg/ml] to prevent non-neuronal tissue to divide and grow.
-
25
On day six and every second day thereafter, replace media with fresh SFM.
Support Protocols
Support Protocol 1: Perforated Patch Clamp Recordings in Nasal Explants
Introduction
Neurons in nasal explants are characteristically discernable as plump phase-bright cells with 20× or 40× phase objectives. Most cellular migration has ended by 6 div making neurons accessible to electrophysiological approaches and spontaneous activity can be recorded (Fueshko and Wray, 1994; Kusano et al., 1995; Constantin and Wray, 2008a). Gramicidin perforated patch clamping maintains both the intracellular chloride gradient and the intracellular signaling cascades, which are disrupted in whole cell recordings (Rhee et al., 1994; Ebihara et al., 1995). GnRH neurons, the focus of this protocol, have long dendrites that receive extensive ionotropic and metabotropic input (Krsmanovic et al., 2009; Campbell and Suter, 2010), however, the technical difficulty of in vivo recordings combined with the added difficulty of perforated patching makes this an extremely impractical application for slice work. Thus, GnRH neurons generated from nasal explants are a valuable means of circumventing this problem. The following protocol outlines the basic steps for successful perforated patch clamp recordings of GnRH neurons in nasal explants maintained for 6-24 div. Subpopulations of GABAergic and glutamanergic neurons in the periphery and olfactory neurons in the remaining olfactory pits of the nasal explants (Fueshko et al., 1998; Constantin, 2011) may also be readily patched, although transgenic reporter animals will greatly facilitate this. Familiarity with electrophysiology is assumed.
Materials
-
Extracellular solution (ECS)
In (mM): NaCl (135), KCl (3), CaCl2 (2), MgCl2 (1), HEPES acid free (10), glucose (20), pH 7.4 with NaOH
-
Intracellular solution (ICS):
In (mM): KCl (150), MgCl2 (2), HEPES, acid free (10), glucose (10), pH 7.3 with KOH
Gramicidin (Sigma Cat. No. G5002)
Dimethyl Sulfoxide (DMSO) (Sigma Cat. No. D8418)
All chemicals from Sigma or Tocris
-
Equipment
Note: A standard electrophysiological set up meeting the requirements and specifications described in “The Axon Guide” (Sherman-Gold, 1993) is assumed.Gravity fed profusion system
3-5 cc syringe to apply suction for filling electrode tip
1 cc syringe and >4 cm needle for backfilling electrodes
0.22 μm sterile filters for syringe (Millipore, SLGS0250S)
Axopatch Amplifier (Axon Instruments)
Nikon inverted microscope (Nikon, 2000E)
Digidata (Axon Instruments)
MWO-3 Three-axis Oil Hydraulic Micromanipulator (Narishige Co, LTD)
P-97 Flaming/Brown Micropipette (Sutter Instruments)
Borosilicate glass (1.5mm OD/0.86mm ID) (Harvard Apparatus Cat. No. 30-0058)
pClamp Acquisition and Analysis Software (Axon Instruments)
Steps and Annotations
Preparing electrodes
-
1
Pull electrodes from Borosilicate glass.
Electrodes should have a short blunt taper, which will allow gramicidin to diffuse easily into the tip. This shape is easiest to accomplish using a 4-step program on a Sutter Instruments Flaming/Brown Micropipette puller (or equivalent). Keep electrodes free of dust or contaminants. -
2
Chloride ground wire and electrode wire when needed.
Chloride wires in 100% Clorox bleach for ∼10-20 min.
Preparing electrodes solutions
-
3
Thaw 6 ml intracellular solution.
-
4
5mg gramicidin /0.2 ml DMSO
Add no more than 6μL of 5 mg/2 ml gramicidin stock to 3 ml intracellular solution (final concentration 10-50 μg/ml) and vortex ∼1 min.Make fresh solution every 2-3 hours.Filter the remaining 3 ml of intracellular solution.Fill 1 cc syringe with vortexed gramicidin containing intracellular solution.
Before recording
-
5
Choose an explant with GnRH neurons that have migrated onto the monolayer of fibroblasts/olfactory ensheathing cells.
-
6
Place the explant in the recording chamber and cover with ECS.
-
7
Run the bath (gravity fed perfusion and peristaltic pump) with ECS solution for 30 min while the explant equilibrates to the new environment.
Patching
-
8
Find a plump phase bright neuron.
Make sure that the cell is accessible from the angle the electrode will approach, and is far enough into the periphery that the electrode is not hung up on the plasma clot around the explant cartilage. -
9
Using light suction, fill the tip of the electrode with filtered intracellular solution and then backfill (using 1cc syringe and long syringe needle) with gramicidin containing intracellular solution before attaching it to the electrode holder.
If the perforation is especially slow, the amount of filtered intracellular solution in the tip of the electrode may need to be decreased or the concentration of gramicidin slightly increased. -
10
Descend into the bath with slight positive pressure to keep cellular debris clear of the tip.
-
11
Check the electrode resistance. The resistance should be 6-8 MΩ in ECS solution.
-
12
Approach the cell at 10× or 20× magnification until the electrode is centered over the cell and descend.
-
13
Change to a 40× magnification before patching.
-
14
Cancel the liquid junction potential after electrode is in the bath using pClamp software or Amplifier.
-
15
Place the electrode tip against the cell membrane so a slight dimple in the membrane is visible.
-
16
Apply small pulses of suction to the electrode (using a 3 or 5 cc syringe) until the seal reaches 1GΩ or higher.
Most cells will spontaneously seal with the first pulse of suction. -
17
Release the suction pressure once the seal is above 1GΩ.
Note: Perforated patch clamp should not have the same force of suction applied as that of whole cell recordings. A good cell should form a 1GΩ seal in less than 3 min.
Gaining access/Recording
-
18
Monitor and record the access resistance (Ra) at the time of patching, and throughout the recording.
Complete perforation and stabilization takes 15-30 min. If the cell appears to perforate and then transients disappear the cell should be discarded. -
19
Compensate capacitance transients (see Axopatch manual or pClamp manual).
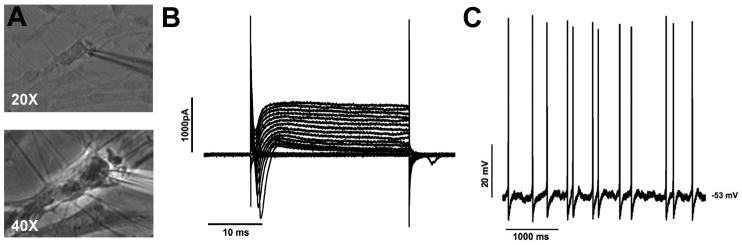
The length of GnRH processes makes space clamping difficult, especially in perforated patch clamp recordings. Cells may not be fully space clamped when access resistance has stabilized (Fig 3B). This makes perforated patch clamping unsuitable for some experimental questions. -
20
Check the perforation periodically by running voltage steps. The transient current will increase in amplitude and decrease in duration as access resistance decreases.
Access resistance stabilizes at or below 100 MΩ (ideal access resistance is 3× to 4× the electrode resistance but may not be obtainable). -
21
Many of the phase-bright plump bipolar cells in the nasal explants are GnRH neurons. However, to confirm the identity of the cells that are patched, explants can be made from transgenic lines expressing GFP or another readily identifiable marker for the cell type of interest. Alternatively, fill electrode with 0.2% biocytin, or similar cell marker (see Pinault, 1996), and rupture the patch at the end of the recording, running a pulse protocol for 2-5 min after going whole cell.
GnRH neurons are generally easy to recognize morphologically under explant conditions, but periodically (10-20%) of cells should be filled to confirm selections are correct if transgenic animals are not available. This is also useful if the cell morphology or contacts are of interest. -
22
Most GnRH neurons are spontaneously active. Action potentials will be detected as soon as the perforation (electrical access) begins.
Figure 3.
A Electrode patched onto GnRH neuron (20× and 40× magnification). B An example of voltage currents obtained from a perforated patched GnRH neuron held at -65 mV and stepped from -100 mV to 90 mV in 10 mV steps. C Spontaneous action potentials (same cell as in B) recorded in I-clamp with no holding current (Vm = -53 mV).
Support Protocol 2: Calcium Imaging in Nasal Explants
Introduction
Calcium imaging experiments in brain slices of a variety of animals has been proven to be difficult. Low level of fluorescence emitted by the cells and the fact that only few neurons are accessible in any given slice makes simultaneous recordings nearly impossible (Jasoni et al., 2007). Synchronized events can more easily be studied in culture models. Therefore, nasal explants offer one the ability to image large numbers of neurons simultaneously and study mechanisms that affect the whole population or specific, identifiable subpopulations (Constantin et al., 2010; Klenke et al., 2010). Transgenic mouse models expressing fluorescent tags, identifying specific neuronal populations, has aided this process extensively. The support protocol presented here introduces the reader briefly to a calcium imaging technique used in our laboratory, to study the regulation of calcium activity in GnRH neurons. If calcium imaging is of special interest, we refer the reader to “The Practical Guide to the Study of Calcium in Living Cells” (Nuccitelli, 1994).
Materials
-
Solutions
Serum Free Medium (SFM, see below)
-
Calcium Green-1 AM probe
Stock solution: 2.7 mM Calcium Green-1 AM probe (Molecular Probes Cat. No. C3012) diluted in 80% DMSO and 20% pluronic F-127 solution (Molecular Probes Cat. No. 753272)
Working solution: Dilution of 1:200 with SFM (final concentration: 13.5 μM)
40 mM KCl-SFM solution
-
Equipment
Recording/Perfusion chamber (Warner Instruments, RC-22 No. 640228)
Peristaltic pump (Spectra Spectra Hardware, Inc., Westmoreland City, PA)
Inverted Microscope (Nikon TE2000) with 20× fluorescence objective
CCD camera (Retiga, Qimaging, Burnaby, Canada)
Imaging software (iVision 4.5, BioVision,)
Loading GnRH-1 neurons with calcium Green-1
-
1
Place nasal explants in a fresh tissue culture dish (35×10 mm) and gently apply 140 μl of calcium Green-1 working solution directly on the explant.
Make sure that the fluid stays on the cover slip, forming a bubble around the explant and does not disperse into the dish. -
2
Incubate the explant at 37 °C in a 5% CO2 humidified incubator for 20 min.
-
3
Gently wash the explant with SFM and incubate as in step 2 for 10 min, repeat SFM wash and incubate for an additional 10 min.
Mounting and imaging nasal explants
-
4
Place nasal explants into a perfusion chamber and cover it with SFM.
-
5
Place perfusion chamber onto inverted imaging microscope and connect to peristaltic pump and gravity fed perfusion system to ensure continuous superfusion with medium or drug solutions.
-
6
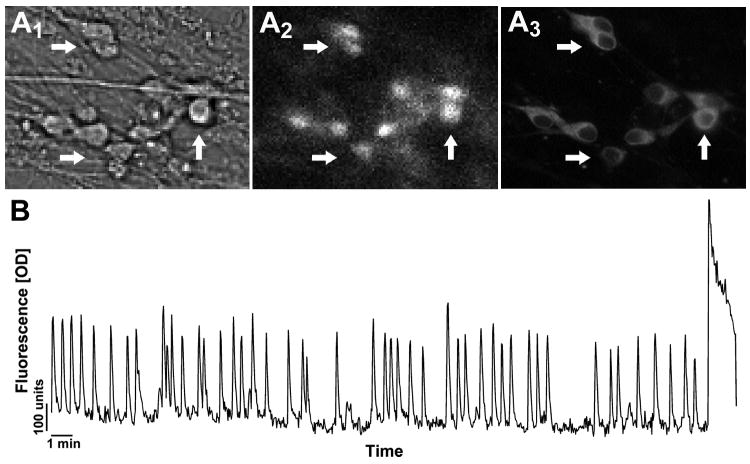
Determine in the bright field setting of the microscope the area of GnRH neurons of interest (Fig 4A1).
-
7
Visualize the calcium Green-1 (Fig 4A2) with a 20× fluorescent objective and a camera connected to a computer.
The excitation wavelengths are provided via a medium-width excitation bandpass filter at 465-495 nm, and the emission is monitored through a 40-nanometer bandpass centered on 535 nm. -
8
The imaging experiments will be piloted by imaging software (e.g. iVision by BioVision).
-
9
Depending on experimental design, pictures can be acquired at a range of time intervals and length.
During our experiments, we acquire pictures every 2 sec. for a length of 33 min. -
10
The fluctuations in calcium oscillations are analyzed a posteriori with iVision software: Identify the individual GnRH neurons and mark them as regions of interest. The calcium Green-1 fluorescence intensity is than analyzed and plotted by the iVision program.
-
11
The iVision output is then analyzed with MATLAB (Mathworks, Natick, MA) (Fig 4B).
-
12
Use immunohistochemistry to stain the explants for GnRH, to verify the identity of the imaged neurons (Fig 4A3).
Figure 4.
Calcium Green-1 imaging of GnRH neurons 6-10 days in vitro. A Cells were identified by their bipolar morphology (A1), loaded with fluorescent calcium-sensitive dye (A2), and their identity verified post-imaging by immunocytochemistry (A3). Arrows indicate identical cells in all fields. B Representative recording showing spontaneous baseline oscillations in intracellular calcium levels in a GnRH neuron during 33-min in serum free media (Y-scale = OD units).
Example of experimental design
-
13
Depending on the experimental questions, we divide our recording time into three to four periods.
For example: the first period is a SFM control period (5 min), followed by a pretreatment period (e.g. pharmacological blocker; 5 min), a treatment period (e.g. agonist/antagonist; 5 min) and a SFM washout period (17 min). -
14
Terminate the experiment by adding 20 μl of a 40mM KCl (diluted in SFM) to depolarize the cells to ensure the viability of the recorded cells.
Reagents and Solutions
For preparing cultures
Gey's + glucose buffer (store at 4 °C for up to one month)
-
1 ml 50% glucose/100 ml Gey's balanced salt solution (Gibco Invitrogen Cat. No. RR080018L1).
Filter after use.
Serum Free Media (SFM) (store at 4°C for up to 1 week)
50% F12 Nutrient Mixture with L-glutamine (GIBCO Invitrogen, Cat. No. 11765)
10 mg/ml Bovine Serum Albumin (BSA) (Gemini, Cat. No. 700-102P)
25% Basal Medium Eagle (2× BME) (Gibco Invitrogen, Cat. No. 900-907)
25% Culture grade water (Gibco Invitrogen, Cat No. 15230-170)
Glutamine (2×10-3 M) (GibcoBRL 25030-081)
Putrescine (1×10-4 M) (Sigma Cat. No. P7505)
Transferin (0.1 mg/ml) (Sigma T-0178)
Selenium (3×10-8 M); make stock solution (1×10-2) in DMEM (without glutamine)
Insulin (0.005 mg/ml) (Sigma I-5500)
Vitamin C (3.8×10-5 M) (Sigma A-4034)
PSN Antibiotics (0.5% (v/v) 100× PSN) (GIBCO Invitrogen Cat. No. 15640)
Glucose (5-7.5 mg/ml) (Sigma G-7021) in culture grade water
1× DMEM high glucose w/o glutamine (GIBCO, cat. No. 10313)
Stock solutions of glutamine (0.2 M), putrescine (1 × 10-4 M), and transferrin (100μg/ml) are made up in DMEM
Plasma and Thrombin for plating explants (store at −20 °C until use)
-
Chicken Plasma, dilute in culture grade water (Cocalico Biologicals Incorporated (Cat. No. 30-0300-5L). Store up to 3 months at -20.
Test plasma dilutions from 1:5 to 1:50 on explants. Appropriate concentration will produce explants that do not float within 30-45 min of feeding and also do not produce excessive holes in the explants by 6 div, but allow thinning of tissue. -
Thrombin: (Sigma T-4265). Store up to 6 months at -20.
Reconstitute using 1:1 (vol/vol) G+G solutionWhen ready to plate explants, dilute 200 μl of frozen Thrombin with 800 μl G+G.
Commentary
Background Information
The nasal explants described here maintain GnRH neurons, olfactory neurons, olfactory ensheathing cells and a variety of other neuronal and non-neuronal cell types in a state similar to that found in vivo. Some structural and organizational characteristics of the original tissue are still in place, which provide an opportunity to study an organized, but isolated, neuronal system. These explants can be maintained for over 30 days in vitro allowing the investigator to study migratory and post-migratory cell processes (Giacobini et al., 2004; Constantin et al., 2010; Klenke et al., 2010).
In the olfactory placode, GnRH neurons migrate along peripherin positive olfactory axons towards the olfactory bulb. Olfactory ensheathing cells (positive for p75 and GFAP) migrate and surround these cells making a continuum from the nose to the brain (for reviews see Su and He, 2010; Wray, 2010). Similarly, nasal explants show peripherin positive axons extending away from the olfactory pits, where the sensory neurons reside (Fueshko et al., 1998). GnRH neurons migrate on these axons towards the periphery of the explant (Fig. 1K, L?). Morphologically distinct olfactory ensheathing cells, positive for p75 nerve growth factor, and glial fibrillary acidic protein (GFAP), also migrate out with the olfactory axons (Forni et al., 2011). Thus, like the developing olfactory placode, nasal explants maintain a microenvironment where sensory neurons, olfactory ensheathing cells, and GnRH neurons intermingle. These GnRH neurons express a similar suite of receptors as adult GnRH neurons in vivo and exhibit cell activity and pulsatile secretion patterns like GnRH cells in vivo (reviewed in Constantin, 2011). Olfactory ensheathing cells are often cultured to study their nerve regenerating permissive properties (Su and He, 2010; King-Robson, 2011), and their axon/glial interactions (Thyssen et al., 2010). Thus, nasal explants may be a useful tool in studying the molecular interactions of these cells.
The accessibility of multiple cell types in nasal explants present in a structurally organized in vitro system allows the experimenter a range of parameters to study. Developmental onset of phenotypic gene expression patterns can be evaluated. Receptor-mediated changes in transcription and translation of specific genes can be analyzed in the cell types of interest using for example single-cell PCR techniques or immunohistochemistry combined with in situ-hybridization. Maturational processes can be observed either in situ (over days in vitro) or post-hoc in explants fixed at different developmental ages (Constantin et al., 2010). Pharmacological manipulations and their outcome on gene transcription and protein presence may be also investigated over developmental changes that occur pre- and post-migration or within the 30 days maintained in culture. Further, cellular receptor signaling pathways or channel mediated intracellular events can be analyzed in target cells using pharmacological manipulations (Klenke et al., 2010). Functional activity of neurons and/or ensheathing cells may be determined using electrophysiological recordings or calcium imaging techniques, as described in our supplementary protocols (Constantin and Wray, 2008b). Electroporation of nasal explants can be used to introduce gene silencing agents, foreign genes, or other pharmacological agents, into the cells to observe their effects on cell specific outcomes. All of these approaches may also be applied to understand factors influencing neuronal migration and the intracellular mechanisms involved (Giacobini et al., 2004).
In optimal nasal explants the plated tissue thins from the inside out, with cells migrating into the periphery, resulting in a monolayer one to three cells thick in the periphery. This thinning process occurs during the first few days of culture allowing cell migration to be visualized in live recordings using phase-contrast microscopy or utilizing transgenic tissue and/or cell loading dyes with fluorescent microscopy. Depending on the intended use, a particular substrate for culturing the explants may be better suited for some applications than others. For example, in both of the support protocols provided here, permanox is generally used as it is easier to handle and is less sensitive to subtle changes in chicken plasma concentration than glass. Nevertheless, in experiments that require higher resolution microscopy, glass is preferable.
The main limitation of nasal explants is the loss of the central nervous system, the target tissue of olfactory axons, olfactory ensheathing cells and GnRH neurons. However, minor variations in culture conditions can be optimized for co-culture with specific target tissues to study directional migration or the influence of tissue derived factors. Further, co-cultures can be used to determine possible indirect interactions between tissues.
This protocol is useful in generating primary cell cultures of neurons and glia cells mimicking an in vivo state. Depending on the research focus this protocol may be modified to enhance a particular cell population of interest. Explants can be maintained for up to a month, making them an ideal model to answer developmental and physiological questions. Especially when used with transgenic animals or cell transfection techniques, the protocols provided here are powerful tools for targeting specific cell physiology and interactions in vitro while maintaining a semi-complex environment.
Critical Parameters and Troubleshooting
Assuming proper solutions and incubator conditions, the key to successful nasal explant cultures is making consistently accurate and precise cuts in the nasal area. It is also essential that the tissue is handled as little as possible since contact with the tissue can result in damage.
The most common problems that arise can be categorized as one of three issues: 1) No neurons present, 2) neurons present but not migrating, or 3) disorganized explants with holes/uneven spreading. These are described below, but a complete table of common problems and their solutions is listed in Table 1.
Table 1. List of Potential Problems and Solutions.
| Problem | Possible Reasons | Solution |
|---|---|---|
| Under or oversized embryos | Strain of mouse/time of day embryos were collected | Adjust embryo collection time by ∼1-3 hrs. |
| Explants appear “junky” with many dead cells | Insufficient SFM coverage/too much SFM | Media should completely cover permanox/glass cover slip but no higher than highest point of the explant. |
| Few/no neurons | Cuts are in wrong location or uneven | Correct cutting procedure (see text). |
| Holes in outgrowing cell carpet | Heparin composition may differ between plasma batches, which can affect the uniform outgrowth of the cells. Explants are left too long after plating before being covered with media. |
For each new batch of plasma dilution tests should be run to test the correct concentration to use. Add media earlier. |
| Contamination by microorganisms | The regular skin flora of the experimenter, non-sterile preparation, handling of the cultures, and/or too frequent opening of the incubators can lead to bacterial or yeast contamination in the cultures. | Ensure that all instruments are sterile and use clean bench conditions. Remove contaminated cultures promptly and sterilize tools and incubators whenever necessary. |
Making consistent cuts requires practice, but in general the most common mistakes arise from clipping off the developing VNO. This results from a) cutting too superficially (a “thin” version of Fig. 1G), which will remove most of the developing olfactory tissue and GnRH neurons or b) taking off too much with the 4th ventral cut (Fig. 1H). Both of these mistakes will result in few if any neurons in the explant.
If very few cells migrate out, explants should be stained for a neuronal marker (e.g. TUJ1) to determine if neurons are present in large clumps around the nasal cartilage. If the latter, several reasons are possible. Most commonly, the cuts are made unevenly (visible as highly unsymmetrical explants) or the explant has been plated incorrectly (with the 4th dorsal cut side up, Fig 2E2). Alternative reasons may be that the explants were handled too much during plating resulting in tissue damage, or insufficient tissue has been cut off from the nasal area. When insufficient tissue is removed, the majority of cell types spreading out from the explant will be non-neuronal, the explants will have many layers of cells (“3D”) and the explant itself will remain thick.
Explants that appear highly disorganized and/or non-symmetric result when the cuts are uneven. All of the cuts should be made in one motion (without hesitation which will cause rough edges and greater tissue damage). The angle of the blade may need to be adjusted to the user's comfort so that the blade can be held perpendicular to the plate. Failure to do so tends to shift the tissue away from where the pressure is applied, thus causing asymmetric cuts. If the dissected heads are left at room temperature, they will be too soft to hold their shape while the noses are cut. If this appears to be the case, the dissected heads should be returned to the refrigerator in G+G solution for a while longer. When learning to make explants, the cutting procedure can be very slow, causing the embryo heads to reach room temperature before all of the noses have been cut. In this case taking half of a litter at a time (leaving the rest at 4 °C) may solve the problem.
Anticipated Results
At 3 div, explants should be symmetrical with a layer of migrating fibroblasts in a halo around the explant and the first GnRH neurons and olfactory axons starting to emerge (Fig 2F). Explants that are distorted and very asymmetrical may be discarded at this point. A reasonable yield is 50% symmetrical explants showing some neurons along the extended olfactory axons at 3 div, but with experience this should be closer to 75%. By 6 div, explants should have many visible olfactory axons extending onto the fibroblast layer with plump phase-bright neurons along the fibers. By 7 div, some explants may have retracted tissue indicating that explants were not well attached to the cover slip or damaged in making. If these holes are large, explants should be discarded.
Ideal explants will have olfactory axons and neurons migrating away from the dorsal aspect of the main tissue mass. These cells will bilaterally dispersed, away from the nasal mesenchyme (NMC), in a radial fashion (Fig 1K) with small clusters of GnRH neurons distributed along peripherin positive olfactory fibers (Fig 1L). Among the fibroblasts, olfactory ensheathing cells will form a surface layer through which the olfactory axons extend away from the NMC. GnRH neuron migration ends by 6 div, however some spreading of non-GnRH neurons will continue.
Time Considerations
Note: Based on a single experienced experimenter doing 6 uteri.
Total time: ∼5 hrs
Uteri dissection ∼10 min
Embryo dissection and head removal ∼30-45 min/6 uteri
Heads should be maintained at 4 °C for a total time of 1-1.5 hrs.
Nasal cuts: This is the longest and most important part of the procedure. For 6 uteri this may take 1.5 hrs for an experienced explant maker, fewer embryos should be used when learning the procedure.
Once explants are cut and in Gey's + glucose, total time at 4 °C should be 30-60 min.
Plating explants: ∼20 min
Once plated, explants should sit for 30-45 min before being bathed in SFM. This will allow the explants to adhere to the coverslips.
Note: Too short of a time will cause the explant to float off the dish, whereas too long (> 45 min) will cause the explant to dry out.
Note: When heads or nasal explants are at 4 °C in Gey's + glucose, 15-35 min breaks are normal, and can be slightly longer if needed.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neuronal Disease and Stroke, NIH.
Literature Cited
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Suter KJ. Redefining the gonadotrophin-releasing hormone neurone dendrite. J Neuroendocrinol. 2010;22:650–658. doi: 10.1111/j.1365-2826.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- Constantin S. Physiology of the gonadotrophin-releasing hormone (GnRH) neurone: studies from embryonic GnRH neurones. J Neuroendocrinol. 2011;23:542–553. doi: 10.1111/j.1365-2826.2011.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Klenke U, Wray S. The calcium oscillator of GnRH-1 neurons is developmentally regulated. Endocrinology. 2010;151:3863–3873. doi: 10.1210/en.2010-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology. 2008a;149:279–290. doi: 10.1210/en.2007-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology. 2008b;149:3500–3511. doi: 10.1210/en.2007-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol. 1995;484:77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31:6915–6927. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166:331–348. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- Fueshko SM, Key S, Wray S. GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J Neurosci. 1998;18:2560–2569. doi: 10.1523/JNEUROSCI.18-07-02560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24:4737–4748. doi: 10.1523/JNEUROSCI.0649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7:45–48. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:860–867. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Robson J. Encouraging regeneration in the central nervous system: is there a role for olfactory ensheathing cells? Neurosci Res. 2011;69:263–275. doi: 10.1016/j.neures.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151:2736–2746. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, Wray S. Midline nasal tissue influences nestin expression in nasal-placode-derived luteinizing hormone-releasing hormone neurons during development. Dev Biol. 2000;227:343–357. doi: 10.1006/dbio.2000.9896. [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Hu L, Leung PK, Feng H, Catt KJ. The hypothalamic GnRH pulse generator: multiple regulatory mechanisms. Trends Endocrinol Metab. 2009;20:402–408. doi: 10.1016/j.tem.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci U S A. 1995;92:3918–3922. doi: 10.1073/pnas.92.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B, DelConte C, Garcia-Castro MI. Embryonic Pax7-expressing progenitors contribute multiple cell types to the postnatal olfactory epithelium. J Neurosci. 2010;30:9523–9532. doi: 10.1523/JNEUROSCI.0867-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R. A Practical Guide to the Study of Calcium in Living Cells. Academic Press; San Diego: 1994. [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ebihara S, Akaike N. Gramicidin perforated patch-clamp technique reveals glycine-gated outward chloride current in dissociated nucleus solitarii neurons of the rat. J Neurophysiol. 1994;72:1103–1108. doi: 10.1152/jn.1994.72.3.1103. [DOI] [PubMed] [Google Scholar]
- Sherman-Gold R. The Axon Guide for Electrophysiology and Biophysics Laboratory Techniques. Axon Instruments, Inc; Sunnyvale, CA: 1993. [Google Scholar]
- Su Z, He C. Olfactory ensheathing cells: biology in neural development and regeneration. Prog Neurobiol. 2010;92:517–532. doi: 10.1016/j.pneurobio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Terasawa E. Luteinizing hormone-releasing hormone (LHRH) neurons: mechanism of pulsatile LHRH release. Vitam Horm. 2001;63:91–129. doi: 10.1016/s0083-6729(01)63004-8. [DOI] [PubMed] [Google Scholar]
- Thyssen A, Hirnet D, Wolburg H, Schmalzing G, Deitmer JW, Lohr C. Ectopic vesicular neurotransmitter release along sensory axons mediates neurovascular coupling via glial calcium signaling. Proc Natl Acad Sci U S A. 2010;107:15258–15263. doi: 10.1073/pnas.1003501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316. doi: 10.1016/s0091-3022(02)00001-8. [DOI] [PubMed] [Google Scholar]
- Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. J Neuroendocrinol. 2010;22:743–753. doi: 10.1111/j.1365-2826.2010.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]