Abstract
Background & Aims
Proliferation of liver stem/progenitor cells (LPCs), which can differentiate into hepatocytes or biliary epithelial cells, is often observed in chronically inflamed regions of liver in patients. We investigated how inflammation might promote proliferation of LPCs.
Methods
We examined the role of interleukin (IL)-22, a survival factor for hepatocytes, on proliferation of LPCs in patients with chronic hepatitis B virus (HBV) infection and in mice. Proliferation of LPCs in mice was induced by feeding a diet that contained 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC).
Results
Hepatic expression of IL-22 was increased in patients with HBV and correlated with the grade of inflammation and proliferation of LPCs. Mice on the DDC diet that overexpressed an IL-22 transgene specifically in liver (IL-22TG), or that were infected with an IL-22–expressing adenovirus, had increased proliferation of LPCs. Signal transducer and activator of transcription (STAT) 3, a component of the IL-22 signaling pathway, was activated in LPCs isolated from DDC-fed IL-22TG mice. Deletion of STAT3 from livers of IL-22TG mice reduced proliferation of LPCs. Moreover, the receptors IL-22R1 and IL-10R2 were detected on EpCAM+CD45– LPCs isolated from DDC-fed wild-type mice. Culture of these cells with IL-22 activated STAT3 and led to cell proliferation, but IL-22 had no effect on proliferation of STAT3-deficient EpCAM+CD45– LPCs. IL-22 also activated STAT3 and promoted proliferation of cultured BMOL cells (a mouse LPC line).
Conclusion
In livers of mice and patients with chronic HBV infection, inflammatory cells produce IL-22, which promotes proliferation of LPCs via STAT3. These findings link inflammation with proliferation of LPCs in patients with HBV infection.
Keywords: immune regulation, viral hepatitis, oval cells, signal transduction
Introduction
Following injury to the liver (mechanical, chemical or other injury types), the liver can regain its original mass through the proliferation of healthy liver epithelial cells, including mature hepatocytes and biliary epithelial cells.1, 2 However, in cases of severe or chronic liver injury in which unhealthy hepatocytes lose the capacity to re-enter the cell cycle and proliferate, a distinct population of adult liver stem/progenitor cells (LPCs) is activated and induced to compensate for liver function, owing to the bipotential capacity of these cells to differentiate into hepatocytes and biliary epithelial cells.3-6 LPCs have been classically termed oval cells in rodents because of their unique “oval-like” shape and large nuclear-to-cytoplasmic ratio. LPCs are often observed in rodent models of liver injury induced by injecting rats with 2-acetylaminoflurene and carrying out a 2/3 partial hepatectomy7 or by feeding mice with a choline-deficient ethionine-supplemented (CDE) diet8 or a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet.9 In humans, the proliferation of LPCs is often observed in severe forms of chronic liver disease, such as alcoholic and nonalcoholic fatty liver diseases, chronic viral hepatitis and cirrhosis, and is also termed ductular reaction because of the formation of ductular structures, which strongly correlates with liver disease severity, including fibrosis and inflammation.10-15
Accumulating evidence from animal models has suggested that several types of inflammatory cells (e.g., T cells, NK cells, and macrophages)16, 17 and their associated cytokines (e.g., IL-6, TNF-α, TWEAK, IFN-γ, and IL-15)18-23 play an important role in promoting LPC proliferation; however, the mechanism by which inflammation promotes LPC proliferation in human chronic liver diseases remains elusive, in part because of the lack of small animal models with chronic viral hepatitis and liver inflammation. For example, many cytokines, including interleukin-22 (IL-22), are present in markedly elevated levels in patients with viral hepatitis24 but are not upregulated in the animal models mentioned above that have been used for the study of LPC proliferation.
IL-22, which is a member of the IL-10 family, plays an important role in controlling bacterial infection, homeostasis, and tissue repair by binding a cell surface IL-22 receptor complex that is composed of IL-10R2 and IL-22R1.25 IL-10R2 is ubiquitously expressed, whereas the expression of IL-22R1 is primarily restricted to epithelial cells, such as keratinocytes, bronchial and intestinal epithelial cells, and hepatocytes (IL-22R1 is not expressed on immune cells).25 IL-22 binding to IL-10R2 and IL-22R1 predominantly activates signal transducer and transcription factor 3 (STAT3), but many other signaling pathways are activated to a lesser extent, such as ERK1/2, JNK, and p38 MAPK, in epithelial cells.25 Many types of cells have been shown to produce IL-22, including activated T cells, NK cells and NKT cells.26, 27 Although many cytokines (e.g., IL-6, IL-21, and IL-23) and transcriptional factors (e.g., STAT3, retinoic acid receptor-related orphan receptor, the basic leucine zipper transcription factor ATF-like, and the aryl hydrocarbon receptor) have been shown to stimulate IL-22 production in helper T cells, the exact mechanisms by which IL-22 expression is regulated are not completely understood.26, 27 Interestingly, immune cell-derived IL-22 targets epithelial cells rather than immune cells.25 We and others have demonstrated that IL-22 protects mice against various types of liver injury by directly preventing hepatocyte death.28, 29 Because LPCs have the bipotential ability to differentiate into hepatocytes and biliary epithelial cells,3-6 we hypothesized that IL-22 may also target LPCs, thereby regulating their proliferation and survival. In the present paper, we demonstrated that IL-22-producing cells and LPCs colocalize in the inflammatory regions and have a strong positive correlation in patients with chronic hepatitis B virus (HBV) infection. Using a murine model of LPC activation that is induced by feeding with a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet and an in vitro LPC culture model, we further demonstrated that LPCs express both IL-22R1 and IL-10R2 and that IL-22 can directly promote LPC proliferation in a STAT3-dependent manner.
Materials and methods
Human samples
Liver samples from 64 patients with chronic HBV were obtained either from biopsy or from the explanted liver during liver transplantation. The patient information is listed in supplemental Table 1. The evaluation of disease severity followed the Scheuer criteria. The study protocol for the use of human samples was approved by the local ethics committee, and all of the patients provided written informed consent.
Animal experiments
The generation of liver-specific IL-22 transgenic mice (IL-22TG) was performed as previously described.24 AlbCre+STAT3flox/flox mice have also been described previously and were referred to as hepatocyte-specific STAT3KO or STAT3Hep-/- mice.30 Because the STAT3 gene is also deleted in the LPCs from AlbCre+STAT3flox/flox mice (see the Results section), we referred to the AlbCre+STAT3flox/flox mice as liver-specific STAT3 knockout (STAT3LKO) mice in the present study. IL-22TGSTAT3LKO double mutant mice (IL-22TGAlbCreSTAT3flox/flox) were generated by crossing IL-22TGSTAT3flox/flox mice with AlbCreSTAT3flox/flox mice. IL-22 knockout mice on a C57BL6 background were kindly provided by Dr. Rachel R. Caspi (NEI, NIH), with the permission of Dr. Wenjun Ouyang and a signed Material Transfer Agreement from Genentech (San Francisco, California), and were further backcrossed to a C57BL6 background for at least 5 generations in our facility.
For the DDC diet model, 6-8-week-old mice were fed a 0.1% DDC-containing diet (Bioserve, Frenchtown, NJ) for various time periods. For the CDE diet model, mice were fed a choline-deficient chow diet (choline-deficient diet combined with normal powdered chow in a 1:1 mixture, from ICN, Costa Mesa, CA) and drinking water containing 0.15% ethionine (Sigma-Aldrich, St. Louis, MO) for 4 weeks as previously described.8 Bromodeoxyuridine (BrdU) (50 mg/kg) was given by intraperitoneal injection 2h before sacrifice. All of the animal experiments were approved by the NIAAA animal care and use committee.
Statistical analysis
The data are expressed as the means ± SD. To compare the values obtained from three or more groups, we used one-factor analysis of variance (ANOVA) followed by Tukey's post hoc test. To compare the values obtained from two groups, Student's t test was performed. Statistical significance was set at the P < 0.05 level.
Results
Positive correlation between IL-22+ inflammatory cells and CK19+ LPCs in patients with chronic HBV
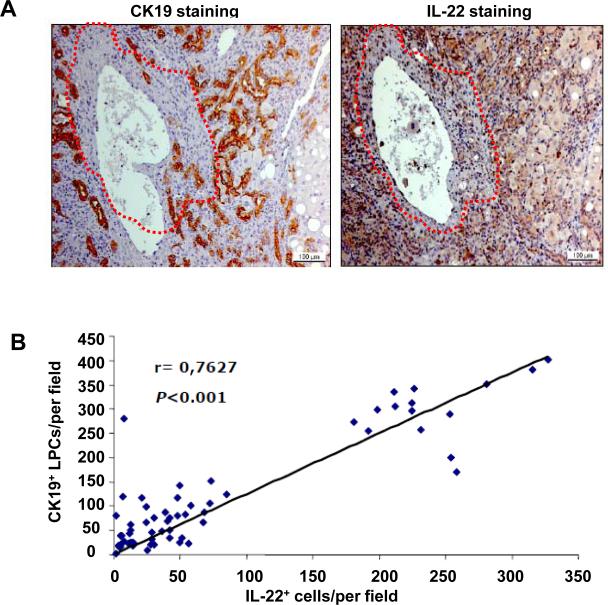
It is well known that LPC proliferation (ductular reaction) often occurs in the inflammatory regions of the liver in patients with chronic viral hepatitis,10-15 and we have previously demonstrated that IL-22+ inflammatory cells are primarily concentrated in these inflammatory regions in chronic HBV and HCV patients.24 To test whether IL-22+ cells and LPC activation colocalize in HBV-infected patients, immunohistochemical analyses were performed on serial sections of 64 HBV-infected liver samples using an anti-IL-22 or anti-CK19 (a marker of LPCs) antibody. Fig. 1A shows that a large number of CK19+ LPCs were found in areas that were enriched in IL-22+ cells (outside the dotted lines), whereas only a few CK19+ LPCs were observed in IL-22-negative regions (within the dotted lines). Among the 64 HBV liver samples, 15 were collected from explanted livers with liver failure (massive/submassive hepatic necrosis), and the remaining 49 samples were collected through biopsies from patients with chronic HBV infection. In general, the samples from the patients with liver failure had higher numbers of CK19+ LPCs compared with the patients with chronic HBV infection. The correlation between the number of CK19+ LPCs and the number of IL-22+ cells, from all 64 samples, was highly significant (Fig. 1B, P<0.001).
Fig. 1. Positive correlation between IL-22+ inflammatory cells and CK19+ LPCs in patients with chronic HBV.
(A) Representative immunostaining with anti-IL-22 and anti-CK19 antibodies of serial liver sections from an HBV patient. Please note that most IL-22+ inflammatory cells and CK19+ LPCs are colocalized outside the dotted line. (B) Spearman's nonparametric rank correlation coefficient between the number of IL-22+ inflammatory cells and CK19+ LPCs from 64 HBV patients.
To determine the types of inflammatory cells that are responsible for IL-22 production, double immunostaining was performed. As illustrated in supplemental Fig. S1, most IL-22+ and CD3+ cells colocalized in the HBV liver samples, which indicated that the CD3+ T cells are the major source of IL-22-producing cells in HBV-infected livers.
IL-22TG mice are more susceptible to DDC-induced LPC activation and proliferation
To determine the role of endogenous IL-22 in LPC proliferation in the DDC model, we measured the serum levels of IL-22 in DDC-fed mice. As illustrated in supplemental Fig. S2a, the serum levels of IL-22 were not elevated at 2 or 4 weeks post-DDC feeding and were only slightly elevated 12 weeks post-DDC feeding. Furthermore, feeding with a DDC diet for 2 weeks induced a similar number of LPCs in both the WT and the IL-22KO mice (supplemental Figs. S2b-d). In addition, IL-22KO and WT mice had a comparable number of BrdU+ hepatocytes after partial hepatectomy (supplemental Fig. S2e). These findings suggest that endogenous IL-22 does not contribute to LPC proliferation in the DDC model and liver regeneration in the partial hepatectomy model.
Because we observed high IL-22 levels in the HBV-infected livers, we used IL-22TG mice, which express high levels of IL-22 in the liver and mimic the elevated hepatic IL-22 levels associated with HBV, to examine the effects of IL-22 on LPC proliferation in the DDC model. Although IL-22TG mice are completely resistant to Con A-induced T cell hepatitis and liver injury,24 feeding with a DDC diet caused similar grades of necrosis and inflammation in the WT and IL-22TG mice (Fig. 2A). Serum ALT and AST levels were slightly higher in the IL-22TG mice from 4 to 6 weeks than in the WT mice (supplemental Fig. S3).
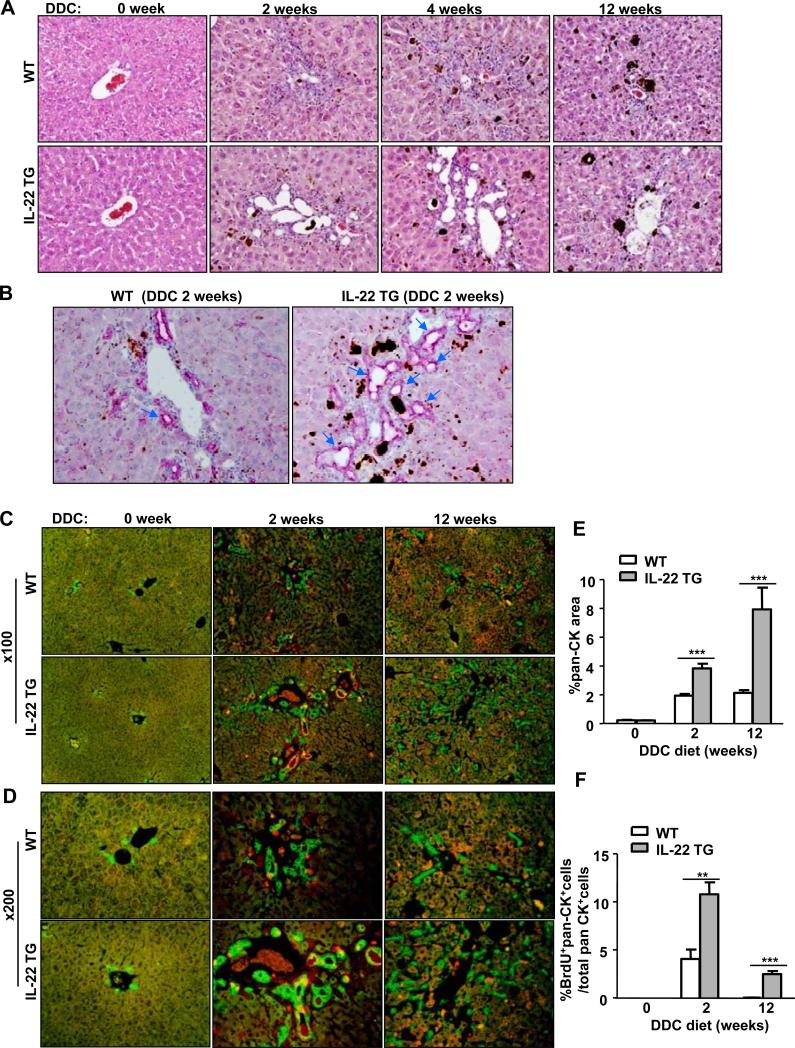
Fig. 2. The IL-22TG mice have increased LPC proliferation after being fed a DDC diet compared with the WT mice.
The mice were fed a DDC diet for up to 12 weeks. (A) H&E staining of liver tissues from DDC-fed mice at different time periods. (B) Representative immunohistochemical analyses with an anti-pan-CK antibody of liver tissues from mice fed a DDC diet for 2 weeks. The arrows indicate ductular reactions. (C, D) Pan-CK (green)/BrdU (red) double staining of liver tissues from mice fed a DDC diet for 2 or 12 weeks with a BrdU injection 2h before sacrifice. (E, F) The number of pan-CK+ and BrdU+pan-CK+ double positive cells was quantified. **P<0.01 and ***P<0.001. Representative photographs from three independent experiments with similar results are shown.
We also examined LPCs in DDC-fed WT and IL-22TG mice by H&E staining and immunofluorescence staining with anti-pan-CK, which is a marker for LPCs and bile duct cells.32 In addition, we investigated LPC proliferation by double staining with anti-pan-CK and anti-BrdU antibodies. As shown in Figs. 2A-D, feeding the WT mice for 2 and 4 weeks (short-term) induced LPC activation and proliferation around the portal regions, which was demonstrated by the appearance of oval-shaped cells on H&E staining (Fig. 2A) and by the detection of pan-CK+ LPC accumulation (Figs. 2B-D). Long-term DDC feeding (12 weeks) induced the accumulation of LPCs in the parenchymal regions in the WT mice (Figs. 2A-D). Interestingly, feeding with a DDC diet for 2 or 4 weeks induced the formation of many large bile ducts (ductular reactions) in the IL-22TG mice (Figs. 2A-B), whereas a long-term (12 weeks) DDC diet caused massive LPC proliferation in the parenchymal regions rather than inducing ductular reactions in the IL-22TG mice (Figs. 2C-D). In addition, double immunohistochemical analyses showed that the number of pan-CK+BrdU+ LPCs was significantly higher in the DDC-fed IL-22TG mice compared with the DDC-fed WT mice (Figs. 2C, 2D, and 2F), which suggested that IL-22 promotes LPC proliferation in vivo.
To further confirm the role of IL-22 on LPC proliferation, we utilized another well-established LPC model induced by feeding a CDE diet.8 As illustrated in supplemental Fig. S4, the number of pan-CK+ and pan-CK+BrdU+ LPCs was markedly higher in the IL-22TG mice compared with the WT mice, which further supported the notion that IL-22 stimulates LPC growth in vivo.
STAT3 is required for the stimulatory role of IL-22 on LPC proliferation in IL-22TG mice
STAT3 is the major downstream transcriptional factor of IL-22. To determine whether STAT3 may contribute to the enhanced LPC proliferation in IL-22TG mice, we generated IL-22TG and liver-specific STAT3KO double mutant (IL-22TGSTAT3LKO) mice in which the expression of recombinase Cre was controlled by an albumin promoter. Because albumin is also expressed in LPCs and AlbCre expression has been shown to delete the floxed genes in LPCs,33, 34 we hypothesized that the STAT3 gene might be deleted in LPCs from the double mutant mice. To confirm this hypothesis, we performed STAT3 and pSTAT3 immunostaining in the liver tissues from DDC-fed WT, IL-22TG, and double mutant mice. As shown in supplemental Fig. S5, STAT3 expression was detected in the LPCs from the DDC-fed WT and IL-22TG mice (STAT3 expression was much higher in the IL-22TG mice), whereas LPCs from the DDC-fed double mutant mice had no STAT3 protein expression. Furthermore, pSTAT3 was detected in the nuclei of LPCs and hepatocytes in the WT and IL-22TG mice after DDC feeding (higher levels of pSTAT3 staining were observed in the LPC nuclei of the IL-22TG mice). No pSTAT3 staining was detected in the LPCs from the DDC-fed double mutant mice.
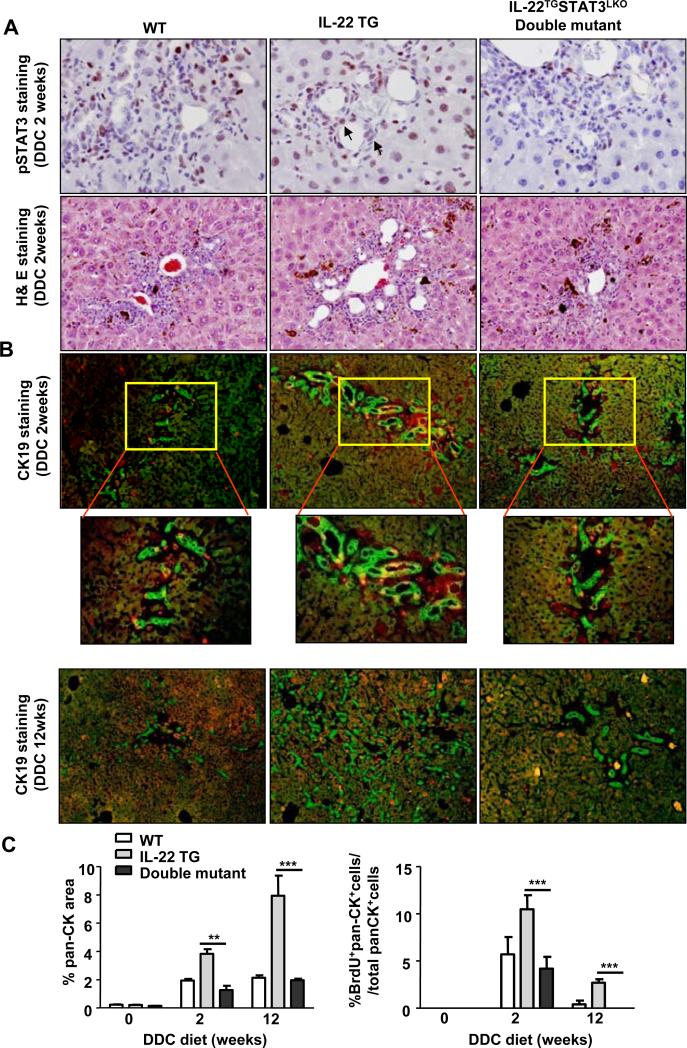
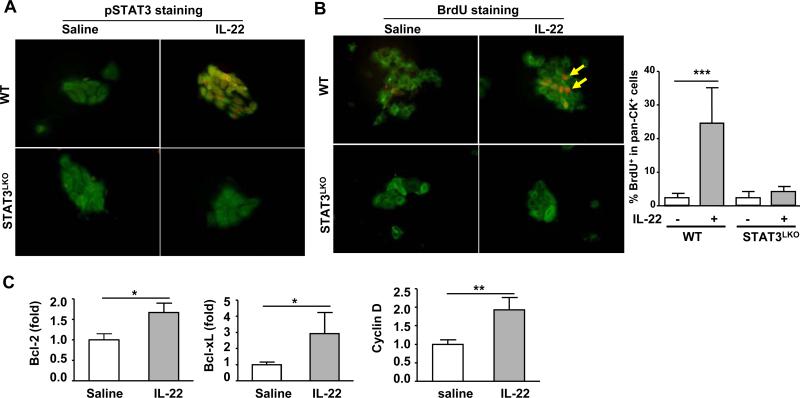
H&E staining showed that the IL-22TG mice fed DDC for 2 weeks had more ductular reactions than did WT mice, and these reactions were diminished in double mutant mice (Fig. 3A). Immunofluorescence staining demonstrated that after 2 or 12 weeks of DDC feeding, the double mutant mice had significantly lower numbers of pan-CK+ and BrdU+pan-CK+ LPCs compared with the IL-22TG mice (Figs. 3B-C). Collectively, liver-specific STAT3 deficiency abolished the increased LPC proliferation in the IL-22TG mice on the DDC diet.
Fig. 3. STAT3 contributes to the increased susceptibility of the IL-22TG mice to DDC-induced LPC proliferation.
WT, IL-22TG, and IL-22TGSTAT3LKO double mutant mice were fed a DDC diet for 2 or 12 weeks. (A) Immunohistochemical staining with an anti-pSTAT3 antibody and H&E staining. The arrows indicate pSTAT3+ LPC nuclei. (B) Pan-CK (green)/BrdU (red) double staining of liver tissues with a BrdU injection 2h before sacrifice. (C) Quantification of pan-CK+ and BrdU+pan-CK+ areas. **P<0.01 and ***P<0.001. Representative photographs from three independent experiments with similar results are shown.
Overexpression of IL-22 via injection of Ad-IL-22 promotes LPC proliferation in WT but not in STAT3LKO mice after DDC feeding
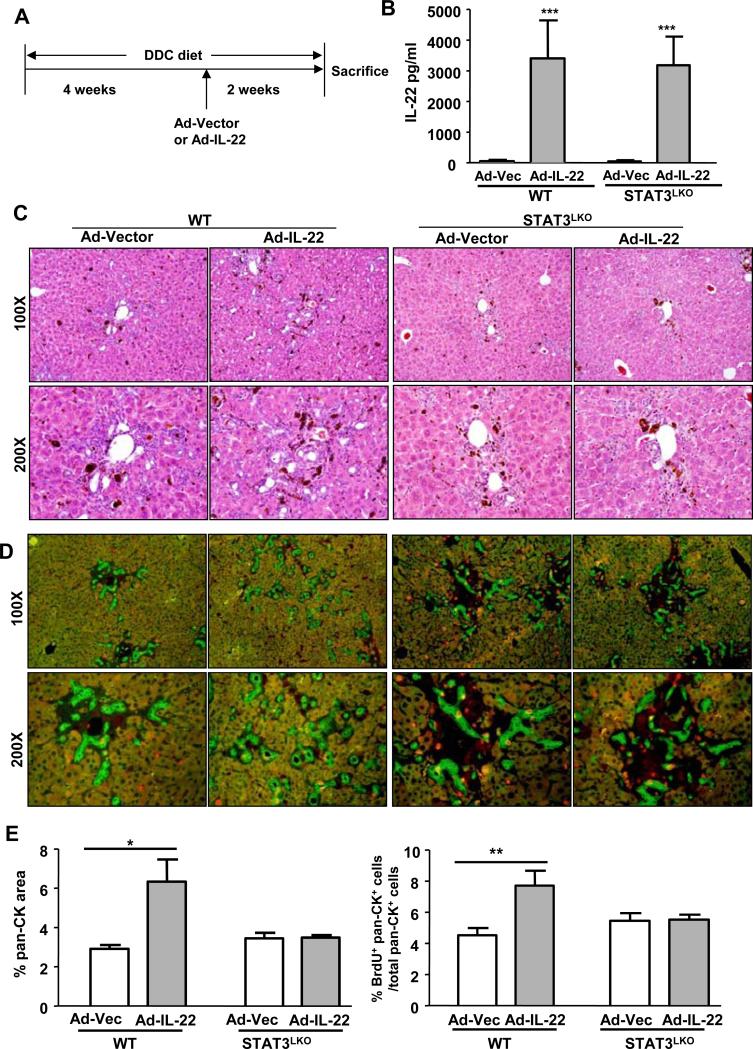
To strengthen the evidence for the participation of IL-22 and STAT3 in LPC proliferation, WT and STAT3LKO mice were fed a DDC diet for 4 weeks and then treated with Ad-IL-22 or Ad-vector control viruses for an additional 2 weeks (Fig. 4A). Serum levels of IL-22 were elevated in the Ad-IL-22-treated WT and STAT3LKO mice but not in the Ad-vector-treated mice (Fig. 4B). In the WT mice, overexpression of IL-22 via infection with Ad-IL-22 markedly increased DDC-induced LPC proliferation, which was demonstrated by the appearance of oval cell reactions observed by H&E staining (Fig. 4C) and pan-CK+BrdU+ cell accumulation (Fig. 4D). However, these changes were not observed in the Ad-IL-22-treated STAT3LKO mice that were fed a DDC diet (Figs. 4C-D). In addition, H&E staining also showed that Ad-IL-22 infection for 2 weeks induced the formation of significant ductular reactions in the WT mice, such reactions were markedly reduced in the STAT3LKO mice (Fig. 4C).
Fig. 4. Injection of Ad-IL-22 stimulates LPC proliferation in the WT but not in the STAT3LKO mice.
WT and STAT3LKO mice were fed a DDC diet for 4 weeks followed by injection with an Ad-vector or Ad-IL-22 virus. The mice were then maintained on a DDC diet for 2 more weeks and sacrificed. (A) A schematic diagram of the experimental design. (B) Serum IL-22 levels. (C) H&E staining. (D) Double staining with pan-CK (green)/BrdU (red) antibodies. (E) Quantification of the pan-CK+ area and BrdU+pan-CK+ double area. *P<0.05 and **P<0.01. Representative photographs from three independent experiments with similar results are shown.
IL-22 promotes the proliferation of primary mouse LPCs, which express IL-22R1 and IL-10R2, in a STAT3-dependent manner
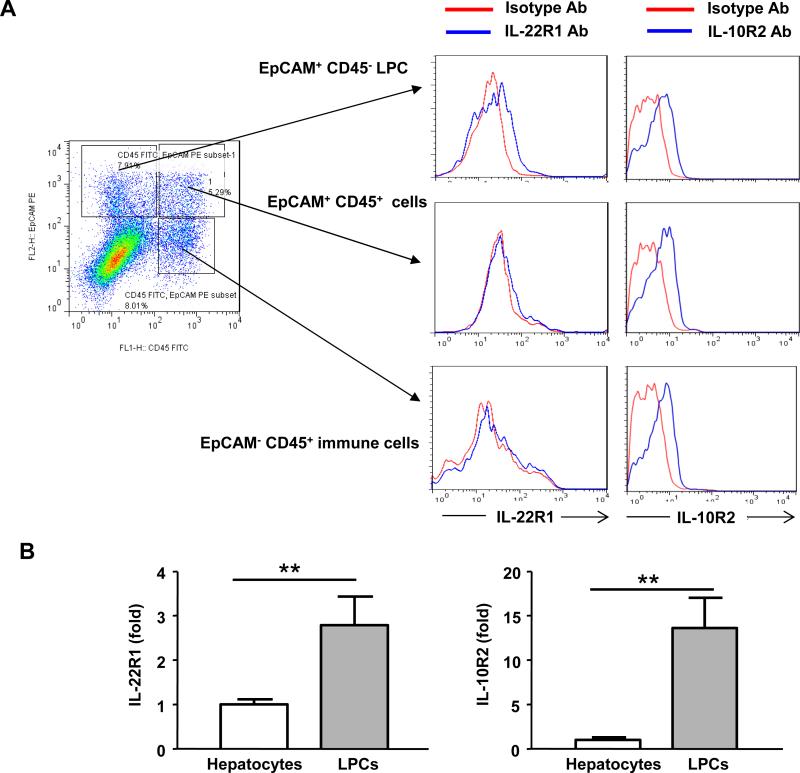
To determine whether IL-22 can directly target LPCs, we examined IL-22R1 and IL-10R2 expression in LPCs. Liver nonparenchymal cells that contain LPCs were isolated from DDC-fed mice and subjected to flow cytometric analyses with anti-EpCAM (LPC cell marker),35 anti-CD45 (lymphocyte marker), and anti-IL-22R1 or anti-IL-10R2 antibodies. As illustrated in Fig. 5, EpCAM+CD45- LPCs, but not CD45+ or CD45+EpCAM+ lymphocytes, were positive for IL-22R1 staining compared with the isotype control. Interestingly, IL-10R2 expression was detected in the EpCAM+CD45- LPCs and in the EpCAM+CD45+ and EpCAM-CD45+ immune cells.
Fig. 5. The expression of IL-22R1 and IL-10R2 on LPCs.
(A) Liver nonparenchymal cells from the DDC-fed mice were isolated and analyzed by flow cytometry with FITC-anti-CD45, PE-anti-EpCAM, and APC-anti-IL-22R1 or APC-anti-IL-10R2 antibodies. Data are representative of three different experiments. (B) Real-time PCR analyses of IL-22R1 and IL-10R2 expression in primary mouse hepatocytes and purified LPCs from the DDC-treated mice. **P<0.01.
We also used real-time PCR analyses to compare the expression of IL-22R1/IL-10R2 in hepatocytes and freshly purified LPCs from the DDC-fed mice. The LPCs were enriched by two-step digestion and Percoll gradient centrifugation and further purified by MACS negative and positive selection with anti-CD45 and anti-EpCAM antibodies (supplemental Fig. S6a). Interestingly, pan-CK staining demonstrated that the purity of the LPCs (EpCAM+CD45-) was 70-80% after 12h of culture (supplemental Fig. S6b). As illustrated in Fig. 5B, the mRNA levels of IL-22R1 and IL-10R2 were significantly higher in the LPCs compared with the hepatocytes.
We also examined the influence of IL-22 on the proliferation of purified mouse LPCs from DDC-fed mice in vitro. After in vitro treatment with IL-22 for 30 min, pSTAT3 staining was detected in the nuclei of pan-CK+ LPCs from the WT mice but not from the STAT3LKO mice (Fig. 6A). The BrdU incorporation assay showed that IL-22 treatment markedly increased WT LPC proliferation, which was demonstrated by the increase in BrdU+pan-CK+ LPCs (Fig. 6B). Very few BrdU+pan-CK+ cells were observed in the IL-22-treated LPCs from the STAT3LKO mice, suggesting that STAT3 contributes to the IL-22 promotion of LPC proliferation in vitro. In addition, real-time PCR revealed that IL-22 treatment upregulated the expression of several STAT3 downstream genes in the WT LPCs, including cyclin D, Bcl2, and Bcl-xL (Fig. 6C).
Fig. 6. IL-22 promotes LPC proliferation in vitro in a STAT3-dependent manner.
LPCs were purified from the DDC-fed WT or STAT3LKO mice and were then cultured in vitro. (A) These purified LPCs were serum starved for 4h and were then treated with IL-22 (100 ng/ml) in vitro for 30 min. Pan-CK (green) and pSTAT3 (red) double staining were performed. (B) Serum-starved LPCs were also treated with IL-22 (100 ng/ml) in vitro, followed by incubation with BrdU for 24h. Pan-CK (green) and BrdU (red) double staining were performed. The arrows indicate positive staining. The number of pan-CK+BrdU+ LPCs was counted and shown in the right panel. (C) Real-time PCR analysis of several antiapoptotic and cell-cycle associated genes from purified LPCs treated with IL-22 for 6h. *P<0.05, **P<0.01, and ***P<0.001. Data are representative of three different experiments.
LPCs isolated from DDC-fed WT mice did not produce IL-22 and treatment with an IL-22 neutralizing antibody had no effect on proliferation of these cells (supplemental Fig. S6c-e). In contrast, LPCs isolated from the DDC-fed IL-22TG mice produced IL-22 and proliferated much faster than did those from the DDC-fed WT mice. Such proliferation was markedly attenuated after treatment with an IL-22 neutralizing antibody (supplemental Fig. S6c-e).
IL-22 promotes cell proliferation and inhibits apoptosis of the mouse LPC cell line BMOL
The effects of IL-22 on cell proliferation were also examined in BMOL cells, which is a well-established mouse LPC line.36 Western blot analyses in Fig. 7A show that IL-22 treatment induced STAT3 activation in a dose-dependent manner. In agreement with the above findings that IL-22 promotes primary mouse LPC proliferation, treatment with IL-22 also markedly stimulated BMOL cell proliferation (Fig. 7B).
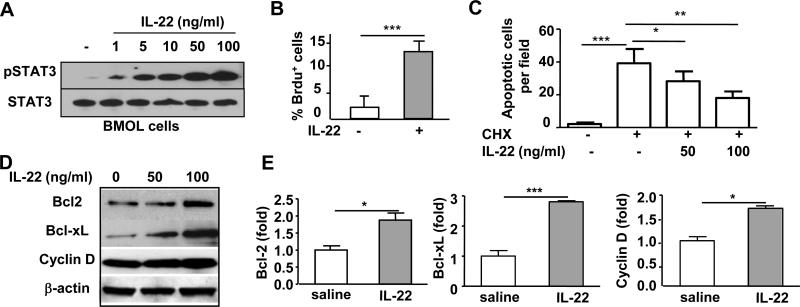
Fig. 7. IL-22 promotes the proliferation and prevents the apoptosis of BMOL cells in vitro.
(A) BMOL cells were treated with IL-22 for 30 min prior to western blot analysis of pSTAT3 and STAT3. (B) BMOL cells were starved overnight and then treated with IL-22 for 24h, and BrdU was added 2h before harvest. BrdU+ cells were detected by immunostaining. (C) BMOL cells were pretreated with IL-22 for 2h prior to treatment with CHX for 6h. Cell apoptosis was then determined. (D, E) BMOL cells were treated with IL-22 for 24h prior to being subjected to western blot or RT-PCR analysis. *P<0.05, **P<0.01, and ***P<0.001.
Because IL-22 is a well-documented survival factor for hepatocytes,28 we hypothesized that IL-22 may also protect against LPC apoptosis. As illustrated in Fig. 7C, treatment with cycloheximide (CHX) significantly induced BMOL cell apoptosis, which was markedly blocked by IL-22 pretreatment in a dose-dependent manner.
To further understand the mechanisms by which IL-22 promotes BMOL cell proliferation and survival, we examined the expression of several IL-22 downstream genes in BMOL cells. As shown in Figs. 7D-E, IL-22 treatment upregulated the mRNA and protein expression of Bcl-2, cyclin D, and Bcl-xL in BMOL cells. In contrast, the expression of IL-20, which is the IL-22 downstream mediator in keratinocytes,37 was not elevated in BMOL cells after IL-22 stimulation (supplemental Fig. S7). Furthermore, IL-20 treatment did not affect the expression of Bcl-2, cyclin D, or Bcl-xL in BMOL cells (supplemental Fig. S7). In addition, hepatic IL-20 expression was not increased in the DDC-fed IL-22TG mice compared with the DDC-fed WT mice (data not shown). These findings suggest that Bcl-2, Bcl-xL, and cyclin D, but not IL-20, are the genes downstream from IL-22 that contribute to the mitogenic and cytoprotective effect of IL-22 on LPCs.
Discussion
Many mitogenic factors have been identified as promoters of LPC proliferation, including IL-6, TNF-α, TWEAK, IFN-γ, and IL-15.18-23 In the current study, we provided in vivo and in vitro evidence that IL-22 is another important factor that promotes LPC proliferation. Although serum and hepatic IL-22 levels are not elevated and although endogenous IL-22 does not contribute to LPC proliferation in the DDC model, hepatic IL-22 levels are markedly elevated in patients with viral hepatitis 24 and the overexpression of IL-22 in transgenic mice or after administration of Ad-IL-22 markedly enhanced LPC proliferation in the DDC-fed mice. This suggests that IL-22 promotes LPC proliferation in vivo. In addition, the LPCs that were isolated from the DDC-fed mice expressed much higher levels of both IL-22R1 and IL-10R2 than did the hepatocytes. This finding is not surprising because IL-22R1 is known to be expressed on epithelial cells,25 and LPCs are the progenitor cells for liver epithelial cells, including hepatocytes and biliary epithelial cells. IL-10R2 is known to be ubiquitously expressed on many types of cells.25 We also observed that in vitro IL-22 challenge increased the proliferation of LPCs and the LPC cell line BMOL cells and protected against CHX-induced BMOL cell apoptosis, which indicated that IL-22 can directly target and enhance the proliferation of LPCs in vitro.
We have previously demonstrated that IL-22 promotes hepatocyte proliferation and survival via the activation of STAT3.28 Activation of the STAT3 signaling pathway has been implicated in the proliferation of LPCs18, 38 and likely also contributes to the mitogenic effect of IL-22 on LPCs. Indeed, the deletion of STAT3 in the LPCs abolished IL-22-mediated LPC proliferation in vivo in mice with IL-22 overexpression (transgenic or Ad-IL-22 injection) (Figs. 3-4) and with IL-22 stimulation of LPC proliferation in vitro (Fig. 6). Moreover, IL-22 treatment is known to upregulate the expression of several antiapoptotic and mitogenic genes, including Bcl-2, Bcl-xL, and cyclin D in hepatocytes.28 These genes were also elevated in the LPCs after IL-22 treatment and likely contribute to IL-22 promotion of LPC proliferation and survival. A recent study suggested that IL-20 is an important IL-22 downstream mediator in keratinocytes.37 However, IL-20 is not elevated in BMOL cells after IL-22 stimulation (supplemental Fig. S7). Collectively, Bcl-2, Bcl-xL, and cyclin D, but not IL-20, contribute to the mitogenic of IL-22 on LPCs.
Another interesting finding is that short-term (2-4 week) DDC feeding induced the formation of many bile ducts (ductular reactions), whereas long-term (12 week) DDC feeding induced massive LPC proliferation and accumulation in the IL-22TG mice. Currently, the mechanisms underlying these reactions are unknown. It is well documented that activated oval cells proliferate and form duct-like structures around the periportal region. Ultimately, the oval cells spread into the liver acini, and the duct-like structures disappear.39 In the present study, the LPCs in the IL-22TG mice that were fed the DDC diet showed a much stronger capability to proliferate. Compared with the WT mice, the stronger proliferation of the LPCs in the IL-22TG mice form more duct-like structures at the early stage of DDC feeding and then spread into the liver acini at the later stage of feeding. Because pan-CK and CK19 markers are also expressed on mature biliary epithelial cells and no specific cell markers exist to distinguish LPCs from biliary epithelial cells in the DDC-fed mice, we cannot rule out the possibility that some of the proliferating cells were biliary epithelial cells in the IL-22TG mice, especially after short-term DDC feeding. Interestingly, without DDC feeding, pan-CK+ and CK19+ cells were not increased in the livers of the IL-22TG mice or the mice that received the Ad-IL-22 injection. Massive ductular reactions and LPC proliferation were only observed in these mice after being fed by a DDC diet. This finding suggests that IL-22 is able to stimulate the proliferation of DDC-induced LPCs rather than directly initiating normal healthy mature biliary epithelial cell proliferation.
Chronic HBV infection is associated with the accumulation of IL-22-producing inflammatory cells in the liver.24 In the current study, we demonstrated that the majority of IL-22+ and CD3+ cells colocalize in HBV patients (supplemental Fig. S1). This suggests that CD3+ T cells are the major cell types that produce IL-22 in the HBV-infected livers. In addition to T cells, activated NKT and NK cells have also been shown to produce IL-22 to a lesser extent.26, 27 However, there are currently no good antibodies to perform double immunostaining for IL-22 and NKT cells or for IL-22 and NK cells, and we cannot rule out the possibility that NKT and NK cells may also contribute to IL-22 production in HBV-infected livers.
Although IL-22 has been reported to have no antiviral activity against HBV replication,40 IL-22 appears to be a mediator of the inflammatory response following recognition of HBV by T cells in the liver.41 In addition, IL-22 is a well-documented survival factor for hepatocytes via the upregulation of antiapoptotic and antioxidant genes.28 In the present study, immunohistochemical analyses of serial liver sections revealed that CK19+ LPCs and IL-22+ inflammatory cells colocalized in the inflammatory regions of HBV-infected livers and had a strong positive correlation, which suggested that IL-22 promotes LPC proliferation. In addition, LPC proliferation is known to play an important role in liver repair when hepatocyte proliferation is suppressed in severe forms of chronic liver diseases, such as cirrhosis.3-6 Thus, in addition to the ability of IL-22 to promote hepatocyte survival and proliferation, the accumulation of IL-22-producing inflammatory cells may also play a critical role in restoring functional liver mass via the stimulation of LPC proliferation in chronic HBV infection. Although we did not perform the correlation studies between LPC proliferation and IL-22 in patients with chronic HCV infection, it is likely that IL-22 will also play an important role in promoting LPC proliferation in HCV patients with high levels of IL-22.24
Supplementary Material
Acknowledgments
This work was supported by the intramural program of NIAAA, NIH (B.G), the German Research Foundation programs “SFB TRR77 Liver Cancer” and “Do373/8-1” (SD) and the Federal Ministry of Education and Research grants “The Virtual Liver” (SD) and “Cell Therapy in Liver Regeneration” (SD and HW).
Abbreviations
- CK19
cytokeratin 19
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- IL-22
interleukin-22
- IL-22TG mice
IL-22 liver-specific transgenic mice
- LPC
liver progenitor cell
- STAT3LKO
liver-specific STAT3 knockout
- pan-CK
pan-cytokeratin
- STAT3
signal transducer and activator of transcription 3
Footnotes
No conflicts of interest exist for any of the authors.
Author contributions: DF designed and performed the experiments, analyzed the data, and wrote the manuscript; XK, HW, OP, and HW performed the experiments; SD and EG designed the study and edited the manuscript; BG designed the study, analyzed the data, and wrote the manuscript.
Additional methods are described in the Supporting Information.
References
- 1.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 3.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–87. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 4.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, Reid LM. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–45. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roskams T, Katoonizadeh A, Komuta M. Hepatic progenitor cells: an update. Clin Liver Dis. 2010;14:705–18. doi: 10.1016/j.cld.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Greenbaum LE, Wells RG. The role of stem cells in liver repair and fibrosis. Int J Biochem Cell Biol. 2011;43:222–9. doi: 10.1016/j.biocel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–40. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 8.Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, Yeoh GC. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519–22. doi: 10.1053/jhep.2001.26751. [DOI] [PubMed] [Google Scholar]
- 9.Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, Greenbaum LE. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–9. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowes KN, Croager EJ, Abraham LJ, Olynyk JK, Yeoh GC. Upregulation of lymphotoxin beta expression in liver progenitor (oval) cells in chronic hepatitis C. Gut. 2003;52:1327–32. doi: 10.1136/gut.52.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–11. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, Hines IN, Rippe RA, Spahr L, Rubbia-Brandt L, Diehl AM. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–43. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–41. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viebahn CS, Yeoh GC. What fires prometheus? The link between inflammation and regeneration following chronic liver injury. Int J Biochem Cell Biol. 2008;40:855–73. doi: 10.1016/j.biocel.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–63. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 16.Strick-Marchand H, Masse GX, Weiss MC, Di Santo JP. Lymphocytes support oval cell-dependent liver regeneration. J Immunol. 2008;181:2764–71. doi: 10.4049/jimmunol.181.4.2764. [DOI] [PubMed] [Google Scholar]
- 17.Viebahn CS, Benseler V, Holz LE, Elsegood CL, Vo M, Bertolino P, Ganss R, Yeoh GC. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500–7. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Yeoh GC, Ernst M, Rose-John S, Akhurst B, Payne C, Long S, Alexander W, Croker B, Grail D, Matthews VB. Opposing roles of gp130-mediated STAT-3 and ERK-1/ 2 signaling in liver progenitor cell migration and proliferation. Hepatology. 2007;45:486–94. doi: 10.1002/hep.21535. [DOI] [PubMed] [Google Scholar]
- 19.Knight B, Yeoh GC, Husk KL, Ly T, Abraham LJ, Yu C, Rhim JA, Fausto N. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. J Exp Med. 2000;192:1809–18. doi: 10.1084/jem.192.12.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, Knight B. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang B, Bissell DM, Burkly LC. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115:2330–40. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, McCall S, Choi SS, Sicklick JK, Huang J, Qi Y, Zdanowicz M, Camp T, Li YX, Diehl AM. Interleukin-15 increases hepatic regenerative activity. J Hepatol. 2006;45:410–8. doi: 10.1016/j.jhep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Knight B, Lim R, Yeoh GC, Olynyk JK. Interferon-gamma exacerbates liver damage, the hepatic progenitor cell response and fibrosis in a mouse model of chronic liver injury. J Hepatol. 2007;47:826–33. doi: 10.1016/j.jhep.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS, Young HA, Gao B. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252–61. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 26.Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–79. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Rutz S, Ouyang W. Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr Opin Immunol. 2011;23:605–12. doi: 10.1016/j.coi.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 29.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horiguchi N, Lafdil F, Miller AM, Park O, Wang H, Rajesh M, Mukhopadhyay P, Fu XY, Pacher P, Gao B. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology. 2010;51:1724–34. doi: 10.1002/hep.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, Theise ND. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology. 2005;41:1252–61. doi: 10.1002/hep.20696. [DOI] [PubMed] [Google Scholar]
- 33.Thompson MD, Wickline ED, Bowen WB, Lu A, Singh S, Misse A, Monga SP. Spontaneous repopulation of beta-catenin null livers with beta-catenin-positive hepatocytes after chronic murine liver injury. Hepatology. 2011;54:1333–43. doi: 10.1002/hep.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, Thorgeirsson SS. Hepatocyte growth factor (HGF) /c-Met signaling is required for stem cell mediated liver regeneration. Hepatology. 2011 doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–60. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 36.Tirnitz-Parker JE, Tonkin JN, Knight B, Olynyk JK, Yeoh GC. Isolation, culture and immortalisation of hepatic oval cells from adult mice fed a choline-deficient, ethionine-supplemented diet. Int J Biochem Cell Biol. 2007;39:2226–39. doi: 10.1016/j.biocel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Docke WD, Asadullah K, Volk HD, Sterry W, Sabat R. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–81. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez A, Factor VM, Schroeder IS, Nagy P, Thorgeirsson SS. Activation of NF-kappaB and STAT3 in rat oval cells during 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Hepatology. 2004;39:376–85. doi: 10.1002/hep.20040. [DOI] [PubMed] [Google Scholar]
- 39.Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology. 2006;43:S89–98. doi: 10.1002/hep.21047. [DOI] [PubMed] [Google Scholar]
- 40.Dambacher J, Beigel F, Zitzmann K, Heeg MH, Goke B, Diepolder HM, Auernhammer CJ, Brand S. The role of interleukin-22 in hepatitis C virus infection. Cytokine. 2008;41:209–16. doi: 10.1016/j.cyto.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.