Abstract
Animal models with complex cortical development are useful for improving our understanding of the wide spectrum of neurodevelopmental challenges facing human preterm infants. Magnetic resonance imaging (MRI) techniques can define both cerebral injury and alterations in cerebral development with translation between animal models and the human infant. We hypothesized that the immature ferret would display a similar sequence of brain development (both grey (GM) and white matter (WM)) to that of the preterm human infant. We describe postnatal ferret neurodevelopment with conventional and diffusion MRI. The ferret is born lissencephalic with a thin cortical plate and relatively large ventricles. Cortical folding and WM maturation take place during the first month of life. From the mid-second through the third week of postnatal life, the ferret brain undergoes a similar, though less complex, pattern of maturational changes to those observed in the human brain during the second half of gestation. GM anisotropy decreases rapidly in the first three weeks of life, followed by an upward surge of surface folding and WM anisotropy over the next two weeks.
Preterm birth is a major public health challenge in the developed world with a high rate of neurodevelopmental impairments displayed in surviving children (1). The precise “lesion(s)” responsible for these impairments are not yet fully understood and will likely be characterized through a combination of human and animal studies.
Numerous immature animal models, including the rat, mouse, rabbit, lamb and baboon, have been studied in relationship to the mechanisms and patterns of brain injury and altered brain development (2-8). The ferret is unique amongst these models in that the ferret is born lissencephalic, with cortical folding and white matter maturation taking place during the first month of life. The advantages of the ferret model include: (i) a long period of forebrain neurodevelopment, facilitating high temporal resolution of brain development during vulnerable intervals; (ii) complex gyral folding, early myelination, and cortical maturation that occur postnatally; (iii) body size that is compatible with small animal magnetic resonance imaging (MRI) scanners, but still large enough to easily administer interventions; and (iv) lower cost than larger immature animal models that require intensive care support following preterm delivery. The ferret has been used to study brain development for more than two decades, with most early studies focusing on development of the visual system (9). More recent works have characterized behavioral changes during ferret development (10, 11).
To enhance our understanding of the normal sequence of brain development in the immature ferret, we aimed to characterize postnatal neurodevelopment with conventional and diffusion magnetic resonance imaging. The knowledge gained from MR imaging in neurodevelopmentally complex animal models, like the ferret, will facilitate the translation of basic science research to clinical application in preterm infants at risk for neurodevelopmental impairment. We hypothesized that the immature ferret would display a similar sequence of brain development (both grey and white matter) to that of the preterm human infant. Prior work using conventional MRI of the neonatal ferret has illuminated key neuroanatomical changes during gyrogenesis (12). The addition of diffusion tensor imaging and analyses utilizing cortical surface models complement conventional MRI and allow further insights into structural and microstructural changes that occur during early brain development.
Methods
Subjects
Ferret litters were bred by and obtained from a commercial vendor (Marshall BioResources, North Rose, NY) following a 41-day gestation. They were delivered to a dedicated animal facility at Washington University on postnatal day 3 (P3) or later. Brains were obtained via extraction following sacrifice at carefully timed ages. Each ferret brain was fixed by intracardiac perfusion with 4% paraformaldehyde (pH 7.4) after clearing the vascular system with 0.1M phosphate-buffered saline containing heparin (3 units/mL). Following extraction, the brains were placed in 4% paraformaldehyde and stored at 4 degrees Celsius. Thirty-five ferret brains were fixed and imaged. The ferret brains were aimed to be spread across early development (up to P35) with more kits at the weekly intervals in the first 3 weeks of life at P7-8; P14-15; and P20-21. Additionally, 32 in vivo image acquisitions were performed to evaluate longitudinal changes in individual ferrets prior to sacrifice. Procedures were reviewed by the Washington University Animal Studies Committee and performed in accordance with the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals.
In vitro MR image acquisition
Spin-echo images were acquired of 35 fixed ferret brains from P4 through adulthood, with P4 (n=2), P6 (n=2), P8 (n=5), P10 (n=2), P13 (n=1), P15 (n=3), P17 (n=1), P20 (n=2), P21 (n=4), P24 (n=1), P28 (n=2), P31 (n=1), P37 (n=1), P59 (n=1), P66 (n=1), P83 (n=1), adult (n=5). Imaging was performed using a 33-cm clear bore, 4.7-Tesla magnet controlled by a Varian INOVA console. Data were acquired using single-turn RF coils matched in size to each brain. The brains were placed in modified 6, 10, 20, 35, and 60-ml syringes, corresponding to brain size, and immersed in 4% phosphate-buffered paraformaldehyde during imaging.
Contrast between grey matter, white matter and cerebrospinal fluid (CSF) in conventional MR images is generally provided by differences in the time constants of transverse relaxation, (T2, spin-spin relaxation time), and longitudinal relaxation (T1, spin-lattice relaxation time). In the developing brain, these time constants change as the brain matures due to changes in water content and myelination (13). In the current study, brain tissue T2 values were measured at 4.7 Tesla in both grey and white matter by measuring signal intensity as a function of echo time (TE) using a Hahn spin echo approach. The data points were fit to an exponential curve (14). The T2 time constant decreases with age.
T2-weighted images were acquired with TE ≈ the average T2 from both grey and white matter determined that age to optimize contrast-to-noise ratio in the images. Voxel size was gradually increased as total brain size increased, from 150 μm × 150 μm × 150 μm (isotropic) at P4 to 350 μm isotropic in the adult.
Diffusion weighted images were acquired with acquisition parameters chosen for immature brains (<5 weeks old): TE = 67 ms, TR = 3.4-5.8 s, 250-300 μm isotropic voxel size, diffusion gradient duration = 9.5 ms, diffusion gradient amplitude = 20 Gauss/cm, and delay between diffusion gradients = 50 ms (b = 200 – 12,100 s/mm2). The diffusion gradient orientation was varied over 25 directions (15). A reference image was obtained with b = 0 s/mm2. For more mature brains (>5 weeks old), the spin-spin relaxation time constant was shorter, and therefore acquisition parameters were TE = 35 ms, 250-350 μm isotropic voxel size, b = 2,700 s/mm2, and gradient orientation was varied over 22 directions.
In vivo MR image acquisition
We obtained MR images on 32 separate occasions on live ferrets (P4 – adult). Seven kits were serially imaged, with one kit scanned weekly from P7 through 42. Each ferret was initially anesthetized with 2-4% isoflurane in a vented anesthesia chamber. After induction of anesthesia, the snout was placed in a nose cone with an attached palate bar or tooth bar (if teeth were present). Anesthesia was subsequently maintained via the nose cone with 1-2% isoflurane in O2 (1.5 L/min). The animal was held still in the prone position secured by molded head supports. The animal’s pulse rate and oxygen saturation were monitored with an MRI-compatible pulse-oximeter (Nonin Medical, Plymouth, MN) attached to a hind paw. Body temperature was maintained with circulating warm water. The entire assembly (animal, restraint, water, oximeter probe, and RF coils) was contained in a plastic bed inserted in the bore of the MR scanner (Figure 1). Anesthesia time ranged from 150-250 minutes. Images were acquired using an 11.7-Tesla magnet controlled by a Varian INOVA console with a two-coil system consisting of a surface receive coil placed just above the skull and a volume-type transmit coil. Receive coil sizes were matched to the ferret brains to optimize signal-to-noise ratio. Serially-imaged kits were returned to their mothers following recovery from anesthesia.
Figure 1.

(A) In vivo imaging apparatus with postnatal day 28 (P28) male ferret. (B) T2-weighted horizontal MR slices at the level of the centrum semi-ovale in a male ferret imaged weekly from P7 through P42.
MR image processing
Conventional image analysis
Conventional T2-weighted images were qualitatively evaluated for developmental landmarks in the white and grey matter. Surface folding index (SFI) was calculated after the method of Dieni et al(4). Using T2-weighted images, the pial boundaries of the cerebral cortex of horizontal slices at the level of the mid-caudate (excluding basal ganglia and diencephalon) were traced using Adobe Photoshop CS3 Extended version 10.0.1 (Adobe Systems Incorporated, San Jose, CA). The length of the pial boundary (L) was measured, as was the area (A) enclosed by that boundary. The SFI was calculated using Equation 1 (note that a normalization constant, (4π)-1, was used so that a circle has an SFI of 1).
| Equation 1 |
Diffusion analysis
Raw data from diffusion studies were phase-corrected using Bayesian probability analysis (16) so that noise was Gaussian and centered about zero. A local diffusion tensor was estimated for each voxel, and relative anisotropy (RA) and apparent diffusion coefficient (ADC) were derived from the standard deviation (RA) and mean (ADC) of the eigenvalues of this diffusion tensor. Regions of interest (ROIs) were placed on horizontal slices in the white matter (WM) and grey matter (GM) lateral to the caudate nucleus, and in the corpus callosum and anterior commissure using Analyze version 7.0 (Rochester, MN). ROIs were also placed in the caudate nucleus and in frontal, somatosensory, and occipital WM tracts on horizontal slices superior to the level of the caudate nucleus.
Surface based analysis
Analysis of cortical surfaces provides a way to describe spatial variations in geometry and microstructure. Using Caret software (http://brainvis.wustl.edu/caret) (17), we generated surface based renderings (triangular faces and their vertices defined in 3-dimensional space) from T2-weighted image volumes. RA estimated on each voxel was mapped onto the generated surface. Curvature was estimated by fitting a second order polynomial to the surface near each vertex. The true and exposed cortical surface areas were determined using Caret (18). Mid-cortical and CSF/cortical grey matter tracings were used to determine true surface area for fixed and in vivo brains, respectively. Area ratios were calculated from these surface areas to quantify the extent of cortical packing for 10 fixed brains (P4, 6, 10, 13, 17, 21, 31, 66, and two adults) and four sets of in vivo images of a single ferret kit imaged on P7, 14, 21, and 28.
Results
Gross examination
The P4 ferret brain is nearly lissencephalic and bright white. The cortex undergoes complex folding during the first 4 weeks of postnatal life. In the 5th and 6th weeks of life, as kits are weaned, cortical tissue continues to increase in volume, but also darkens slightly, progressing toward the color of light brown found in the adult brain. Additionally, from the 5th week to adulthood the brain’s shape changes. The rostal portion extends forward and narrows, the gyri become less rounded, and the sulcal spaces narrow. Brain weight increases linearly over the first 5 weeks of life and then begins to plateau (Figure 2). There is no notable difference in size or shape of the body or brain between males and females in the first 6 weeks of life during the period of our major observations.
Figure 2.
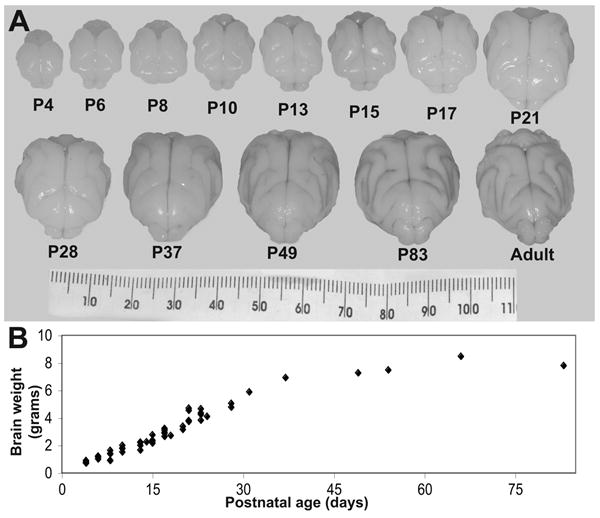
(A) Photographs of fixed ferret brains from postnatal day 4 (P4) through adulthood. (B) Plot of brain weights from P4-83.
Conventional Imaging
The youngest ferret imaged (P4) displayed a simple, smooth, thin cortical plate. The ventricles were relatively large and the subventricular zone appeared prominent (Figure 3). At P10, the cortex displayed simple folding with the signal in both the white and grey matter becoming more homogeneous. The volume of the ventricles decreased slightly, and the subventricular zone remained prominent. By P17, the cortex appeared thicker and more complex with widespread folding and the ventricles and the subventricular zone were difficult to visualize.
Figure 3.

T2-weighted horizontal mid-caudate level MR slices of fixed ferrets from postnatal day 4 (P4) through adulthood. Note the increase in cortical folding occurring prior to myelination. The reversal in image intensity occurs at P37.
At P21, T2 hyper-intensity appeared within the maturing region of the caudal white matter and T2 hypo-intensity, consistent with areas of myelination, emerged within the posterior limb of the internal capsule and centrum semi-ovale. Over the following week, T2 hyper-intensity extended rostrally while regions of T2 hypo-intensity (myelination) expanded throughout the cerebral white matter. At P37, grey/white contrast diminished, emerging into a reversal of T2 contrast characteristics. Thus, cortical grey matter became hyper-intense, the deep nuclear grey matter remained hypo-intense, and the sulcal depths increased and became narrower. Over the next week (P42) grey/white contrast fully reversed compared to the immature brain. From then, myelination within the white matter continued to become more hypo-intense into adulthood with caudal brain narrowing.
Quantitatively, the surface folding index (SFI) increased gradually over the first 3 weeks of postnatal life, then rapidly during the 4th and 5th weeks of life.
Diffusion Weighted Imaging
During the first 21 days of postnatal life, ferret cortical gray matter anisotropy rapidly decreased, with minor increases in WM anisotropy. An upward surge in WM anisotropy occurred from 21 to 35 days of age. Subsequent changes in WM anisotropy occurred more slowly. (Figure 4)
Figure 4.

Diffusion anisotropy in cortical grey (+) and white (○) matter, along with the surface-folding index (■) in ferrets from postnatal day 4 through 37. Note the earliest change is a decrease in grey matter occurring prior to the increase in WM anisotropy. Surface folding parallels the change in WM anisotropy. The locations of regions of interest used for anisotropy are shown in a horizontal slice from a P8 brain.
Surface-based analysis
The mean curvature values and area ratios calculated with CARET software (17) are depicted in Figures 5A and 5B, respectively. Minimal curvature is present at P4. The cortical curvature increases during each of the first four weeks of development, as shown at P10, 17, and 24 in Figure 5A. The area ratio, a measure of cortical packing, increases linearly for the first four weeks of life, then begins to plateau.
Figure 5.
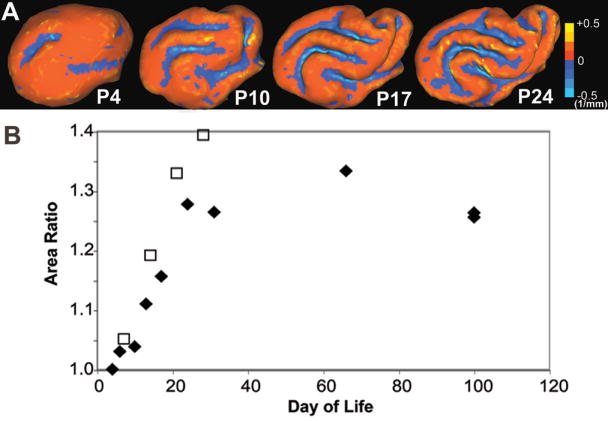
(A) Curvatures are mapped on 3D cortical surfaces at postnatal day 4, 10, 17, and 24. (B) Area ratios quantify the degree of cortical packing in 10 fixed (◆) and 4 in vivo (□) ferret brains from postnatal day 4 through adulthood and postnatal day 7 through 28, respectively.
Comparison to human development
From these preliminary data, it appears the ferret brain undergoes a similar (though less complex) sequence of development between postnatal days 10 and 21 to the preterm human brain between 25 and 40 weeks gestation. Representative 3-D reconstructions of ferret cortical surfaces, together with human cortical surface representations at comparable stages are shown in Figure 6.
Figure 6.

3D reconstructions of (A) postnatal day 4, 10, 17, and adult ferret brains and (B) human brains at 25, 30, 33, and 39 weeks gestation and adult maturation.
Discussion
The MR data from our study demonstrate that despite the human brain being far more complex than the ferret brain, the pattern of changes has both qualitative and quantitative similarities. These data confirm the value of the immature ferret as a model for studies of brain injury and development, relevant to both the preterm and term-born human infant. In addition, MR imaging can be an important translational tool between the human and animal model.
There are notable variations in the number of ferret kits that are imaged at each time point. Due to kit availability, higher numbers of subjects were imaged at some time points with a single kit at others. However, as the ferret kit brains displayed smooth and consistent transitions during early development, extrapolations of observations between the ages was felt to be appropriate.
Comparison of MR imaging features with histology during ferret brain development
Production of the neurons and glia of the ferret forebrain cortex takes place in the subventricular zone, with neurons and glia migrating to the developing cortical plate. Maturation of individual cortical layers generally proceeds with an inside to outside (layer VI to layer II) sequence. Maturation also proceeds in a rostrocaudal and laterodorsal direction (19, 20). Cortical neuron production begins on approximately embryonic day 20 (E20) and continues for 5 weeks (21). By E29 neurons are present along the entire cortical plate surface and somatosensory neurogenesis completes rostrally by E36 (19). Histologically, neurogenesis in the somatosensory cortex is complete by P2, while neuron production in the visual cortex continues through the second week of postnatal life (22).
The major features of postnatal brain maturation observed on MR imaging in the ferret begin with early development (P4-10), characterized by commencement of gyral folding, loss of cortical GM anisotropy, and moderate increases in curvature. The next phase in development (P10-28) is characterized on MR imaging by marked increases in WM anisotropy, surface folding index, and curvature. These maturational changes in the second and third postnatal weeks of ferret life are similar to the changes occurring in second half of human gestation (23). The layers of cortex become increasingly histologically distinct over the first four weeks of life (22). The decrease in signal intensity of the cortical ribbon on T2-weighted images in the fourth week of life corresponds temporally with the neuronal cell differentiation into larger, less densely spaced cells observed in previous histological examinations (12, 19, 20).
MR imaging also delineates a third phase of neurodevelopment in this model, which occurs from the fifth to seventh week of postnatal life. During this phase, myelination and a reversal of grey/white contrast on T2-weighted images occur, accompanied by no major changes in the surface folding index, curvature, anisotropy, or brain size. The decrease in white matter signal intensity on T2-weighted imaging, occurring in the fifth and sixth weeks of life, corresponds to maturation of myelin. By 2 months of age, cortical cytoarchitecture closely resembles its adult form (21). Following this phase, the brain elongates rostrally, the sulcal spaces continue to narrow, and the gyri become less rounded and more squarely shaped, but no significant changes of our measured indices occur.
This long interval of neuromaturation is in contrast to the rapid maturation of rodents (21).
Application of MRI in human and immature animal model models
MR imaging studies in the prematurely born infant have documented common, qualitative abnormalities, including white matter signal abnormalities, ventriculomegaly, and thinning of the corpus callosum (24). These abnormalities, when present at term equivalent, have been correlated with adverse neurodevelopmental outcomes at two years of age (25). Additionally, diffusion weighted imaging has been shown to reflect WM and GM microstructural maturation in human brain from 26 weeks gestation through infancy. The two quantitative measures from diffusion imaging that have been shown to be sensitive to brain injury in both the acute and chronic phases in preterm (26) and term-born infants (27, 28) are apparent diffusion coefficient (ADC) and relative anisotropy (RA). Another quantitative measure, surface folding index, quantifies the extent of gyral folding and has been used as a marker for injury and development in animal models (29). Surface based morphometry was recently used to quantify changes in gyrification occurring in preterm human infants from 26 to 36 weeks gestation (30). Thus, a combination of these analysis techniques was undertaken in this study.
Conventional MR imaging methods have been applied to several immature animal models, including baboons, lambs, rabbits, rats, ferrets, and mice expanding our knowledge of conventional MRI characteristics of brain development and injury (2-4, 8, 12). However, apart from the rabbit (31), baboon (7), and rodent (32), few quantitative measures have been made in these studies. Normative values of anisotropy and diffusivity are currently used to assess for the presence and degree of brain injury. Application of these quantitative measures to both human and animal studies can enhance the translational understanding of the model with the human disease process.
Future of the ferret model of neurodevelopment
The future of the ferret as a neurodevelopmental animal model remains promising. This model displays complex gyral folding and myelination, a long interval of neurodevelopment, and numerous similarities to the preterm human brain. We are currently developing an injury model using chronic hypoxia to generate mild, diffuse patterns of brain injury. Additionally, behavioral testing with water mazes will strengthen our understanding of both development and injury. Future analysis will combine functional neurodevelopmental testing (water maze performance) with both imaging and histologic markers of development and injury. The immature ferret model can be used to expand our knowledge about the effects of current and future interventions used in the care of neonates on brain development and injury. Improvements in understanding these effects and the mechanisms leading to brain injury in preterm infants will help us refine neonatal care to limit the frequency and severity of adverse neurological outcomes.
Acknowledgments
Financial Support: Green Foundation (JJN), National Science Foundation Grant DMS-0540701 (PVB), AAP Marshall Klaus Perinatal Research Grant (ARB)
Abbreviations
- ADC
apparent diffusion coefficients
- E
embryonic day
- GM
grey matter
- P
postnatal day
- RA
relative anisotropy
- SFI
surface folding index
- WM
white matter
References
- 1.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 2.Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- 3.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 4.Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S. The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropathol Exp Neurol. 2004;63:1297–1309. doi: 10.1093/jnen/63.12.1297. [DOI] [PubMed] [Google Scholar]
- 5.Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, Rees SM. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–949. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischaemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;422:85–88. doi: 10.1111/j.1651-2227.1997.tb18353.x. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 2007;27:12506–12515. doi: 10.1523/JNEUROSCI.3063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodygensky GA, Inder TE, Neil JJ. Application of magnetic resonance imaging in animal models of perinatal hypoxic-ischemic cerebral injury. Int J Dev Neurosci. 2008;26:13–25. doi: 10.1016/j.ijdevneu.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- 10.Christensson M, Garwicz M. Ontogenesis of within-session locomotor habituation in the open field. Neuroreport. 2005;16:1319–1323. doi: 10.1097/01.wnr.0000175614.93312.53. [DOI] [PubMed] [Google Scholar]
- 11.Christensson M, Garwicz M. Time course of postnatal motor development in ferrets: ontogenetic and comparative perspectives. Behav Brain Res. 2005;158:231–242. doi: 10.1016/j.bbr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Neal J, Takahashi M, Silva M, Tiao G, Walsh CA, Sheen VL. Insights into the gyrification of developing ferret brain by magnetic resonance imaging. J Anat. 2007;210:66–77. doi: 10.1111/j.1469-7580.2006.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin E, Kikinis R, Zuerrer M, Boesch C, Briner J, Kewitz G, Kaelin P. Developmental stages of human brain: an MR study. J Comput Assist Tomogr. 1988;12:917–922. doi: 10.1097/00004728-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hahn EL. Spin Echoes. Phys Rev. 1950;80:580–594. [Google Scholar]
- 15.Batchelor PG, Atkinson D, Hill DL, Calamante F, Connelly A. Anisotropic noise propagation in diffusion tensor MRI sampling schemes. Magn Reson Med. 2003;49:1143–1151. doi: 10.1002/mrm.10491. [DOI] [PubMed] [Google Scholar]
- 16.Neil JJ, Bretthorst GL. On the use of Bayesian probability theory for analysis of exponential decay data: an example taken from intravoxel incoherent motion experiments. Magn Reson Med. 1993;29:642–647. doi: 10.1002/mrm.1910290510. [DOI] [PubMed] [Google Scholar]
- 17.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 19.McSherry GM, Smart IH. Cell production gradients in the developing ferret isocortex. J Anat. 1986;144:1–14. [PMC free article] [PubMed] [Google Scholar]
- 20.Smart IH, McSherry GM. Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J Anat. 1986;147:27–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson CA, Peduzzi JD, Hickey TL. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci. 1989;9:1242–1253. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noctor SC, Scholnicoff NJ, Juliano SL. Histogenesis of ferret somatosensory cortex. J Comp Neurol. 1997;387:179–193. [PubMed] [Google Scholar]
- 23.McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, Almli CR, Shiran SI, Conturo TE, Neil JJ. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12:1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 24.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 25.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 26.Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 27.McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, Shimony JS, Shiran SI, Neil JJ. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology. 2002;59:824–833. doi: 10.1212/wnl.59.6.824. [DOI] [PubMed] [Google Scholar]
- 28.Ward P, Counsell S, Allsop J, Cowan F, Shen Y, Edwards D, Rutherford M. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics. 2006;117:e619–e630. doi: 10.1542/peds.2005-0545. [DOI] [PubMed] [Google Scholar]
- 29.Rees S, Stringer M, Just Y, Hooper SB, Harding R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Brain Res Dev Brain Res. 1997;103:103–118. doi: 10.1016/s0165-3806(97)81787-7. [DOI] [PubMed] [Google Scholar]
- 30.Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Huppi PS. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18:1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- 31.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun SW, Neil JJ, Song SK. Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med. 2003;50:743–748. doi: 10.1002/mrm.10605. [DOI] [PubMed] [Google Scholar]


