Abstract
Background
Interferon-β1a (IFNB) and glatiramer acetate (GA) are distinct therapies which are both partially effective for relapsing MS. It is not known if combining the two treatments would be more effective.
Objective
To review the rationale, design, and baseline characteristics of the CombiRx study of combined treatment with IFNB and GA.
Methods
The key inclusion criteria included a diagnosis of relapsing MS, at least 2 episodes of MS activity in the previous 3 years, expanded disability status scale of 0 to 5.5, and no prior treatment with either IFNB or GA. Subjects were randomized to IFNB+GA, IFNB monotherapy, or GA monotherapy in a 2:1:1 ratio.
Results
From 2005 to 2009, we enrolled 1008 subjects. The participants were 72.4% female and 87.6% Caucasian with a mean age of 37.7 years. The median duration of symptoms was 2 years at entry into the study, and the mean EDSS was 2.1. On the baseline MRI, the mean total lesion load was 12.2 ml, and 40% of the participants had enhancing lesions.
Conclusion
We have recruited a population of patients with clinical and MRI characteristics typical for early MS. The study results will aid in deciding on the optimum early treatment. This trial should serve as a model for future studies of combination therapy.
Keywords: interferon beta-1a, glatiramer acetate, combination therapy, clinical trial, multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system that affects approximately 350,000-400,000 persons in the United States and 2.5 million persons worldwide. The typical form of the disease begins in young adulthood, has a relapsing-remitting course, and often causes substantial neurologic disability over time (Compston and Coles, 2008; Noseworthy et al., 2000). There are several treatments approved for relapsing-remitting MS (RRMS), but none are ideal. Interferon-β (IFNB) has a good safety profile and reduces relapse rate by 32% (Jacobs et al., 1996). Glatiramer acetate (GA) likewise is safe for long-term use and reduces relapse rate by 29% (Ford et al., 2010; Johnson et al., 1995). More recently developed treatments, such as natalizumab and fingolimod, may have better efficacy but have safety concerns and side effects which limit their use.
As more agents have been tested in MS, it appears that no single therapy is likely to have the desired combination of efficacy and safety. One reasonable approach to this problem is combination therapy (Tullman and Lublin, 2005). The concurrent use of two effective drugs with different mechanisms of action could have an additive or synergistic benefit without additional side effects. IFNB and GA are an obvious choice for combination therapy, since both have good safety, modest efficacy as monotherapy, and probable different mechanisms of action.
An ideal study of a combination therapy should compare the combination to either drug as monotherapy. It should also be randomized and blinded and have an adequate sample size and duration to discern meaningful differences in the treatment groups. We report here the design and baseline characteristics of such a study of the combination of IFNB and GA. Future reports will correlate these baseline characteristics with the genetic studies, biomarkers, and clinical course collected as part of this study.
2. Materials and methods
2.1 Study design
CombiRx is a 3-arm, randomized, double-blind, placebo-controlled, multi-center, Phase-III trial of combination therapy utilizing a partial 2×2 factorial design with a 2:1:1 randomization balance (Table 1). With the partial factorial design, there is no arm with no active treatment. The monotherapy arms have a matched placebo injection. Participants were followed for a minimum of 36 months and up to 7 years, if they continued into the extension phase of the trial (Figure 1). The major inclusion and exclusion criteria are given in Table 2. Diagnosis of MS was made according to the McDonald criteria (McDonald et al., 2001; Polman et al., 2005). Participants had to have experienced 2 clinical relapses in the prior three years or one clinical relapse with subsequent MRI activity.
Table 1.
Treatment combinations in CombiRx Primary Trial
| Active IFNB | Placebo IFNB | |
|---|---|---|
| Active GA | Arm 1 (500): Active IFNB + Active GA | Arm 2 (250): Active IFNB + Placebo GA |
| Placebo GA | Arm 3 (250): Active IFNB + Placebo GA | NA |
Figure 1.
Study timeline and assessments
Table 2.
Major inclusion and exclusion criteria
| Inclusion Criteria |
| Age 18 and 60 years, inclusive |
| Expanded Disability Status Scale (EDSS) score of 0 to 5.5, inclusive |
| Diagnosis of relapsing-remitting MS by either the Poser or McDonald criteria |
| At least 2 exacerbations in the prior three years; one exacerbation may be an MRI change meeting the McDonald MRI criteria for dissemination in time |
| Exclusion Criteria |
| Any prior use of interferon beta or glatiramer acetate |
| Acute exacerbation within 30 days of screening |
| Steroids for acute exacerbations (>100 mg/day) within 30 days of Screening Visit or chronic systemic steroid use |
| Evidence of progressive MS |
| IVIg, azathioprine, methotrexate, cyclosporine, mitoxantrone, cyclophosphamide, mycophenolate or plasma exchange in the twelve weeks prior to study drug dosing or 4 aminopyridine in the four weeks prior to study dosing |
| Any previous treatment with natalizumab, cladribine, T cell vaccine, alemtuzumab, daclizumab, rituximab, altered peptide ligand or total lymphoid irradiation |
| Any prior history of seizure or significant cardiac, hepatic, pulmonary, or renal disease; immune deficiency; or other serious medical conditions |
2.2 Randomization
Eligible participants were randomized using a distributed Data Entry System (DES), where the sites and participants were masked to the assigned treatment arm. Participants were randomized to one of the three treatment arms in a 2:1:1 ratio (combination:single agent:single agent) within site using a permuted block design, with block sizes of 4 or 8.
2.3 Medications
All participants receive at least one active medication, and all participants take the same number of injections. Interferon-β1a is given 30 μg intramuscularly once a week, and glatiramer acetate is given at 20 mg subcutaneously daily. Matched placebo preparations were provided for the active medications by their respective manufacturer.
2.4 Outcome Measures
The primary objective of the core study is to determine whether combined treatment is more effective than either agent alone in treating RRMS, as determined by the number of relapses during 36 months of follow up. The primary analysis will compare the relapse hazard rate between the treatment groups using a Cox Proportional Hazards Model with Anderson-Gill Modification. Secondary outcome measures include confirmed progression on the expanded disability status scale (EDSS), change in the Multiple Sclerosis Functional Composite (MSFC), and change in the MRI as measured by the Z4 composite (see below for description) and measures of disease progression in the extension phase of the trial.
2.5 Relapse Definition
For this study, a protocol defined relapse is the appearance of a new symptom or worsening of an old symptom, attributable to MS; accompanied by a change in the neurologic examination (demonstrated by a 0.5 or greater increase in the EDSS or a 2 point change in one functional system or a 1 point change on two functional systems, excluding bladder and cognitive changes); lasting at least 24 hours in the absence of fever; preceded by stability or improvement for at least 30 days; and confirmed by the examining physician within 7 days of onset. Relapses meeting the above criteria but not seen within 7 days are non-protocol defined relapses. Episodes which the treating physician felt were MS relapses but without a change in the EDSS are defined as suspect relapses. Only protocol defined relapses are used in the primary analysis.
2.6 Participant Assessments
After enrollment, participants return to clinic every 3 months for EDSS, MSFC, multiple sclerosis quality of life index (MSQLI), and Rankin assessments. Patient care was directed by the treating physician, and the EDSS was performed by the examining physician. Both physicians were blinded to treatment assignment, and the examining physician was instructed not to discuss treatment side effects. MRI was performed at entry and at months 6, 12, 24, and 36 and yearly through the extension (Figure 1). Low contrast visual acuity was assessed using Low Contrast Sloan Letter charts with 100%, 2.5% and 1.25% contrast.
2.7 Study Management
The Clinical Coordinating Center (CCC) is located at the Mount Sinai School of Medicine, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis and is responsible for overall study management including regulatory and finance. The Statistical and Data Management Center (SDMC) is located at the University of Alabama at Birmingham, School of Public Health, Department of Biostatistics and is responsible for design and management of the case report forms, data entry system and data collection and analysis. The MRI Analysis Center (MRI-AC) is located at the University of Texas Health Science Center at Houston. All centers received local IRB or WIRB approval. Drug distribution and participant supplies were managed by The Health and Human Services, Supply Service Center (HHS SSC) in Perry Point, Maryland.
2.8 MRI Acquisition and Analysis
MRI of the brain with and without contrast was acquired using a standardized protocol. Imaging data from each site were sent to the MRI Analysis Center where semi-automated processing was used to extract the enhancing lesion volume, the T2 lesion volume, T1 hypointense lesion volume, and normalized CSF. These four values were combined in a composite Z score (Poonawalla et al., 2010; Wolinsky et al., 2000). The individual measures were transformed into dimensionless values representing the number of standard deviations from the mean and added together. This z-transformation allows meaningful combination of measure with different units and values, and improves the correlation with EDSS.
2.9 Baseline Statistical Analysis
Summary statistics are presented as mean ± SD, mean (median) and %, where applicable. A repeated measures mixed model was used for comparison of MSFC components across screening visits 1, 2, and baseline. P-values < 0.05 were considered meaningful. Analyses were performed using JMP v8 and SAS v9.2 (Cary, NC).
2.10 Role of Funding Agency
The study was funded by the National Institutes of Health with medications and matched placebos provided by Biogen Idec and Teva Pharmaceuticals. Design, analysis, and decision to publish results are the responsibility of the CCC, SDMC, MRI-AC. Additional funding was provided by the NINDS NIH Intramural Program for the Biomarker MS ancillary study and the National Multiple Sclerosis Society for the Contrast Sensitivity ancillary project. The trial is listed on www.clincaltrials.gov, NCT00211887.
3. Results
3.1 Screening & Enrollment
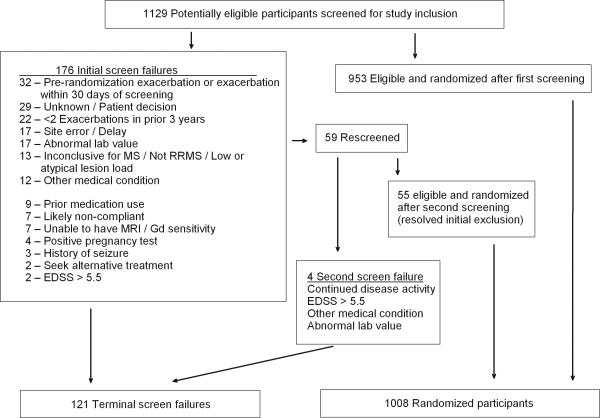
The CombiRx trial began screening in January 2005 and closed enrollment in April 2009, slightly exceeding planned enrollment of 1000 with 1008 randomized participants. Recruitment was slower than anticipated, and was likely affected by competition from multiple other studies in the target patient population and the approval of natalizumab. During the maximum 45-day screening period, 1129 individuals were assessed for eligibility (Figure 2). Of these, 953 were eligible and enrolled; with 172 initially failing screening. CombiRx allowed for re-screening if the criterion initially excluding the individual could be resolved, including stabilization of neurological symptoms with a minimum of 30-days between the first and second screening assessment. Ongoing or new disease activity accounted for 18% of the initial screening failures, 17% occurred in individuals who either declined to participate or did not return for enrollment (unknown reason), and 12% did not meet the disease activity criteria of 2 or more exacerbations in the prior 3 years. Of the 59 individuals who were rescreened, 55 were ultimately randomized with 29% stabilizing disease activity, 24% due to a delay in the initial screening process exceeding the 45 day window and 11% experiencing an additional relapse to qualify for the disease activity requirement.
Figure 2.
Screened and randomized participants with reasons for screen failure
3.2 Baseline Characteristics
Out of the randomized participants (Table 3), 72.4% were female, 87.6% Caucasian, and 7.0% African American, with a mean age of 37.7 years. The majority of participants (77.8%) met the Poser Criteria (Poser et al., 1983) for definite MS, with the remainder (22.2%) meeting the McDonald Criteria (Table 4) (McDonald et al., 2001; Polman et al., 2005). The majority of subjects had experienced 2 clinical attacks, with only 13% being diagnosed on the basis of a single clinical event with subsequent MRI evidence of dissemination in time. The median number of relapses was 2, with a mean of 2.5. Only 9% of participant had more than 3 relapses. The majority (97%) had experienced at least 1 relapse in the prior 12 months.
Table 3.
Demographics and Diagnosis
| N | % | |
|---|---|---|
| Female | 730 | 72.4 |
| Caucasian | 886 | 87.9 |
| African American | 73 | 7.2 |
| Non-Hispanic | 903 | 89.6 |
| Mean±SD | Med (Min, Max) | |
|---|---|---|
| Age (years) | 37.7±9.7 | 37.0 (18-61) |
| BMI | 28.7±6.9 | 27.4 (16.1-60.0) |
| N | % | |
|---|---|---|
| Poser Criteria | 784 | 77.9 |
| McDonald Criteria | 224 | 22.1 |
| 2 or more clinical, 1 lesion | 92 | 9.1 |
| 1 clinical, 2 or more lesions | 81 | 8.0 |
| 1 clinical, 1 lesion | 50 | 5.0 |
Table 4.
Baseline Disease, Clinical, and MRI Characteristicsa
| Mean±SD | Median (Min, Max) | |
|---|---|---|
| Years since diagnosis | 1.2±3.3 | 0.0 (0-36) |
| Years of symptom | 4.3±5.6 | 2.0 (0-39) |
| Relapses prior 12 Months | 1.7±0.8 | 2.0 (0-6) |
| Relapses prior 3 Years | 2.5±1.0 | 2.0 (1-15) |
| EDSS | 2.1±1.2 | 2.0 (0-6.5)b |
| Rankin Score (1007) | 1.0±0.7 | 1.0 (0-3) |
| MSFC (1000) | ||
| 9 Hole Peg Test | 20.2±4.7 | 19.1 (11.5-86.5) |
| Timed 25Foot Walk | 5.0±1.6 | 4.7 (2.3-21.7) |
| PASAT-3 | 49.8±10.4 | 53.0 (0-60) |
| Contrast Letter Acuity (996), # correct | ||
| CLA 100% | 56.5±6.3 | 59.0 (0-60) |
| CLA 2.50% | 37.7±10.4 | 39.0 (0-60) |
| CLA 1.25% | 27.3±11.9 | 29.0 (0-60) |
| MRI characteristics | ||
| Number T2 Lesions | 87.6±56.1 | 71 (6-379) |
| Volume T2 Lesionsc | 10.5±11.5 | 6.4 (0.1-76.2) |
| Number Post Gd T1 Hypointense Lesions | 63.7±58.0 | 44 (0-423) |
| Volume Post Gd T1 Hypointense Lesions | 1.7±2.2 | 0.9 (0-12.4) |
| Burden of Disease (T2 + T1) | 12.2±13.2 | 7.5 (0.1-87.4) |
| Number Gd Lesions, all subjectsd | 1.7±4.2 | 0 (0-41) |
| Volume Gd Lesionse | 0.3±0.5 | 0.1 (0.002-4.3) |
| Number (%) subjects with Gd Lesion | 399 (39.6) |
N = 1008, unless noted
Two participants with protocol violation EDSS of 6 and 6.5 at entry
all volumes are in ml
1 participant received partial Gadolinium
in subjects with Gd lesions
The exclusion of patients previously treated with either interferon or glatiramer acetate ensured that the majority of subjects were newly diagnosed (Table 5). The majority of participants (87%) were enrolled within 1 year of a clinical diagnosis with a median duration of symptoms of 2 years. As expected for recently diagnosed MS, this cohort had minimal disability; the mean EDSS score at screening median score of 2.0. The baseline averages (mean±SD) for the MSFC components were 5.0±1.6 seconds for Timed 25 foot walk, 20.2±4.7 seconds for the 9-Hole Peg test and 49.9±10.4 correct answers out of a possible 60 on the 3 Second PASAT. In addition, participants had an average of 56.7% correct on the 100% Contrast Letter Acuity test, 38.7% at 2.5% contrast and 27.3% at 1.25% contrast. The median Rankin Score at baseline was a 1, indicating “No significant disability despite symptoms, able to carry out all usual duties and activities.” The majority of participants (74.2%) were employed outside of the home, 11.0% were actively employed within the home (self-employed/homemaker) and 6.3% were not employed due to MS.
To reduce the practice effect, each participant had 2 screening visits with assessment of the MSFC before the baseline measurements. Small but significant practice effects were seen on the 9-hole peg test and the 3 second PASAT, with minimal effect on the 25 foot timed walk (Figure 3).
Figure 3. MSFC components at screening and baseline visits.
There was consistent, significant improvement from screening visit 1 to baseline on both the 9-Hole Peg Test (p<0.0001) and the PASAT (p<0.0001) and the 25-foot timed walk was significantly improved at baseline over screening visits 1 and 2 (p=0.0053).
All participants had an MRI prior to study entry (Table 5). The average (median) total lesion volume was 12.2 (7.5) ml, the T2 hyperintense lesion volume was 10.5 (6.4) ml, and the T1 hypointense lesion volume was 1.7 (0.8) ml. Forty percent of participants had gadolinium enhancing (Gd) lesions at time of baseline MRI. For those with Gd lesions, the average (median) number of lesions was 4.3 (3) with a volume of 0.3 (0.1) ml.
3.3 Family history
A significant number of participants, 24.7%, had a family member with MS and 3.3% reported two or more affected relatives. The reported prevalence of MS in the mothers of participants was 3.5%. For other relatives, 8.6% of subjects reported a cousin with MS, 3.8% reported a sibling with MS, 4.1% reported an affected aunt, and 1.9% reported an affected uncle. These percentages are neither age-adjusted nor corrected for the number of relatives at risk.
4. Discussion
We have successfully enrolled a large cohort of MS patients previously untreated with IFNB or GA in an NIH funded trial. The patient population enrolled is representative of recently diagnosed MS patients in North America, and is similar to that of recent large pharmaceutical company sponsored studies of clinically isolated syndrome (CIS) (Comi et al., 2009; Kappos et al., 2006) or RRMS (Mikol et al., 2008; O'Connor et al., 2009). The mean age, disease duration, EDSS, and T2 lesion volume is slightly higher in this study compared to the CIS groups and very similar to the RRMS trials. The percent with Gd lesions on MRI is similar to both CIS and RRMS and the proportions of Caucasians and females are as expected. The results of this trial should be broadly applicable to early MS patients and useful for treatment decisions in clinical practice.
The MSFC has been developed as an outcome measure for clinical trials in MS (Cutter et al., 1999), and is included as one of our secondary endpoints. The MSFC is potentially more responsive and less subjective than the EDSS, but there are marked practice effects with significant improvement over the first assessments. Trials using the MSFC have included pre-baseline sessions to attenuate this practice effect, but the optimum number and scheduling for these sessions has not been determined. This study included two screening visits before baseline with the baseline visit occurring 7 to 45 days after the first screening visit. We observed substantial improvement between the first and second screening visits, with a smaller change between the second screening visit and baseline. The most noticeable changes were in the PASAT. These findings are concordant with results reported in other clinical trials (Cohen et al., 2008; Cohen et al., 2001).
The familial incidence reported here suggests these patients were drawn from a high risk population. The 3.5% prevalence of MS in mothers of patients is very close to the 3.7% rate reported in Canada, but is higher than the rate found in England or Australia (O'Gorman et al., 2011; Robertson et al., 1996; Sadovnick et al., 1988). We did not record the ages and total numbers of siblings and cousins, so the risk for those groups cannot be rigorously compared to previous studies. Reasonable estimates for the unknown data support the impression that the familial recurrence risk in this population is similar to that in Canada.
As the field of MS therapeutics evolves, it is possible that combination treatment will become increasingly common. An ideal combination would utilize two drugs, each of which is partially effective as monotherapy, with well-defined and complementary mechanisms of action, an additive or synergistic effect on disease, and differing or minimal adverse effects. The main attraction of combination treatment is the potential for increased efficacy, particularly for a disease such as MS, where existing treatments are only partially effective. The rationale for testing the combination of IFNB and GA was that both were partially effective, safe, and they likely had differing mechanisms of action.
In addition to the potential for greater benefit, combination therapy has potential pitfalls. The combination could cause an unexpected adverse effect or the two drugs could work against each other. For example, it would not make sense to combine a treatment which induces proliferation of regulatory leukocytes with a cytotoxic agent. In the CombiRx study, the two agents have multiple suggested mechanisms by which they might benefit MS, and it remains to be seen whether the combination will be additive, synergistic, or antagonistic. The pilot trial suggested that the combination would be safe and not antagonistic (Lublin et al., 2002), while in vitro and animal studies gave mixed results (Brod et al., 2000; Dhib-Jalbut et al., 2002; Milo and Panitch, 1995).
In addition to concerns about the usefulness of combined IFNB and GA in RRMS, practical barriers also exist. The requirement for two separate injection schedules can be problematic for patients and the high cost of the drugs places a burden on patients and their insurance. This type of study is very important to demonstrate if there is any added benefit and the magnitude of that benefit.
For combination treatment to be worthwhile, the combination should be more effective than either treatment given as monotherapy without a major increase in risk. The CombiRx study includes patients on IFNB monotherapy, GA monotherapy, and combined IFNB and GA. Many of the published studies of combination treatment in MS have only two groups—a standard treatment and the standard treatment in combination with a second agent. Such studies cannot be definitive, since it remains unknown whether the combination would be better than the second agent as monotherapy. Such a study design would be justifiable for a pilot study of a rescue therapy for active MS, but not for deciding optimum treatment for recently diagnosed patients.
In summary, we have successfully designed and enrolled a rigorous study of the combination of two of the existing first-line therapies for MS. This study was investigator initiated and managed, and NIH funded, with provision of medications by the pharmaceutical companies. The results will be available in 2012, and are eagerly awaited. The impact of this trial on clinical practice will depend on how the results of combination therapy compare to other treatments developed since this study was designed. Although efficacy and safety are our primary considerations when selecting therapy, the injection schedule for combination treatment may be a barrier to patient acceptance when oral treatments are available. Regardless of whether this particular combination proves beneficial, this study will serve as a model for future combination studies. It is also a model for future investigator driven studies of questions which are of great importance for MS treatment, but which lie outside the interests of any single pharmaceutical company.
MSARD Highlights.
Available therapies for MS are only partially effective.
Combining therapies might be more effective.
We compared the combination of interferon and glatiramer to either agent alone.
We present the baseline characteristics of our study population.
Acknowledgements
The study is funded by The National Institutes of Health, The National Institute of Neurological Disorders and Stroke (Phase III study: UO1 NS045719, planning grant: R21 NS41986) and is listed on www.clinicaltrials.gov NCT00211887. Materials were provided to the study by Biogen Idec and Teva Pharmaceutical. Contrast Letter Acuity funding provided by the National Multiple Sclerosis Society. We would like to thank Theresa McVie, MS for assistance with preparation of tables and data analysis; CCC, SDMC and MRI-AC staff; and the following site investigators and staff who have enrolled participants: M Agius, Sacramento CA; K Bashir, Birmingham AL; R Baumhefner, Los Angeles CA; G Birnbaum, Golden Valley MN; G Blevins, Edmonton AB Canada; R Bomprezzi, Phoenix AZ; A Boster, Columbus OH; T Brown, Kirkland WA; J Burkholder, Canton OH; A Camac, Lexington MA; D Campagnolo, Phoenix AZ; J Carter, Scottsdale AZ; B Cohen, Chicago IL; J Cooper, Berkeley CA; J Corboy Aurora CO; A Cross, Saint Louis MO; L Dewitt, Salt Lake City UT; J Dunn, Kirkland WA; K Edwards, Latham NY; E Eggenberger, East Lansing MI; J English, Atlanta GA; W Felton, Richmond VA; P Fodor, Colorado Springs CO; C Ford, Albuquerque NM; M Freedman, Ottawa Ontario Canada; S Galetta, Philadelphia PA; G Garmany, Boulder CO; A Goodman, Rochester NY; M Gottesman, Mineola NY; C Gottschalk, New Haven CT; M Gruental, Albany NY; M Gudesblatt, Patchogue NY; R Hamill, Burlington VT; J Herbert, New York NY; R Holub, Albany NY; W Honeycutt, Maitland FL; B Hughes, Des Moines IA; G Hutton, Houston TX; D Jacobs, Philadelphia PA; K Johnson, Baltimore MD; L Kasper, Lebanon NH; J Kattah, Peoria IL; M Kaufman, Charlotte NC; M Keegan, Rochester NY; O Khan, Detroit MI; B Khatri, Milwaukee WI; M Kita, Seattle WA; B Koffman, Toledo OH; E Lallana, Lebanon NH; N Lava, Albany NY; J Lindsey, Houston TX; P Loge, Billings MT; S Lynch, Kansas City KS; F McGee, Richmond VA; L Mejico, Syracuse NY; L Metz, Calgary AB Canada; P O'Connor, Toronto ON Canada; K Pandey, Albany NY; H Panitch, Burlington VT; J Preiningerova, New Haven CT; K Rammohan, Columbus OH; C Riley, New Haven CT; P Riskind, Worcester MA; L Rolak, Marshfield WI; W Royal, Baltimore MD; S Scarberry, Fargo ND; A Schulman, Richmond VA; T Scott, Pittsburgh PA; C Sheppard, Uniontown OH; W Sheremata, Miami FL; L Stone, Cleveland OH; W Stuart, Atlanta GA; S Subramaniam, Nashville TN; V Thadani, Lebanon NH; F Thomas, Saint Louis MO; B Thrower, Atlanta GA; M Tullman, New York NY; A Turel, Danville PA; T Vollmer, Phoenix AZ; S Waldman, La Habra CA; B Weinstock-Guttman, Buffalo NY; J Wendt, Tucson AZ; R Williams, Billings MT; D Wynn, Northbrook IL; M Yeung, Calgary AB Canada.
Abbreviations
- DES
Data Entry System
- EDSS
expanded disability status scale
- GA
glatiramer acetate
- IFNB
interferon-β1a
- MSFC
Multiple Sclerosis Functional Composite
- MSQLI
multiple sclerosis quality of life index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brod SA, Lindsey JW, et al. Combination therapy with glatiramer acetate (copolymer-1) and a type I interferon (IFN-alpha) does not improve experimental autoimmune encephalomyelitis. Ann Neurol. 2000;47:127–31. [PubMed] [Google Scholar]
- Cohen JA, Calabresi PA, et al. Avonex Combination Trial in relapsing--remitting MS: rationale, design and baseline data. Mult Scler. 2008;14:370–82. doi: 10.1177/1352458507083189. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Cutter GR, et al. Use of the multiple sclerosis functional composite as an outcome measure in a phase 3 clinical trial. Arch Neurol. 2001;58:961–7. doi: 10.1001/archneur.58.6.961. [DOI] [PubMed] [Google Scholar]
- Comi G, Martinelli V, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1503–11. doi: 10.1016/S0140-6736(09)61259-9. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cutter GR, Baier ML, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(Pt 5):871–82. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S, Chen M, et al. Effect of combined IFNbeta-1a and glatiramer acetate therapy on GA-specific T-cell responses in multiple sclerosis. Mult Scler. 2002;8:485–91. doi: 10.1191/1352458502ms862oa. [DOI] [PubMed] [Google Scholar]
- Ford C, Goodman AD, et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis : results from the 15-year analysis of the US prospective openlabel study of glatiramer acetate. Mult Scler. 2010;16:342–50. doi: 10.1177/1352458509358088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–94. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Brooks BR, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45:1268–76. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- Kappos L, Polman CH, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–9. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- Lublin F, Baier M, et al. Results of the extension of a trial to assess the longer term safety of combining interferon beta-1a and glatiramer acetate. Neurology. 2002;58(Suppl. 3):A85. [Google Scholar]
- McDonald WI, Compston A, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Mikol DD, Barkhof F, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008 doi: 10.1016/S1474-4422(08)70200-X. [DOI] [PubMed] [Google Scholar]
- Milo R, Panitch H. Additive effects of copolymer-1 and interferon beta-1b on the immune response to myelin basic protein. J Neuroimmunol. 1995;61:185–93. doi: 10.1016/0165-5728(95)00085-g. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, et al. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- O'Connor P, Filippi M, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8:889–97. doi: 10.1016/S1474-4422(09)70226-1. [DOI] [PubMed] [Google Scholar]
- O'Gorman C, Freeman S, et al. Familial recurrence risks for multiple sclerosis in Australia. J Neurol Neurosurg Psychiatry. 2011;82:1351–4. doi: 10.1136/jnnp.2010.233064. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Poonawalla AH, Datta S, et al. Composite MRI scores improve correlation with EDSS in multiple sclerosis. Mult Scler. 2010;16:1117–25. doi: 10.1177/1352458510374892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser CM, Paty DW, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Robertson NP, Fraser M, et al. Age-adjusted recurrence risks for relatives of patients with multiple sclerosis. Brain. 1996;119(Pt 2):449–55. doi: 10.1093/brain/119.2.449. [DOI] [PubMed] [Google Scholar]
- Sadovnick AD, Baird PA, et al. Multiple sclerosis: updated risks for relatives. Am J Med Genet. 1988;29:533–41. doi: 10.1002/ajmg.1320290310. [DOI] [PubMed] [Google Scholar]
- Tullman MJ, Lublin FD. Combination therapy in multiple sclerosis. Curr Neurol Neurosci Rep. 2005;5:245–8. doi: 10.1007/s11910-005-0053-9. [DOI] [PubMed] [Google Scholar]
- Wolinsky JS, Narayana PA, et al. Linomide in relapsing and secondary progressive MS: part II: MRI results. MRI Analysis Center of the University of Texas-Houston, Health Science Center, and the North American Linomide Investigators. Neurology. 2000;54:1734–41. doi: 10.1212/wnl.54.9.1734. [DOI] [PubMed] [Google Scholar]