Abstract
The syntheses and photophysical properties of 1-(5-methylhexyl)-2,3,7,8-tetrahydro-1H-naphtho[2,1-e]indol-9(6H)-one (7a) and 1-(5-methylhexyl)-2,3,8,9-tetrahydro-1H-naphtho[2,1-e]indol-6(7H)-one (7b) are reported. They are prepared in eight steps from the corresponding bromonaphthylamines. These fluorescent compounds have PRODAN-like cores, and they are structurally similar to cholesterol. Compound 7a is the first reported PRODAN derivative where both the amino and carbonyl groups are constrained to be coplanar with the naphthalene core. Comparing the photophysical behavior of these compounds with related compounds indicates that locking the amino group in a five-membered ring enhances their desirable properties as solvent polarity sensors.
Keywords: PRODAN, fluorescent cholesterol model, solvatochromism
1. Introduction
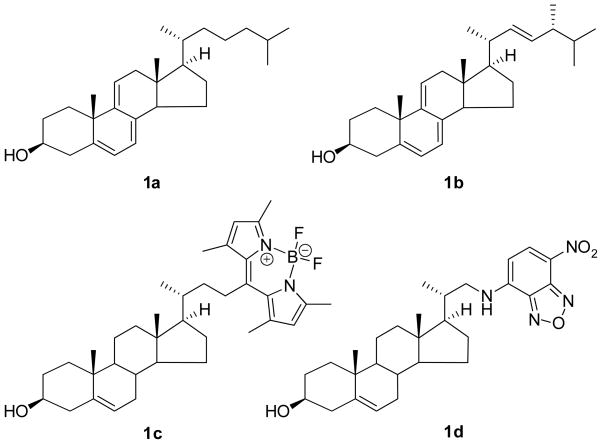
Cholesterol is a singularly important biomolecule in animals. Its primary functions are to modify the structure and permeability of cell membranes and to serve as a precursor to a number of hormones. Determining the interaction between cholesterol and other biological structures is difficult because cholesterol is not readily detected. While it contains a single alkene, it is not amenable to analysis by electronic spectroscopy. One way to study the interaction between a non-fluorescent substrate like cholesterol with host systems is with suitable fluorescent model compounds. Several fluorescent cholesterol analogs have been prepared (Fig. 1). [1–3] These molecules fall into two extremes. Cholestatriene 1a and the related ergosterol derivative 1b conserve most of the structure of cholesterol, but the triene chromophore has a relatively low fluorescent quantum yield, and the absorption and emission maxima are bracketed between 300–400 nm. The BODIPY and benzoxadiazol derivatives, 1c and 1d, are on the other extreme. They add a significant structural perturbation to the cholesterol moiety, but the chromophores have high fluorescent quantum yields and they absorb and emit in the visible region (430–510 nm). However, the highly polar nature of the fluorophores strongly affects interactions with supramolecular structures such as lipid bilayers. As a result, the suitability of these two derivatives as a cholesterol mimic has been questioned. [1, 4]
Figure 1.
Fluorescent cholesterol models
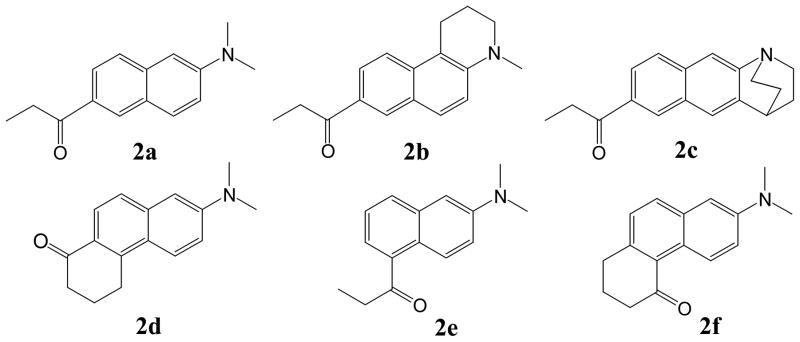
PRODAN (6-propionyl-2-(dimethylamino)naphthalene, Fig. 2) is widely used as a fluorescent probe in the studies of biomolecules due to its optimal photophysical properties. It was first prepared 1979 by Weber and Farris who examined its interaction with bovine serum albumin (BSA).[5] Despite having a carbonyl group, the fluorescence quantum yield for PRODAN is nearly unity in polar solvents. The position of the fluorescence emission maximum shifts to progressively lower energy as the solvent polarity increases. This behavior is attributed to an increase in the molecular dipole moment of the excited state resulting from an intramolecular charge transfer (ICT). Twisting of the amino group has often been proposed to explain the solvatochromism (TICT hypothesis).[6–8] We have shown that PRODAN derivatives that enforce planarity of either the 3° amino group (2b) or the carbonyl group (2d) behave like PRODAN, whereas the derivative with a twisted amino group (2c) behaves differently.[9–11] Derivatives where both groups are constrained to be planar have not been reported. The PRODAN regioisomer 2e and its constrained carbonyl derivative 2f are nearly as solvatochromic as PRODAN. Their quantum yields are slightly smaller, and they decrease markedly with increased solvent polarity.[12]
Figure 2.
PRODAN (2a) and geometrically constrained derivatives (2b–d) and regioisomers (2e–f).
This paper reports the preparation and photophysical properties of two fluorescent cholesterol models that have a PRODAN-like fluorophore. While the structural perturbations are greater than in 1a and 1b, they are not as extreme as they are in 1c and 1d, and they preserve most of the desirable photophysical properties of 1c and 1d.
2. Experimental
2.1 General Methods
NMR spectra are recorded on a Varian Mercury 400-Vx system. High resolution ESI-MS are acquired with a Bruker Apex-Qe instrument. Fluorescence emission data are collected using a SLM-Aminco SPF-500 spectrometer as the excitation source and sample holder. The emitted light is collected with a fiber optic cable and detected with an Ocean Optics Maya spectrometer. Solvents used for photophysical characterization are spectrophotometric grade from Acros. Relative fluorescence quantum yields in toluene are determined using anthracene (Φ = 0.30) and 9,10-diphenylanthracene (Φ = 0.90) as references using the method of standard additions. PM6/COSMO semiempirical calculations are performed using AMPAC 9.0 from Semichem, Inc (www.semichem.com). Calculations incorporate the following keywords: PM6; SDCI = 8; singlet; qcscf; cosmo; tight; truste; micros = 0; root = 1 (or 2); scfcrt = 0. The values for dielec, iofr and rsolv are 2.3741, 1.4961 and 1, resp., for toluene and 35.688, 1.3442 and 1, resp., for acetonitrile.
2.2 Materials
Bromonaphthalenamines 3a and 3b are prepared through a Bucherer reaction from the corresponding bromonaphthols.[13,14]. 5-Methylhexyl methanesulfonate is made from 5-methylhexan-1-ol and methanesulfonyl chloride and distilled under vacuum before use.
2.3 Synthesis of Fluorescent Cholesterol Models and Intermediates
2.3.1 N-(6-bromonaphthalen-2-yl)methanesulfonamide (4b)
Pyridine (10 mL) is added with stirring to a solution of 6-bromonaphthalen-2-amine (3b, 8.88 g, 40.0 mol) in CH2Cl2 (100 mL) cooled to 0 °C. Next, a solution of methanesulfonyl chloride (8.70 g, 75.9 mmol) in CH2Cl2 (10 mL) is added dropwise. The mixture is stirred at 0 °C for 1 hr, then stirred at room temperature for 2 hr. The mixture is poured into aq. NaHCO3 (15 g, 300 mL), and the layers are stirred together rapidly for 30 min. The solid that forms is collected by suction filtration. The two filtrate layers are separated, and the aqueous layer is extracted with CH2Cl2 (2 × 50 mL). The combined organic layers are washed with 10% HCl (3 × 50 mL), dried over CaCl2, filtered, and concentrated in vacuo. The slurry of the resulting solid in water is stirred rapidly and suction filtered. This solid is combined with the first solid and they are recrystallized from ethanol (100 mL) and water (50 mL) giving N-(6-bromonaphthalen-2-yl)methanesulfonamide (7.87 g, 26.2 mmol, 66%) after drying under vacuum at 100°C, m.p. 169–170 °C. 1H NMR (CDCl3) δ 7.93 (s, 1H), 7.71 (m, 2H), 7.65 (d, J= 8.6 Hz, 1H), 7.52 (d, J= 8.1 Hz, 1H), 7.45 (d, J= 8.1 Hz, 1H), 3.00 (s, 3H); 13C NMR (CDCl3) δ 136.07, 132.28, 131.52, 129.85, 129.55, 129.06, 128.40, 121.41, 118.65, 115.96, 39.10. Found [M+Na]+ 321.95097. C11H10BrNO2SNa+ requires 321.95078.
2.3.2 N-(5-Bromonaphthalen-2-yl)methanesulfonamide (4a)
This isomer (4.52 g, 15.1 mol, 81%) is made from 5-bromonaphthalen-2-amine (3a, 4.21 g, 18.7 mmol) using the procedure above, m.p. 164°C–165°C. 1H NMR (CDCl3) δ 9.67 (s,1H), 8.16 (d, J=9.1 Hz, 1H), 7.76 (s, 1H), 7.75 (d, J=8.0 Hz, 1H), 7.68 (d, J=7.6 Hz, 1H), 7.54 (d, J=9.1 Hz, 1H)meta, 7.30 (dd, J=8.0, 7.6 Hz, 1H), 3.01 (s, 3H); 13C NMR (CDCl3) δ 136.83, 135.31, 129.14, 129.06, 128.79, 127.61, 127.18, 122.61, 121.69, 116.07, 39.32. Found [M+Na]+ 321.95096. C11H10BrNO2SNa+ requires 321.95078.
2.3.3 7-Bromo-3H-benzo[e]indole (5b)
Potassium carbonate (2.35 g, 17.0 mmol) is added to a solution of N-(6-bromonaphthalen-2-yl)methanesulfonamide (4b, 7.87 g, 26.2 mmol) in DMF (25 mL) under Ar followed by 2-bromo-1,1-diethoxyethane (9.4 mL, 62.5 mol). The reaction mixture is heated overnight at 110 °C with stirring. Another portion of 2-bromo-1,1-diethoxyethane (2.0 mL, 13.3 mol) is added, and heating and stirring is continued overnight. When TLC analysis shows that the reaction is complete, the reaction is allowed to cool. The inorganic solids are removed by suction filtration, and the solid is washed with a small amount of CH2Cl2. The volatile solvent is removed in vacuo, and the higher boiling materials are removed by vacuum distillation (0.1 Torr, up to 145°C). The residue is taken up in CH2Cl2 (50 mL), and boron trifluoride etherate (4.0 mL, 31.6 mmol) is added. The reaction is stirred at room temperature overnight and monitored by TLC. The next day two more portions of boron trifluoride etherate (1.0 mL ea, 15.8 mmol total) are added, and the reaction is stirred at room temperature overnight. The following day the reaction mixture is poured slowly into aq. NaHCO3 (15 g, 200 mL) with vigorous stirring. After the bubbling ceases, the mixture is diluted with CH2Cl2 (100 mL), and the layers are separated. The aqueous layer is extracted with CH2Cl2 (2× 50 mL). The combined organic layers are washed with H2O (200 mL), dried over CaCl2, and concentrated in vacuo. A solution of 5% methanolic KOH (400 mL) is added, and the reaction is heated at reflux overnight. The reaction is allowed to cool, and the contents are poured into H2O (600 mL). The methanol is allowed to evaporate overnight. The resulting solid is collected by suction filtration, washed with water and air-dried. The filtrate is acidified with acetic acid (50 mL) and charged with NaCl (100 g). The resulting solid is collected by suction filtration, washed with water, and air-dried giving unreacted N-(6-bromonaphthalen-2-yl)methanesulfonamide (0.49 g, 1.6 mmol). The first solid is purified by vacuum sublimation (0.1 Torr, T ~ 200°C) giving 7-bromo-3H-benzo[e]indole (3.20 g, 13.0 mmol, 76% over three steps), m.p. 122–124 °C. 1H NMR (CDCl3) δ 8.11 (d, J= 8.6 Hz, 1H), 8.05 (d, J= 1.9 Hz, 1H), 7.61 (dd, J= 8.6, 1.9 Hz, 1H), 7.56 (d, J= 9.0 Hz, 1H), 7.49 (d, J= 9.0 Hz, 1H), 7.31 (m, 1H), 7.07 (m, 1H); 13C NMR (CDCl3) δ 132.38, 130.68, 128.97, 126.84, 125.00, 122.91, 122.84, 122.12, 116.94, 114.00, 102.09. Found [M-H]− 243.97656. C12H7BrN− requires 243.97674.
2.3.4 6-bromo-3H-benzo[e]indole (5a)
This isomer (0.78 g, 3.17 mmol, 40% overall conversion) is made from N-(5-bromonaphthalen-2-yl)methanesulfonamide (4a, 2.50g, 8.33 mmol) using the procedure above, m.p. 93°C–96°C. 1H NMR (CDCl3) δ 8.50 (br s, 1H), 8.21 (d, J=7.7 Hz, 1H), 8.03 (d, J=8.9 Hz, 1H), 7.71 (d, J=7.4 Hz, 1H), 7.61 (d, J=8.9 Hz, 1H), 7.37 (dd, J=7.7, 7.4 Hz, 1H), 7.31 (m, 1H), 7.08 (m, 1H); 13C NMR (CDCl3) δ 129.73, 127.86, 127.81, 126.35, 123.76, 123.26, 123.23, 123.17, 123.10, 122.12, 114.26, 102.39. Found [M-H]− 243.97657. C12H7BrN− requires 243.97674.
2.3.5 7-bromo-3-(5-methylhexyl)-2,3-dihydro-1H-benzo[e]indole (6b)
Sodium hydride (250 mg, 6.3 mmol, 60% in oil, washed with hexanes) is added in one portion to a solution of 7-bromo-3H-benzo[e]indole (5b, 1.30 g, 5.28 mmol) in DMF (25 mL). After the reaction is complete, 5-methylhexyl methanesulfonate (1.00 g, 5.2 mmol) is added in one portion. The reaction is stirred under N2 overnight and monitored by TLC. The next day additional sodium hydride (120 mg, 3.0 mmol) is added followed in 10 min by 5-methylhexyl methanesulfonate (0.40 g, 2.1 mmol). Stirring is continued overnight. The next day the reaction mixture is diluted with hexanes (75 mL) and CH2Cl2 (25 mL). The organic layer is washed with water (3 × 100 mL), then dried over CaCl2, and conc. in vacuo. The excess 5-methylhexyl methanesulfonate is removed by vacuum distillation (0.1 Torr, up to 190°C) leaving 7-bromo-3-(5-methylhexyl)-3H-benzo[e]indole (1.63 g) which is used without further purification. This solid is stirred vigorously with acetic acid (40 mL) while NaCNBH3 (3.30 g, 52.5 mmol) is added in several portions. The reaction is left to stir under N2 overnight. The contents of the reaction are poured slowly into aq. NaHCO3 (60 g, 500 mL), and the resulting mixture is stirred rapidly for 1 hr. The yellow precipitate that forms is collected by suction filtration and washed with water giving crude 7-bromo-3-(5-methylhexyl)-2,3-dihydro-1H-benzo[e]indole (1.63 g, 4.71 mmol, 89%) after air-drying. A portion was purified by vacuum sublimation for analysis, m.p. 50–51 °C. 1H NMR (CDCl3) δ 7.85 (d, J= 1.6 Hz, 1H), 7.53 (d, J= 8.8 Hz, 1H), 7.41 (dd, J= 8.9, 1.6 Hz, 1H), 7.39 (d, J= 8.9 Hz, 1H), 6.92 (d, J= 8.8 Hz, 1H), 3.52 (t, J= 8.8 Hz, 2H), 3.22 (t, J= 8.8 Hz, 2H), 3.14 (t, J= 7.4 Hz, 2H), 1.60 (m, 2H), 1.56 (m, 2H), 1.40 (m, 1H), 1.25 (m, 2H), 0.89 (d, J= 6.6 Hz, 6H); 13C NMR (CDCl3) δ 151.05, 130.58, 129.60, 129.59, 129.02, 127.53, 123.99, 121.11, 114.65, 111.81, 53.63, 49.93, 39.04, 28.19, 27.95, 27.15, 25.23, 22.84. Found [M]+ 345.10837. C19H24BrN+ requires 345.10866.
2.3.6 6-Bromo-3-(5-methylhexyl)-2,3-dihydro-1H-benzo[e]indole (6a)
This isomer (900 mg, 2.60 mmol, 86%) is made from 6-bromo-3H-benzo[e]indole (5a, 0.78 g, 3.17 mmol) using the procedure above, except that the final purification is by vacuum distillation. 1H NMR (CDCl3) δ 8.04 (d, J=9.0 Hz, 1H), 7.44 (d, J=8.3 Hz, 1H), 7.42 (d, J=7.6 Hz, 2H), 7.16 (dd, J=8.3, 7.6 Hz, 1H), 6.95 (d, J=9.0 Hz, 1H), 3.53 (t, J=8.8 Hz, 2H), 3.21 (t, J=8.8 Hz, 2H), 3.15 (t, J=7.5 Hz, 2H), 1.58 (m, 3H), 1.39 (m, 2H), 1.24 (m, 2H), 0.90 (d, J=6.7 Hz, 6H); 13C NMR (CDCl3) δ 151.46, 132.32, 127.92, 126.74, 126.21, 125.46, 123.93, 122.21, 121.38, 111.87, 53.72, 49.76, 39.09, 28.24, 27.93, 27.35, 25.27, 22.91. Found [M]+ 345.10878. C19H24BrNa+ requires 345.10866.
2.3.7 1-(5-methylhexyl)-2,3,7,8-tetrahydro-1H-naphtho[2,1-e]indol-9(6H)-one (7b)
A solution of 7-bromo-3-(5-methylhexyl)-2,3-dihydro-1H-benzo[e]indole (6b, 600 mg, 1.73 mmol) in DMAC (8 mL, freshly distilled from CaH2) under N2 is charged with NiCl2(PPh3)2 (120 mg, 0.18 mmol) and stirred for 15 minutes until the solution becomes homogeneous. A solution of 4-ethoxy-4-oxobutylzinc bromide (5.0 mL, 0.5 M solution in THF, 2.5 mmol) is added to the mixture. Stirring is continued for four hours. More NiCl2(Ph3)2 (140 mg, 0.21mmol) is added. After 15 minutes of stirring, a solution of 4-ethoxy-4-oxobutylzinc bromide (3.0 mL, 1.5 mmol) is added. Stirring is continued overnight. The following day the mixture is poured into water (200 mL) and stirred for 1 hour. Salt is added to the mixture, and then the precipitated solid is collected with suction filtration. The solid is washed with water, air-dried and dried under vacuum overnight. The residue is covered with polyphosphoric acid (~ 5 mL), heated to 110°C and stirred for two hours. The reaction is allowed to cool and then poured into aq. NaHCO3 (30 g, 300 mL). The precipitated solid is collected with suction filtration and air-dried. The solid is purified through column chromatography using a gradient elution (hexanes, ethyl acetate). A final vacuum sublimation (0.1 Torr, T ~ 200°C) gave 1-(5-methylhexyl)-2,3,7,8-tetrahydro-1H-naphtho[2,1-e]indol-9(6H)-one (200 mg, 0.60 mmol, 34%), m.p. 54–55°C. 1H NMR (CDCl3) δ 9.22 (d, J= 9.3 Hz, 1H), 7.67 (d, J= 8.5 Hz, 1H), 7.22 (d, J= 8.5, 1H), 7.02 (d, J= 9.3 Hz, 1H), 3.50 (t, J= 8.7 Hz, 2H), 3.24 (t, J= 8.7 Hz, 2H), 3.15 (t, J= 7.4 Hz, 2H), 3.04 (t, J= 6.1 Hz, 2H), 2.75 (t, J= 6.6 Hz, 2H), 2.15 (tt, J= 6.6, 6.1 Hz, 2H), 1.58 (m, 3H), 1.40 (m, 2H), 1.24 (m, 2H), 0.89 (d, J= 6.4 Hz, 6H); 13C NMR (CDCl3) δ 201.02, 150.43, 142.56, 130.7, 128.76, 128.18, 127.51, 126.98, 124.92, 121.56, 113.01, 53.58, 50.05, 41.43, 39.08, 31.62, 28.20, 27.95, 27.56, 25.26, 23.40, 22.85. Found [M+Na]+ 358.21448. C23H29NONa+ requires 358.21414.
2.3.8 1-(5-methylhexyl)-2,3,8,9-tetrahydro-1H-naphtho[2,1-e]indol-6(7H)-one (7a)
This isomer (420 mg, 1.26 mol, 48%) is made from 7-bromo-3-(5-methylhexyl)-2,3-dihydro-1H-benzo[e]indole (6a, 900 mg, 2.60 mmol) using the procedure above, m.p. 158–160°C. 1H NMR (CDCl3) δ 8.01 (d, J=9.2 Hz, 1H), 7.93 (d, J=9.2 Hz, 1H), 7.40 (d, J=8.7 Hz, 1H), 6.93 (d, J=8.7 Hz, 1H), 3.61 (t, J=8.8 Hz, 2H), 3.27 (m, 4H), 3.21 (t, J=7.3 Hz, 2H), 2.68 (t, J=6.5 Hz, 2H), 2.24 (t, J=6.0 Hz, 2H), 1.62 (m, 2H), 1.56 (m, 1H), 1.39 (m, 2H), 1.25 (m, 2H), 0.89 (d, J=6.8, Hz, 6H). 13C NMR (CDCl3) δ 198.4, 152.81, 144.54, 133.37, 126.42, 126.08, 124.58, 123.64, 121.77, 121.04, 110.33, 53.26, 48.99, 39.03, 38.49, 28.19, 27.89, 27.05, 26.15, 25.20, 23.12, 22.82. Found [M+Na]+ 358.21425. C23H29NONa+ requires 358.21414.
3. Results and Discussion
3.1 Synthesis of fluorescent cholesterol models
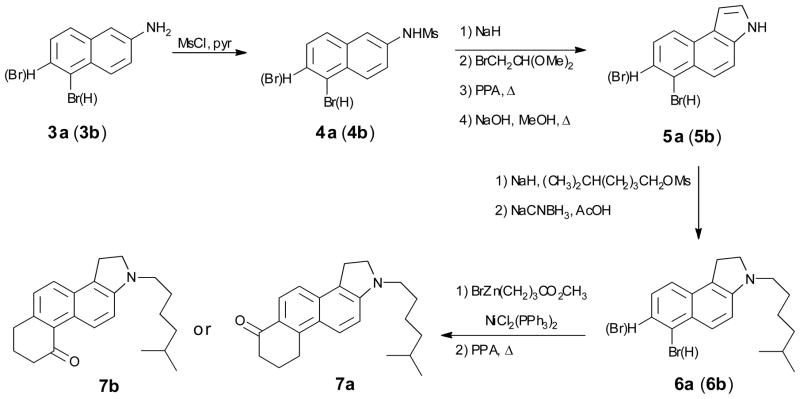
The reaction sequence for the preparation of 7a and 7b is shown in Scheme 1. Because the starting material is a naphthalene derivative, the synthesis results in the formation of a five-membered ring containing the amine and a six-membered ring containing the carbonyl. The creation of the indole ring follows the procedure of Sundberg and Laurino.[15] Alkylation of the indole anion is exceptionally facile: it does not require heating and the anion is too weak a base to promote an elimination reaction with the mesylate. Reduction of the indole to the indoline must be carried out before subsequent generation of the carbonyl group. Creation of the cyclohexanone substructure is accomplished through a Negishi coupling followed by electrophilic cyclization directly from the ester. Prior hydrolysis of the ester is not necessary. For 7a substitution occurs at the only available ortho postion, whereas with 7b substitution occurs exclusively at the α-position of the naphthalene ring.
Scheme 1.
Preparation of 7a and 7b
3.2 Absorption and steady-state fluorescence studies
The photophysical behavior of 7a and 7b in a range of aprotic solvents and two alcohols is reported in Table 1. The properties of related PRODAN derivatives are presented for comparison. In aprotic solvents fusing the amino group in a five-membered ring leads to a ~14 nm bathochromic shift in the absorption for 7a vs. 2d and to a ~24 nm shift in 7b vs. 2f. The effects on the fluorescence are more striking. In 7a the emission maximum is shifted by ~33 nm. For 7b the average shift is by 84 nm, but the magnitude increases from 58 nm to just over 100 nm as the solvent polarity increases. The effect of the fused ring is also seen in the relative fluorescence intensities. For 7a the intensity is relatively constant for all solvents. This behavior contrasts with that of PRODAN where the intensity drops precipitously in very nonpolar solvents. Relative fluorescence quantum yields for 7a are 0.70 ± 0.05 and 0.89 ± 0.04 in toluene and ethanol, respectively. For 7b the falloff in emission intensity with increasing solvent polarity is much more pronounced than in 2f. The relative fluorescence quantum yields for 7b are 0.28 ± 0.03 in toluene and 0.016 ± 0.004 in ethanol. The effects of protic solvents on the emission maximum in 7a are similar to those with 2a and 2d. The position of the maximum suffers an additional bathochromic shift due to hydrogen-bonding interactions. Alcohol solvents quench the fluorescence of 7b. The peak maximum undergoes a slight hypsochromic shift in isopropanol, and a much larger shift in ethanol, indicating a different emitting species.
Table 1.
Photophysical characteristics of cholesterol models 7a and 7b, PRODAN (2a) and related derivatives 2d and 2f.a
| solvent | 2a | 2d | 7a | 2f | 7b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| abs | em | I/Imax | abs | em | I/Imax | abs | em | I/Imax | abs | em | I/Imax | abs | em | I/Imax | |
| C6H12 | 343 | 392 | 0.01 | 347 | 407 | 0.01 | 362 | 433 | 0.77 | 397 | 449 | 0.72 | 418 | 507 | 1.00 |
| PhMe | 349 | 416 | 0.32 | 353 | 417 | 0.15 | 368 | 452 | 0.74 | 403 | 478 | 1.00 | 426 | 554 | 0.58 |
| PhCl | 354 | 425 | 0.63 | 357 | 423 | 0.32 | 370 | 462 | 0.89 | 402 | 498 | 0.96 | 427 | 573 | 0.39 |
| EtOAc | 352 | 430 | 0.71 | 353 | 429 | 0.33 | 366 | 467 | 0.69 | 396 | 506 | 0.59 | 417 | 586 | 0.21 |
| CH2Cl2 | 358 | 440 | 0.75 | 358 | 440 | 0.65 | 373 | 472 | 0.93 | 398 | 511 | 0.84 | 426 | 595 | 0.30 |
| EtCOMe | 367 | 443 | 0.83 | 356 | 443 | 0.55 | 368 | 481 | 0.52 | 398 | 517 | 0.30 | 420 | 612 | 0.06 |
| CH3CN | 368 | 456 | 0.85 | 356 | 448 | 0.79 | 370 | 489 | 0.90 | 396 | 523 | 0.24 | 419 | 625 | 0.03 |
| DMSO | 369 | 464 | 0.87 | 363 | 453 | 0.84 | 376 | 472 | 1.00 | 407 | 538 | 0.25 | 428 | 643 | 0.03 |
| iPrOH | 367 | 480 | 0.99 | 368 | 482 | 1.00 | 376 | 512 | 0.96 | 406 | 520 | 0.12 | 417 | 637 | <0.01 |
| EtOH | 369 | 494 | 1.00 | 369 | 489 | 0.82 | 374 | 522 | 0.94 | 406 | 495 | 0.05 | 410 | 539 | 0.02 |
Maximum absorbance (abs) and fluorescence (em) in nm. I/Imax is the normalized, absorption-adjusted emission intensity over the range of solvents.
3.3 Computational studies
The electronic structures for the optimized ground and excited states of 7a and 7b are calculated using the PM6 semiempirical method with a conductor like screening model (COSMO) to account for solvent effects. For these calculations the long alkyl chain is treated as a methyl group (7a′ and 7b′), and configurational interaction is limited to singly and doubly excited microstates using eight orbitals bracketing the HOMO. This method and CI level were chosen because they best reproduce the absorption and fluorescence data shown in Table 2. For both chromophores the dipole moment doubles in the relaxed excited state. The ground and excited state dipole moments are not collinear: they form angles of 25° and 70° for 7a′ and 7b′, respectively. The direction of the ground state dipole moments mostly derive from the carbonyl groups, whereas in the excited state the electron donation from the amino group becomes important. The change in the orientation of the ground and excited state dipole moments can be partially ascribed to the fixed positions of the carbonyl groups. The different angles for 7a′ and 7b′ result from the opposing directions of the carbonyl groups in addition to other electronic differences (e.g., direct resonance in 7a′ but not 7b′). As with PRODAN, 7a′ has two closelying Franck-Condon excited states within 0.2 eV, whereas in 7b′ the S2 state is >0.5 eV higher in energy. For PRODAN, and by analogy 7a′, the consequence of these two states is an internal conversion to a charge-transfer state (ICT) with a different electronic configuration.[16][17] For 7b′ the relaxed excited state has the same electronic configuration (singly occupied HOMO and LUMO) as the ground state as is seen with 2f.[12]
Table 2.
Photophysical parameters for 7a and 7b from semiempirical calculations and solvatochromism analysis.
| Solvent | μ(D)a | μ*(D)a | μ*/μ | ∠μ*μ(°) (Δμ)b | Abs (nm) (obs) | Em (nm) (obs) | Stokes (cm−1) | μ*(D)c (Δμ)d | μ*(D)e | |
|---|---|---|---|---|---|---|---|---|---|---|
| 7a | PhMe | 6.0 | 12.7 | 2.1 | 24 (7.6) | 399 (368) | 483 (452) | 4360 (5050) | 12.4 (8.7) | 10.2 |
| CH3CN | 7.3 | 15.2 | 2.1 | 25 (9.1) | 403 (370) | 516 (489) | 5430 (6580) | 13.1 (8.7) | 11.5 | |
| 7b | PhMe | 4.9 | 10.7 | 2.2 | 70 (10.1) | 422 (426) | 548 (554) | 5450 (5420) | 13.5 (11.8) | 10.2 |
| CH3CN | 6.2 | 12.2 | 2.0 | 69 (11.5) | 418 (419) | 648 (625) | 8490 (7870) | 14.0 (11.8) | 11.5 |
From semiempirical calculations.
Using δμ= μ2+μ*2 − 2μμ*cos θ.
From Eq. 3 using the slopes from the Lippert-Mataga and Mataga plots and the value of μ from semiempirical calculations and an Onsager radius of 5.1Å.
From Eq. 1. using the value of μ from semiempirial calculations and an Onsager radius of 5.1Å.
From Eq. 4 using the value of μ from semiempirial calculations and an Onsager radius of 5.1Å.
3.4 Solvatochromic analysis
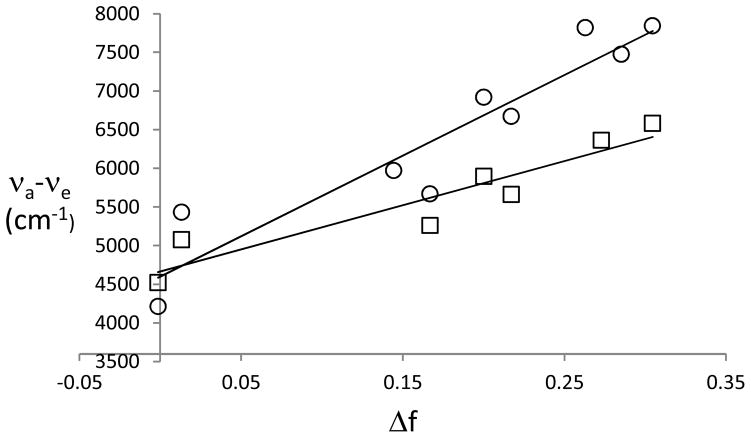
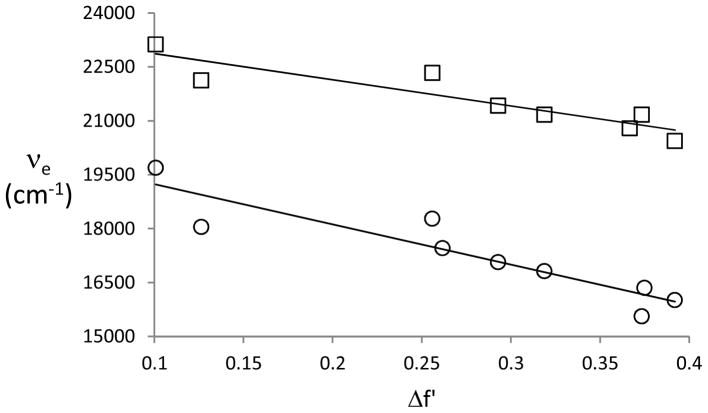
Analysis of the solvatochromic data using various theoretical models allows for the determination of the excited state dipole moments for 7a and 7b. In the Onsager model a combination of Lippert-Mataga (Fig. 3, Eq. 1) and Mataga plots (Fig. 4, Eq. 2) is typically sufficient to predict the ratio of the dipole moments of the excited and ground states (μ*/μ) without requiring the solute radius. However, this approach gives rise to very large values for the dipole moment ratios (5–10). Among the assumptions made in applying the Onsager model are a spherical fluorophore and collinear ground and excited dipole moments. Neither is accurate in the present case (cf. Table 2). The requirement of a spherical shape is typically ignored. Eq. 3 (where 2πε0hc = 1.105 × 10−35 C2) is used to calculate the excited state dipole moments when the collinearity condition is not satisfied. This expression only involves scalar terms. [18] Here the value of the Onsager radius is required, and a value of 5.1Å is calculated for 7a and 7b from the mass-density formula assuming a density of 0.95 g mL−1.[19] Only aprotic solvents are used in these analyses. As shown in Table 1, protic solvents give rise to unusual Stokes shifts because of the H-bonding interactions with the carbonyl group. The slopes of the best fit lines are greater in magnitude for 7b than for 7a in both the in the Lippert-Mataga (mL–M) plots (5700 cm−1 for 7a and 10400 cm−1 for 7b) and for the Mataga (mM) plots (−7300 cm−1 and −11200 cm−1, respectively). The dipole moments for the excited states derived from these plots are shown in Table 2. For 7a these values are slightly smaller than those predicted by the semiempirical calculations, whereas for 7b they are slightly larger.
Figure 3.
Lippert-Mataga plots for 7a (□) and 7b (○).
Figure 4.
Mataga plots for 7a (□) and 7b (○).
| (1) |
| (2) |
| (3) |
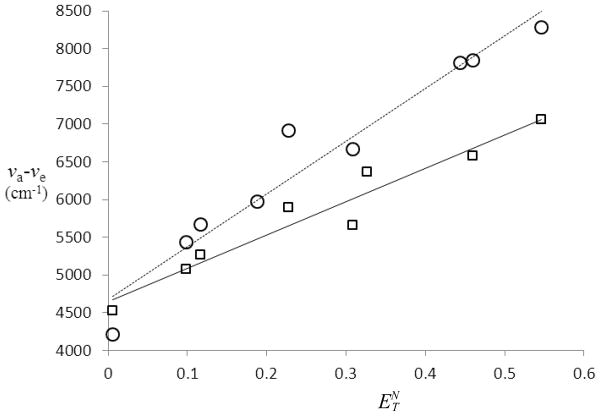
Another empirical correlation for the determination of the excited state dipole moment has been developed by Ravi using Reichart’s solvent parameter.[20,21] In this method the Stokes shift is plotted against as shown in Fig. 5. The slope of the best-fit line (mR) is related to the change in the dipole moments between the ground and excited states (Δμ= μ*−μ) by Eq. 4. In this expression the subscript B refers to the betaine dye used to develop the scale. For this pyridinium phenolate the Onsager radius (aB) is 6.2Å, and the change in dipole moments (ΔμB) is 9.0 D. The calculated excited state dipole moments from this analysis are shown in Table 2. That the slope for 7b is greater than that for 7a indicates that the former undergoes a greater change in dipole moment upon excitation.
Figure 5.
Plot of Stokes shifts for 7a (□) and 7b (○) vs.
| (4) |
Insight into how solvent affects the photophysical behavior of these compounds can be made through multiparameter regression analysis with empirical solvent parameter scales. The recent generalized treatment of Catalán divides the effects into fours scales: polarizability (SP), dipolarity (SdP), acidity (SA) and basicity (SB).[22] In this analysis the Stokes shift is treated as a function of the solvent parameters by Eq. 5. The SA term is deliberately ignored because protic solvents are not included. A second analysis is reported for both compounds with fewer solvent parameters. Results for the regression analysis are shown in Table 3. In general, the parameter fittings with 7a are not as good as with 7b. Solvent basicity does not seem to play a significant factor in either compound. For 7b the Stokes shift appears to be primarily dependent on solvent dipolarity. This singular dependence on dipolarity was also reported with 2f. [12] For 7a both dipolarity and polarizability are important.
Table 3.
Regression Results for Eq. 5
| SP | SdP | SB | νo | R2 | |
|---|---|---|---|---|---|
| 7a | −4540 ± 1380 | 1950 ± 340 | −690 ± 540 | 7860 ± 770 | 0.85 |
| −4090 ± 1090 | 1700 ± 300 | 7440 ± 990 | 0.83 | ||
| 7b | −990 ± 1070 | 3410 ± 300 | 510 ± 480 | 4920 ± 810 | 0.96 |
| 3560 ± 260 | 4300 ± 180 | 0.96 |
| (5) |
4. Conclusions
Fluorescent cholesterol model compounds 7a and 7b have been prepared in eight steps from 6- and 5-bromo-2-naphthylamine. They show similar solvatochromic behavior as with simpler, structurally-related compounds 2d and 2f. For 7a the effect of fusing the amino group into a five-membered ring decreases the fluorescence deactivation pathways especially in low polarity solvents. It must emit from a planar charge-transfer excited state. For 7b the ring fusion leads to progressively larger Stokes shifts with increasing solvent polarity. These greater shifts coincide with increased non-radiative decay that is characteristic of charge-transfer excited states. The absorption and emission profiles for these compounds make them potentially useful probes in biological systems as cholesterol models.
Supplementary Material
Acknowledgments
This research was supported by Grant 1R15 089925-01 from the NIH/NHLBI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scheidt HA, Müller P, Herrmann A, Huster D. J Biol Chem. 2003;278:45563. doi: 10.1074/jbc.M303567200. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Mintzer E, Bittman R. J Org Chem. 2006;71:1718. doi: 10.1021/jo052029x. [DOI] [PubMed] [Google Scholar]
- 3.Kao YJ, Soutar AK, Hong KY, Pownall HJ, Smith LC. Biochemistry. 1978;17:2689. doi: 10.1021/bi00606a036. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JE, Epand RF, Epand RM, Li Z, Bittman R, Yip CM. Biophys J. 2006;90:2170. doi: 10.1529/biophysj.105.073510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber G, Farris FJ. Biochemistry. 1979;18:3075. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- 6.Parusel A. J Chem Soc, Faraday Trans. 1998;94:2923. [Google Scholar]
- 7.Parusel ABJ, Schneider FW, Köhler G. J Mol Struct: THEOCHEM. 1997;398:341. [Google Scholar]
- 8.Parisio G, Marini A, Biancardi A, Ferrarini A, Mennucci B. J Phys Chem B. 2011;115:9980. doi: 10.1021/jp205163w. [DOI] [PubMed] [Google Scholar]
- 9.Davis BN, Abelt CJ. J Phys Chem A. 2005;109:1295. doi: 10.1021/jp046050y. [DOI] [PubMed] [Google Scholar]
- 10.Everett RK, Nguyen HAA, Abelt CJ. J Phys Chem A. 2010;114:4946. doi: 10.1021/jp1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo BC, Abelt CJ. J Phys Chem A. 2003;107:10938. [Google Scholar]
- 12.Abelt CJ, Sun T, Everett RK. Photochem Photobiol Sci. 2011 doi: 10.1039/c0pp00377h. [DOI] [PubMed] [Google Scholar]
- 13.Sanguinet L, Twieg RJ, Wiggers G, Mao G, Singer KD, Petschek RG. Tetrahedron Lett. 2005;46:5121. [Google Scholar]
- 14.Everett R, Hamilton J, Abelt C. Molbank. 2009:M602. doi: 10.3390/M602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundberg RJ, Laurino JP. J Org Chem. 1984;49:249. [Google Scholar]
- 16.Zachariasse KA. Chem Phys Lett. 2000;320:8. [Google Scholar]
- 17.Zachariasse KA, Druzhinin SI, Bosch W, Machinek R. J Am Chem Soc. 2004;126:1705. doi: 10.1021/ja037544w. [DOI] [PubMed] [Google Scholar]
- 18.Kawaki A. Solvent-Shift Effect on Electronic Spectra and Excited-State Dipole Moments. In: Rabek JF, editor. Progress in Photochemistry and Photophysics. Vol. 5. CRC; Boca Raton, FL: 1990. pp. 1–47. [Google Scholar]
- 19.Roesch N, Zerner MC. J Phys Chem. 1994;98:5817. [Google Scholar]
- 20.Ravi M, Soujanya T, Samanta A, Radhakrishnan T. J Chem Soc, Faraday Trans. 1995;91:2739. [Google Scholar]
- 21.Reichardt C, Welton T. Solvents and Solvent Effects in Organic Chemistry. Wiley-VCH Verlag GmbH; 2011. [Google Scholar]
- 22.Catalán J. J Phys Chem B. 2009;113:5951. doi: 10.1021/jp8095727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.