Abstract
Objectives
The purpose of this study was to assess the prevalence of the re-entry circuit within the interventricular septum in post-infarction patients referred for ventricular tachycardia (VT) ablation.
Background
Post-infarction ventricular tachycardia can involve the endocardial myocardium, the intramural myocardium, the epicardium, or the His Purkinje system.
Methods
Among 74 consecutive patients with recurrent post-infarction VT, 33 patients (45%) were identified in whom the critical part of the VT involved the interventricular septum. A total of 206 VTs were induced in these 33 patients. In 46 of the 206 VTs, a critical component was identified in the interventricular septum. The critical isthmus of the re-entry circuit was identified by entrainment mapping, activation mapping, or pace-mapping.
Results
In 32 of 46 VTs (70%), the critical component of the re-entry circuit was confined to the endocardium. In 9 of 46 VTs (20%), the critical component involved the Purkinje system, and in 5 of 46 VTs (11%), an intramural area was critical. Entrainment and/or pace-mapping helped to identify critical areas of endocardial VTs as well as VTs involving the Purkinje fibers, but neither of these mapping techniques localized intramural VTs. Electrocardiographic characteristics were specific for each of the septal locations. All VTs mapped to the interventricular septum were acutely successfully ablated. VTs recurred in 9 of 33 patients with septal VTs during a mean follow-up period of 40 ± 20 months.
Conclusions
Post-infarction VT involving the interventricular septum can involve the endocardial muscle, Purkinje fibers, or intramural muscle fibers. Electrocardiographic characteristics differ depending on the type of tissue involved.
Keywords: post-infarction, septum, ventricular tachycardia
The septum is frequently involved in myocardial infarction and contains myocardial and Purkinje fibers that both can give rise to ventricular arrhythmias (1–3). The purpose of this study was to characterize post-infarction ventricular tachycardias (VTs) involving the interventricular septum.
Methods
Patient characteristics
The subjects of this study were 33 consecutive patients (30 men; mean age 64 ± 9 years; ejection fraction 0.29 ± 0.15) referred for radiofrequency ablation of recurrent VT in whom the left ventricular septum was demonstrated by entrainment mapping, activation mapping, or pace-mapping to be critically involved in the re-entry circuit. The patients were selected from a consecutive group of 74 patients who were referred for ablation of post-infarction VT. All patients had a history of ≥1 myocardial infarction (anterior in 15, inferior in 14, and both anterior and inferior in 4). Catheter ablation was performed because of recurrent VT causing frequent implantable cardioverter-defibrillator (ICD) discharges in 20 patients and because of recurrent VT in 13 patients. All patients had failed therapy with ≥1 antiarrhythmic drug, including amiodarone in 21 of 33 patients. Patient characteristics are given in Table 1.
Table 1.
Characteristics of Patients With and Without Evidence of Septal VTs
| Nonseptal VTs (n = 41) | Septal VT (n = 33) | p Value | |
|---|---|---|---|
| No. of induced VTs (exit site identified) | 248 (172) | 160 | — |
| No. of septal VTs | — | 46 | — |
| Age, yrs | 69 ± 10 | 64 ± 9 | 0.03 |
| Women/men | 7/34 | 4/29 | NS |
| Ejection fraction | 30 ± 11 | 29 ± 15 | NS |
| QRS during VT, ms (exit site identified) | 207 ± 38 (203 ± 38) | 187 ± 45 | 0.003 (0.009) |
| VT morphology | |||
| LBBB (exit site identified) | 71 (51) | 21 | 0.03 |
| RBBB (exit site identified) | 177 (121) | 25 | (0.04) |
| VT cycle length, ms (exit site identified) | 370 ± 93 (386 ± 101) | 404 ± 100 | 0.02 (NS) |
| Infarct localization | 0.003 | ||
| Inferior | 33 | 18 | |
| Anterior | 8 | 19 | |
| Low-voltage area (−1.0 mV), cm2 | Median = 73; IQR = 45 | Median = 90; IQR = 86 | 0.10 |
| Coronary artery related to infarction | <0.0001 | ||
| LAD | 8 | 19 | |
| LCx | 19 | 0 | |
| RCA | 16 | 18 | |
Values are n (%), mean ± SD, or n, except for the low-voltage area, which was presented as median with interquartile range (IQR) because data were not normally distributed. Nonseptal ventricular tachycardias (VTs): no evidence of septal involvement based on pace-mapping or entrainment mapping.
LAD = left anterior descending artery; LBBB = left bundle branch block; LCx = left circumflex artery; RBBB = right bundle branch block; RCA = right coronary artery; VT = ventricular tachycardia.
A total of 206 VTs (VT cycle length, 375 ± 98 ms) were induced in these patients. Forty-six VTs (1.4 VTs per patient, VT cycle length: 404 ± 100 ms) were mapped to the interventricular septum (Figs. 1A to 1C). Based on far-and near-field ICD electrograms and available 12-lead electrocardiogram recordings (4), 21 of the VTs were clinically relevant in that they caused either recurrent VT or recurrent ICD discharges. All VTs were mapped and targeted for ablation. Twenty-one VTs had a left bundle branch block morphology, and 25 had a right bundle branch block morphology.
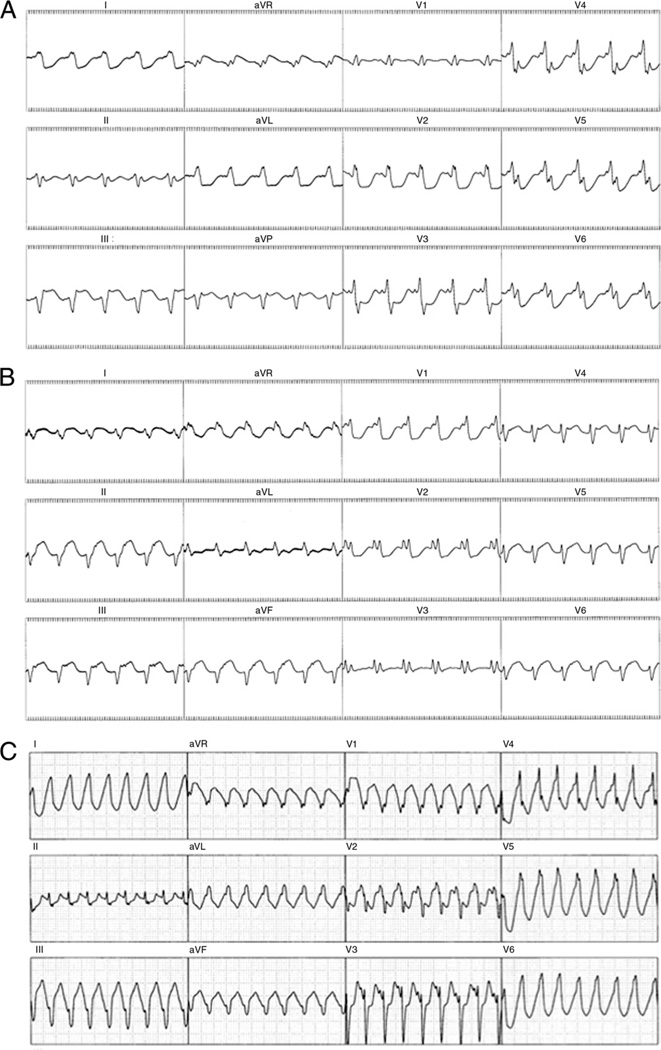
Figure 1. 12-Lead Electrocardiogram of 3 Different Ventricular Tachycardias.
(A) Ventricular tachycardia involving the left ventricular endocardium. (B) Ventricular tachycardia involving the Purkinje system.
(C) Ventricular tachycardia involving the intramural septum.
Electrophysiologic study and mapping
After informed consent was obtained, 2 multipolar electrode catheters were introduced into a femoral vein and positioned in the right ventricular apex and the His bundle position. Programmed right ventricular stimulation was performed using 1 to 4 extrastimuli (5). Left ventricular mapping was performed using femoral artery access and a retrograde aortic approach. In 3 patients, a transseptal approach was performed due to aortic valve replacement, aortic atheromas, or peripheral vascular disease. An electroanatomic mapping system (CARTO, Biosense Webster, Inc., Diamond Bar, California) was used in all patients with a 7-F mapping/ablation catheter that had a 4-mm tip and a 2-mm ring electrode separated by 1 mm or an irrigated 3.5-mm-tip catheter (Thermocool, Biosense Webster, Inc.). Electrograms were filtered at 50 to 500 Hz. The intracardiac electrograms and leads V1, I, II, and III were displayed on an oscilloscope and recorded at a speed of 100 mm/s. The recordings were stored on optical disk (EP Med Inc., New Berlin, New Jersey). Systemic heparinization was maintained throughout the procedure to maintain an activated clotting time of >250 s.
Mapping protocol
A voltage map was generated during sinus rhythm. An electrogram amplitude <1.0 mV was defined as low voltage (6,7). The degree of septal involvement was expressed as the area and percentage of septal involvement (Fig. 2). If a VT was hemodynamically tolerated, activation mapping was performed (Fig. 2). During the activation map, entrainment mapping was performed to identify critical sites of the re-entry circuit (8). A mean of 114 ± 86 sites was collected to construct the activation maps. Entrainment mapping was performed at all sites displaying abnormal electrograms and at sites of earliest endocardial activation. In patients with tolerated VTs in whom the earliest activation was located at the left ventricular septum (Fig. 3A), activation mapping was performed from the right ventricular side to assess the site of earliest activation (Figs. 3B and 3C). Entrainment mapping was repeated at the site of earliest right ventricular activation. Sites were assessed for the presence of Purkinje potentials during VT and sinus rhythm.
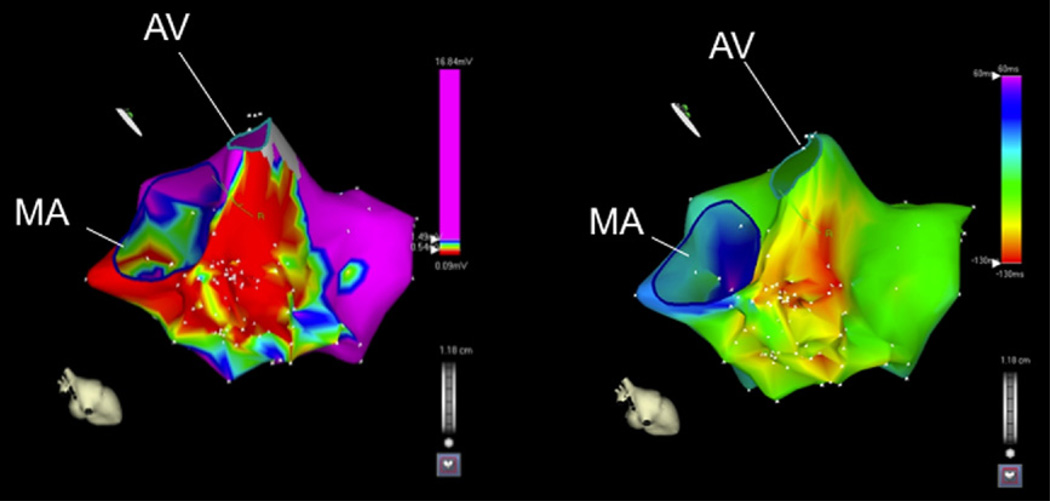
Figure 2. Voltage Sinus Rhythm Map and Activation Map of an Intramural Ventricular Tachycardia.
(A) Voltage map of the left ventricle in a patient with a previous inferoseptal myocardial infarction. The area of low voltage includes mainly the basal left ventricular septum. This patient had an intramural ventricular tachycardia circuit. (B) Activation map during ventricular tachycardia indicating that the earliest area of activation is in the basal left ventricular septum. AV = aortic valve; MA = mitral annulus.
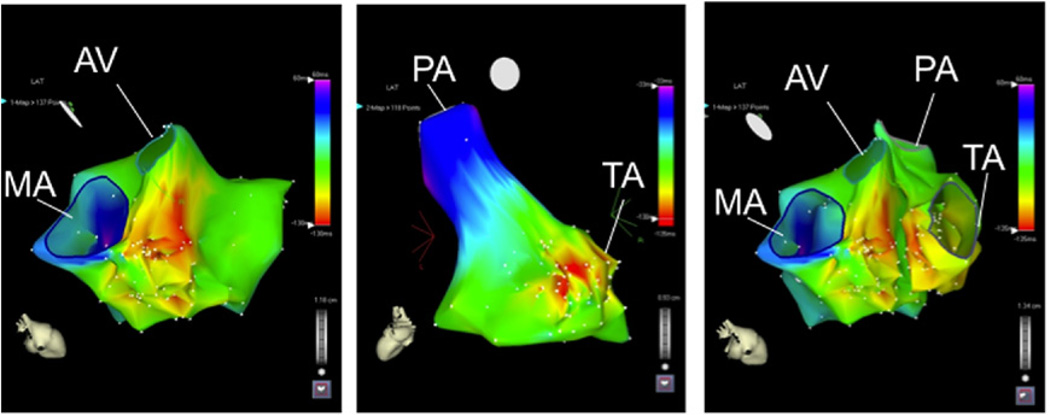
Figure 3. Septal Ventricular Activation Maps of an Intramural Ventricular Tachycardia.
(A) Activation map of the left ventricular septum indicating the earliest activation during ventricular tachycardia is located at the basal septum. The ventricular tachycardia originated from an intramural circuit. (B) Activation map of the right ventricular septum indicating that the earliest breakthrough is in the mid septum. The earliest activation is −25 ms preceding the QRS for the right septum and −24 ms for the left septum. (C) Merged right- and left-sided activation maps. AV = aortic valve; MA = mitral annulus; PA = pulmonary artery; TA = tricuspid annulus.
Pace-mapping was performed in areas with a voltage of ≤1.5 mV (9) for VTs that were not hemodynamically tolerated.
Radiofrequency ablation
Radiofrequency ablation was performed at the critical isthmus of the VT re-entry circuit. An isthmus was defined as a site showing concealed entrainment with matching stimulus-QRS and electrogram-QRS intervals (10) or where VT terminated during pacing without global capture (11). If no concealed entrainment could be identified and there were no matching pace-maps, radiofrequency energy was delivered at the site of the earliest endocardial activation. If there was insufficient power delivery (<20 W) with a 4-mm-tip catheter, an irrigated-tip catheter (Thermocool, Biosense Webster, Inc.; n = 2) or an 8-mm-tip catheter (Navistar, Biosense Webster Inc.; n = 1) was used. If VT terminated after 20 s of radiofrequency energy delivery, the application of radiofrequency delivery was continued for another 60 to 120 s. Applications of radiofrequency energy were titrated to maintain a target temperature at the electrode-tissue interface of 60°C for the 4-mm-tip catheter and an impedance decrease of 10 Ω for irrigated-tip catheters. With the 8-mm-tip catheter, the power was set at 70 W.
Programmed stimulation was repeated post-ablation at 2 right ventricular sites. A VT was considered successfully ablated if the VT terminated during radiofrequency energy delivery and could not be induced subsequently.
During follow-up, patients received the same antiarrhythmic drugs that had failed before the ablation procedure.
Classification of VTs
ENDOCARDIAL MYOCARDIAL VT
An isthmus was defined as endocardial if there was: 1) concealed entrainment at the left ventricular endocardium and a matching stimulus-QRS and electrogram QRS interval; or 2) if VT terminated during pacing without global capture; or 3) if there was a match in ≥10 of 12 leads with the spontaneous VT during pace-mapping. To rule out the possibility of Purkinje fiber involvement, the absence of Purkinje potentials was mandatory during sinus rhythm or during VT at the effective ablation site.
VTs WITH INVOLVEMENT OF THE PURKINJE SYSTEM
VTs were classified as involving the Purkinje system if the following criteria were met: 1) a Purkinje potential was present during sinus rhythm and during VT at the critical site (Fig. 4); and/or 2) concealed entrainment with matching stimulus-QRS and electrogram-QRS intervals was present or if VT terminated during pacing without global capture (Fig. 4) at a site with a Purkinje potential. In the presence of Purkinje potentials, changes in the intervals separating the potentials preceded changes in the R-R intervals. Purkinje potentials (Fig. 4) were defined as sharp, high-frequency potentials preceding the QRS onset during sinus rhythm. Bundle branch re-entry was ruled out by standard criteria (12).
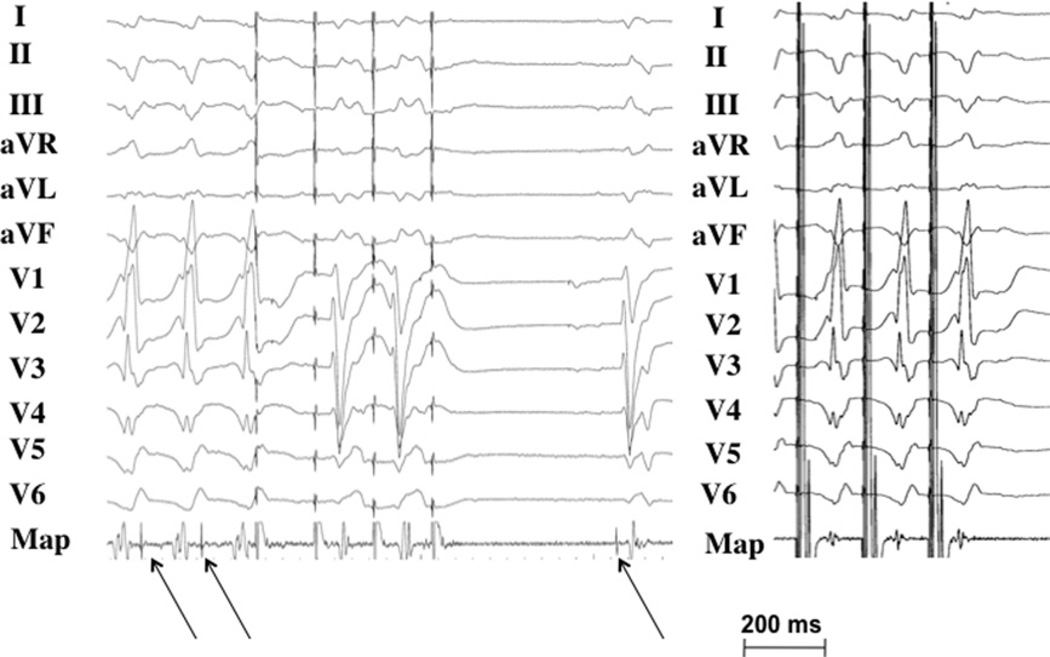
Figure 4. Critical Site of a VT Originating From the Purkinje System.
Nonglobal capture at a site where Purkinje fibers are present during ventricular tachycardia and sinus rhythm (arrows).
A pace-map during sinus rhythm (right panel shows a match with the targeted ventricular tachycardia).
INTRAMURAL VTS
VTs were classified as intramural if the following criteria were met: 1) there was focal type of endocardial activation at both the right and left ventricular endocardium during activation mapping; and 2) the VT could be entrained, but concealed entrainment could not be documented. At the effective site, pace-mapping during sinus rhythm showed a pace-map of <10 of 12 matching leads compared with the spontaneous VT.
Cardiac magnetic resonance imaging
Before the electrophysiology procedure, all patients without contraindications to magnetic resonance imaging (MRI) underwent delayed-enhancement (DE) MRI. The studies were performed on a 1.5-T MRI scanner (Signa Excite CV/i, General Electric, Milwaukee, Wisconsin) with a 4- or 8-element phased-array coil placed over the chest of patients in the supine position. Images were acquired with electrocardiography gating during breath-holds. Dynamic short- and long-axis images of the heart were acquired using a segmented k-space, steady-state, free-precession pulse sequence (repetition time of 4.2 ms, echo time of 1.8 ms, 1.4 × 1.4-mm in-plane spatial resolution, 8-mm slice thickness). Fifteen min after administration of 0.20 mmol/kg of intravenous gadolinium diethylenetriamine tetraacetic acid (Magnevist, Berlex Pharmaceuticals, Wayne, New Jersey), 2-dimensional delayed-enhancement imaging was performed using an inversion-recovery sequence (13) (repetition time of 6.7 ms, echo time of 3.2 ms, 1.4 × 1.4-mm in-plane spatial resolution, 8-mm slice thickness) in the long and short axis of the left ventricle at matching cine-image slice locations. The inversion time (250 to 350 ms) was optimized to null the normal myocardium.
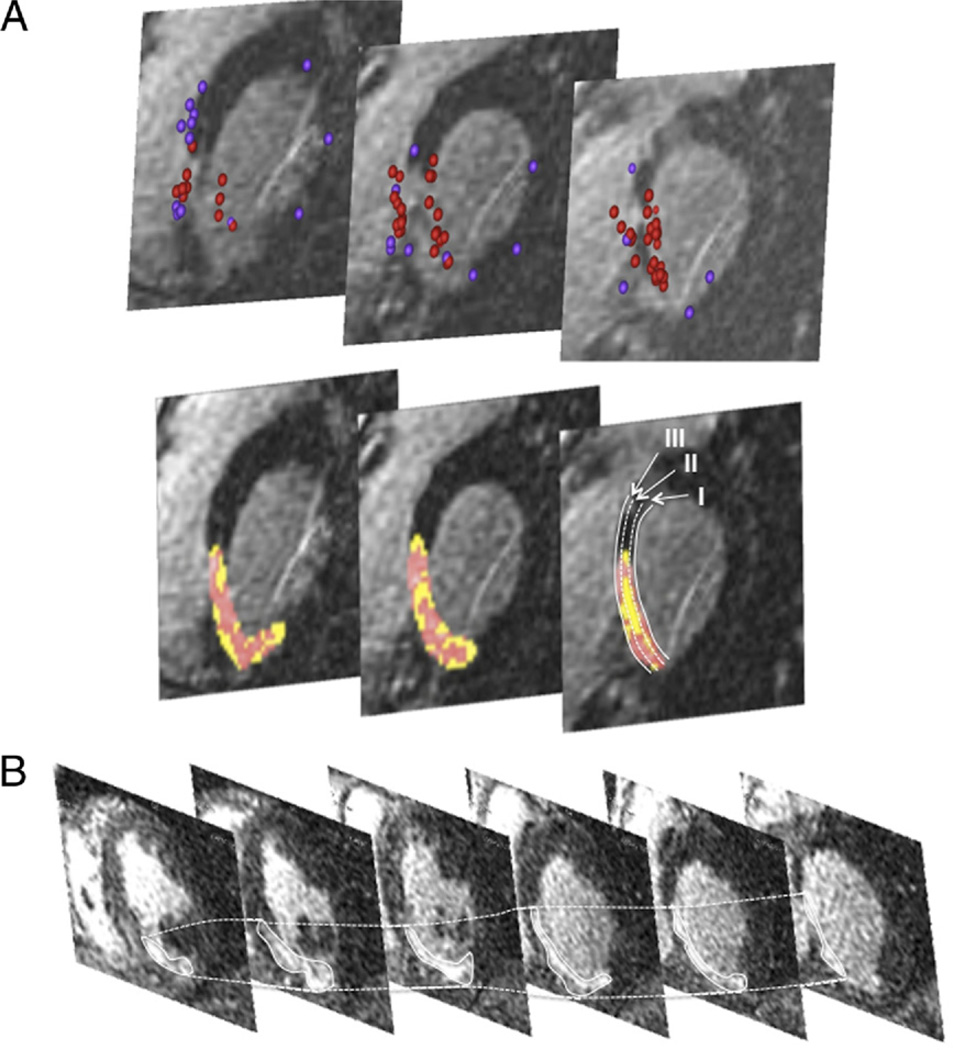
All DE-MRI was analyzed offline with specialized post-processing software (Osirix, University of Geneva, Geneva, Switzerland; Matlab, Mathworks, Natick, Massachusetts). For each subject, manual tracing of the endocardial contour of DE images was performed on a stack of 15 to 20 short-axis images, from base to apex of the left and right ventricles (Figs. 5A and 5B). The area of DE was then automatically determined by a region-growing algorithm as the area encompassing pixels with values of ≥50% of the maximal signal intensity within scar using the traditional method (14). Then areas of DE were analyzed for heterogeneity (15,16). These findings were correlated with the VT classification.
Figure 5. Stack of Short-Axis Delayed-Enhanced Magnetic Resonance Images.
(A) Magnetic resonance imaging with delayed enhancement (stack of short-axis views from a basal [right] to an apical direction) in a patient with intramural ventricular tachycardia. This patient had an inferoseptal myocardial infarction. The scar extends from the basal septum to the midinferior septum. Mapping points (upper panel) obtained during electroanatomic mapping were projected onto the magnetic resonance imaging (red tags indicate low-voltage sites, purple tags indicate normal voltage sites) confirming the presence of endocardial scar tissue. The lower panel shows the automated color coding depending on the signal intensity. The septum was divided into an endocardial third (I), an intramural third (II), and an epicardial third (III). The scar was also separated into core infarct (red) and peri-infarct zone (yellow). (B) Delayed-enhancement magnetic resonance imaging (stack of short-axis views from a basal [right] to an apical direction) in a patient with an inferoseptal infarction and endocardial ventricular tachycardia. No intramural ventricular tachycardia circuit was present in this patient. Compared with A, the intramural segment lacks areas with tissue heterogeneity.
Detailed analysis of the septal component of the scar was performed. The septum was divided into a left ventricular endocardial third, an intramural third, and an epicardial third (Fig. 5A). The septal component of each scar was also separated into core infarct (signal >3 SDs from normal) and peri-infarct zone (signal 2 to 3 SDs from normal). The reported data were obtained from the full myocardial thickness in the region of the septum spanned by the scar. The volume of core infarct and peri-infarct zones in the short-axis segment where the critical site was recorded as well as in the preceding and the subsequent short-axis slice was assessed and reported depending on the mechanism of the VT.
Follow-up
All patients were seen every 6 to 12 months in an ICD clinic. All but 1 patient without a previously implanted ICD underwent ICD implantation before hospital discharge. One patient refused ICD implantation.
Statistical analysis
Continuous variables are summarized as mean ± 1 SD and compared between 2 groups using the Student t test and across multiple groups using 1-way analysis of variance. For continuous variables that are highly skewed, the Kruskal-Wallis test was used. Discrete variables were compared using the Fisher exact test or by chi-square analysis. A p value <0.05 was considered statistically significant. In unpaired statistical comparisons in which characteristics of different VT types were compared, the Bonferroni correction was used to adjust for multiple comparisons. No corrections were made for multiple VTs within individuals when the VTs were the unit of analysis.
Results
Septal versus nonseptal VTs
Patients with VTs involving the septum more often had anterior wall myocardial infarctions than patients without septal involvement. Their septal scar burden was substantially higher than in patients in whom septal VTs could not be identified. Furthermore, they more often had VT morphologies with left bundle branch block compared with others and the QRS width was narrower compared with VTs with a nonseptal exit. Scar size as assessed by measurement of the endocardial low-voltage area was no different in patients with VTs involving the septum compared with other patients (Table 1).
Characteristics of endocardial septal VTs
In this series of VTs with septal involvement, the endocardium was the most prevalent location (70%) of the critical component of the re-entry circuit (Table 2). VTs involving the endocardium of the septum were distinct from other VTs involving the septum in that their QRS complexes were significantly broader and left bundle branch block morphologies were seen as frequently as right bundle branch block morphologies. Patients with VTs in this anatomic location had more total scar tissue in the left ventricle and the septum compared with the other patients in this series. Pacemapping and entrainment mapping helped to identify critical areas for all VTs in the endocardial septal location.
Table 2.
Comparison of Septal VT Localization
| Variables | Endocardial Myocardium (n = 20) |
Purkinje Related (n 8) |
Intramural Myocardium (n = 5) |
p Value |
|---|---|---|---|---|
| No. of VTs | 32 | 9 | 5 | — |
| Age, yrs | 65 ± 10 | 62 ± 7 | 64 ± 7 | NS |
| Women/men | 3/17 | 0/8 | 0/5 | NS |
| QRS width, ms | 204 ± 38* | 137 ± 15 | 155 ± 37† | <0.0001 |
| QRS: V1−/V2+ | 4/32 (13%) | 0/9 (0%) | 4/5 (80%) | 0.0003 |
| Morphology | 0.03 | |||
| LBBB | 16/32 | 1/9 | 4/5 | |
| RBBB | 16/32 | 8/9 | 1/5 | |
| Axis | NS | |||
| Superior | 21/32 | 9/9 | 3/5 | |
| Inferior | 11/32 | 0/9 | 2/5 | |
| VT cycle length, ms | 404 ± 97 | 415 ± 127 | 385 ± 78 | NS |
| Pace-map match score at effective site | 11.1 ± 0.9* | 12.0 ± 0.0‡ | 8.8 ± 0.5† | <0.0001 |
| CE | (10/10) | 9/9 | 0/5 | — |
| Ejection fraction, % | 26 ± 15 | 29 ± 11 | 43 ± 14† | <0.05 |
| Low-voltage area (<1.0 mV), cm2§ | Median = 109; IQR = 53 | Median = 55; IQR = 61‡ | Median = 22; IQR = 8† | 0.002 |
| Low-voltage area (<1.0 mV), cm2, within left ventricular septum§ | Median = 32; IQR = 32 | Median = 27; IQR = 15 | Median = 13; IQR = 16 | NS |
| Septal involvement to whole septal area, %§ | Median = 55; IQR = 30 | Median = 50; IQR = 47‡ | Median = 16; IQR = 26† | 0.04 |
| Infarct localization | 0.05 | |||
| Inferior | 11 | 6 | 0 | |
| Anterior | 11 | 3 | 5 | |
| Coronary artery | <0.05 | |||
| LAD | 11 | 3 | 5 | |
| RCA | 12 | 6 | 0 | |
| LCx | 0 | 0 | 0 | |
| Septal origin | ||||
| Basal/nonbasal | 8/24 | 4/5 | 5/0 | 0.005 |
| Anterior/posterior | 19/13 | 1/8 | 2/3 | 0.04 |
Values are n, mean ± SD, or n (%).
QRS: V1−/V2+ = QRS morphology with QS complex in V1 and a broad R-wave in V2. P values were adjusted for multiple comparisons. *Endocardial VTs versus Purkinje-related VTs: p < 0.05.
Intramural versus endocardial VTs: p < 0.05.
Purkinje-related VTs versus intramural VTs: p < 0.05.
CE = concealed entrainment; IQR = interquartile range; other abbreviations as in Table 1
Characteristics of VTs involving the Purkinje system
Distinguishing features of these included the narrowest QRS complex of all septal post-infarction VTs and a higher prevalence of right bundle branch block morphology mimicking fascicular VT. Entrainment mapping and pace-mapping both indicated the critical site of the re-entry circuit. Matching pace-maps with the targeted VT were identified at all critical sites where Purkinje potentials were recorded.
Characteristics of intramural VTs
The least common location of septal VTs was intramural (11%). VTs originating from the intramural myocardium were distinct from other septal VTs in that they most often had a left bundle branch block morphology with a Q wave in V1 and a broad R-wave in V2 (Fig. 1). The site of origin was in the basal septum in all instances. Although these VTs could be induced with programmed stimulation and showed classic entrainment with pacing, concealed entrainment could not be demonstrated from the endocardium. Furthermore, pace-mapping failed to identify the exit site of these VTs, and none of the pace-maps had a matching QRS complex with the targeted VT in ≥10 of 12 leads when pacing was performed at the effective ablation site. The QRS width was significantly narrower than in the endocardial VTs, but similar to the VTs involving the Purkinje system (p = 0.009 for endocardium vs. intramural; p = 0.4 for Purkinje related vs. intramural). Intramural VTs tended to occur more often in patients with septal involvement associated with anterior infarction– than Purkinje-related VTs in which inferior infarctions predominated (p = 0.07). Patients with intramural VTs had the highest left ventricular ejection fraction and the least amount of total and septal scar as assessed by voltage mapping of the endocardium.
Magnetic resonance imaging
In patients with endocardial VTs, the peri-infarct zone throughout the septum represented 4.4 ± 4.4% of the scar, and in patients with Purkinje fiber–related VT, the peri-infarct zone composed 9.7 ± 4.4% of the scar, and in the only patient with intramural VTs, the peri-infarct zone represented 36% of the scar or 7 times the SD from the mean of the patients with endocardial VTs (Fig. 5A, Table 3). Patients with endocardial VTs and patients with VTs involving the Purkinje fiber system had a similar amount of core infarct and peri-infarct volume throughout the septal scar. Patients with endocardial nonseptal VTs had a similar core infarct and peri-infarct volume compared with patients with endocardial septal VTs.
Table 3.
Comparison of Magnetic Resonance Imaging Data
| Variables | n | Normal Volume, cm3 | CI Volume, cm3 | Peri-infarct Zone Volume, cm3 | PIZ/CI Ratio |
|---|---|---|---|---|---|
| Endocardial VT | 8 | 0.07 ± 0.07 | 1.96 ± 1.90 | 0.09 ± 0.09 | 0.05 ± 0.06 |
| Purkinje VT | 2 | 0.06 ± 0.08 | 1.77 ± 0.46 | 0.18 ± 0.08 | 0.10 ± 0.02 |
| Intramural VT | 1 | 0.04 | 1.21 | 0.68 | 0.56 |
Volumes and ratios between patients with endocardial VT and Purkinje fiber–related VT were not statistically different. The PIZ/CI ratio was >5 SD higher in the patient with intramural VT compared with patients with Purkinje–related or endocardial VTs.
CI = core infarct; PIZ = peri-infarct zone; VT = ventricular tachycardia.
Mapping and ablation
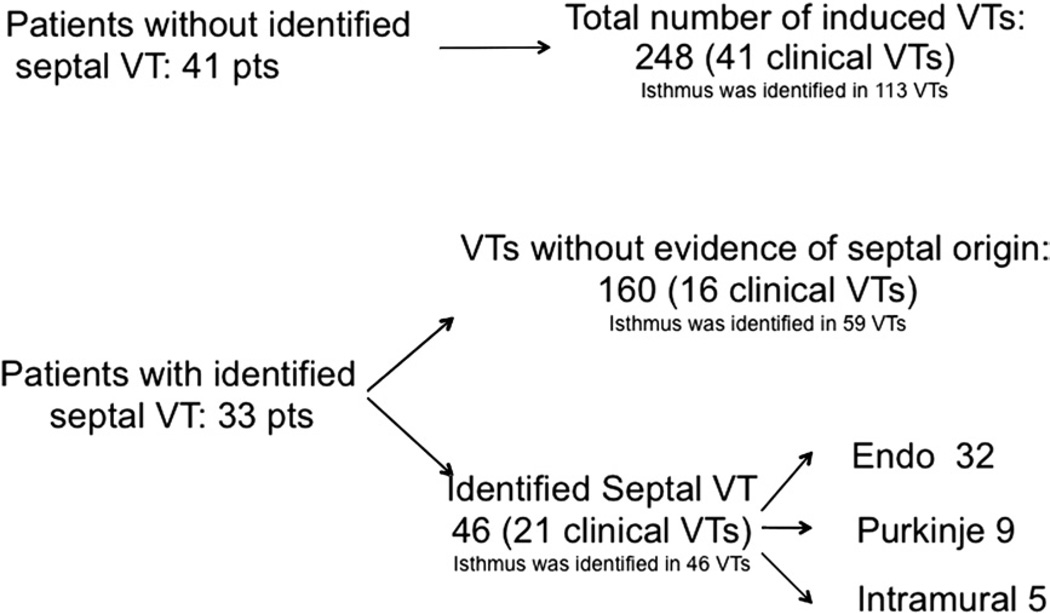
All clinically relevant VTs were successfully ablated. All 46 VTs localized to the septum were effectively ablated. Among the remaining 160 VTs, a critical isthmus was identified for 59 VTs, and these VTs were effectively ablated (Fig. 6). After ablation, 22 of 33 patients (67%) were noninducible with programmed ventricular stimulation. In 100 of the 105 VTs, when pacing was performed at a VT isthmus, there was either a 10–11/12 (n = 66) or a 12/12 pace-map (n = 34) at the critical area. There was no difference with respect to ablation outcome if 10 or 12 leads matched with the morphology of the targeted VT. In 10 of 11 patients, at least 1 VT remained inducible at the end of the procedure; in these inducible VTs, an isthmus could not be identified during endocardial left ventricular mapping.
Figure 6. Ablation Outcome.
Schema indicating the different types of ventricular tachycardia (VT) and their clinical relevance as well as whether critical components were identified.
Endo = endocardial.
The mean fluoroscopy time was 60 ± 23 min, and the mean procedure time was 380 ± 113 min. A mean of 57 ± 43 min of radiofrequency energy was delivered per patient. One patient required 2 procedures to eliminate an intramural VT. In 6 patients, a 4-mm-tip conventional catheter was used for the ablation procedure. In 2 patients with intramural circuits, the 4-mm-tip catheter failed to eliminate the intramural VT. In 1 of these patients, an 8-mm-tip catheter was used, and in the other patient, an irrigated-tip catheter was used to eliminate the intramural circuit. In the remaining 27 patients, irrigated-tip catheters were used.
Follow-up
Treatment with amiodarone was continued post-ablation in 17 of 33 patients. During follow-up of 40 ± 20 months, 1 patient died suddenly, 1 patient died secondary to a stroke, and 1 patient died secondary to myocardial infarction. Nine of the 33 patients (27%) had recurrent VT. Based on ICD electrogram analysis that was available in 5 of 9 patients, none of the recurring VTs was a septal VT. Six of 9 patients needed repeat ablation procedures, and all but 1 patient had no ventricular arrhythmias subsequently. The remaining patients were controlled with adjustment of antiarrhythmic medications.
Discussion
Main findings
This study describes the distinctive characteristics of post-infarction VTs involving the interventricular septum. The critical component of the re-entry circuit can involve the deep intramural myocardium, the Purkinje system, or the endocardium. Approximately 70% of the induced VTs had critical components in the septal endocardium. The remainder was split between VTs involving the Purkinje fiber system and intramural circuits. VTs involving the Purkinje system had the narrowest QRS complexes and displayed a right bundle branch block morphology. Intramural VTs had a left bundle branch block with a QS complex in V1 and a broad R-wave in lead V2. Endocardial VTs from the interventricular septum had the broadest QRS width. Patients with endocardial VTs had the most extensive scarring and the lowest ejection fraction.
Mechanism of septal post-infarction VT
Intramural VTs could be entrained, but a protected area within the scar tissue could not be identified by concealed entrainment. Endocardial breakthrough points were juxtaposed on the left and right ventricular septum, consistent with a deep focus within the septum. Similar findings were described by Kaltenbrunner et al. (17) using intraoperative right and left ventricular mapping. In the latter study, intramural VTs had the lowest surgical success rate. In all intramural VTs in the present study, a conventional 4-mm-tip catheter failed to eliminate the VT, and either an irrigated-tip catheter or an 8-mm-tip catheter was required to reach the VT focus. Pace-mapping at the earliest site of endocardial activation resulted in a very different QRS morphology compared with the targeted VT morphology. Capture of the myocardium surrounding the catheter tip generates a QRS complex that reflects local capture and is different from the QRS complex of an intramural VT. Because these VTs could be induced by programmed stimulation and could be entrained, their mechanism was re-entry. DE-MRI may be useful in identifying patients with intramural circuits.
VTs involving the Purkinje fiber system are less frequent than subendocardial VTs. This might be surprising given the extensive supply of the Purkinje fiber system in the septum. The exact nature of the re-entry circuit remains to be demonstrated; however, connections allowing impulse propagation to proceed from the myocardium to the Purkinje fiber system may be required (1). It is possible that the conditions necessary for these connections to occur limit the prevalence of this type of VT.
Infarct anatomy
Intramural VT has been described in post-infarction patients during intraoperative mapping studies (2,17). The interventricular septum has a dual blood supply. The left anterior descending artery supplies the anterior and basal septum, whereas the right coronary artery supplies the posterior septum. Furthermore, transmurality is often incomplete in the setting of anterior wall infarctions affecting the basal septum. This explains why most patients with intramural VT originating from a scar in the basal septum had previous anterior wall myocardial infarctions (18).
Cardiac MRI obtained in a patient with an intramural VT that could not be ablated from the right side or the left side of the septum using a conventional 4-mm-tip catheter identified an intramural area that was spared from scar tissue and that was a potential arrhythmogenic substrate. This area of spared myocardium might indicate an intramural pathway that is shielded from an endocardial infarct core (2) and might be difficult to reach from the left- or right-sided endocardium. Use of an irrigated-tip catheter resulted in successful ablation in this patient.
Inferior wall myocardial infarctions involving the right coronary artery were more prevalent for endocardial VTs and VTs involving the Purkinje fiber system. The scar was larger in these patients compared with patients with intramural VTs. Also, in these patients, a septal scar and critical components of VT were more often in the posterior aspect of the septum. This corresponds to the location of the posterior fascicle. The observation that inferior wall myocardial infarctions are often transmural at the basal and mid-myocardial levels (19) could explain the lower prevalence of intramural circuits in patients with previous inferior wall infarctions. Patients with endocardial VT had a larger area of septal low voltage compared with patients with intramural VTs. It is possible that the larger area alone might be responsible for the development of endocardial VTs over time. In the presence of a less extensive infarction in which more Purkinje fibers survive, these fibers can also be involved in the re-entry circuit (1). The dual blood supply of the posterior fascicle (20) may result in more surviving Purkinje fibers in the setting of an inferior wall infarction. This could make it more likely for posterior Purkinje fibers to participate in VTs compared with patients with anterior wall infarction.
Study limitations
A limitation is that only 7 of the patients with septal VTs underwent MRI. Furthermore, because most VTs were not hemodynamically tolerated, activation mapping could not always be performed, and the prevalence of intramural VTs may have been underestimated. A VT circuit confined to the right ventricle or a circuit in the epicardial part of the septum was not ruled out in all patients and may account for some of the VTs for which no critical isthmus could be identified. Because the entire VT circuit was not mapped, septal involvement cannot be excluded for VTs labeled as nonseptal.
Clinical implications
Septal VTs have distinctive electrocardiographic characteristics that help to focus attention on an area of interest within the left ventricular septum. The QRS complex of septal VTs is narrower and more often shows left bundle branch block morphology compared with nonseptal VTs. The presence of a left bundle branch block pattern with a broad R-wave in V2 suggests an intramural location of the VT circuit, in which case, activation mapping rather than pace-mapping or entrainment mapping will be needed to identify the endocardial breakthrough site where ablation is most likely to be effective. A relative narrow QRS complex mimicking fascicular tachycardia suggests participation of the Purkinje system.
Acknowledgments
Drs. Bogun and Oral received a grant from the Leducq Foundation. Dr. Desjardins received an NIH grant (K23 EB006481). Dr. Bogun participated in a catheter study from Biosense Webster. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- DE
delayed enhancement
- ICD
implantable cardioverter-defibrillator
- MRI
magnetic resonance imaging
- VT
ventricular tachycardia
REFERENCES
- 1.Bogun F, Good E, Reich S, et al. Role of Purkinje fibers in post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;48:2500–2507. doi: 10.1016/j.jacc.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 2.Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL, Cain ME. Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation. 1992;86:1872–1887. doi: 10.1161/01.cir.86.6.1872. [DOI] [PubMed] [Google Scholar]
- 3.de Bakker JM, Coronel R, Tasseron S, et al. Ventricular tachycardia in the infarcted, Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990;15:1594–1607. doi: 10.1016/0735-1097(90)92832-m. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K, Liu TY, Scott C, et al. The value of defibrillator electrograms for recognition of clinical ventricular tachycardias and for pace mapping of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2010;56:96–79. doi: 10.1016/j.jacc.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Hummel D, Strickberger S, Daoud E, et al. Results and efficency of programmed ventricular stimulation with four extrastimuli compared with one, two, and three extrastimuli. Circulation. 1994;90:2827–2832. doi: 10.1161/01.cir.90.6.2827. [DOI] [PubMed] [Google Scholar]
- 6.Callans DJ, Ren JF, Michele J, Marchlinski FE, Dillon SM. Electro-anatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction. Correlation with intracardiac echocardiography and pathological analysis. Circulation. 1999;100:1744–1750. doi: 10.1161/01.cir.100.16.1744. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins B, Crawford T, Good E, et al. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6:644–651. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogun F, Bahu M, Knight B, et al. Comparison of effective and ineffective target sites which demonstrate concealed entrainment in patients with coronary artery disease undergoing radiofrequency ablation of ventricular tachycardia. Circulation. 1997;95:183–190. doi: 10.1161/01.cir.95.1.183. [DOI] [PubMed] [Google Scholar]
- 9.Bogun F, Good E, Reich S, et al. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–2019. doi: 10.1016/j.jacc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 10.Bogun F, Kim HM, Han J, et al. Comparison of mapping criteria for hemodynamically tolerated, postinfarction ventricular tachycardia. Heart Rhythm. 2006;3:20–26. doi: 10.1016/j.hrthm.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Bogun F, Krishnan SC, Marine JE, et al. Catheter ablation guided by termination of post-infarction ventricular tachycardia by pacing with non-global capture. Heart Rhythm. 2004;1:422–426. doi: 10.1016/j.hrthm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Merino JL, Peinado R, Fernandez-Lozano I, et al. Bundle-branch reentry and the postpacing interval after entrainment by right ventricular apex stimulation: a new approach to elucidate the mechanism of wide-QRS-complex tachycardia with atrioventricular dissociation. Circulation. 2001;103:1102–1108. doi: 10.1161/01.cir.103.8.1102. [DOI] [PubMed] [Google Scholar]
- 13.Simonetti OP, Kim RJ, Fieno DS, et al. An improved mr imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 14.Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–2389. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 17.Kaltenbrunner W, Cardinal R, Dubuc M, et al. Epicardial and endocardial mapping of ventricular tachycardia in patients with myocardial infarction. Is the origin of the tachycardia always subendocardially localized? Circulation. 1991;84:1058–1071. doi: 10.1161/01.cir.84.3.1058. [DOI] [PubMed] [Google Scholar]
- 18.Ideker RE, Wagner GS, Ruth WK, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. II. Correlation with quantitative anatomic findings for anterior infarcts. Am J Cardiol. 1982;49:1604–1614. doi: 10.1016/0002-9149(82)90235-1. [DOI] [PubMed] [Google Scholar]
- 19.Roark SF, Ideker RE, Wagner GS, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. III. Correlation with quantitative anatomic findings for inferior infarcts. Am J Cardiol. 1983;51:382–389. doi: 10.1016/s0002-9149(83)80069-1. [DOI] [PubMed] [Google Scholar]
- 20.Rotman M, Wagner GS, Wallace AG. Bradyarrhythmias in acute myocardial infarction. Circulation. 1972;45:703–722. doi: 10.1161/01.cir.45.3.703. [DOI] [PubMed] [Google Scholar]