Abstract
OBJECTIVE
It is known that toll-like receptor (TLR)4 plays an important role in atherosclerosis. Since both microvascular (MIC) and macrovascular (MAC) endothelial cells (ECs) are present in atherosclerotic lesions, this study compared TLR4-triggered inflammatory response and crosstalk with mononuclear cells between MIC and MAC ECs.
METHODS AND RESULTS
ELISA, real-time PCR and gene expression profiling showed that TLR4 activation by LPS stimulated a much higher expression of inflammatory genes including cytokines, chemokines, growth factors and adhesion molecules in MIC ECs than MAC ECs. Furthermore, co-culture studies showed that TLR4 activation in MIC ECs, but not MAC ECs, induced a crosstalk with U937 mononuclear cells through MIC EC-released IL-6 to upregulate matrix metalloproteinase (MMP)-1 expression in U937 cells. To explore molecular mechanisms underlying the different responses to TLR4 activation between MIC and MAC ECs, we showed that MIC ECs had a higher expression of TLR4 and CD14 and a higher TLR4-mediated NFκB activity than MAC ECs.
CONCLUSIONS
This study showed that TLR4 activation triggers a more robust inflammatory response in MIC ECs than MAC ECs. Given the importance of inflammatory cytokines and MMPs in plaque rupture, MIC ECs may play a key role in plaque destabilization through a TLR4-dependent mechanism.
Keywords: Toll-like receptor, Inflammation, Endothelial Cells, Gene Expression, Interleukin 6
Atherosclerosis is an inflammatory disease 1, 2. In atherosclerotic lesions, both immune cells (monocytes, macrophages and lymphocytes) and vascular cells (endothelial cells and smooth muscle cells) release inflammatory cytokines 3, 4 that contribute not only to plaque development, but also to ultimate plaque rupture and acute coronary syndrome 5–7. Among cells involved in atherosclerosis, endothelial cells (ECs) play a unique role in the vascular homeostasis and inflammation not only by serving as a barrier between circulating blood and vascular wall, but also by participating in angiogenesis, thrombosis and vascular remodeling 8–11. The role of endothelial dysfunction in atherogenesis is well known 12.
Vascular ECs represent a highly heterogeneous population of cells with their biological functions 9. Based on their location in the vascular systems, ECs are separated into macrovascular endothelial cells (MAC ECs) that line large- or medium-sized vessels and microvascular endothelial cells (MIC ECs) that line small size vessels and capillaries. While acute coronary syndromes are considered as macrovascular diseases as they occur in large/medium sized vessels, microvascular system frequently occurs inside atherosclerotic lesions 13. MIC ECs constitute the microvascular system that are largely developed from the vasa vasorum and penetrate into atherosclerotic lesions to supply oxygen and nutrients 13. Studies have indicated that MIC ECs in advanced plaques contribute to intraplaque hemorrhage, lipid core expansion, and plaque rupture 13–15. However, the underlying mechanisms have not been fully understood.
Toll-like receptors (TLRs) are receptors for the innate immune response that plays a crucial role in inflammation-associated diseases 16. In recent years, studies have well documented a role of TLR4 in diabetes 17, 18 and vascular diseases such as atherosclerosis 19, 20. Although it is known that TLR4 is expressed by a variety of cell types including immune cells and vascular cells 21, the studies on TLR4 have been largely focused on immune cells such as monocytes, macrophages and lymphocytes. Indeed, the information concerning the role of TLR4 in inflammatory response by vascular ECs, in particular MIC ECs, is relatively scarce.
Previous studies have shown that MAC and MIC ECs have many different biological properties. For example, pulmonary MAC ECs and MIC ECs have different signal transduction and barrier properties 22, 23. In diabetic rats, cardiac MIC ECs had reduced abilities for angiogenesis when compared with cardiac MAC ECs 24. Furthermore, human umbilical vein ECs secreted substantially higher levels of matrix metalloproteinases (MMPs) compared to ECs isolated from dermal capillaries 25. Clearly, these studies compared the phenotypes between MIC and MAC ECs and provided insight into the relationship between vascular locations and specific biological functions of ECs. More importantly, defining the differences in specific phenotypes involved in inflammation between MIC and MAC ECs may help find specific targets to inhibit inflammatory response. Given the crucial role of ECs in atherosclerosis, it is interesting to delineate and compare the responses from MIC and MAC ECs to TLR4 activation. In this study, we demonstrated a marked difference between MIC and MAC ECs in inflammatory response to TLR4 activation and its consequences.
METHODS
Cell Culture
Human aortic ECs and human adult dermal microvascular ECs as cell models for MAC ECs and MIC ECs, respectively, as well as the corresponding cell culture media were purchased from Invitrogen (Carlsbad, CA). Human aortic ECs were grown in Medium 200 with low serum growth supplement (LSGS) containing 2% fetal bovine serum, 1 μg/ml of hydrocortisone, 10 ng/ml of human epidermal growth factor (hEGF), 3 ng/ml of basic fibroblast growth factor (bFGF) and 10 μg/ml of heparin. Human adult dermal microvascular ECs were grown in Medium 131 with microvascular growth supplement containing 5% fetal bovine serum, 1 μg/ml of hydrocortisone, 1 ng/ml of hEGF, 10 μg/ml of heparin, 0.08 mM of dibutyl cyclic AMP. Human cardiac microvascular ECs (Lonza, Allendale, NJ) were grown in EGM™-2 media containing 5% FBS, 0.04% hydrocortisone, 0.4% bFGF, 0.1%vescular endothelial growth factor, 0.1% insulin like growth factor-1 and 0.1% 0.5 hEGF. The ECs were maintained at 37 °C with 5% CO2 and 95% humidity. The ECs between passage 4 and 8 were used for the experiments. For all experiments, 100% confluent cultures of MAC and MIC ECs were used. The 6-well Corning Transwell plates (Sigma, St. Louis, MO) that have 2-compartments separated by a polycarbonate membrane with 0.4 μm pores were used for coculture of ECs and U937 cells or human monocytes, in which MIC or MAC ECs were grown to 100% confluence in the lower compartment and U937 cells or human monocytes were grown (1 × 106 cells/well) in the upper compartment. U937 mononuclear phagocytes 26 were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in a 5% CO2 atmosphere in RPMI 1640 medium (GIBCO, Invitrogen Cop. Carlsbad, CA) containing 10% fetal calf serum, 1% MEM non-essential amino acid solution, and 0.6 g/100 ml of HEPES. Human monocytes were isolated as described previously 27 from blood obtained from healthy donors and treated in the medium that was same as that used for U937 cells. The blood donation for monocyte isolation was approved by university Institution Review Board (IRB).
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-6 and MMP-1 in medium were quantified using sandwich ELISA kits according to the protocol provided by the manufacturer (R&D System, Minneapolis, MN).
Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from cells using the RNeasy minikit (Qiagen, Santa Clarita, CA). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad; Hercules, CA) using 20 μl of reaction mixture containing 0.5 μg of total RNA, 4 μl of 5x iScript reaction mixture, and 1 μl iScript reverse transcriptase. The complete reaction was then cycled for 5 minutes at 25°C, 30 minutes at 42°C and 5 minutes at 85°C using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription reaction mixture was then used for PCR amplification in the presence of the primers (Table 1). The Beacon Designer Software (PREMIER Biosoft International; Palo Alto, CA) was used for primer designing (Supplementary Table 1). Primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Real-time PCR was carried out in duplicates using 25 μl of reaction mixture that contained 1.5 μl of RT mixture, 0.2 μM of both primers, and 12.5 μl of iQ™ SYBR Green Supermix (Bio-Rad; Hercules, CA). The real-time PCR was performed using the iCycler™ real-time detection system (Bio-Rad; Hercules, CA) with a two-step method. The reaction was activated with hot-start enzyme (95°C for 3 min) and cDNA was then amplified for 40 cycles consisting of denaturation (95°C for 10 sec) and annealing/extension (56°C for 45 sec). A melt-curve was then performed (55°C for 1 min and then temperature was increased by 0.5°C every 10 sec) to detect the formation of primer-derived trimers and dimmers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control was amplified with the primers (Table 1). Data were analyzed with the iCycler iQ™ I software. The average Ct (threshold cycle) of fluorescence units was used for analysis. Quantification was calculated using the Ct of cytokine cDNA relative to that of GAPDH cDNA in the same sample.
Table 1.
Comparison of fold increase in inflammatory gene expression in response to LPS between microvascular and macrovascular endothelial cells
| Genes | Microvascular ECs | Macrovascular ECs | ||||||
|---|---|---|---|---|---|---|---|---|
| Ct in control cells | Ct in LPS- treated cells | ΔCt | Fold increase | Ct in control cells | Ct in LPS- treated cells | ΔCt | Fold increase | |
| IL-6 | 26.7 | 19.9 | 6.8 | 111.4 | 25.6 | 24 | 1.6 | 3.0 |
| IL-8 | 19.4 | 15.9 | 3.5 | 11.3 | 20.9 | 19.1 | 1.8 | 3.5 |
| TNFα | 29 | 27.7 | 1.3 | 2.5 | 30.7 | 30.1 | 0.6 | 1.5 |
| TNFAIP2 | 24.4 | 21.5 | 2.9 | 7.5 | 25.9 | 25.2 | 0.7 | 1.6 |
| IL-1β | 24.3 | 22.5 | 1.8 | 3.5 | 27.6 | 24.4 | 3.2 | 9.2 |
| CXCL1 | 21.6 | 17 | 4.6 | 24.3 | 22.4 | 19.2 | 3.2 | 9.2 |
| CXCL10 | 28.5 | 16.1 | 12.4 | 5404.7 | 29.8 | 26.2 | 3.6 | 12.1 |
| CXCL3 | 26.6 | 19.7 | 6.9 | 119.4 | 26.7 | 22 | 4.7 | 26.0 |
| CXCL5 | 26.9 | 19.5 | 7.4 | 168.9 | 29.6 | 26.7 | 2.9 | 7.5 |
| CXCL6 | 25.9 | 17.4 | 8.5 | 362.0 | 24.6 | 22 | 2.6 | 6.1 |
| CXCL9 | 28.4 | 25.3 | 3.1 | 8.6 | 29.6 | 28.9 | 0.7 | 1.6 |
| MCP-1 | 20.4 | 16.9 | 3.5 | 11.3 | 23.6 | 19.5 | 4.1 | 17.1 |
| ICAM-1 | 21.4 | 17.2 | 4.2 | 18.4 | 24.6 | 21.7 | 2.9 | 7.5 |
| VCAM-1 | 27.3 | 23.0 | 4.3 | 19.7 | 29.0 | 25.9 | 3.1 | 8.6 |
| E-Selectin | 28.0 | 23.7 | 4.3 | 19.7 | 29.4 | 25.3 | 4.1 | 17.1 |
| HGF | 28.3 | 26.9 | 1.4 | 2.6 | 27.1 | 26.7 | 0.4 | 1.3 |
| VEGFA | 26 | 24.9 | 1.1 | 2.1 | 25 | 24.6 | 0.4 | 1.3 |
| VEGFC | 24.2 | 21.2 | 3 | 8.0 | 24.7 | 24.1 | 0.6 | 1.5 |
| GADPH | 15.8 | 15.8 | 0 | 0 | 15.6 | 15.5 | 0.1 | 1.1 |
| β actin | 14.7 | 14.8 | −0.1 | 0.9 | 15.0 | 15.3 | −0.3 | 0.8 |
Note: TNFAIP2, tumor necrosis factor-alpha-induced protein 2; HGF, hepatocyte growth factor; VEGFA, vascular endothelial growth factor A; VEGFC, vascular endothelial growth factor C; ΔCt, the difference of two Cts. The data are representative of those from two experiments with similar results.
PCR Arrays
First-strand cDNA was synthesized from RNA using RT2 First Strand Kit (SuperArray Bioscience Corp., Frederick, MD). Human pathway RT2 Profiler™ PCR Arrays (SuperArray Bioscience Corp.) were performed using 2X SuperArray RT2 qPCR master mix and the first strand cDNA by following the instruction from the manufacturer.
Blocking Studies
For studies in which TLR4 or CD14 was blocked, MIC or MAC ECs were treated with 100 ng/ml of LPS in the absence or presence of 5 or 10 μg/ml of anti-TLR4 or anti-CD14 antibodies (R&D Systems) for 24 h. For blocking studies using neutralizing anti-cytokine antibodies, medium conditioned by MIC or MAC ECs incubation in the absence or presence of 100 ng/ml of LPS was collected after 24 h. U937 cells were then incubated with 75% of fresh medium containing 100 ng/ml of LPS and 25% of the MIC EC-conditioned medium in the absence or presence of 1 or 2.5 μg/ml of anti-IL-6, IL-1α, IL-1β or TNFα antibody (R&D System) for 24 h. After the incubation, MMP-1 in culture medium was quantified using ELISA.
Immunoblot
Fifty μg of cytoplasma protein in each sample was electrophoresed in a 10% polyacrylamide gel. After transferring proteins to a PVDF membrane, TLR4, MD-2 and CD14 were immunoblotted with primary antibodies (TLR4 and MD-2 antibodies from Abcam, Cambridge, MA; CD14 antibody from Santa Cruz Biotechnology, Inc., Santa Cruz, CA; GAPDH antibody from Cell Signaling Technology, Danvers, MA) and HRP-conjugated secondary antibody (EMD Chemicals, Inc., Gibbstown, NJ). TLR4, MD-2 and CD14 were detected by incubating the membrane with enhanced chemiluminescence (ECL) Plus Lumigen™ PS-3 detection reagent (GE Healthcare, UK) for 1 min and exposing it to x-ray film for 1 to 5 min.
Transfection and Luciferase Activity Assay
Endothelial cells were transfected with 1 μg of NFκB or AP-1 Cignal Reporter Assay kit (Qiagen Inc., Valencia, CA) using Fugene 6 as transfection reagent (Roche Diagnostics Corp., Indianapolis, IN) for 8 h. The constitutively expressing renilla luciferase constructs were used as control. The cells were then treated with fresh medium containing 100 ng/ml of LPS for 24 h. After the treatment, the cells were rinsed with cold PBS and lysed with Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Both firefly and renilla luciferase levels were measured in a luminometer using the dual-luciferase reporter assay reagents (Promega) according to the instruction from the manufacturer. The firefly luciferase levels were normalized to the renilla luciferase levels.
Statistic Analysis
Data were presented as mean ± SD. Comparison between treatments was performed using the one-way analysis of variance (ANOVA). A value of P< 0.05 was considered significant.
RESULTS
A Marked Difference in IL-6 Secretion in Response to TLR4 Activation between MIC ECs and MAC ECs
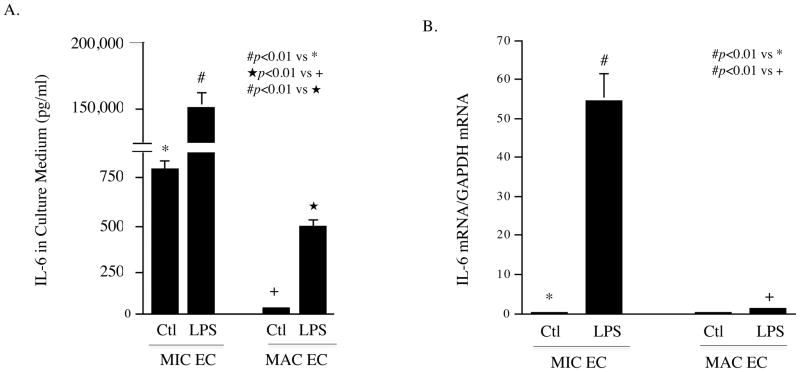
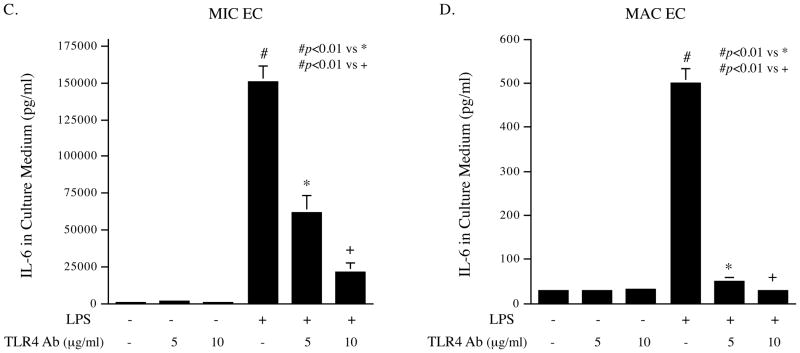
To determine the difference in TLR4-mediated upregulation of cytokine production between MIC and MAC ECs, we first focused on the secretion of IL-6, a key inflammatory cytokine, in response to LPS, a potent ligand for TLR4 16. Results showed that the baseline level of IL-6 released by untreated human dermal MIC ECs was much higher than that by untreated human aortic MAC ECs and, in response to LPS, IL-6 secreted by MIC ECs was much higher than that by MAC ECs (Fig. 1A). The amount of IL-6 secreted by LPS-treated MIC ECs and MAC ECs was 185- and 17-fold of that by untreated cells (150,916 vs 814 pg/ml for MIC ECs and 501 vs 29 pg/ml for MAC ECs), respectively. Similarly, quantification of IL-6 mRNA showed that MIC ECs had much higher IL-6 mRNA expression in response to LPS than MAC ECs (Fig. 1B). Furthermore, IL-6 secretion by human cardiac MIC ECs in response to LPS was also determined and results showed that cardiac MIC ECs released similar amount of IL-6 as dermal MIC ECs (Supplementary Fig. 1A). To exclude the possibility that different culture conditions for dermal MIC and aortic MAC ECs are responsible for the difference in IL-6 secretion, aortic MAC ECs were cultured in Medium 131 that was used for dermal MIC ECs. Results showed that the culture in Medium 131 did not significantly increase IL-6 secretion by aortic MAC ECs (Supplementary Fig. 1B). To ensure that the stimulatory effect of LPS on IL-6 expression was a result of TLR4 activation, we treated ECs with LPS in the presence or absence of anti-TLR4 antibody. Results showed that 10 μg/ml of TLR4 antibody effectively antagonized the stimulatory effect of LPS on IL-6 secretion from either MIC or MAC ECs (Fig. 1C and D). In contrast, antibody against TLR2 had no effect on LPS-stimulated IL-6 secretion (data not shown). Taken together, these results demonstrated that while TLR4 activation led to an increase in IL-6 secretion by both MIC and MAC ECs, the difference in the amount of IL-6 released by MIC and MAC ECs was tremendous.
Figure 1.
Stimulation of IL-6 expression by TLR4 activation in MIC and MAC ECs. A and B: 100% confluent cultures of MIC or MAC ECs were treated with or without 100 ng/ml of LPS for 24 h. After the treatment, IL-6 in culture medium was quantified with ELISA (A) and IL-6 mRNA in cells was quantified with real-time PCR (B). C and D: MIC ECs (C) or MAC ECs (D) were treated with or without 100 ng/ml of LPS in the absence or presence of 5 or 10 μg/ml of anti-TLR4 antibody (Ab) for 24 h. After the treatment, IL-6 in culture medium was quantified with ELISA. The presented data were from representative of three experiments with similar results.
TLR4 Activation in MIC ECs Led to a Higher Expression of Inflammatory Molecules than that in MAC ECs
To further compare the inflammatory responses of MIC and MAC ECs to TLR4 activation, PCR array analysis for gene expression profile of inflammatory cytokines, chemokines, growth factors and adhesion molecules was performed. As shown in Table 1, TLR4 activation triggered a much stronger response in MIC ECs than MAC ECs for the expression of many of the tested genes. For example, TLR4 activation increased chemokine CXCL10 expression by 5,405- and 12-fold in MIC ECs and MAC ECs, respectively. Table 2 summarizes the fold increases in the expression of cytokines, chemokines, growth factors and adhesion molecules observed in MIC ECs as compared to those in MAC ECs after TLR4 was activated. To confirm the findings from the above PCR arrays, several genes such as IL-6, CXCL-1 and CXCL-10 were quantified using real-time PCR and the results were consistent with those observed from PCR array (Supplementary Table 2). Furthermore, TLR4 blockade significantly inhibited LPS-stimulated expression of MCP-1, ICAMP-1 and E-selectin, the representatives of the inflammatory molecules stimulated by LPS in MIC ECs (Supplementary Fig. 2), indicating again that TLR4 activation is responsible for the upregulation of inflammatory molecules by LPS.
Table 2.
Comparison of stimulated inflammatory gene expression in response to LPS between microvascular and macrovascular endothelial cells
| Genes | Microvascular ECs | Macrovascular ECs | Comparison between microvascular ECs and macrovascular ECs | |||
|---|---|---|---|---|---|---|
| Ct in control cells | Ct in LPS- treated cells | Ct in control cells | Ct in LPS- treated cells | ΔCt between LPS- treated microvascular and macrovascular ECs | Fold increase in microvascular ECs compared to macrovascular ECs | |
| IL-6 | 26.7 | 19.9 | 25.6 | 24 | 4.1 | 17.1 |
| IL-8 | 19.4 | 15.9 | 20.9 | 19.1 | 3.2 | 5.3 |
| TNFα | 29 | 27.7 | 30.7 | 30.1 | 2.4 | 5.3 |
| TNFAIP2 | 24.4 | 21.5 | 25.9 | 25.2 | 3.7 | 13.0 |
| IL-1β | 24.3 | 22.5 | 27.6 | 24.4 | 1.9 | 3.7 |
| CXCL1 | 21.6 | 17 | 22.4 | 19.2 | 2.2 | 4.6 |
| CXCL10 | 28.5 | 16.1 | 29.8 | 26.2 | 10.1 | 1097.5 |
| CXCL3 | 26.6 | 19.7 | 26.7 | 22 | 2.3 | 4.9 |
| CXCL5 | 26.9 | 19.5 | 29.6 | 26.7 | 7.2 | 147.0 |
| CXCL6 | 25.9 | 17.4 | 24.6 | 22 | 4.6 | 24.3 |
| CXCL9 | 28.4 | 25.3 | 29.6 | 28.9 | 3.6 | 12.1 |
| MCP-1 | 20.4 | 16.9 | 23.6 | 19.5 | 2.6 | 6.1 |
| ICAM-1 | 21.4 | 17.2 | 24.6 | 21.7 | 4.5 | 22.6 |
| VCAM-1 | 27.3 | 23.0 | 29.0 | 25.9 | 2.9 | 7.5 |
| E-Selectin | 28.0 | 23.7 | 29.4 | 25.3 | 1.6 | 3.0 |
| HGF | 28.3 | 26.9 | 27.1 | 26.7 | −0.2 | 0.9 |
| VEGFA | 26 | 24.9 | 25 | 24.6 | −0.3 | 0.8 |
| VEGFC | 24.2 | 21.2 | 24.7 | 24.1 | 2.9 | 7.5 |
| GADPH | 15.8 | 15.8 | 15.6 | 15.5 | −0.3 | 0.8 |
| β actin | 14.7 | 14.8 | 15.0 | 15.3 | 0.5 | 1.4 |
Note: TNFAIP2, tumor necrosis factor-alpha-induced protein 2; HGF, hepatocyte growth factor; VEGFA, vascular endothelial growth factor A; VEGFC, vascular endothelial growth factor C; ΔCt, the difference of two Cts. The data are representative of those from two experiments with similar results.
TLR4 Activation in MIC ECs Triggered an IL-6-mediated Crosstalk with U937 Mononuclear Cells to Upregulate MMP-1 Expression by U937 Mononuclear cells
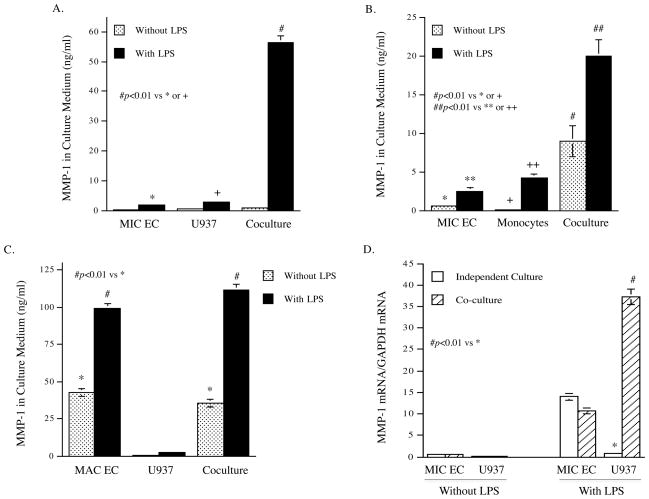
Data from our and other laboratories have shown that inflammatory cytokines such as IL-6, IL-1β and TNFα are powerful stimulators of MMP expression by mononuclear cells 28–30. Since the above studies showed that TLR4 activation led to a robust upregulation of inflammatory cytokine expression by MIC ECs, we proposed that inflammatory cytokines released by MIC ECs in response to TLR4 activation were capable of stimulating MMP expression by mononuclear cells. Using a coculture system, we found that TLR4 activation in the coculture of MIC ECs and U937 mononuclear cells led to a striking augmentation of MMP-1 secretion that was 10-fold of the combined amount of MMP-1 secreted from independent cultures of MIC ECs and U937 cells (Fig. 2A). Similar augmentation of MMP-1 production was also observed in coculture of MIC ECs and human peripheral blood monocytes treated without or with LPS (Fig. 2B). For MAC ECs, while unstimulated cells had a high production of MMP-1, TLR4 activation further stimulated MMP-1 secretion by 2-fold (Fig. 2C). However, coculture of MAC ECs and U937 cells did not augment TLR4 activation-stimulated MMP-1 secretion (Fig. 2C).
Figure 2.
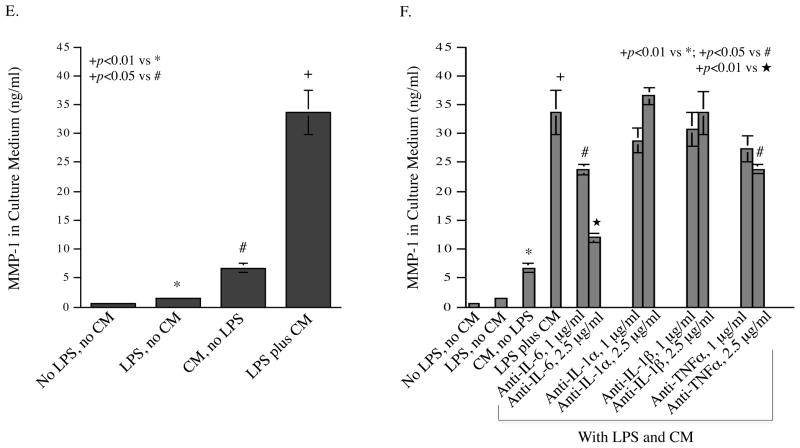
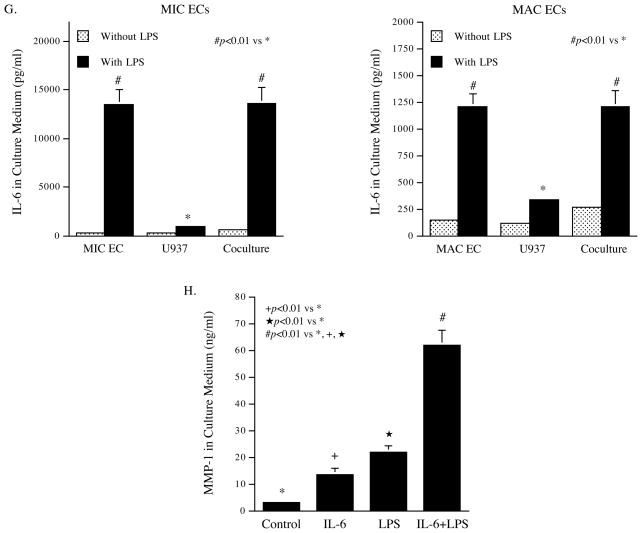
Augmentation of LPS-stimulated MMP-1 production by coculture of MIC ECs with mononuclear cells. A and B: MIC ECs and U937 cells (A) or human monocytes (B) were cultured independently or together (coculture) in the absence or presence of LPS (100 ng/ml for U937 cells and 10 ng/ml for human monocytes) for 24 h. After the treatment, MMP-1 in culture medium was quantified using ELISA. C: MAC ECs and U937 cells were cultured independently or together (coculture) in the absence or presence of 100 ng/ml of LPS for 24 h. After the treatment, MMP-1 in culture medium was quantified using ELISA. D: The expression of MMP-1 mRNA by MIC ECs and U937 cells in the independent culture or the coculture. The MIC ECs and U937 cells from the above studies were used for RNA isolation. MMP-1 mRNA was quantified using real-time PCR. E: Addition of MIC EC-conditioned medium (CM) to U937 cells increased LPS-stimulated MMP-1 secretion by U937 cells. CM was collected after 24 h incubation of MIC ECs without or with 100 ng/ml of LPS. U937 cells were treated with or without 100 ng/ml of LPS in the absence or presence of MIC EC-CM (25% of total medium) for 24 h. After the treatment, MMP-1 in medium was quantified using ELISA. F: The role of inflammatory cytokines released by MIC ECs in the augmentation of LPS-stimulated MMP-1 production by U937 cells. U937 cells were incubated with fresh medium (75% of total medium) containing 100 ng/ml of LPS and the MIC EC-CM (25% of total medium) in the absence or presence of 1.0 or 2.5 μg/ml of anti-IL-6, IL-1α, IL-1β or TNFα antibody for 24 h. After the incubation, MMP-1 in culture medium was quantified using ELISA. G: Quantification of IL-6 in medium of independent culture of MIC ECs, MAC ECs or U937 cells or in medium of the coculture of MIC ECs or MAC ECs with U937 cells in the absence or presence of 100 ng/ml of LPS. MIC ECs or MAC EC and U937 cells were cultured independently or together (coculture) in the absence or presence of 100 ng/ml of LPS for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. H: Synergistic effect of IL-6 and LPS on MMP-1 production by U937 cells. U937 cells were treated with 10 ng/ml of IL-6, 100 ng/ml of LPS or both for 24 h and MMP-1 in culture medium was quantified using ELISA. The presented data (mean ± SD) were from representative of three experiments with similar results.
To determine which cells, MIC ECs or U937 cells, in the coculture contributed to increased MMP-1 production, MMP-1 mRNA expression in cocultured MIC ECs or U937 cells was quantified and compared to that in the independent culture. Results showed that TLR4 activation led to a remarkable increase in MMP-1 mRNA expression in U937 cells, but not MIC ECs (Fig. 2D), indicating that U937 cells in the coculture were the major contributors to the increased MMP-1 production.
To investigate whether MIC ECs interacted with U937 mononuclear cells through released agent (s) that increased MMP-1 production by U937 cells, we treated U937 cells with MIC EC-conditioned medium and LPS, and found that the addition of the conditioned medium to U937 cells markedly augmented LPS-stimulated MMP-1 secretion (Fig. 2E), suggesting that soluble factor (s) released by MIC ECs had a synergistic stimulation with TLR4 activation on MMP-1 secretion by U937 cells. To identify the soluble factor (s), we performed blocking studies using neutralizing antibodies against to IL-6, IL-1α, IL-1β, or TNFα. Results showed that among these antibodies, anti-IL-6 antibody significantly inhibited the augmentation of MMP-1 production by U937 cells incubated with MIC EC-conditioned medium and LPS (Fig. 2F), suggesting that IL-6 released by MIC ECs in response to TLR4 activation is the major soluble factor responsible for the increased MMP-1 secretion by U937 cells. Additionally, anti-TNFα antibody at 2.5 μg/ml also inhibited MMP-1 secretion with a less percentage reduction as compared to anti-IL-6 antibody, indicating a minor role of TNFα in MMP-1 secretion. Quantification of IL-6 in medium from the coculture of MIC ECs or MAC ECs with U937 cells showed that IL-6 released by the coculture of MIC ECs and U937 cells was much more than that released by the coculture of MAC ECs and U937 cells (Fig. 2G). Moreover, studies showed that LPS and IL-6 had a synergistic effect on MMP-1 expression by U937 cells (Fig. 2H).
MIC ECs Had a Significantly Higher Expression of TLR4 and CD14 than MAC ECs
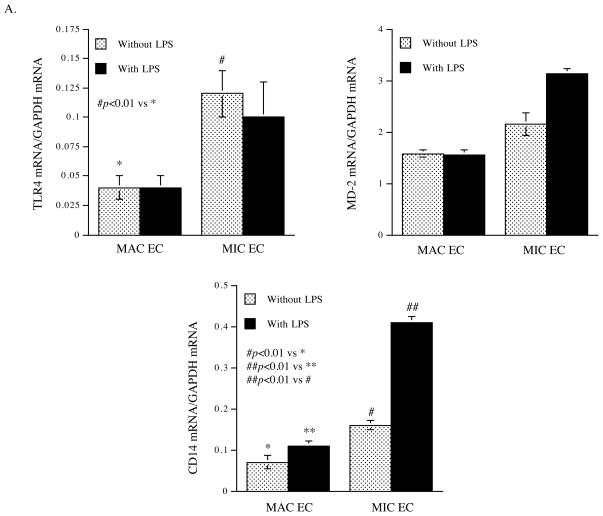
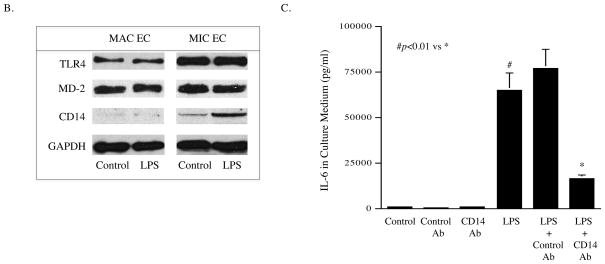
To unravel the mechanisms by which MIC ECs have stronger inflammatory response to TLR4 activation than MAC ECs, we compared the expression of TLR4, CD14 and MD-2 between MIC and MAC ECs in the absence or presence of TLR4 activation. Results showed that the baseline of TLR4 expression in MIC ECs was about 3-fold of that in MAC ECs, and TLR4 activation by LPS did not change TLR4 expression (Fig. 3A and B). While MD-2 expression had no significant difference between MIC and MAC ECs, the baseline expression of CD14 in MIC ECs was significantly higher than MAC ECs, and TLR4 activation further increased CD14 expression in MIC ECs (Fig. 3A and B). These findings suggest that the higher expression of TLR4 and CD14 by MIC ECs when compared to MAC ECs may lead to the stronger inflammatory response to TLR4 activation in MIC ECs. The role of CD14 in LPS-stimulated IL-6 secretion by MIC ECs was confirmed by the observation that neutralizing anti-CD14 antibody effectively blocked the stimulatory effect of LPS (Fig. 3C).
Figure 3.
The expression of TLR4, MD-2 and CD14 by MIC and MAC ECs in the absence and presence of LPS. A and B: MIC or MAC ECs were treated without or with 100 ng/ml of LPS for 24 h. After the treatment, the mRNA and protein expression of TLR4, MD-2 and CD14 were quantified using real-time PCR (A) and immunoblotting (B), respectively. C: The effect of CD14 brocade on LPS-stimulated IL-6 secretion by MIC ECs. Confluent MIC ECs were treated with 100 ng/ml of LPS in the absence or presence of 10 μg/ml of anti-CD14 antibodies for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. The presented data (mean ± SD) were from representative of three experiments with similar results.
TLR4 Activation Led to a Higher NFκB Transcriptional Activity in MIC ECs than that in MAC ECs
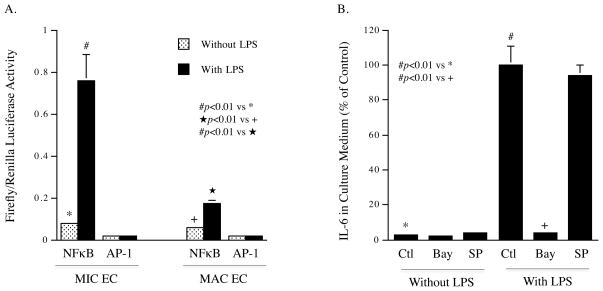
It is known that TLR4 activation leads to increased NFκB transcriptional activity that is responsible for the transcriptional upregulation of inflammatory cytokines 16. In this study, we used luciferase reporter constructs to determine the NFκB transcriptional activity in MIC and MAC ECs in response to TLR4 activation. Results showed that TLR4 activation increased NFκB transcriptional activity by 10- and 3-fold, respectively, in MIC and MAC ECs (Fig. 4A). Interestingly, TLR4 activation did not increase AP-1 transcriptional activity in either MIC or MAC ECs. To confirm the role of NFkB in TLR4-mediated inflammatory response by MIC ECs, MIC ECs were treated with LPS in the presence of Bay117085, a specific inhibitor of NFkB signaling pathway, or SP600125, a specific inhibitor of JNK signaling pathway that is involved in AP-1 activation. Results showed that Bay117085 abolished LPS-stimulated IL-6 secretion, but SP600125 had no effect (Fig. 4B), which is consistent with the results presented in Fig. 4A.
Figure 4.
The NFκB and AP-1 transcriptional activities in MIC and MAC ECs treated with or without LPS. A. MIC or MAC ECs were transfected with NFκB promoter- or AP-1 promoter-luciferase reporter constructs for 18 h and then treated with or without 100 ng/ml of LPS for 24 h. After the treatment, Firefly luciferase activities were assayed and normalized to Renilla luciferase activities. B. MIC ECs were treated with or without 100 ng/ml of LPS in the absence or presence of 5 μM of Bay117085 or SP600125 for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. The amount of IL-6 released by cells treated with LPS in the absence of pharmacological inhibitors was considered as 100%. The presented data (mean ± SD) were from representative of three experiments with similar results.
DISCUSSION
Previous studies have demonstrated neovascularization in the intima and media of atherosclerotic lesions13, 14. The observation that total microvessel content correlated with intimal thickness 31 suggests that microvascular system nurtures cells in atherosclerotic lesions. In addition, studies also indicate that microvascular system facilitates leukocyte recruitment32 as O’Brien et al. 33, 34 have demonstrated that the expression of VCAM-1, ICAM-1 and E-selectin was 2 to 3-fold higher on neovessels than that on arterial luminal endothelial cells. Furthermore, it was reported that while atherosclerotic plaques with moderate and severe inflammation had significantly increased neovessel content, ruptured plaques exhibited the highest degree of neovascularization35. Studies using ultrasound image or biomarker detection have also clearly demonstrated that neovascularization is associated with plaque destabilization36–38.
Although the link between neovascularization and plaque vulnerability has been well documented, the mechanisms underling the link have not been well understood. In the present study, we demonstrated that TLR4 activation in MIC ECs triggered a robust upregulation of inflammatory cytokines, chemokines, growth factors and adhesion molecules. Furthermore, we showed that the consequence of the increased cytokine production by MIC ECs was a crosstalk with mononuclear cells through MIC EC-released IL-6, leading to an upregulated MMP-1 expression by mononuclear cells. Given the crucial role of inflammatory cytokines and MMPs in plaque rupture39, our study has elucidated a novel mechanism potentially involved in neovascularization-associated plaque vulnerability.
To investigate the mechanisms by which MIC ECs exhibited a much stronger inflammatory response than MAC ECs, we first excluded the possibility that the different culture conditions for MIC and MAC ECs are the cause by the following studies. Firstly, we showed that both dermal MIC and cardiac MIC ECs had a similar response to LPS although their culture media have different compositions. Secondly, we showed that MAC ECs did not have a remarkable increase in IL-6 secretion in response to LPS when their medium was switched from Medium 200 to Medium 131, which was used for MIC ECs. Further investigations on the TLR4 receptor system and transcriptional activation indicate that the major causes for the powerful inflammatory response by MIC ECs are likely to be the higher levels of TLR4 and CD14 expression and NFkB transcriptional activity.
Our study showed that MIC ECs had significantly higher expression levels of TLR4 and CD14 than MAC ECs. It is known that CD14, a co-receptor for TLR4, is critical for TLR4 activation 40. Higher TLR4 and CD14 expression by MIC ECs is likely to lead to a stronger TLR4 signaling and TLR4-mediated vascular inflammation. The role of TLR4 expression in pathogenesis of acute coronary syndrome is indicated by the pathological studies that showed the local expression of TLR4 at the site of ruptured plaques in patients with acute myocardial infarction41. Another recent study showed that combined interference of TLR2 and TLR4 mRNA expression synergistically stabilized atherosclerotic plaques in apolipoprotein E-knockout mice 42. Thus, TLR4 expression has been implicated in the pathogenesis of not only atherogenesis, but also plaque destabilization. Our present study showed that TLR4 activation in the coculture of MIC ECs and mononuclear cells triggered a powerful upregulation of MMP-1 expression by mononuclear cells via a synergy between IL-6 stimulation and TLR4 activation, supporting the role of TLR4 in plaque destabilization. In addition to the higher levels of TLR4 and CD14 expression in MIC ECs, our data also showed that NFκB transcriptional activity was stimulated by LPS to a higher level in MIC ECs than that in MAC ECs. Considering the importance of NFκ B activity in inflammatory responses, the high NFκB transcriptional activity in MIC ECs, which may be related to the increase in TLR4 and CD14 expression, is likely to play an essential role in the robust responsiveness to LPS by MIC ECs.
It is noteworthy that the current study is an in vitro study that reported the difference of TLR4 and CD14 expression between cultured MIC and MAC ECs. However, no study has been published so far to show the difference of TLR4 and CD14 expression between MIC and MAC ECs in atherosclerotic lesions. Obviously, the findings from in vivo studies are essential since it would validate our in vitro observations. Currently, a study with atherosclerosis-prone apolipoprotein E-deficient mice to compare the expression of TLR4 and CD14 by MIC and MAC ECs in advanced aortic atherosclerotic plaques is in progress in our laboratory. Furthermore, it would be even more important to compare the expression of TLR4 and CD14 by MIC and MAC ECs in human atherosclerotic plaques since the findings are more relevant to human cardiovascular disease.
It is interesting to find that among the tested inflammatory cytokines, IL-6 was stimulated to the highest level in MIC ECs by TLR4 activation (Table 1). The extremely high increase in IL-6 secretion by MIC ECs in response to TLR4 activation leads to a powerful stimulation of MMP-1 secretion from U937 mononuclear cells, indicating that IL-6 released by MIC ECs plays a major role in the crosstalk between MIC ECs and U937 mononuclear cells. These observations, along with our previous reports that IL-6 also serves as a mediator for the crosstalk between fibroblasts and U937 cells to upregulate MMP-1 expression43, clearly indicate that IL-6 is a vital soluble factor released by MIC ECs or fibroblasts to interact with monocytes for stimulation of MMP-1 that is known to promote matrix degradation and plaque destabilization39.
The difference in IL-6 secretion by MIC and MAC ECs in response to TLR4 activation was striking: IL-6 secretion by MIC ECs and MAC ECs in response to TLR4 activation was 185- and 17-fold higher, respectively, of that by control cells. The remarkable difference in IL-6 secretion may explain why the coculture of MIC ECs and U937 cells, but not that of MAC ECs and U937 cells, led to a crosstalk with U937 cells and stimulation of MMP-1 production. Our previous study has shown that IL-6 at concentration of 1,000 pg/ml is required to stimulate MMP-1 expression by U937 monocytes28. Thus, 501 pg/ml of IL-6 released by MAC ECs in response to TLR4 activation is insufficient to stimulate MMP-1 expression by U937 cells.
Another interesting observation from this study is that TLR4 activation stimulated a gene cluster in the chemokine family including chemokine (C-X-C motif) ligand (CXCL)1, CXCL3, CXCL6, CXCL9, CXCL10 and monocyte chemotactic protein-1 (MCP-1) to higher levels in MIC ECs than MAC ECs (Table 2). Surprisingly, in response to TLR4 activation, CXCL5 and CXCL10 expression by MIC ECs was 147- and 1097-fold, respectively, much higher than that by MAC ECs, suggesting that MIC ECs are more capable of recruiting mononuclear cells than MAC ECs. In addition, it is known that some chemokines also play a crucial role in angiogenesis44. The finding that MIC ECs had a high expression of chemokines in response to TLR4 activation also suggests that TLR4 activation may contribute to neovasculization in atherosclerosis. Moreno et al.14 have postulated that TLR4 activation is a potential mechanism in addition to hypoxia in neovascularization in atherosclerosis.
Vascular ECs express adhesion molecules that facilitate the adhesion and translocation of mononuclear cells through the EC monolayer. Our present study showed that in response to TLR4 activation, MIC ECs had a higher mRNA level of intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and E-selectin than MAC ECs had (Table 2), which is consistent with the findings from pathological studies on human atherosclerotic plaques described by O’Brien et al., who demonstrated that the expression by ECs in the neovessels of VCAM-1, ICAM-1 and E-selectin was significantly higher than that observed in arterial luminal endothelial cells34. The increase in the levels of adhesion molecules in response to TLR4 activation by MIC ECs indicates that MIC ECs may play a more important role in the accumulation of mononuclear cell in atherosclerotic plaque and plaque destabilization.
The role of TLR4 activation by LPS in atherosclerosis has been implicated in Chlamydia pneumonia-associated atherosclerosis45. About 70% of patients with acute myocardial infarction (AMI) show a seroresponse to a chlamydial LPS epitope46. Besides Chlamydia pneumonia, specific infectious agents including the periodontal disease pathogen Porphyromonas gingivalis have been implicated in the progression of atherosclerosis as reported in recent years 47. It has been shown that Porphyromonas gingivalis-derived LPS stimulates inflammatory response by engaging TLR448. In addition to bacteria-derived LPS as an exogenous ligand for TLR4, other molecules produced endogenously, such as minimally modified low-density lipoprotein, also engage TLR4 to stimulate inflammatory response by macrophages49, 50.
In conclusion, the present study demonstrated that TLR4 activation stimulated a stronger inflammatory response in MIC ECs than that in MAC ECs. It also showed that MIC ECs have an increased expression of TLR4 and CD14 as well as a higher level of NFκB transcriptional activity when compared to MAC ECs as the potential underlying mechanisms. The findings from this study indicate that MIC ECs may play an important role in plaque destabilization through TLR4-dependent mechanisms.
Supplementary Material
Acknowledgments
We thank Dr. Gabriel Virella for his comments on the study.
SOURCES OF FUNDING
This work was supported by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs, NIH grant DE016353 (to Y.H.) and DK081352 (to M.L-V.).
Footnotes
DISCLOSURES
None.
References
- 1.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: Transition from theory to practice. Circulation journal: official journal of the Japanese Circulation Society. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 2.Shibata N, Glass CK. Regulation of macrophage function in inflammation and atherosclerosis. Journal of lipid research. 2009;50 (Suppl):S277–281. doi: 10.1194/jlr.R800063-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaves DR, Channon KM. Inflammation and immune responses in atherosclerosis. Trends in immunology. 2002;23:535–541. doi: 10.1016/s1471-4906(02)02331-1. [DOI] [PubMed] [Google Scholar]
- 4.Moreno PR, Purushothaman KR, Fuster V, O’Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: Implications for plaque vulnerability. Circulation. 2002;105:2504–2511. doi: 10.1161/01.cir.0000017265.52501.37. [DOI] [PubMed] [Google Scholar]
- 5.Fuster V, Corti R, Fayad ZA, Schwitter J, Badimon JJ. Integration of vascular biology and magnetic resonance imaging in the understanding of atherothrombosis and acute coronary syndromes. Journal of thrombosis and haemostasis. 2003;1:1410–1421. doi: 10.1046/j.1538-7836.2003.00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen SD, Andersen HR, Falk E. What an interventional cardiologist should know about the pathophysiology of acute myocardial infarction. Seminars in interventional cardiology. 1999;4:11–16. [PubMed] [Google Scholar]
- 7.Shin J, Edelberg JE, Hong MK. Vulnerable atherosclerotic plaque: Clinical implications. Current vascular pharmacology. 2003;1:183–204. doi: 10.2174/1570161033476727. [DOI] [PubMed] [Google Scholar]
- 8.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. Journal of leukocyte biology. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: Directing innate and adaptive immunity, coagulation, and inflammation. Journal of immunology (Baltimore, Md : 1950) 2007;178:6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 10.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nature reviews Immunology. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 11.Szekanecz Z, Koch AE. Endothelial cells in inflammation and angiogenesis. Current drug targets Inflammation and allergy. 2005;4:319–323. doi: 10.2174/1568010054022187. [DOI] [PubMed] [Google Scholar]
- 12.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction. Part ii: Association with cardiovascular risk factors and diseases. A statement by the working group on endothelins and endothelial factors of the european society of hypertension. Journal of hypertension. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Moulton KS. Angiogenesis in atherosclerosis: Gathering evidence beyond speculation. Current opinion in lipidology. 2006;17:548–555. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- 14.Moreno PR, Purushothaman KR, Zias E, Sanz J, Fuster V. Neovascularization in human atherosclerosis. Current molecular medicine. 2006;6:457–477. doi: 10.2174/156652406778018635. [DOI] [PubMed] [Google Scholar]
- 15.Ribatti D, Levi-Schaffer F, Kovanen PT. Inflammatory angiogenesis in atherogenesis--a double-edged sword. Annals of medicine. 2008;40:606–621. doi: 10.1080/07853890802186913. [DOI] [PubMed] [Google Scholar]
- 16.Stoll LL, Denning GM, Weintraub NL. Endotoxin, tlr4 signaling and vascular inflammation: Potential therapeutic targets in cardiovascular disease. Current pharmaceutical design. 2006;12:4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 17.Grieco FA, Vendrame F, Spagnuolo I, Dotta F. Innate immunity and the pathogenesis of type 1 diabetes. Seminars in immunopathology. 2011;33:57–66. doi: 10.1007/s00281-010-0206-z. [DOI] [PubMed] [Google Scholar]
- 18.Zipris D. Toll-like receptors and type 1 diabetes. Advances in experimental medicine and biology. 2010;654:585–610. doi: 10.1007/978-90-481-3271-3_25. [DOI] [PubMed] [Google Scholar]
- 19.Michelsen KS, Arditi M. Toll-like receptor signaling and atherosclerosis. Current opinion in hematology. 2006;13:163–168. doi: 10.1097/01.moh.0000219662.88409.7c. [DOI] [PubMed] [Google Scholar]
- 20.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 21.Cole JE, Mitra AT, Monaco C. Treating atherosclerosis: The potential of toll-like receptors as therapeutic targets. Expert review of cardiovascular therapy. 2010;8:1619–1635. doi: 10.1586/erc.10.149. [DOI] [PubMed] [Google Scholar]
- 22.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: Differential regulation of ca2+ and permeability. The American journal of physiology. 1998;274:L810–819. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- 23.Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. American journal of physiology Lung cellular and molecular physiology. 2006;291:L30–37. doi: 10.1152/ajplung.00317.2005. [DOI] [PubMed] [Google Scholar]
- 24.Wang XH, Chen SF, Jin HM, Hu RM. Differential analyses of angiogenesis and expression of growth factors in micro- and macrovascular endothelial cells of type 2 diabetic rats. Life sciences. 2009;84:240–249. doi: 10.1016/j.lfs.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Jackson CJ, Nguyen M. Human microvascular endothelial cells differ from macrovascular endothelial cells in their expression of matrix metalloproteinases. The international journal of biochemistry & cell biology. 1997;29:1167–1177. doi: 10.1016/s1357-2725(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 26.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (u-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 27.Seager Danciger J, Lutz M, Hama S, Cruz D, Castrillo A, Lazaro J, Phillips R, Premack B, Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Samuvel DJ, Sundararaj KP, Lopes-Virella MF, Huang Y. Il-6 and high glucose synergistically upregulate mmp-1 expression by u937 mononuclear phagocytes via erk1/2 and jnk pathways and c-jun. Journal of cellular biochemistry. 2010;110:248–259. doi: 10.1002/jcb.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang CH, Chen CF, Chen WM, Fong YC. Il-6 increases mmp-13 expression and motility in human chondrosarcoma cells. The Journal of biological chemistry. 2011;286:11056–11066. doi: 10.1074/jbc.M110.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Yin P, Di D, Luo G, Zheng L, Wei J, Zhang J, Shi Y, Xu N. Il-6 regulates mmp-10 expression via jak2/stat3 signaling pathway in a human lung adenocarcinoma cell line. Anticancer research. 2009;29:4497–4501. [PubMed] [Google Scholar]
- 31.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. The American journal of pathology. 1993;143:164–172. [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer OJ, van der Wal AC, Teeling P, Becker AE. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: A prominent role for neovascularization? Cardiovascular research. 1999;41:443–449. doi: 10.1016/s0008-6363(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. The Journal of clinical investigation. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of e-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 35.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 36.Giannarelli C, Ibanez B, Cimmino G, Garcia Ruiz JM, Faita F, Bianchini E, Zafar MU, Fuster V, Garcia MJ, Badimon JJ. Contrast-enhanced ultrasound imaging detects intraplaque neovascularization in an experimental model of atherosclerosis. JACC Cardiovascular imaging. 2010;3:1256–1264. doi: 10.1016/j.jcmg.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Kyriakakis E, Cavallari M, Andert J, Philippova M, Koella C, Bochkov V, Erne P, Wilson SB, Mori L, Biedermann BC, Resink TJ, De Libero G. Invariant natural killer t cells: Linking inflammation and neovascularization in human atherosclerosis. European journal of immunology. 2010;40:3268–3279. doi: 10.1002/eji.201040619. [DOI] [PubMed] [Google Scholar]
- 38.Sirol M, Moreno PR, Purushothaman KR, Vucic E, Amirbekian V, Weinmann HJ, Muntner P, Fuster V, Fayad ZA. Increased neovascularization in advanced lipid-rich atherosclerotic lesions detected by gadofluorine-m-enhanced mri: Implications for plaque vulnerability. Circulation Cardiovascular imaging. 2009;2:391–396. doi: 10.1161/CIRCIMAGING.108.801712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 40.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunological reviews. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa Y, Satoh M, Itoh T, Minami Y, Takahashi Y, Akamura M. Local expression of toll-like receptor 4 at the site of ruptured plaques in patients with acute myocardial infarction. Clinical science (London, England: 1979) 2008;115:133–140. doi: 10.1042/CS20070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang JM, Wang Y, Qi LH, Gao F, Ding SF, Ni M, Liu CX, Zhang C, Zhang Y. Combinatorial interference of toll-like receptor 2 and 4 synergistically stabilizes atherosclerotic plaque in apolipoprotein e-knockout mice. Journal of cellular and molecular medicine. 2011;15:602–611. doi: 10.1111/j.1582-4934.2010.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by u937 macrophages in coculture: Cross-talking between fibroblasts and u937 macrophages exposed to high glucose. The Journal of biological chemistry. 2009;284:13714–13724. doi: 10.1074/jbc.M806573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiefer F, Siekmann AF. The role of chemokines and their receptors in angiogenesis. Cellular and molecular life sciences. 2011;68:2811–2830. doi: 10.1007/s00018-011-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saikku P. Epidemiologic association of chlamydia pneumoniae and atherosclerosis: The initial serologic observation and more. The Journal of infectious diseases. 2000;181 (Suppl 3):S411–413. doi: 10.1086/315625. [DOI] [PubMed] [Google Scholar]
- 46.Sessa R, Nicoletti M, Di Pietro M, Schiavoni G, Santino I, Zagaglia C, Del Piano M, Cipriani P. Chlamydia pneumoniae and atherosclerosis: Current state and future prospectives. International journal of immunopathology and pharmacology. 2009;22:9–14. doi: 10.1177/039463200902200102. [DOI] [PubMed] [Google Scholar]
- 47.Gibson FC, 3rd, Genco CA. Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: Disparate diseases with commonalities in pathogenesis through tlrs. Current pharmaceutical design. 2007;13:3665–3675. doi: 10.2174/138161207783018554. [DOI] [PubMed] [Google Scholar]
- 48.Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, Schifferle RE, Hajishengallis G, Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize tlr4, induce formation of tlr2/1/cd36 complexes in lipid rafts and trigger tlr2-induced inflammatory responses in human vascular endothelial cells. Cellular microbiology. 2007;9:2030–2039. doi: 10.1111/j.1462-5822.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 49.Chavez-Sanchez L, Madrid-Miller A, Chavez-Rueda K, Legorreta-Haquet MV, Tesoro-Cruz E, Blanco-Favela F. Activation of tlr2 and tlr4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Human immunology. 2010;71:737–744. doi: 10.1016/j.humimm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified ldl binds to cd14, induces macrophage spreading via tlr4/md-2, and inhibits phagocytosis of apoptotic cells. The Journal of biological chemistry. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.