Abstract
Vibrio parahaemolyticus is a significant cause of gastroenteritis worldwide. Characterization of this pathogen has revealed a unique repertoire of virulence factors that allow for colonization of the human host and disease. The following describes the known pathogenicity determinants while establishing the need for continued research.
Keywords: Vibrio parahaemolyticus, Gastroenteritis, Hemolysin, Type III secretion system, Pathogenesis
1. Introduction
Vibrio parahaemolyticus is a Gram-negative, halophilic bacterium that thrives in warm climates within marine or estuarine environments. It is commonly found free swimming, attached to underwater surfaces, or commensally associated with different shellfish species [1]. While some strains of V. parahaemolyticus are strictly environmental, many strains are pathogenic to humans. Virulent strains can cause distinct diseases, including wound infections, septicemia, or more commonly acute gastroenteritis which is acquired through the consumption of raw or undercooked seafood, especially shellfish. Since its discovery in 1950, Vibrio parahaemolyticus has become a leading cause of seafood-derived food poisoning throughout the world [2, 3]. The global dissemination of this pathogen underscores the importance of understanding its many virulence factors and their effects on the human host. This review will focus on the current knowledge of different Vibrio parahaemolyticus strains and their wide repertoire of toxins and type 3 secretion system effectors.
2. Vibrio parahaemolyticus
In the fall of 1950 in the southern suburbs of Osaka, Japan, an outbreak of acute gastroenteritis sickened 272 individuals. Twenty of these people ultimately died. While the source of the illness was believed to be shirasu, a small partially dried sardine, the etiologic agent was unknown. An intense investigation began with filtered homogenates from shirasu passed through a guinea pig model to eliminate poisoning by chemicals as a possible cause of the disease. When the test animals developed peritonitis, the homogenates were inoculated to various growth media. Along with other known bacterial organisms, two species of unidentified Gram-negative rods were also isolated. Since these organisms could not be separated by isolation streaking, they were inoculated intraperitoneally into mice. When disease symptoms developed several hours later, ascitic fluid was collected and streaked onto blood agar. One of the organisms was identified as Proteus morganii. The other organism was previously unclassified, and was named Pasteurella parahaemolytica. Further testing showed that this organism alone was pathogenic to mice and could be isolated from the stool samples of afflicted individuals from the original outbreak [4–7]. P. parahaemolytica was reclassified as Vibrio parahaemolyticus when it was found to grow preferentially on high salt media [4].
V. parahaemolyticus is a Gram-negative, halophilic, mesophilic, small rod that may have a single curve to its shape [3, 8]. It exists as either a swimmer cell with a single polar flagella, or a swarmer cell covered in lateral flagella (Fig. 1) [1]. Depending on environmental conditions, V. parahaemolyticus can produce a capsule, with over 70 different K antigens detected in various strains [7].
Fig. 1. Virulence Associated Factors of V. parahaemolyticus.
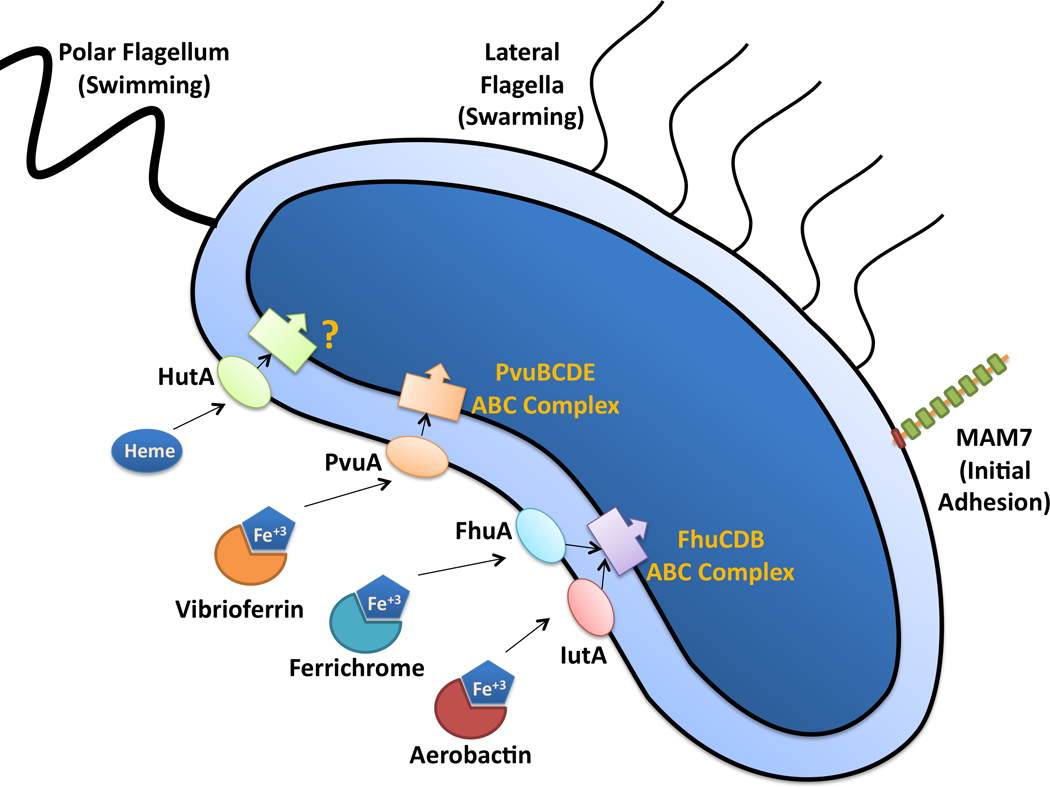
A single flagellum at one pole of the bacterium is sheathed to extend the bacterial membrane and is required for swimming motility in liquid environments. During growth on semi-solid surfaces, flagella are produced along the lateral side of the bacterium, which aid in swarming motility. MAM7 is a multivalent adhesion protein that binds to fibronectin and phosphatidic acid, and it is required for the initial attachment to host cells. As shown simplistically, V. parahaemolyticus can utilize the siderophores vibrioferrin, ferrichrome, and aerobactin, along with heme, to scavenge iron from the environment. These iron transporters are internalized by different membrane receptors on the outer membrane of the bacteria and transported to the cytoplasm by different ABC complexes.
V. parahaemolyticus is found free living in brackish and estuarine waters, and requires salinity for survival [9]. In winter months when water temperatures are unfavorable, V. parahaemolyticus may be undetectable. It has been proposed that the organism survives in marine sediment, and is reintroduced to the water column when temperatures rise [3]. Infaunal burrows have been demonstrated to contain high levels of V. parahaemolyticus, likely acting as a source for these organisms to be distributed through an estuary environment during favorable conditions [10].
In locations where water temperatures do not drop below 15 °C, V. parahaemolyticus may be detected year round [3] with the number of organisms detected in water, sediment and oysters increasing as water temperatures rise [11]. Due to filter feeding, oysters may have a concentration of V. parahaemolyticus up to 100-fold higher than surrounding waters. Up to 100% of oysters may be contaminated with V. parahaemolyticus and/or V. vulnificus during summer months, which increases the chances of infection [6]. V. parahaemolyticus is disseminated throughout the world, and recently, due to warming ocean temperatures, has been detected in coastal waters as far north as the southern coast of Alaska [8, 12, 13].
In Japan, V. parahaemolyticus accounts for 20–30% of all food poisoning cases and is the leading cause of foodborne illness [3]. The high rate of infection is attributed to the overall high seafood diet as well as the common practice of eating seafood raw. Gastroenteritis caused by V. parahaemolyticus is generally associated with the consumption of raw or undercooked seafood including fish, crab and other crustaceans, and mollusks. Since V. parahaemolyticus is sensitive to heat, cooking generally kills the bacterium rendering contaminated food safe to eat. In the United States, the first case of gastroenteritis caused by V. parahaemolyticus occurred in 1971 in Maryland and was associated with contaminated crabmeat. V. parahaemolyticus is the primary cause of US Vibrio-associated foodborne illness. The majority of these infections are a result of consuming Vibrio-contaminated raw oysters [2].
An increase in non-cholera Vibrio infections that began in the mid 1990s has been associated with the detection of a new clonal group that includes three new serotypes: O3:K6, O4:K68, and O1:K untypeable [6]. Since 1996, the most common serotype of V. parahaemolyticus has been O3:K6. While non-pandemic variants for this serotype were detected in Japan as early as 1983, the first reported illness caused by this strain was first observed in a Japanese traveler returning from Indonesia in 1995. An outbreak of diarrheal disease then occurred in Calcutta, India in February of 1996 with 50–80% of the isolates confirmed as O3:K6 [7]. V. parahaemolyticus has now been detected in North and South America, Europe, Africa, and Asia with outbreaks of disease occurring worldwide [7]. To date, at least 12 pathogenic serotypes of V. parahaemolyticus have been identified [14]. Many strains have been sequenced including the O3:K6 serotype strains RimD 2210633 and AQ3810; the O4:K12 strain Vp10329; and the O4:K68 serotype strains AN-5034, K5030 and Peru-466 (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi) [14–16]. To understand the biology of Vibrio parahaemolyticus, numerous clinical and environmental strains without a sequence have been utilized to understand the biology of V. parahaemolyticus [2, 17, 18]. The RimD 2210633 strain was the first fully sequenced and annotated genome [15] and is therefore the focus of most studies.
3. Disease
Infection with V. parahaemolyticus can cause three distinct medical conditions: gastroenteritis, wound infections, and septicemia. Acute gastroenteritis presents with abdominal cramping, diarrhea, nausea, vomiting, low-grade fever, headache, and occasional bloody diarrhea different than that seen in other enteric infections. Infection occurs 4 to 96 hours after consumption of contaminated food, and lasts up to three days. The illness is self-resolving in immunocompetent individuals and can be sufficiently treated with oral rehydration alone [2, 7].
Wound infection is common among fishermen and is generally acquired when small wounds occur in or around seawater [19]. This form of V. parahaemolyticus infection is sometimes limited to cellulitis, but may progress to necrotizing fasciitis, an uncommon infection of soft tissues characterized by a rapid spread of bacteria with associated inflammation and necrosis of tissues.
Septicemia occurs when V. parahaemolyticus enters the blood stream of the patient and is disseminated throughout the body. Systemic immune activation leads to inflammation and increased vascular permeability. This can result in hypovolemic shock, multisystem organ failure and death. The subpopulation of patients most at risk for septicemia includes those with underlying medical conditions including liver disease, diabetes, cancer, and recent gastric surgery [2]. Immunocompromised individuals and those with liver failure due to liver cirrhosis or hepatitis virus infection seem to be at greatest risk of septicemia [19, 20]
4. Cellular factors associated with V. parahaemolyticus pathogenicity
Genomic analysis has demonstrated that a common Vibrio progenitor gave rise to V. parahaemolyticus, V. cholerae, and the other Vibrio species. The acquisition of a Type III Secretion System (T3SS) similar to that found in Yersinia species and herein referred to as T3SS1, was the basis of a V. parahaemolyticus ancestor. The acquisition by some strains of a second Type III Secretion System (T3SS2), and thermostable direct hemolysin (TDH) and TDH related hemolysin (TRH) genes has lead to a number of V. parahaemolyticus species with varying degrees of pathogenicity. This evolution is separate from that of V. cholerae, which also acquired T3SS2 as well as the phage-encoded cholera toxin (CTX) in some strains, but does not possess T3SS1 [21]. In addition to Type III Secretion and TDH genes, V. parahaemolyticus possesses flagella for swimming and swarming, as well as the ability to produce a capsule. These are both factors that likely aid in environmental survival as well as colonization of the human host. Gene loci for two separate Type VI Secretion Systems (VP1386-VP1420, T6SS1 and VPA1030-VPA1043, T6SS2, occurring on chromosomes 1 and 2, respectively) have been identified in RimD2210633 [15, 16]. Type VI secretion is present in many Gram-negative pathogens, and may be involved in the modulation of eukaryotic signaling [22], but currently it has not been investigated in V. parahaemolyticus. Additionally, activation of these genes is not altered under T3SS-inducing conditions meant to mimic the environment encountered within a human host [23]. As such, these systems in V. parahaemolyticus will not be discussed further here.
4.1. Flagella, capsule, and biofilm regulation
A common cellular factor associated with many intestinal pathogens is the presence of one or more flagella. V. parahaemolyticus possesses two different types of flagella with distinct functions. A polar flagellum is constitutively expressed and is used for swimming. The whip of the flagellum is made of six different flagellin proteins and is sheathed, which may aid in attachment. The energy to rotate this flagellum is provided by a sodium motive force, which is advantageous in salt water with an average pH of 8. V. parahaemolyticus is capable of swimming at speeds up to 60 µm/s when expressing this single flagellum [1].
A decrease in flagellar rotation speed as a consequence of increased viscosity of the growth environment, or growth under iron-limiting conditions, induces a switch to a swarmer cell type. This entails the production of a number of non-sheathed peritrichous flagella which allows the bacteria to swarm over solid or semi-solid substrates. These flagella are different than the single polar flagellum in that they are unsheathed, made from a single flagellin protein, and are powered by the proton motive force [1].
The switch from a swimmer to a swarmer cell is highly regulated. The activation of OpaR, a V. harveyii LuxR homolog and the regulator of capsule production, blocks production of the lateral flagella (laf) genes, which are necessary for lateral flagella production [24]. Inactivation of OpaR switches the cell from opaque (OP) to the translucent (TR) form that lacks a capsule and can swarm effectively over surfaces or through viscous liquids. ScrG and ScrC, both GGDEF and EAL-domain containing proteins, regulate c-di-GMP levels in the cell. A decrease in c-di-GMP levels leads to lateral flagella synthesis and swarming. ScrG can also block CpsA synthesis, which is necessary for capsule synthesis, as well as decrease biofilm formation and adherence to surfaces [25, 26]. A decrease in c-di-GMP levels may also enhance expression of other virulence factors, including T3SS proteins [27].
4.2. Adhesion to host cells
During infection, a variety of bacterial adhesion factors are expressed to provide the contact with the host cell necessary for secretion of effector and toxin proteins. In a recent study, an outer membrane protein from V. parahaemolyticus was described that was necessary and sufficient for initial contact of this pathogen with multiple cultured host cell lines. This multivalent adhesion molecule (MAM) consists of six (MAM6) or seven (MAM7) mammalian cell entry (mce) domains, and has homologs encoded in a number of Gram-negative animal pathogens.
This work demonstrated that MAM7 bound to both fibronectin and phosphatidic acid, and that blocking the binding of either of these substrates could prevent adhesion of MAM7 to host cells. Heterologous expression of MAM7 was sufficient for attachment of a non-pathogenic E. coli strain to host cells, which could in turn block attachment and attenuate cytotoxicity of V. parahaemolyticus and a number of other MAM7-expressing Gram-negative bacterial pathogens. Furthermore, MAM7 was necessary for attachment early in the infection, as well as for T3SS-mediated cell death in some cell types. The characterization of MAM7 provides insight into the initial events of bacterial and host cell interactions, and provides a potential therapeutic target for not only V. parahaemolyticus, but many other Gram-negative pathogens as well. [28]
4.3. Iron acquisition
Iron is an essential element for all organisms but usable forms of iron are scarcely found in most environments. Therefore, V. parahaemolyticus, and other bacteria utilize several methods of iron acquisition to obtain this element from the environment (Fig. 1). In humans, iron is associated with different molecular complexes such as transferrin, lactoferrin, and hemoglobin. During infection, bacteria utilize iron chelators known as siderophores to scavenge iron [29]. V. parahaemolyticus produces a siderophore known as vibrioferrin which is believed to be synthesized and secreted by proteins from the pvsABCDE operon. For vibrioferrin uptake, the bacteria utilize a siderophore membrane receptor, termed PvuA, which is coupled to an inner membrane ATP-binding cassette (ABC). The ABC transporter system is comprised of proteins from the pvuBCDE gene cluster and is required for transporting the siderophore across the inner membrane [30].
V. parahaemolyticus can also acquire ferric-bound siderophores produced by different fungal and bacterial species or through the uptake of heme. Ferrichrome and aerobactin are two siderophores produced by other organisms that can be retrieved by V. parahaemolyticus with the membrane receptors FhuA and IutA, respectively. Both receptors are believed to be coupled to the FhuCDB ABC complex [31]. While it is known that V. parahaemolyticus can utilized heme as an iron source, little is known about the proteins involved in this process. V. parahaemolyticus was found to contain a homologue of the V. cholerae heme membrane receptor hutA, but its function remains uncharacterized [32].
4.4. Toxins
Nearly all V. parahaemolyticus strains isolated from clinical samples possess β-hemolytic activity attributed to TDH or TRH (Fig. 2). These isolates are able to lyse human erythrocytes when plated on a high-salt media called Wagatsuma agar, a process termed the Kanagawa phenomenon (KP) [33]. The KP test is commonly used to identify pathogenic V. parahaemolyticus in seafood as well as patient samples. The reproducibility of the KP test is dependent on pH, media salinity, and erythrocyte type. As such, identification of pathogenic serovars by this method is not always accurate. Identification of the tdh gene in samples has been shown to more accurately predict virulence, as it is a genetic test rather than a phenotypic test [34]. The tdh gene is encoded and co-regulated with T3SS2 genes [23]. The identification of TDH may actually serve to identify V. parahaemolyticus strains with T3SS2, the expression of which may be a significant factor in determining if a serovar can cause pandemic outbreaks of disease.
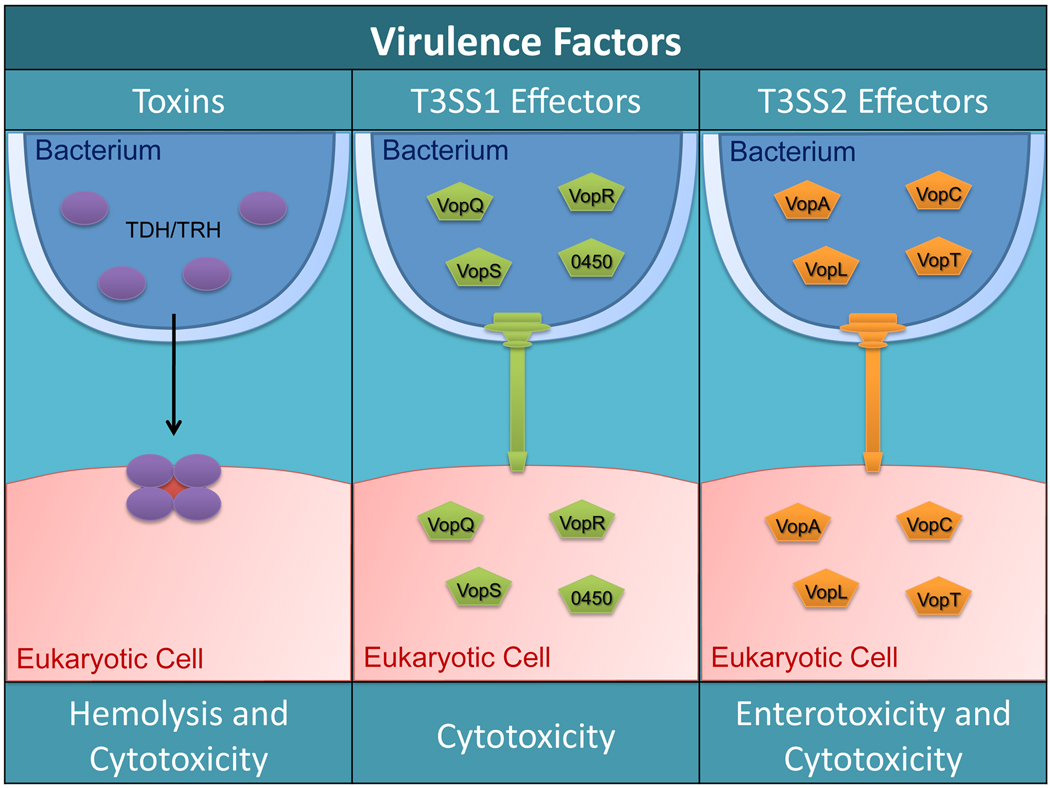
Fig. 2. Known toxins and type 3 secreted effectors of V. parahaemolyticus.
The TDH and TLH toxins are secreted from the bacteria and form tetrameric pore complexes in the host membrane. These pores allow ions to flow freely across the host membrane which leads to hemolysis or cytotoxicity (left panel). V. parahaemolyticus translocates T3SS1 effectors (VopQ, VopR, VopS, and VPA0450) into host cells to cause cytotoxicity in different cell types such as macrophages and HeLa cells (middle panel). T3SS2 effectors (VopA, VopC, VopL, and VopT) are translocated into host cells to cause cytotoxicity of colon epithelial cells or enterotoxicity within the host (right panel).
V. parahaemolyticus RimD 2210633 possesses two copies of the TDH toxin, vpa1314 (tdhA) and vpa1378 (tdhS) [15]. TDH is a reversible amyloid toxin [35] that has been shown to associate with cholesterol and sphingolipid-enriched lipid rafts. Disruption of these lipid microdomains abrogated cytotoxicity in nucleated cells, but not hemolytic activity against erythrocytes, indicating two potential activities for this toxin [36]. The x-ray crystallographic structure of TDH showed that this protein forms a homotetramer with a central pore 23 Å in diameter. This relatively large pore size helps explain previously observed low ion selectivity, allowing both water and ions to flow through a membrane with little impedance [37]. This alteration in ion flux from affected cells in the intestine may be the mechanism for the diarrhea observed during infection [38]. While TDH was considered to be a major virulence factor for V. parahaemolyticus pathogenesis, deletion of both tdhA and tdhS did not affect cytotoxicity towards cultured Hela cells and only showed partial fluid accumulation in a rabbit ileal loop model [39]. This indicates the existence of other virulence factors [40–42]. The cell death of cultured cells infected with TDH-deficient strains was later correlated with functional T3SSs [39].
TRH is predicted to act in a similar manner to lyse cells based upon high sequence homology (68%) between the trh and tdh genes. In a recent study, TRH and TDH were shown to cause similar levels of hemolysis in vitro and size exclusion analysis revealed that TRH also exists in a tetramer complex [43]. Future studies will unravel the similarities and differences of these two toxins. Another toxin, thermolabile hemolysin (TLH), is found in all strains of V. parahaemolyticus, but very little is known about its function. The tlh gene was found to be strongly upregulated in a genomic screen performed under conditions meant to mimic the intestinal environment of the human host [23]. Therefore, this gene may be equally important as the tdh and trh genes in the process of human infection. Table 1 lists the sequenced strains of V. parahaemolyticus and the presence of tdh and trh genes, as well as T3SSs.
Table 1.
Occurrence of virulence factors in sequenced pandemic strains
| Strains | TDH | TRH | T3SS1 | T3SS2 | Ref. |
|---|---|---|---|---|---|
| O3:K6 | |||||
| RimD 2210633 | + | − | + | + | [15] |
| AQ3810 | + | − | + | + | [16] |
| O4:K12 | |||||
| Vp10329 | + | + | + | + | [14] |
| O4:K68 | |||||
| AN-5034 | + | − | + | + | a |
| K5030 | + | − | + | + | a |
| Peru-466 | + | − | + | + | a |
http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi -- accessed 05/23/2011
4.5. The Type III Secretion Systems of V. parahaemolyticus
The T3SS is a bacterial organelle evolved to deliver proteins, termed effectors, directly into the cytoplasm of a eukaryotic cell [44]. Made up of 20–30 proteins, the secretion apparatus consists of a basal body that spans both the inner and outer bacterial membranes, a needle that acts as a conduit between the bacterial and eukaryotic cells, and a translocon pore that is inserted into the eukaryotic cell membrane [45]. Structurally, the apparatus bears resemblance to a flagellar system from which it may have evolved. Some secretion apparatus proteins have homology to flagellar export proteins, with core transmembrane proteins showing the highest level of conservation [46].
The T3SS allows bacteria to translocate effectors from their cytoplasm directly to the cytoplasm, or cytoplasmic face of the host cell membrane, without release of the effectors to the cytoplasm [47]. The specific complement of effectors within a pathogen determines not only its lifestyle, but also the disease that it causes. There are over 100 different characterized effector proteins [47]. While their activities and targets vary, effectors identified thus far tend to manipulate a limited set of eukaryotic systems. Common targets of T3SS effectors include the actin cytoskeleton, innate immune signaling, and autophagy. These systems can be up- or down-regulated depending on the specific needs of the pathogen [40, 48–52].
Translocation occurs in an ATP-dependent manner. Effectors, which are generally bound to chaperones in a quiescent, partially folded state, are unfolded and threaded through the needle. The chaperone remains in the bacterial cytoplasm [44, 53, 54]. Upon entering the host cell cytoplasm, the effectors refold into an active state where they manipulate eukaryotic signaling cascades to facilitate infection and disrupt the host immune response [55].
Genomic sequencing of V. parahaemolyticus RimD 2210633 revealed two pathogenicity islands with one on each of the two chromosomes [15] (Table 1). The first island encodes T3SS1 and some of its cognate effectors [56, 57]. The second island, termed Vp-PAI and now also referred to as VPaI-7, encodes T3SS2, its known effectors, as well as the tdh genes (Fig. 2). Both islands have a number of uncharacterized hypothetical proteins [15, 23, 56, 58]. Based on G+C content, T3SS1 was ancestrally acquired, while T3SS2 was obtained through a relatively recent lateral gene transfer [15]. The presence of two T3SS2 gene clusters (T3SS2α and T3SS2β) in different V. parahaemolyticus strains indicates this acquisition has occurred at least twice [21].
Several V. parahaemolyticus strains were created from RimD 2210633 to enable the study of each T3SS and the characterization of these hypothetical proteins. The POR1 strain maintains both functional T3SSs, but has tdhAS deleted. POR2 is constructed from the POR1 background, and has a deletion of vcrD1, an inner membrane structural ring for T3SS1, which prevents the formation of the T3SS1 needle. As a result, POR2 only secretes effectors from T3SS2. POR3 is similar to POR2, but has a deletion of vcrD2 instead of vcrD1, and can only secrete effectors from T3SS1 (Table 2) [42].
Table 2.
V. parahaemolyticus strains used to study effectors are derived from the RimD2210633 clinical isolate
| TDH | T3SS1 | T3SS2 | |
|---|---|---|---|
| RimD 2210633 | + | + | + |
| POR1 | − | + | + |
| POR2 | − | − | + |
| POR3 | − | + | − |
4.5.1. T3SS1
T3SS1 is present in all V. parahaemolyticus strains and is a defining characteristic of this species [21]. This system causes cytotoxicity in cultured human cells, but does not appear to contribute to enterotoxicity during infection as determined by a rabbit ileal loop model [40–42, 48]. T3SS1 is similar to the Yersinia ysc T3SS based on the number of genes, gene identity, and gene order [15, 56, 59]. T3SS1 can be induced by growing liquid cultures at 37 °C in low calcium media, which is a similar condition used to induce the Yersinia secretion system.
Transcription of the T3SS1 genes is regulated by three interacting proteins (ExsC, ExsD, and ExsE) that control the activity of ExsA, a member of the AraC family of transcriptional activators. Under non-inducing conditions, ExsA is bound to ExsD, an anti-activator, and rendered inactive. ExsE, a substrate for T3SS1, is bound to its chaperone ExsC, which is an anti-anti-activator of the system. When low calcium conditions are encountered ExsE is secreted and causes the release of ExsC, which binds to ExsD. By sequestering ExsD, ExsA is released and is able to activate transcription of T3SS1 genes. This regulatory system requires low-level expression of T3SS1 genes to allow for the initial secretion of ExsE [60]. This may be accomplished through leaky regulation of the T3SS1 genes, or additional regulatory systems that allow for some expression under inhibitory conditions. Preliminary evidence indicates the Heat-stable Nucleoid Structuring protein (H-NS) may negatively regulate T3SS1. H-NS is commonly found in Gram-negative bacteria and is known for genome-wide repression of protein expression by binding to curved DNA normally found at active sites of transcription [61]. It is not currently understood how this protein fits into the regulatory cascade to modulate T3SS1 gene expression [60].
While the regulation of T3SS1 is just now being elucidated, the characterization of three T3SS1 effectors, VopQ, VopS and VPA0450, support an infection model that involves the induction of autophagy, followed by membrane blebbing, cell rounding, and then cell lysis. Rapid induction of autophagy by VopQ causes the target cell to digest itself and prevents phagocytosis of the infecting bacteria. Detachment of the plasma membrane from the actin cytoskeleton by VPA0450 destabilizes the cell. The collapse of the actin cytoskeleton by VopS leads to cell rounding and shrinkage (Table 3). Finally, cells lyse and release their contents [62, 63].
Table 3.
Known T3SS effectors target diverse substrates to drive cytotoxicity and alter immune function
| T3SS | Effector | Domain | Activity | Cellular consequence |
Ref. |
|---|---|---|---|---|---|
| T3SS1 | VopQ (VP1680) | Unique to VopQ | Unknown target or mechanism | Induction of autophagy | [48, 56] |
| VopR (VP1683) | Unknown | Unknown target or mechanism | Unknown | [56] | |
| VopS (VP1686) | Fic domain | AMPylates Rho-family GTPases | Collapse of the actin cytoskeleton | [52, 56] | |
| VPA0450 | Inositol polyphosphate 5-phosphatase | Removes D5 phosphate from PtdIns(4,5)P2 | Destabilization of plasma membrane | [56, 63] | |
| T3SS2 | VopC (VPA1321) | Cytotoxic Necrotizing Factor-1 homolog | Deamidation of Rho GTPases, making them constitutively active (hypothesized) | Disregulation of actin network, inhibition of apoptosis (hypothesized) | [71] |
| VopT (VPA1327) | ADP-Ribosyltransferase | ADP-ribosylation of Ras | Induction of cytotoxicity | [71] | |
| VopA/P (VPA1346) | Acetyltransferase | Inhibition of MAPK signaling | Lack of innate immune activation and cytokine production | [71–73] | |
| VopL (VPA1370) | WH2, and proline rich region | Nucleation of actin polymerization | Alteration of cell shape, possible loss of tight junction integrity | [40] |
An in silico screen for chaperone proteins previously identified four potential T3SS1 effectors. VP1680, VP1683, and VP1686, now referred to as VopQ, VopR and VopS, respectively, are located within the T3SS1 gene locus. VPA0450 is unlinked to T3SS1, and is located on the second chromosome [57]. Secretion of VopQ, VopS, and VPA0450 was confirmed by a secretion assay using POR1 grown under inducing conditions [52, 56, 62, 63]. VopR was not detected as it has a pI outside the range of the 2-D gel used to separate the secreted proteins prior to mass spectrometer analysis [56]. The target and mechanism of action for VopR (VP1683) has not been determined.
Burdette, et al., showed in previous work that VopQ (VP1680) induced PI3-kinase independent autophagy upon infection with POR3 or transfection of vopQ into Hela cells [48]. Microinjection of recombinant VopQ into Hela cells induced autophagy in less than thirty minutes as measured by the conversion of LC3-I to LC3-II and the formation of LC3-GFP punctae [62]. In addition to inducing autophagy, VopQ was able to block phagocytosis of V. parahaemolyticus by RAW 264.7 macrophages, possibly through the sequestration of necessary membrane components. VopQ was necessary for rapid host cell lysis during infection as cells infected with a ΔvopQ strain of V. parahaemolyticus lysed 3 hours slower than those infected with a wild type strain [48]. Recent studies have identified VopQ as an activator of the JNK, p38 and ERK MAPK pathways in human intestinal epithelial cell cultures. MAPK activation resulted in the secretion of IL-8 and was necessary for full cytotoxicity [64, 65]. The target of VopQ and the mechanism for this activation were not determined, which leaves the question of whether or not MAPK activation is the intended purpose of VopQ or an off-target effect resulting from unregulated induction of autophagy. The molecular target and mechanism of action for VopQ are still under investigation.
VopS (VP1686) is responsible for the rounding of cells and cytoplasmic dispersion of actin seen during infection of Hela cells with POR3. VopS indirectly targets the actin cytoskeleton by AMPylating Rho-family GTPases [52]. The Fic domain within VopS mediates the direct transfer of adenosine monophosphate from ATP to the switch 1 region of these small G-proteins, which prevents their binding to downstream effectors. This blocks the signaling cascade regulating the actin cytoskeleton, leading to its collapse [66, 67].
VPA0450 has now been shown to be a phosphatidylinositide phosphatase homologous to the inositol polyphosphate 5-phosphatase (IPP5C) domain of the eukaryotic protein synaptojanin. By hydrolyzing phosphatidylinositide (4,5)-bisphosphate (PtdIns(4,5)P2) in the plasma membrane, VPA0450 disrupts the association of actin-binding proteins with the membrane. Alone, VPA0450 is sufficient to induce membrane blebbing. In conjunction with the other T3SS1 effectors, the catalytic activity of VPA0450 drives the rapid lysis of host cells [63].
T3SS1 was initially proposed to kill cells by apoptosis based on Annexin V staining of phosphotidylserine (PS) after three hours of infection with V. parahaemolyticus POR3 [56]. Later work demonstrated that LDH is released in as little as two hours, indicating the Annexin V staining was likely due to cell permeability rather than the flipping of PS to the outer leaflet of the plasma membrane [48]. Additionally, POR3 failed to activate caspases or cleave poly ADP ribose polymerase (PARP), both indicators of apoptosis activation [62]. An additional study has indicated cell death proceeds by oncosis based on the uptake of a membrane impermeable dye as well as protection of cells from cytotoxicity by PEG3350, an osmoprotectant [18]. These studies were performed with V. parahaemolyticus NY-4 which, as an O3:K6 serovar, harbors a gene encoding TDH [2]. The tdh gene would need to be deleted and the osmoprotection assay repeated before oncosis could be identified as a mechanism of cell death during Vibrio infection. Apoptosis has been shown to occur in Epithelioma papulosum cyprini (EPC) cells from a carp fish during infection with a Vibrio alginolyticus strain that harbors the VopQ homolog Va1680 [68]. It is possible that this fish cell line, or fish in general, do not have the target of VopQ, and the cells default to apoptosis when not directed to induce autophagy.
4.5.2. Vp-PAI and T3SS2
T3SS2 is found primarily in clinical isolates, and is associated with pandemic strains of V. parahaemolyticus and large outbreaks of disease [42]. Strains without T3SS2 are generally considered to lack pathogenic potential [69]. T3SS2 is unlike any other specific T3SS, but has closest homology to the Hrp1 system found in Pseudomonas syringae [42, 47]. The activity of T3SS2 has been associated with enterotoxicity in the rabbit ileal loop model [42], as well as disruption of tight junction integrity in cultured cell monolayers [41, 69]. While tdh is co-regulated with T3SS2, it is not necessary for the pathogenic effects observed in these model systems [23, 41, 58, 69].
Induction of Vp-PAI gene expression occurs upon contact with bile acids. Specific components of crude bile, including deoxycholate, taurodeoxycholate, and glycodeoxycholate, were able to induce a number of genes, which were mostly found within Vp-PAI [23]. The transcriptional regulators, and putative environmental sensors of this genomic island, were identified as the ToxR homologs VtrA (VPA1332) and VtrB (VPA1348). Both of these proteins contain an N-terminal OmpR-like winged helix-turn-helix DNA binding domain as well as predicted single transmembrane regions. Epistasis studies demonstrated that VtrA initates expression of VtrB. In turn, VtrB activates transcription of tdhAS, T3SS2 structural genes, and effectors including VopC, VopT, VopA, and VopL (Table 3) [58]. As seen below, some of these effectors may a have redundant activity, and therefore, molecular microbial genetics complemented with biochemistry may be required to elucidate the effector’s biochemical activity and cellular target.
VopC (VPA1321) has homology to cytotoxic necrotizing factor 1 (CNF1) an exotoxin found in some pathogenic E. coli strains. CNF1 has been shown to specifically activate Rho, Rac and Cdc42 by deamidating a glutamine residue in the switch 2 region of each enzyme, preventing hydrolysis of GTP. This causes different effects inside the cell including the induction of numerous actin-dependent phenotypes, the modification of the mitochondrial network, and inhibition of apoptosis [70]. A functional analysis of VopC to determine if it shares catalytic activity with CNF1 has not been performed. As such the activity and phenotypic consequences of VopC translocation into host cells is undetermined.
VopT (VPA1327) has homology to the ADP-ribosyltransferase domain of the Pseudomonas aeruginosa effectors ExoS and ExoT. It has been shown to transfer ADP-ribose to Ras, a small monomeric GTPase. This activity is partially responsible for the cytotoxicity seen during infection of Caco-2 monolayers with V. parahaemolyticus [71]. Ras has been implicated in controlling a variety of cellular processes including cell growth, differentiation, and apoptosis. It is also involved in activating the ERK/MAPK pathway (Ehrhardt, 2002). Therefore, inhibition of Ras by VopT is likely to have numerous effects on host cells during infection.
VopA (VPA1346, also referred to as VopP) was characterized as a YopJ homolog that blocks MAPK signaling by acetylating a conserved serine, threonine, and lysine residue on MAPKKs. This prevents the phosphorylation and activation of the MAPK pathway, which prevents the induction of cytokines. VopA/P differs from YopJ in that it only targets the MAPK pathway, whereas YopJ also blocks NF-κB signaling [72, 73]. Although Ras activation triggers many signaling pathways, VopA/P may be considered to be partially redundant with VopT as both block the activation of MAPK pathways.
VopL (VPA1370) was identified as a protein containing three Wiskott-Aldrich homology 2 (WH2) domains. The WH2 domains are able to bind actin monomers and are thought to position them for elongation of an actin filament. Transfection of VopL into Hela cells resulted in the formation of stress fibers independent of Rho-family GTPase activity [40]. A homolog from V. cholerae, VopF, has similar domain architecture but induces aberrant actin protrusions on the cell surface [74]. Biochemical analysis showed that recombinant VopL was sufficient to nucleate actin polymerization independent of other cellular factors [40]. Depending on the cellular target for VopC, VopL could be potentially redundant with VopC.
While the characterization of each T3SS is progressing, the evolutionary purpose of these T3SSs and their effectors is still unknown. As V. parahaemolyticus is an incidental pathogen of humans, it is unlikely that the T3SS were acquired specifically to induce disease in this host. Rather, T3SS may provide improved fitness in the environment.
While a previous study implicated T3SS1 in survival of V. parahaemolyticus during co-culture with the amoeba Acanthamoeba castellanii [75], recent work has detailed the critical important of T3SS2 for survival from predation by a number of marine protists. V. parahaemolyticus strains lacking the Vp-PAI genes, and specifically T3SS2, were unable to survive in the presence of flagellated and ciliated protists, as well as amoeba. The presence of these genes, however, not only promoted bacterial survival, but resulted in killing of the protists, ascribing a function for T3SS2 in the environment [76]. Survival from protist grazing would allow for enhanced invasion and survival of the estuarine environment by V. parahaemolyticus strains bearing both T3SS1 and 2, while leaving open to question the concerted effects of these systems on a mammalian host.
5. Mammalian model systems to study V. parahaemolyticus pathogenicity
To date, much of the work characterizing how V. parahaemolyticus causes disease has been reductionist in its approach. Many of its toxins and effectors have been identified and characterized. While this has yielded significant and valuable data, how these various bacterial factors work together is also of importance. Disease mediated by V. parahaemolyticus does not occur through the isolated actions of individual effectors. Rather, effectors and toxins work in concert to orchestrate a disease progression that has been difficult to study due to the lack of a relevant in vivo model.
To facilitate the study of the T3SSs as a whole, in conjunction with the effects of TDH, multiple animal models have been developed. Intraperitoneal injection of V. parahaemolyticus into mice has demonstrated that both T3SS1 and TDH contribute to lethality during systemic infection, while T3SS2 is necessary for fluid accumulation in the rabbit ligated ileal loop [77]. These findings are supported by a study in which piglets infected orogastrically with a V. parahaemolyticus NY-4 strain expressing only T3SS2 resulted in acute, self-limited diarrhea similar to that seen in humans. A different strain with only T3SS1 failed to produce symptoms. When these same strains were used in a lung-inhalation model in mice, The T3SS1-expressing strain caused 80–100% mortality in 12 hours, while the T3SS2-expressing strain did not cause death of the test subjects [78]. These results suggest a role for T3SS2 effectors in causing the initial infection and diarrheal disease, while T3SS1 may be required for survival from immune clearance once V. parahaemolyticus disseminates out of the intestine and into deeper tissues. Continued development of relevant model systems will continue to improve our understanding of how each T3SS contributes to disease.
6. Conclusion
V. parahaemolyticus is a common human pathogen with an arsenal of virulence factors such a toxins and type 3 secreted effectors that alter the homeostasis and integrity of human cells. Other cellular processes such as capsule formation, iron acquisition, and flagellar motility are also required for infection of the host. As the mechanism of each virulence factor is elucidated, a new question arises—how do these factors work in concert to contribute to an overall infection model? While this question can be partially answered in vitro with tissue culture cells, it will ultimately require a relevant in vivo model.
Acknowledgements
K.O., C.A.B. and T.J.C are supported by National Institutes of Health-AID Grants R01-AI056404 and R01-AI087808, and Grant I-1561 from the Welch Research Foundation. C.A.B. is supported by NIGMS training grant 5T32GM008203 in cellular and molecular biology. K.O. is a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease and a W.W. Caruth, Jr. Biomedical Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCarter L. The multiple identities of Vibrio parahaemolyticus. J Mol Microbiol Biotechnol. 1999;1:51–57. [PubMed] [Google Scholar]
- 2.Yeung PS, Boor KJ. Epidemiology, pathogenesis, and prevention of foodborne Vibrio parahaemolyticus infections. Foodborne Pathog Dis. 2004;1:74–88. doi: 10.1089/153531404323143594. [DOI] [PubMed] [Google Scholar]
- 3.Su YC, Liu C. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 2007;24:549–558. doi: 10.1016/j.fm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Joseph SW, Colwell RR, Kaper JB. Vibrio parahaemolyticus and related halophilic Vibrios. Crit Rev Microbiol. 1982;10:77–124. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- 5.Honda T, Iida T, Akeda Y, Kodama T. Sixty years of Vibrio parahaemolyticus. Microbe. 2008;3:462–466. [Google Scholar]
- 6.Morris JG., Jr Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin Infect Dis. 2003;37:272–280. doi: 10.1086/375600. [DOI] [PubMed] [Google Scholar]
- 7.Nair GB, Ramamurthy T, Bhattacharya SK, Dutta B, Takeda Y, Sack DA. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin Microbiol Rev. 2007;20:39–48. doi: 10.1128/CMR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis. 2000;181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 9.Baffone W, Tarsi R, Pane L, Campana R, Repetto B, Mariottini GL, Pruzzo C. Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ Microbiol. 2006;8:1299–1305. doi: 10.1111/j.1462-2920.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 10.Gamble MD, Lovell CR. Infaunal burrows are enrichment zones for Vibrio parahaemolyticus. Appl Environ Microbiol. 2011 doi: 10.1128/AEM.02897-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson CN, Flowers AR, Noriea NF, 3rd, Zimmerman AM, Bowers JC, DePaola A, Grimes DJ. Relationships between environmental factors and pathogenic vibrios in the Northern Gulf of Mexico. Appl Environ Microbiol. 2010;76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasconcelos GJ, Stang WJ, Laidlaw RH. Isolation of Vibrio parahaemolyticus and Vibrio alginolyticus from estuarine areas of Southeastern Alaska. Appl Microbiol. 1975;29:557–559. doi: 10.1128/am.29.4.557-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin JB, DePaola A, Bopp CA, Martinek KA, Napolilli NP, Allison CG, Murray SL, Thompson EC, Bird MM, Middaugh JP. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med. 2005;353:1463–1470. doi: 10.1056/NEJMoa051594. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Escalona N, Strain EA, De Jesus AJ, Jones JL, Depaola A. Genome sequence of a clinical O4:K12 serotype Vibrio parahaemolyticus strain 10329. J Bacteriol. 2011 doi: 10.1128/JB.05044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y, Kimura S, Yasunaga T, Honda T, Shinagawa H, Hattori M, Iida T. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet. 2003;361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 16.Boyd EF, Cohen AL, Naughton LM, Ussery DW, Binnewies TT, Stine OC, Parent MA. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol. 2008;8:110. doi: 10.1186/1471-2180-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bej AK, Patterson DP, Brasher CW, Vickery MC, Jones DD, Kaysner CA. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tlh, tdh and trh. J Microbiol Methods. 1999;36:215–225. doi: 10.1016/s0167-7012(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Konkel ME, Call DR. Type III secretion system 1 of Vibrio parahaemolyticus induces oncosis in both epithelial and monocytic cell lines. Microbiology. 2009;155:837–851. doi: 10.1099/mic.0.024919-0. [DOI] [PubMed] [Google Scholar]
- 19.Hlady WG, Klontz KC. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 20.Blake PA, Merson MH, Weaver RE, Hollis DG, Heublein PC. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med. 1979;300:1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- 21.Okada N, Matsuda S, Matsuyama J, Park KS, de los Reyes C, Kogure K, Honda T, Iida T. Presence of genes for type III secretion system 2 in Vibrio mimicus strains. BMC Microbiol. 2010;10:302. doi: 10.1186/1471-2180-10-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao G, Jiao X, Zhou X, Wang F, Yang Z, Huang J, Pan Z, Zhou L, Qian X. Distribution of genes encoding four pathogenicity islands (VPaIs), T6SS, biofilm, and type I pilus in food and clinical strains of Vibrio parahaemolyticus in China. Foodborne Pathog Dis. 2010;7:649–658. doi: 10.1089/fpd.2009.0441. [DOI] [PubMed] [Google Scholar]
- 23.Gotoh K, Kodama T, Hiyoshi H, Izutsu K, Park KS, Dryselius R, Akeda Y, Honda T, Iida T. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS One. 2010;5:e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaques S, McCarter LL. Three new regulators of swarming in Vibrio parahaemolyticus. J Bacteriol. 2006;188:2625–2635. doi: 10.1128/JB.188.7.2625-2635.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira RB, Antunes LC, Greenberg EP, McCarter LL. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol. 2008;190:851–860. doi: 10.1128/JB.01462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YK, McCarter LL. ScrG, a GGDEF-EAL protein, participates in regulating swarming and sticking in Vibrio parahaemolyticus. J Bacteriol. 2007;189:4094–4107. doi: 10.1128/JB.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krachler AM, Ham H, Orth K. The outer membrane adhesion factor MAM7 initiates host cell binding during infection by Gram-negative pathogens. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kustusch RJ, Kuehl CJ, Crosa JH. Power plays: iron transport and energy transduction in pathogenic vibrios. Biometals. 2011;24:559–566. doi: 10.1007/s10534-011-9437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanabe T, Funahashi T, Nakao H, Miyoshi S, Shinoda S, Yamamoto S. Identification and characterization of genes required for biosynthesis and transport of the siderophore vibrioferrin in Vibrio parahaemolyticus. J Bacteriol. 2003;185:6938–6949. doi: 10.1128/JB.185.23.6938-6949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funahashi T, Tanabe T, Shiuchi K, Nakao H, Yamamoto S. Identification and characterization of genes required for utilization of desferri-ferrichrome and aerobactin in Vibrio parahaemolyticus. Biol Pharm Bull. 2009;32:359–365. doi: 10.1248/bpb.32.359. [DOI] [PubMed] [Google Scholar]
- 32.O'Malley SM, Mouton SL, Occhino DA, Deanda MT, Rashidi JR, Fuson KL, Rashidi CE, Mora MY, Payne SM, Henderson DP. Comparison of the heme iron utilization systems of pathogenic Vibrios. J Bacteriol. 1999;181:3594–3598. doi: 10.1128/jb.181.11.3594-3598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishibuchi M, Kaper JB. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect Immun. 1995;63:2093–2099. doi: 10.1128/iai.63.6.2093-2099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hongping W, Jilun Z, Ting J, Yixi B, Xiaoming Z. Insufficiency of the Kanagawa hemolytic test for detecting pathogenic Vibrio parahaemolyticus in Shanghai, China. Diagn Microbiol Infect Dis. 2011;69:7–11. doi: 10.1016/j.diagmicrobio.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Fukui T, Shiraki K, Hamada D, Hara K, Miyata T, Fujiwara S, Mayanagi K, Yanagihara K, Iida T, Fukusaki E, Imanaka T, Honda T, Yanagihara I. Thermostable direct hemolysin of Vibrio parahaemolyticus is a bacterial reversible amyloid toxin. Biochemistry. 2005;44:9825–9832. doi: 10.1021/bi050311s. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda S, Kodama T, Okada N, Okayama K, Honda T, Iida T. Association of Vibrio parahaemolyticus thermostable direct hemolysin with lipid rafts is essential for cytotoxicity but not hemolytic activity. Infect Immun. 2010;78:603–610. doi: 10.1128/IAI.00946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagihara I, Nakahira K, Yamane T, Kaieda S, Mayanagi K, Hamada D, Fukui T, Ohnishi K, Kajiyama S, Shimizu T, Sato M, Ikegami T, Ikeguchi M, Honda T, Hashimoto H. Structure and functional characterization of Vibrio parahaemolyticus thermostable direct hemolysin. J Biol Chem. 2010;285:16267–16274. doi: 10.1074/jbc.M109.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda T, Ni Y, Miwatani T, Adachi T, Kim J. The thermostable direct hemolysin of Vibrio parahaemolyticus is a pore-forming toxin. Can J Microbiol. 1992;38:1175–1180. doi: 10.1139/m92-192. [DOI] [PubMed] [Google Scholar]
- 39.Park KS, Ono T, Rokuda M, Jang MH, Iida T, Honda T. Cytotoxicity and enterotoxicity of the thermostable direct hemolysin-deletion mutants of Vibrio parahaemolyticus. Microbiol Immunol. 2004;48:313–318. doi: 10.1111/j.1348-0421.2004.tb03512.x. [DOI] [PubMed] [Google Scholar]
- 40.Liverman AD, Cheng HC, Trosky JE, Leung DW, Yarbrough ML, Burdette DL, Rosen MK, Orth K. Arp2/3-independent assembly of actin by Vibrio type III effector VopL. Proc Natl Acad Sci U S A. 2007;104:17117–17122. doi: 10.1073/pnas.0703196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch T, Livingstone S, Buenaventura E, Lutter E, Fedwick J, Buret AG, Graham D, DeVinney R. Vibrio parahaemolyticus disruption of epithelial cell tight junctions occurs independently of toxin production. Infect Immun. 2005;73:1275–1283. doi: 10.1128/IAI.73.3.1275-1283.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park KS, Ono T, Rokuda M, Jang MH, Okada K, Iida T, Honda T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun. 2004;72:6659–6665. doi: 10.1128/IAI.72.11.6659-6665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohnishi K, Nakahira K, Unzai S, Mayanagi K, Hashimoto H, Shiraki K, Honda T, Yanagihara I. Relationship between heat-induced fibrillogenicity and hemolytic activity of thermostable direct hemolysin and a related hemolysin of Vibrio parahaemolyticus. FEMS Microbiol Lett. 2011;318:10–17. doi: 10.1111/j.1574-6968.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 44.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 45.Izore T, Job V, Dessen A. Biogenesis, Regulation, and Targeting of the Type III Secretion System. Structure. 2011;19:603–612. doi: 10.1016/j.str.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Marlovits TC, Stebbins CE. Type III secretion systems shape up as they ship out. Curr Opin Microbiol. 2010;13:47–52. doi: 10.1016/j.mib.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 48.Burdette DL, Seemann J, Orth K. Vibrio VopQ induces PI3-kinase-independent autophagy and antagonizes phagocytosis. Mol Microbiol. 2009;73:639–649. doi: 10.1111/j.1365-2958.2009.06798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 51.Von Pawel-Rammingen U, Telepnev MV, Schmidt G, Aktories K, Wolf-Watz H, Rosqvist R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol Microbiol. 2000;36:737–748. doi: 10.1046/j.1365-2958.2000.01898.x. [DOI] [PubMed] [Google Scholar]
- 52.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 53.Akeda Y, Galan JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- 54.Arnold R, Jehl A, Rattei T. Targeting effectors: the molecular recognition of Type III secreted proteins. Microbes Infect. 2010;12:346–358. doi: 10.1016/j.micinf.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ono T, Park KS, Ueta M, Iida T, Honda T. Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1. Infect Immun. 2006;74:1032–1042. doi: 10.1128/IAI.74.2.1032-1042.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panina EM, Mattoo S, Griffith N, Kozak NA, Yuk MH, Miller JF. A genome-wide screen identifies a Bordetella type III secretion effector and candidate effectors in other species. Mol Microbiol. 2005;58:267–279. doi: 10.1111/j.1365-2958.2005.04823.x. [DOI] [PubMed] [Google Scholar]
- 58.Kodama T, Gotoh K, Hiyoshi H, Morita M, Izutsu K, Akeda Y, Park KS, Cantarelli VV, Dryselius R, Iida T, Honda T. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. PLoS One. 2010;5:e8678. doi: 10.1371/journal.pone.0008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monack DM, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci U S A. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kodama T, Yamazaki C, Park KS, Akeda Y, Iida T, Honda T. Transcription of Vibrio parahaemolyticus T3SS1 genes is regulated by a dual regulation system consisting of the ExsACDE regulatory cascade and H-NS. FEMS Microbiol Lett. 2010 doi: 10.1111/j.1574-6968.2010.02066.x. [DOI] [PubMed] [Google Scholar]
- 61.Dorman CJ. H-NS: a universal regulator for a dynamic genome. Nat Rev Microbiol. 2004;2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 62.Burdette DL, Yarbrough ML, Orvedahl A, Gilpin CJ, Orth K. Vibrio parahaemolyticus orchestrates a multifaceted host cell infection by induction of autophagy, cell rounding, and then cell lysis. Proc Natl Acad Sci U S A. 2008;105:12497–12502. doi: 10.1073/pnas.0802773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broberg CA, Zhang L, Gonzalez H, Laskowski-Arce MA, Orth K. A Vibrio Effector Protein is an Inositol Phosphatase and Disrupts Host Cell Membrane Integrity. Science. 2010;329:1660–1662. doi: 10.1126/science.1192850. [DOI] [PubMed] [Google Scholar]
- 64.Matlawska-Wasowska K, Finn R, Mustel A, O'Byrne CP, Baird AW, Coffey ET, Boyd A. The Vibrio parahaemolyticus Type III Secretion Systems manipulate host cell MAPK for critical steps in pathogenesis. BMC Microbiol. 2010;10:329. doi: 10.1186/1471-2180-10-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimohata T, Nakano M, Lian X, Shigeyama T, Iba H, Hamamoto A, Yoshida M, Harada N, Yamamoto H, Yamato M, Mawatari K, Tamaki T, Nakaya Y, Takahashi A. Vibrio parahaemolyticus Infection Induces Modulation of IL-8 Secretion Through Dual Pathway via VP1680 in Caco-2 Cells. J Infect Dis. 2011;203:537–544. doi: 10.1093/infdis/jiq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luong P, Kinch LN, Brautigam CA, Grishin NV, Tomchick DR, Orth K. Kinetic and structural insights into the mechanism of AMPylation by VopS Fic domain. J Biol Chem. 2010;285:20155–20163. doi: 10.1074/jbc.M110.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woolery AR, Luong P, Broberg CA, Orth K. AMPylation: something old is new again. Front Microbiol. 2010;1:1–6. doi: 10.3389/fmicb.2010.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z, Chen C, Hu CQ, Ren CH, Zhao JJ, Zhang LP, Jiang X, Luo P, Wang QB. The type III secretion system of Vibrio alginolyticus induces rapid apoptosis, cell rounding and osmotic lysis of fish cells. Microbiology. 2010;156:2864–2872. doi: 10.1099/mic.0.040626-0. [DOI] [PubMed] [Google Scholar]
- 69.Caburlotto G, Lleo MM, Hilton T, Huq A, Colwell RR, Kaper JB. Effect on human cells of environmental Vibrio parahaemolyticus strains carrying type III secretion system 2. Infect Immun. 2010;78:3280–3287. doi: 10.1128/IAI.00050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miraglia AG, Travaglione S, Meschini S, Falzano L, Matarrese P, Quaranta MG, Viora M, Fiorentini C, Fabbri A. Cytotoxic necrotizing factor 1 prevents apoptosis via the Akt/IkappaB kinase pathway: role of nuclear factor-kappaB and Bcl-2. Mol Biol Cell. 2007;18:2735–2744. doi: 10.1091/mbc.E06-10-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kodama T, Rokuda M, Park KS, Cantarelli VV, Matsuda S, Iida T, Honda T. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell Microbiol. 2007;9:2598–2609. doi: 10.1111/j.1462-5822.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 72.Trosky JE, Li Y, Mukherjee S, Keitany G, Ball H, Orth K. VopA inhibits ATP binding by acetylating the catalytic loop of MAPK kinases. J Biol Chem. 2007;282:34299–34305. doi: 10.1074/jbc.M706970200. [DOI] [PubMed] [Google Scholar]
- 73.Trosky JE, Mukherjee S, Burdette DL, Roberts M, McCarter L, Siegel RM, Orth K. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J Biol Chem. 2004;279:51953–51957. doi: 10.1074/jbc.M407001200. [DOI] [PubMed] [Google Scholar]
- 74.Tam VC, Suzuki M, Coughlin M, Saslowsky D, Biswas K, Lencer WI, Faruque SM, Mekalanos JJ. Functional Analysis of VopF Activity Required for Colonization in Vibrio cholerae. MBio. 2010;1 doi: 10.1128/mBio.00289-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laskowski-Arce MA, Orth K. Acanthamoeba castellanii promotes the survival of Vibrio parahaemolyticus. Appl Environ Microbiol. 2008;74:7183–7188. doi: 10.1128/AEM.01332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matz C, Nouri B, McCarter L, Martinez-Urtaza J. Acquired Type III Secretion System Determines Environmental Fitness of Epidemic Vibrio parahaemolyticus in the Interaction with Bacterivorous Protists. PLoS One. 2011;6:e20275. doi: 10.1371/journal.pone.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hiyoshi H, Kodama T, Iida T, Honda T. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect Immun. 2010;78:1772–1780. doi: 10.1128/IAI.01051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pineyro P, Zhou X, Orfe LH, Friel PJ, Lahmers K, Call DR. Development of two animal models to study the function of Vibrio parahaemolyticus type III secretion systems. Infect Immun. 2010;78:4551–4559. doi: 10.1128/IAI.00461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]