Abstract
The prognosis of patients diagnosed with malignant gliomas including glioblastoma multiforme (GBM) is poor and there is an urgent need to develop and translate novel therapies into the clinic. Neural stem cells display remarkable tropism toward GBMs and thus may provide a platform to deliver oncolytic agents to improve survival. First we provide a brief review of clinical trials that have used intra-tumoral herpes simplex virus thymidine kinase (HSV/tk) gene therapy to treat brain tumors. Then, we review recent evidence that neural stem cells can be used to deliver HSV/tk to GBMs in animal models. While previous clinical trials used viruses or non-migratory vector-producing cells to deliver HSV/tk, the latter approaches were not effective in humans, primarily because of satellite tumor cells that escaped surgical resection and survived due to low efficiency delivery of HSV/tk. To enhance delivery of HSV/tk to kill gliomas cells, recent animal studies have focused on the ability of neural stem cells, transduced with HSV/tk, to migrate efficiently and selectively to regions occupied by GBM cells. This approach holds the promise of targeting GBM cells that have infiltrated the brain well beyond the original site of the tumor epicenter.
Keywords: Glioblastoma multiforme, tropism, neural stem cells, bystander effect, Herpes Simplex Virus/thymidine kinase
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary central nervous system tumor in adults. GBMs are associated with a shortened life expectancy and high levels of morbidity [1]. The incidence of GBM is approximately 13,000 cases per year with a median survival of approximately 12 months [2]. Conventional treatments including surgical resection, irradiation and chemotherapy may extend survival by weeks, but GBMs are notoriously resistant to adjuvant therapies [3]. Infiltrative GBM cells that escape the surgical debulking of the tumor account for tumor recurrence in virtually all cases. This infiltrative nature allows some cells to migrate outside the range of local treatments such as surgery and radiation therapy. Some factors that may contribute to GBM resistance to therapy include: expression of multidrug resistence genes, the inability of many chemotherapeutic drugs to cross the blood-brain barrier, the narrow therapeutic window between toxicity and efficacy for radiation and chemotherapy, and the continual repopulation of the tumor by aberrant tumor stem cells from the subventricular zone. The infiltrative GBM cells are highly resistant to treatment so that new therapies must target malignant behaviors of these cells whether they reside at the edge of the surgical cavity or have invaded one or both hemispheres.
Recent in vitro and in vivo studies highlight the ability of neural stem cells to migrate selectively to brain tumors and to factors secreted by gliomas and cells in their microenvironment [4–8]. The tropic properties of stem cells, specifically that of neural stem cells (NSCs) [9,10] to target brain tumors (i.e. gliomatropism), suggest that they could serve a role in delivering cytotoxic therapies to gliomas. However, this approach requires a better understanding of migratory NSCs, their derivation, administration, and application techniques before it can become useful clinically. It will be important to characterize the cellular phenotypes of embryonic stem (ES) cell-derived NSC preparations and endogenous NSC’s for more predictable outcomes. Assessing the migratory potential of the sub-population of progenitor cells derived from the NSC’s will ensure reproducibility when considering candidates for optimal drug delivery in vivo. Several groups have shown that the cells derived from ES cells after neural induction are a mixture of true NSCs and progeny of NSCs [9,11]. In fact, ES cell-derived NSCs and subventricular zone (SVZ)-derived NSCs experience different developmental cues and therefore result in many cellular phenotypes, including highly differentiated cells.
Due to their gliomatropic behavior, NSCs are considered advantageous for delivering anti-tumor agents as an adjuvant for cancer therapy. Various prodrug systems have been designed in which a non-toxic compound (prodrug) is activated within the tissues to become a cytolytic agent when acted upon by a converting enzyme. Examples of prodrug/enzyme gene therapy methods, some of which have employed NSCs as a converting enzyme vehicle for intracranial and disseminated tumor treatment, include 5-fluorocytosine/cytosine deaminase, Camptothecin-11/rabbit carboxylesterase, and Ganciclovir (GCV)/Herpes Simplex Virus-thymidine kinase (HSV/tk) [4–6,12–14]. In this review, we focus on HSV/tk/GCV and on the potential use of NSCs as vehicles to deliver chemotherapy directly to brain tumor cells.
HSV/TK/GCV – MECHANISMS OF ACTION
The HSV/tk targeted gene therapy approach for treating malignant gliomas is a well-characterized gene therapy [15–19]. It is known as a suicide gene therapy because upon exposure to GCV it leads to a cascade of events resulting in death of rapidly dividing cells transduced with HSV/tk and the rapidly dividing bystander cells (see below). This form of gene therapy was developed as a human cancer therapy because of its efficacy in preclinical studies [12,20]. The key enzyme, thymidine kinase, has a crucial function in DNA synthesis and is therefore exploited in anti-viral drugs designed to target constantly replicating viruses. Previous therapies involving HSV/tk for cancer treatment used retro-viruses to insert the HSV/tk DNA directly into the genome of cancer cells.
In normal cells, tk functions by catalyzing the phosphorylation of thymidine to produce deoxythymidine mono-, di-, and tri- phosphate (dTMP, dTDP, dTTP), and dTTP inserts itself into nascent DNA during cell division [21]. Unlike human tk, HSV/tk has broad substrate specificity with the ability to phosphorylate pyrimidines (thymidine), pyrimidine analogs (such as azidothymidine or AZT, the popular drug used to combat HIV), purines (guanosine), and purine analogs (such as GCV). Ganciclovir is a commonly used drug for treatment of Herpes infection and is used in combination with the HSV/tk method for cancer therapy as the prodrug. GCV is administered intravenously and freely diffuses across the blood-brain-barrier. Initially, the non-toxic nucleoside analog GCV is referred to as the prodrug, that upon phosphorylation by the enzyme HSV/tk produces a cytotoxic metabolite that accumulates in the cell [20,21].
STEM CELLS AS THERAPEAUTIC PLATFORMS TO DELIVER ONCOLYTIC AGENTS
Neural stem cells are primitive, self-renewing cells that can differentiate to form mature cells of neuronal or glial lineage. Neural stem cells can be obtained from neurogenic regions in the brain such as the subventricular zone (SVZ) or can be derived from induced embryonic stem cells [22]. Neural stem cells are considered to be good candidates as therapeutic vectors for malignant cancer therapy because they possess a number of unique characteristics. As mentioned earlier, it is critically important to identify the migrating population of NSCs, presumably the responsive, therapeutic population, to deliver oncolytic factors to gliomas. The cells observed migrating may not be definitive NSCs, rather they are likely a mixture of definitive NSCs and their progeny. However, in this review we refer to them collectively as NSCs.
Perhaps the most important feature of NSCs in this context is a large migratory capacity that enables them to traverse brain hemispheres. This is a crucial property when considering a cell-based therapeutic method capable of ‘tracking down’ diffuse infiltrative gliomas such as GBM satellite cells by responding to cues from extracellular matrix components, adhesion molecules, and chemokines [10,23]. This ability of transplanted NSCs to migrate towards contralateral tumor insults in the brain has been demonstrated in many studies and was first observed in rat models by Snyder and colleagues [4]. Since then, many stem cell types including embryonic, mesenchymal and neural stem cells have shown glioma tropism in the central nervous system (CNS) as well as the ability to deliver prodrug/enzyme therapies to cancers [4,23–26].
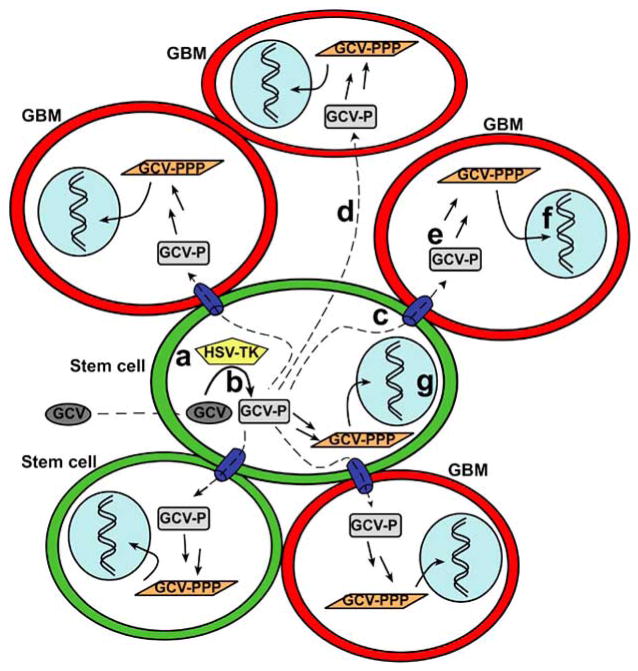
The use of stem cells as therapeutic platforms, requires effectors and target cells to be contiguous since the HSV/tk protein in transduced stem cells converts a prodrug to an oncolytic factor, which is transferred from the stem cell to tumor cells via gap junctions. This is known as the “bystander effect” (Fig. 1). After migration to the glioma, the HSV/tk transduced stem cells form gap-junction channels with adjacent tumor cells, and the gap junctions enable transfer of phosphorylated GCV metabolites from one cell to the other [18,27]. Gap junction formation provides adhesive contacts important for neuronal migration and has been implicated in modulating the adhesiveness and invasion of malignant gliomas [28,29]. Regarding the HSV/tk gene therapeutic approach, intercellular communication via gap junctions has been linked to a strong tumoricidal bystander effect, particularly involving connexin 43 [30].
Fig. 1. Suicide gene therapy and the bystander effect.
(a) Transduced stem cells (green) that express constitutively Herpes Simplex Virus thymidine kinase (HSV-TK) migrate to the vicinity of GBM cells (red). (b) Upon systemic administration of the non-toxic, antiviral drug ganciclovir (GCV), GCV is mono-phosphorylated (GCV-P) by the viral TK enzyme in the stem cells. (c) Gap junctions present between cells facilitate the passive transport of GCV-P to adjacent non-transduced target GBM cells. (d) GCV-P may also be transported to GBM cells via a gap junction-independent pathway. (e) Endogenous mammalian kinases within the GBM cells further phosphorylate GCV-P to its toxic product (GCV-PPP). (f, g) GCV-PPP acts as a purine analog incorporating itself into nascent DNA where it terminates replication, resulting in apoptosis of rapidly dividing cells. The death of non-transduced cells due to the transfer of an oncolytic agent is referred to as the bystander effect and in conjunction with concurrent suicide of transduced cells by the same mechanism involving GCV-PPP, is collectively known as suicide gene therapy. Figure adapted from Hurwitz et al. 2003 [16].
The specific mechanisms whereby GCV/tk delivered by stem cells kills rapidly dividing cells are as follows. Initially, the HSV/tk present in the stem cells converts GCV into monophosphorylated GCV-P that then traverses into neighboring cells through the gap junctions [30]. Alternatively, it is possible for GCV-P to be transferred by efflux from HSV/tk-expressing cells with subsequent uptake by neighboring cells in a gap junction independent manner [31]. After GCV-P is transferred, it is further phosphorylated by host cell thymidine kinase to create the cytotoxic metabolite GCV-triphosphate (GCV-PPP). The GCV-PPP competitively inhibits incorporation of deoxyguanosine triphosphate (dGTP), and cells undergoing DNA replication (e.g., rapidly dividing glioma cells) integrate GCV-PPP into newly synthesized DNA, causing premature chain termination that leads to cell death via apoptosis [12,17,32].
Throughout adulthood in rodents, neuroblasts originating from the SVZ migrate along the rostral migratory stream and enter the olfactory bulb [9,33]. However, in response to cortical injury, SVZ-derived neuroblasts can migrate in many directions through gray matter and sub-cortical white matter, including regions of the cerebral cortex and the corpus callosum and to subcortical structures such as the striatum, septum, thalamus and hypothalamus [33,34]. Regarding experimental gliomas, Glass and colleagues observed that endogenous SVZ neural precursor cells migrate toward a glioblastoma graft. To assess the functional characteristics of the neural precursors in the tumor margin, whole-cell voltage-clamp recordings revealed outward current patterns similar to type 2 neural progenitor cells in the hippocampus [35]. Choosing the appropriate cell type that is suitable for engrafting, navigating, and perhaps integrating into a neural environment is essential for delivering chemotherapy to the GBM in the brain parenchyma. However, little is known of NSC migration in the human brain, so conclusions based on rodent studies should be regarded as indicative but not definitive.
NEURAL STEM CELL HOMING & PATHOTROPISM
The innate attraction and migration to areas of inflammation due to CNS pathologies such as brain tumors make NSCs unique candidates for therapeutic platforms. Therefore, several preclinical studies have explored the use of NSCs as drug-delivery vehicles to treat brain cancer [4,6,36]. The chemokines and receptors that direct NSC pathotropism are not defined completely but involve signals released at the site of insult (cancer, lesion, etc.) and transient reparative signals expressed by cells in the microenvironment [10,37]. In response to inflammatory signals released by microglia, activated astrocytes secrete chemokines that act as chemoattractants for NSC’s [10]. Some chemoattractants that may induce NSC tropism include stem cell factor (SCF), stromal cell derived factor-1 (SDF-1), vascular endothelial growth factor (VEGF), fractalkine, monocyte chemoattractant protein 1 (MCP-1), hepatocyte growth factor (HGF), and many of the CXC motif chemokines [10,37–39].
Stem cell tropism towards various types of cancers involves different subsets of stem cells and occurs following xenotransplantation of stem cells [23–25]. Cross-species interactions may be an important point when considering treatment and tolerance in humans; for instance, human clinical trials have tested mouse fibroblasts as drug delivery biopumps, as described later.
We examined cross-species interactions using ex vivo post-natal day 10 rat organotypic brain slices as a model system for migration. Neuralized mouse embryonic stem cells that were implanted on the slices, migrated and co-localized with human glioblastoma cells (implanted at the same time as the neuralized stem cells but on contralateral sides of the slices) within one-week (Fig. 2). This migration of murine stem cells toward human glioma cells on ex vivo rat slices is similar to in vivo results of transplanted syngeneic NSC migration to induced brain tumors in rodent models [40]. In both in vivo and ex vivo studies, transplanted stem cells surrounded the tumor graft and were not detectable in other areas of the brain, suggesting a very directed migration of the stem cells that resulted in co-localization with the glioma cells (Fig. 2D). We do not know the specific chemoattractants that acted on the implanted cells, but this well-orchestrated movement illustrates the compatibility of signaling mechanisms between species (albeit in the absence of an immune response in these ex vivo studies). Although the transfer of agents between implanted NSCs and glioma cells was not established in our experiments (Figs. 2, 3), the observed tumor infiltration and contiguity of glioma and implanted stem cells is necessary to confer the bystander effect.
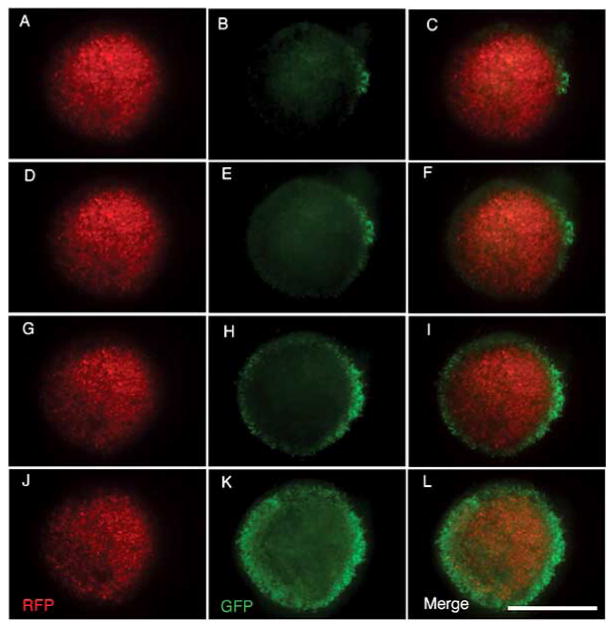
Fig. 2. GFP-expressing neuralized mouse embryonic stem cells (nESCs) co-localize with RFP-expressing human tumor cells in an organotypic brain slice.
Stereoscopic epifluorescent images show tumor mass infiltration by nESCs. GFP-expressing nESCs and RFP-expressing human glioma cells were introduced on the surface of the organotypic rat brain slice. The living brain section was obtained from a 300-micron thick coronal slice just anterior to bregma obtained from a fresh rat brain embedded in agarose and cut with a vibratome. Initially, 10 μl (~8,000 cells/μl) of both cell types were simultaneously implanted at separate locations on the surface of a one-week old slice. Aliquots of the two cell types were applied approximately 10 mm from each other across the width of the organotypic slice. Images shown here were taken one week after implantation of the nESCs and glioma cells. (A–D) Stereomicroscopic epifluoresent images show that stem cells placed distant from the tumor cells co-localized with tumor cells and were not detectable in other regions of the brain slice. Scale bars = 1 mm; Scale bar in C applies to A–C.
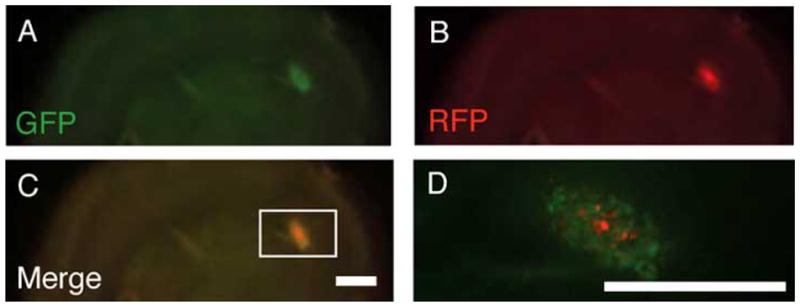
Fig. 3. Time course of tumor infiltration by neuralized embryonic stem cells on an organotypic brain slice.
These stereoscopic epifluorescent images show the progression of tumor mass infiltration by nESCs over the course of a several weeks in an organotypic brain slice culture. GFP-expressing nESCs and RFP-expressing human glioma cells were introduced on the surface of an organotypic rat brain slice as described in Fig. 2. (A–C) Starting at 2 weeks post-implantation, nESCs are found at the tumor mass. At 3 weeks (D–F) and 4 weeks (G–I) post-implantation, the tumor mass becomes infiltrated progressively by nESCs. At 6 weeks (J–L), the tumor mass becomes encapsulated completely by the stem cells, suggesting contiguity between nESCs and glioma cells. Scale bar = 1 mm; Scale bar applies to all panels.
It will be of interest to determine the specific mechanisms that lead to stem cell proliferation and migration (and potential differentiation) when attacking brain tumors. For instance, further studies are needed to analyze whether the stem/progenitor cells proliferate prior to migration, or whether the tumor microenvironment influences cell migration prior to proliferation. Molecular and cellular mechanisms underlying stem/progenitor cell mobilization are relatively unknown and understanding how these cells mobilize may improve strategies for optimizing where and when to administer the cells, and in the context of GCV/tk, how to improve GCV administration for more efficient bystander killing.
Our migration data illustrate that ex vivo culturing provides the opportunity to view cells in a neural environment that closely mimics in vivo conditions, a limitation that in vitro migration assays have yet to achieve. Organotypic cultures further highlight the potent ability of signaling molecules to regulate stem cell migration, and suggest the conservation of stem cell and glioma cell signaling molecules across species (i.e., human and mouse). This may also prove to be a useful model system for investigating pathotropism and for testing various types of stem cells as potential delivery vehicles for prodrug/enzyme therapies.
ADVANCES IN PRE-CLINICAL AND CLINICAL STUDIES
Development of a GCV/tk mediated gene therapy has been a focus of cancer research for many years with the central issue being the effectiveness of transgene delivery to non-transduced cancer cells. In the past, viral vectors have been used predominantly to deliver gene products directly to tumor cells but have met with limited success. Therefore, novel strategies employing cell-mediated delivery of GCV/tk are being investigated.
There has been success in developing and characterizing neural stem cells as vehicles to deliver GCV/tk for cancer gene therapy [6]; recently, pre-clinical studies have reported substantial progress in eliminating brain tumors in rat models. Li and colleagues [6] demonstrated complete elimination of glioma xenografts by HSV/tk engineered neural stem cells in combination with GCV treatment (termed NSC/tk therapy). This study reported that NSC/tk therapy completely abolished tumors that were induced 7 days prior to the intra-tumoral administration of NSCs, and remarkably, rodents remained glioma-free for up to 10 weeks before sacrifice. These encouraging findings show the potential of NSC/tk therapy even though direct intra-tumoral delivery of HSVtk-producing fibroblast vector producer cells was ineffective in human clinical trials. Nonetheless, these exciting results still leave one of the most important questions regarding the feasibility of this application unanswered before translating into clinical trials: Will NSC/tk stem cell therapy target infiltrative GBM cells at some distance from the tumor’s epicenter? Since xenografts, including human orthotopic xenografts, form relatively well-circumscribed GBMs, genetic mouse models that result in spontaneous tumor occurrence may be better suited to addressing this important question.
In a Phase III clinical trial, fibroblasts were used to deliver HSV/tk intra-tumorally to GBM patients as an adjuvant to conventional therapy (surgical resection and radiotherapy) [41]. In this multi-center controlled trial, randomized patients received vector-producing mouse fibroblasts cells (cell line PA317) engineered to deliver the HSV/tk gene in a retrovirus-mediated fashion to tumor cells. The vector producer cells were injected into the wall of the cavity formed by surgical tumor debulking. Study patients then received intravenous GCV and radiation versus radiation alone. Unfortunately median patient survival was not significantly different compared to the radiation-treated control group. Most of the recurrences were adjacent to the wall of the cavity and in patients who succumbed before they were treated with GCV, vector producer cells were seen at autopsy within a few millimeters of needle tracks. It is not known whether the outcome resulted from the use of murine-derived cells, non-migratory fibroblasts, or was a combination of various technical factors. Before an NSC/tk therapy can be employed in a human clinical trial, preclinical testing will need to be completed in genetic mouse models of GBM or human orthotopic xenografts that more closely mimic the human disease. After GCV administration, it is expected that all the transplanted tk+ stem cells and the targeted tumor cells would succumb to self-induced death. Ideally, GCV will need to be administered when the maximum number of stem cells have homed to GBM cells within the brain.
CONCLUSIONS
Clinical studies support the feasibility and biosafety of an HSV/tk-based gene therapy. It remains to be determined in humans if efficient gene transfer to tumor cells can be achieved using stem cells as a delivery platform. Additional research is required to elucidate the utility of transplanted stem cells, before neural, mesenchymal or embryonic cells can be used safely and effectively in the clinical setting. Neural stem cells transduced with HSV/tk combined with GCV administration provide a promising new treatment option that may one day extend significantly the life of patients with GBM.
Acknowledgments
This work was supported by the National Institutes of Health (NS045813 to MDK) and a Flo-Dickey Funk Fellowship (to PR). The authors especially thank Dr. Joseph Loftus at the Mayo Clinic Scottsdale, AZ for providing the SF767 cell line. The authors thank Laura Engel, Jason Kern, Jason Morrison and the Molecular Cytology Core at the University of Missouri for experimental support.
References
- 1.Hassler M, Seidl S, Fazeny-Doerner B, et al. Diversity of cytogenetic and pathohistologic profiles in glioblastoma. Cancer Genet Cytogenet. 2006;166:46–55. doi: 10.1016/j.cancergencyto.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 2.CBTRUS. Statistical report: primary brain tumors in the United States, 1998–2002. 2005. Published by the Central Brain Tumor Registry of the United States. [Google Scholar]
- 3.Noble M. Can neural stem cells be used to track down and destroy migratory brain tumor cells while also providing a means of repairing tumor-associated damage? Proc Natl Acad Sci USA. 2000;97:12393–5. doi: 10.1073/pnas.97.23.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barresi V, Belluardo N, Sipione S, Mudo G, Cattaneo E, Condorelli DF. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;10:396–402. doi: 10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 7.Serfozo P, Schlarman MS, Pierret C, Maria BL, Kirk MD. Selective migration of neuralized embryonic stem cells to stem cell factor and media conditioned by glioma cell lines. Cancer Cell Int. 2006;6:1. doi: 10.1186/1475-2867-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol Ther. 2005;11:926–31. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 10.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 11.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Neural differentiation of mouse embryonic stem cells in vitro and after transplantation into eyes of mutant mice with rapid retinal degeneration. Brain Res. 2004;1014:131–44. doi: 10.1016/j.brainres.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Boucher PD, Im MM, Freytag SO, Shewach DS. A novel mechanism of synergistic cytotoxicity with 5-fluorocytosine and ganciclovir in double suicide gene therapy. Cancer Res. 2006;66:3230–7. doi: 10.1158/0008-5472.CAN-05-3033. [DOI] [PubMed] [Google Scholar]
- 13.Danks MK, Yoon KJ, Bush RA, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 14.Herrlinger U, Woiciechowski C, Sena-Esteves M, et al. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1:347–57. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 15.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–2. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz RL, Chevez-Barrios P, Boniuk M, Chintagumpala M, Hurwitz MY. Retinoblastoma: from bench to bedside. Expert Rev Mol Med. 2003;5:1–14. doi: 10.1017/S1462399403005520. [DOI] [PubMed] [Google Scholar]
- 17.Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990;82:297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- 18.Namba H, Iwadate Y, Kawamura K, Sakiyama S, Tagawa M. Efficacy of the bystander effect in the herpes simplex virus thymidine kinase-mediated gene therapy is influenced by the expression of connexin43 in the target cells. Cancer Gene Ther. 2001;8:414–20. doi: 10.1038/sj.cgt.7700317. [DOI] [PubMed] [Google Scholar]
- 19.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–61. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 20.Kokoris MS, Black ME. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganci-clovir or acyclovir activity. Protein Sci. 2002;11:2267–72. doi: 10.1110/ps.2460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reardon JE. Herpes simplex virus type 1 and human DNA polymerase interactions with 2′-deoxyguanosine 5′-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989;264:19039–44. [PubMed] [Google Scholar]
- 22.Alvarez-Buylla A, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Research Bulletin. 2002;57:751–78. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava AS, Shenouda S, Mishra R, Carrier E. Transplanted embryonic stem cells successfully survive, proliferate, and migrate to damaged regions of the mouse brain. Stem Cells. 2006;24:1689–94. doi: 10.1634/stemcells.2005-0531. [DOI] [PubMed] [Google Scholar]
- 24.Aboody KS, Bush RA, Garcia E, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 26.Chen HI, Bakshi A, Royo NC, Magge SN, Watson DJ. Neural stem cells as biological minipumps: a faster route to cell therapy for the CNS? Curr Stem Cell Res Therapy. 2007;2:13–22. doi: 10.2174/157488807779317044. [DOI] [PubMed] [Google Scholar]
- 27.Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93:1831–5. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–7. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 29.Lin JH, Takano T, Cotrina ML, et al. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302–11. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanson M, Marcaud V, Robin E, Valery C, Sturtz F, Zalc B. Connexin 43-mediated bystander effect in two rat glioma cell models. Cancer Gene Ther. 2002;9:149–55. doi: 10.1038/sj.cgt.7700411. [DOI] [PubMed] [Google Scholar]
- 31.Drake RR, Pitlyk K, McMasters RA, Mercer KE, Young H, Moyer MP. Connexin-independent ganciclovir-mediated killing conferred on bystander effect-resistant cell lines by a herpes simplex virus-thymidine kinase-expressing colon cell line. Mol Ther. 2000;2:515–23. doi: 10.1006/mthe.2000.0192. [DOI] [PubMed] [Google Scholar]
- 32.Beltinger C, Fulda S, Kammertoens T, Meyer E, Uckert W, Debatin KM. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc Natl Acad Sci USA. 1999;96:8699–704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 34.Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 35.Glass R, Synowitz M, Kronenberg G, et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–46. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003;14:1247–54. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 37.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendall SE, Najbauer J, Johnston HF, et al. Neural Stem Cell Targeting of Glioma is Dependent on PI3K Signaling. Stem Cells. 2008;26(6):1575–86. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 39.Yip S, Sabetrasekh R, Sidman RL, Snyder EY. Neural stem cells as novel cancer therapeutic vehicles. Eur J Cancer. 2006;42:1298–308. doi: 10.1016/j.ejca.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Potent bystander effect in suicide gene therapy using neural stem cells transduced with herpes simplex virus thymidine kinase gene. Oncology. 2005;69:503–8. doi: 10.1159/000091032. [DOI] [PubMed] [Google Scholar]
- 41.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]