Abstract
The combination of genomics and high-throughput cDNA sequencing technologies has facilitated the identification of many small RNAs (sRNAs) that play a central role in the post-transcriptional gene regulation of Salmonella enterica serovar Typhimurium. To date, most of the functionally characterized sRNAs have been involved in the regulation of processes which are not directly linked to virulence. Just five sRNAs have been found to affect the ability of Salmonella to replicate within mammalian cells, but the precise regulatory mechanisms that are used by sRNAs to control Salmonella pathogenicity at the post-transcriptional level remain to be identified. It is anticipated that an improved understanding of sRNA biology will shed new light on the virulence of Salmonella.
Keywords: Salmonella Typhimurium, post-transcriptional regulation, sRNA, virulence
Introduction
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a well-characterized enteropathogen which causes both gastroenteritis and serious systemic infections. In humans, salmonellosis is mainly contracted by the ingestion of contaminated food or water. The annual cost of Salmonella infection in the USA is estimated to be US$3 billion.1 Thus, Salmonella continues to have a big impact upon human life, and the control of this bacterium remains a significant challenge for the food industry.
The fact that Salmonella bacteria have been found in a number of different sites in the body during infection and at different stages of food processing reflects the ability of the microbes to thrive in many environmental conditions. Salmonella can sense its environment and rapidly adapt to changing conditions, a process which is mediated by regulation at the transcriptional, post-transcriptional and translational levels. The key players involved in this adaptation process are transcription factors and nucleoid-associated proteins, as well as the more recently identified regulatory small RNAs (sRNAs). Though the first evidence for the existence of bacterial sRNAs was reported in 1967,2 most of the discoveries of bacterial sRNAs have only occurred in the last decade. The identification of sRNAs in Enterobacteriaceae initially focused on non-pathogenic strains of Escherichia coli.3-6 The strategy for finding sRNAs involved bioinformatic screens that were validated by experimental approaches using transcriptomic tools such as tiling microarrays and high-throughput cDNA sequencing (RNA-seq).7 To date, hundreds of sRNAs have been identified in bacteria, but roles in virulence have only been elucidated for a minority. RNAIII of Staphylococcus aureus was the first regulatory sRNA shown to be involved in bacterial pathogenicity by targeting at least five mRNAs that encode virulence factors.8-10 Other examples of virulence-associated sRNAs have been described in a recent review.11
Here we survey the sRNAs identified in S. Typhimurium to date and discuss our current understanding of the role of sRNAs in the control of virulence. We then focus on the regulation of these sRNAs and their target mRNAs.
Small RNAs in bacteria
sRNAs are stable and abundant transcripts of about 50–500 nucleotides in length which are usually non-coding and exhibit a regulatory function. Post-transcriptional gene regulation by sRNAs may occur in different ways by base-pairing interaction with a target RNA resulting in different outcomes or by directly binding to proteins to modulate their function.12-14 Two distinct classes of sRNAs have been identified: trans-encoded RNAs which are transcribed from intergenic regions of the genome, and cis-encoded RNAs which are encoded on the strand complementary to coding sequences or the 5′ or 3′ untranslated region (5′ UTR, 3′ UTR) of transcripts.15-17 The family of trans-encoded sRNAs usually requires the chaperone Hfq to stabilize the often imperfect base-pairing interaction with target mRNA.18 In contrast, cis-encoded sRNAs possess a region of perfect complementarity to their target mRNA and Hfq is not needed for target binding. It is now clear that sRNAs are involved in many key physiological processes including anaerobic growth, nutrient availability, iron homeostasis and the response to oxidative, envelope and osmotic stress.9,19-23
Insights from sRNA research in Salmonella
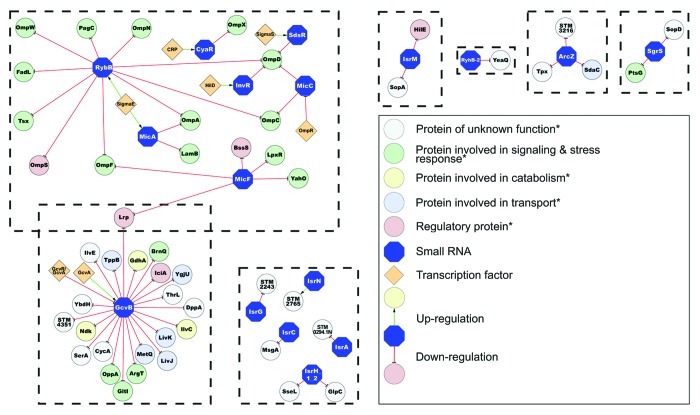
Much of the initial investigation of riboregulation by sRNAs involved non-pathogenic E. coli strains as a model. The more recent use of Salmonella as a model organism allows us to ask new questions about sRNAs involved in virulence in a variety of infection models, in the context of a well-established array of genetic tools. Next to E. coli, Salmonella is now the best-characterized model of sRNA-mediated regulation in Gram-negative bacteria. To date, the largest sRNA regulon has been identified in Salmonella, with the GcvB sRNA controlling expression of ~1% of the S. Typhimurium genome (Fig. 1).24,25 These studies expand our view on the biological significance of sRNAs, establishing them as global gene regulators.
Figure 1. An overview of published small RNA regulatory networks in S. Typhimurium.24,25,29,32,33,36,37,45,56,57,74,93-98
Key mechanistic findings from studies in Salmonella have advanced our basic understanding of sRNA-mediated regulation in bacteria. The distinct modular structure of sRNAs, including the highly conserved target binding region (also referred as to the “seed” region), was demonstrated for the σE-dependent sRNA RybB in Salmonella. Fusion of this seed region to an unrelated sRNA backbone permitted full repression of the RybB regulon.26-28
Current techniques for the discovery of sRNA targets
To complement the identification of new sRNAs, several methods allowing the discovery of sRNAs targets have been developed in Gram-negative bacteria. For trans-encoded sRNAs, the base-pairing interaction with mRNAs is imperfect and often requires Hfq. One example is the Hfq-associated sRNA MicC sRNA which silences S. Typhimurium ompD mRNA and only requires a ≤ 12-bp RNA duplex within the CDS (codons 23–26) for repression.29 Different bioinformatic tools allow the prediction of the binding regions of sRNA and mRNA by combining comparative genomics with a search for certain physical parameters. TargetRNA calculates optimal hybridization scores between an sRNA and all the mRNA in the genome.30 IntaRNA is a method for the prediction of interactions between two RNAs based on minimization of an extended hybridization energy.31 Although most of these software tools can confirm previously known findings, they should be considered as predictive tools that often produce false-positive results and require experimental validation.
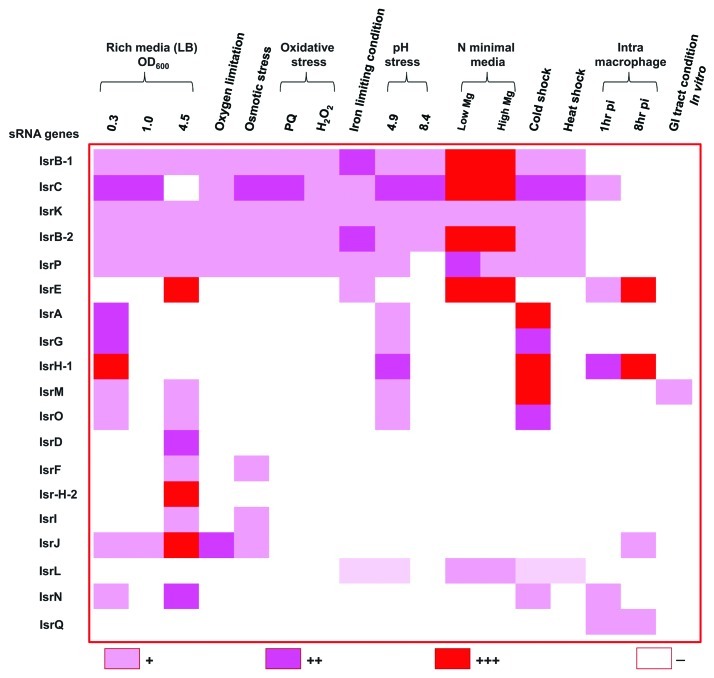
As the interaction of cis-encoded sRNAs involves a perfect match, the identification of their targets is more straightforward. IsrA is a cis-encoded RNA present on the complementary strand to the STM0294.1 gene, which encodes a protein with no clear functional annotation. Padalon-Brauch et al. have shown that IsrA is expressed during exponential phase, osmotic stress, peroxide stress and cold shock, and downregulated during stationary phase (Fig. 2). The expression of STM0294.1 shows the opposite pattern to that of IsrA, and so it has been suggested that IsrA could regulate transcription of STM0294.1.32
Figure 2. Differential expression of S. Typhimurium island-encoded sRNAs during growth under various stress conditions determined by northern blot analysis32 or RT-PCR.57 Expression levels are shown as high, medium, low and no expression.32 Cultures of S. Typhimurium were grown under different conditions (described from left-hand side): Cells grown in LB to an OD600 of 0.3, 1 and 4.5; Oxygen limitation overnight growth without agitation in 50 mL Falcon tubes to an OD600 of 0.9; Osmotic stress—cells grown in LB containing elevated (0.5 M) NaCl levels for 30 min; Oxidative stress using 0.2 mM paraquat (PQ) and 1 mM hydrogen peroxide (H2O2); Iron limiting conditions—addition of 0.2 mM 2,2’ dipyridyl; pH stress – LB at pH 4.9 (adjusted with HCl) and LBK media at pH 8.4; N min low Mg and N min high Mg – N minimal media with 10 µM MgCl2 (low magnesium) and 10 mM MgCl2 (high magnesium); Cold shock at 15°C and heat shock at 42°C; Intra-macrophage 1h and 8h – within activated J774.A1 macrophage cells assayed using gentamycin protection assay;32 In vitro conditions resembling the gastrointestinal tract.57
Pulse-expression of sRNAs has been developed as an efficient method to identify mRNA targets, because classical genetic approaches often result in quite subtle phenotypes for sRNA mutants.33,34 The technique involves the rapid overproduction of an sRNA followed by the use of a microarray to identify bacterial transcripts that were bound by the sRNA and subsequently degraded by an RNaseE-dependent mechanism.33 This approach has been used to elucidate large regulons of as many as 50 genes controlled by a single sRNA (Fig. 1).
The regulatory interaction between sRNAs and candidate mRNA targets must be confirmed within living bacterial cells, and can be done with a GFP-based two-plasmid reporter system. The ablation of GFP fluorescence by expression of the sRNA confirms the direct effect of an sRNA upon its mRNA target. This is measured accurately by flow cytometry, on agar plates or by determining the GFP protein levels by western blotting.35
The attribution of the role of a particular sRNA can be complicated by functional redundancy as it is well known that several sRNAs can silence the same target mRNA. For example, MicC, RybB, InvR and SdsR all negatively regulate the ompD gene (Fig. 1).26,29,36,37 In this case, the effect of deleting one sRNA could be masked by the action of the remaining three sRNAs, and it may be necessary to delete all the sRNAs regulating a particular pathway to observe a clear phenotype. Another example of functional redundancy is the Csr system which modulates carbon metabolism and also regulates SPI1 and SPI2 expression through HilD.38 It comprises two sRNAs, CsrB and CsrC and the RNA chaperone CsrA.39,40 Although the single mutants ΔcsrB or ΔcsrC are not impaired in their ability to infect epithelial cells, a ΔcsrB ΔcsrC mutant shows a significant invasion defect. While complementation with either CsrB or CsrC leads only to partial restoration of wild-type levels of invasion, the presence of both sRNAs expressed in trans is necessary to rescue the invasion defect, illustrating the difficulties in assigning virulence-associated functions to sRNAs.
Conservation of small RNAs between Salmonella and E. coli
The last decade witnessed increasing numbers of sRNAs being discovered in E. coli and Argaman et al. reported that about 24 sRNAs were conserved between Salmonella and E. coli.3,41 This important finding prompted the use of conservation analysis to discover new sRNAs in E. coli.5 The advent of bacterial whole genome sequencing and the use of RNA-seq led to the discovery of the widespread nature of sRNAs, many of which were found to be highly conserved in intergenic regions in bacteria. Approximately 400 sRNAs have now been predicted in about 70 microbial genomes, including those of the Escherichia, Shigella and Salmonella genera,42 and comparative analyses of the genomes of different Salmonella serovars and E. coli have shown the levels of sRNA conservation. Recent studies have reported similar levels (48–67%) of conservation among Salmonella and E. coli species.43,44 The large number of non-conserved small RNAs suggests that species-specific sRNAs could have specialized roles in pathogenicity. However, even highly conserved sRNAs were shown to regulate species-specific virulence factors as demonstrated by Papenfort et al.45 In this study, the Salmonella-specific effector protein SopD was shown to be regulated by the ancestral sRNA SgrS, which is found in both pathogenic and non-pathogenic species.
Expression profiles of S. Typhimurium sRNAs and their role during infection
S. Typhimurium and E. coli diverged from a common ancestor about 100–130 million years ago and share about 71% of their genetic information.46-48S. Typhimurium possesses a unique set of attributes that allow it to survive in the hostile environments associated with each stage of animal infection, and to colonize different intracellular niches within mammalian cells. For instance, once ingested, this bacterium must first cope with an increase in temperature followed by the acidic environment of the stomach. In the intestine, the microorganism is subjected to increased osmolarity, a decrease in oxygen tension, bile and competition with the intestinal microbiota.49Salmonella can subsequently enter and proliferate within non-phagocytic and phagocytic cells, where the pathogen resists intracellular defense mechanisms such as antimicrobial peptides, the acidification of the Salmonella-containing vacuole (SCV) and the production of reactive oxygen and nitrogen species. In response to these stressful conditions, S. Typhimurium must quickly modulate its transcriptional profile, raising the possibility that the rapid gene regulation mediated by sRNAs would be particularly relevant.50 Monitoring sRNA expression could reveal patterns of induction relevant to the strategies used by Salmonella to survive within host cells.
An sRNA involved in Salmonella virulence was first reported in the year 2000.51 Deletion of the bi-functional transfer-mRNA (tmRNA), which rescues ribosomes stalled on defective mRNAs (reviewed in52), resulted in an avirulent Salmonella mutant in mouse infections. Binding of tmRNA to stalled ribosomes requires the small protein SmpB which has been shown to be important for proliferation of Salmonella in macrophages.53,54
In an attempt to find S. Typhimurium specific sRNAs that had not already been characterized in E. coli, the Altuvia lab used a computational approach to identify and validate 19 new sRNAs located in intergenic regions of the Salmonella pathogenicity islands (SPIs).32 The sRNA expression was monitored by northern blot analysis both in media mimicking infection-relevant stress conditions and directly inside macrophages. Many of the island-encoded sRNAs were induced in conditions including stationary phase growth, in minimal medium, upon temperature shock, acidity and oxidative stress (Fig. 2). In macrophages, expression of IsrC and IsrN was induced early during infection and then decreased as the infection progressed, similar to the results shown earlier for OxyS in E. coli.20,55 Conversely, IsrE (RyhB2), RyhB1 and IsrH showed increased levels of expression later during infection. The differential expression patterns suggest a role for sRNAs at different stages of infection. In contrast, IsrH has recently been shown to be downregulated during infection of fibroblasts, in which wild-type Salmonella is non-replicative.56
Two of the island-encoded sRNAs, IsrJ and IsrM, were found to be particularly important for Salmonella proliferation within non-phagocytic cells and/or macrophages.32,57 IsrJ is upregulated under conditions which promote invasion of epithelial cells and is positively regulated by HilA, the central transcriptional activator of SPI1. The deletion of isrJ results in a less invasive mutant strain that is impaired for translocation of the effector protein SptP, which is required for remodelling the host cell cytoskeleton after bacterial entry.32,58
The ΔisrM mutant showed a broad virulence defect, with reduced invasion of epithelial cells, lower intracellular replication/survival in macrophages, and reduced growth in the ileum and spleen of mice.57 IsrM post-transcriptionally represses the expression of virulence factors hilE and sopA. Most SPI1 genes are negatively regulated by HilE through sequestration of HilD, the major transcriptional activator of SPI1, while SopA is a secreted effector protein that is involved in causing inflammation and diarrhea.59,60 IsrM therefore aids in choreographing the expression of virulence factors.
Another study to identify sRNAs required for S. Typhimurium virulence focused on 37 sRNAs that are conserved in both E. coli and S. Typhimurium. Single small RNA deletion mutants were tested by competitive index in the murine infection model. A key finding of this study was that 34 of the tested 37 sRNAs did not play a role in Salmonella virulence (Table 1a).61 Only two sRNA mutants, ΔsroA and ΔistR gave a reproducible attenuated phenotype in mice, with a reduced ability to compete with the wild-type strain (Table 1b). The IstR sRNA, which was originally identified in E. coli by the Altuvia lab in 2004, inhibits the synthesis of an SOS-induced toxic peptide.62 The SroA RNA is assumed to result from attenuated transcription of a riboswitch element of the thiBPQ mRNA that codes for proteins involved in thiamine uptake,63,64 but its function remains unclear. In contrast, one strain lacking the OxyS sRNA was shown to be hyper-virulent. OxyS, a member of the OxyR regulon, is upregulated by micromolar levels of peroxide and coordinates the cellular response to oxidative stress.65
Table 1a. Thirty four S. Typhimurium sRNAs that are not required for murine virulence*.
| sRNA name (Alternative name) | First reported in | Upstream gene |
Downstream gene | Relevant references |
|---|---|---|---|---|
| ArcZ (SraH, RyhA) |
E. coli |
yhbL |
acrB |
74 99 |
| CsrB |
E. coli |
yqcC |
syd |
100 40 |
| CyaR (RyeE) |
E. coli |
yegQ |
SL2113 |
93 |
| DsrA |
E. coli |
yodD |
yedP |
71 75 |
| GcvB (IS145) |
E. coli |
gcvA |
ygdI |
101 24 25 |
| GlmY (SroF, tke1) |
E. coli |
yfhK |
purG |
102 103 |
| GlmZ (SraJ, k19, RyiA) |
E. coli |
yifK |
hemY |
102 103 |
| InvR (STnc270) |
Salmonella |
invH |
SL2880 |
37 |
| IsrB-1 (IS092) |
Salmonella |
SL0946 |
SL0947 |
32 |
| IsrC (IS102) |
Salmonella |
envF |
msgA |
32 |
| IsrE (RyhB-2, RfrB) |
Salmonella |
SL1208 |
yeaQ |
32 |
| MicA (SraD) |
E. coli |
luxS |
gshA |
104 33 96 105 |
| MicC (IS063, tke8) |
E. coli |
nifJ |
ynaF |
29 |
| MicF |
E. coli |
ompC |
yojN |
106 107 95 |
| MicM (RybC, ChiX, SroB) |
E. coli |
ybaK |
ybaP |
63 108 |
| MntS (RybA) |
E. coli |
ybiP |
mntR |
5 |
| OmrA (RygB) |
E. coli |
aas |
galR |
27 |
| OmrB (t59, RygA, SraE) |
E. coli |
aas |
galR |
27 |
| RprA (ISO83) |
E. coli |
ydiK |
ydiL |
72 75 |
| RybB (p25) |
E. coli |
SL0845 |
SL0846 |
5,33,109,28,26 |
| RydB (tpe7, IS082) |
E. coli |
ydiH |
SL1302 |
5 |
| RydC (IS067) |
E. coli |
SL1568 |
cybB |
4,110 |
| SdsR (RyeB, tpke79) |
E. coli |
SL1806 |
SL1807 |
111,36 |
| RyfA (tp1, PAIR3) |
E. coli |
SL2496 |
sseB |
5 |
| RyhB (RyhB-1, SraI, IS176, RfrA) |
E. coli |
yhhX |
yhhY |
5 |
| SgrS (RyaA) |
E. coli |
yobN |
leuD |
112,94,45 |
| SibC (t27, RygC, QUAD1c) |
E. coli |
ygfA |
serA |
113,114 |
| SibD (tp8, RygD, C0730) |
E. coli |
yqiK |
rfaE |
113,114 |
| Spot42 (spf) |
E. coli |
polA |
yihA |
115,116 |
| SraA (psrA/t15) |
E. coli |
clpX |
lon |
3 |
| SraB (pke2) |
E. coli |
SL1126 |
yceD |
3 |
| SraF (tpk1, IS160, PRE-element) |
E. coli |
yceD |
ygjT |
3,117 |
| SraL (RyjA) |
E. coli |
soxR |
SL4203 |
3 |
| SroC | E. coli | gltJ | gltI | 63 |
Table 1b. Five sRNAs involved in virulence of S. Typhimurium.
| sRNA name | Target mRNA | Role in virulence | References |
|---|---|---|---|
| IsrJ |
|
Control of effector protein production |
32 |
| IsrM |
hilE, sopA |
Modulates the expression of SPI1 proteins via hilE; downregulates SopA |
57 |
| IstR |
tisAB |
SOS induced toxic peptide – Inhibits growth allowing DNA repair |
62,61 |
| OxyS |
Regulates about 40 genes; including rpoS |
Inhibits alternate stress adaptation pathways during oxidative stress |
20,32,61 |
| SroA | Riboswitch element of the thiBPQ operon | Putative import of Thiamine and Thiamine pyrophosphate | 61 |
AmgR is a 1.2 Kb antisense transcript encoded on the complementary strand to the mgtCBR operon. The mgtC gene encodes a protein necessary for Salmonella to survive within macrophages, to grow in low Mg2+ environments and for virulence in mice.66 PhoQ, the kinase in the PhoPQ two component regulatory system, senses low levels of Mg2+ and the response regulator PhoP induces transcription of the mgtCBR operon. AmgR regulates expression of the mgtCBR operon by de-stabilizing the mgtC and mgtB transcripts in an RNaseE-dependent manner. An amgR mutant strain was found to be more virulent than the wild-type strain in mice. AmgR is PhoP-dependent and PhoP directly binds the amgR promoter, leading to amgR expression in low Mg2+ conditions. Therefore, PhoP has an apparently paradoxical effect on mgtC expression as it directly activates both mgtC and amgR, but AmgR has a repressive effect on mgtC. This regulatory mechanism may have evolved to titrate the levels of MgtC expressed at appropriate times during infection.67
The published roles of sRNAs in the virulence of S. Typhimurium are summarized in Tables 1a and 1b, and it is likely that the list of sRNAs that are required for infection will increase in the future. The expression profiles derived from northern blot and RT-PCR analyses of 19 island-encoded sRNAs are shown in Figure 2, and it is apparent that the levels of sRNAs vary in different environmental conditions. The recent profiling of 13 sRNAs during infection of fibroblasts showed that the levels of sRNAs varied during an infection time-course.56 The levels of regulatory sRNAs within bacterial cells are likely to give clues to their function, and so expression profiling should be a useful discovery tool in the future.
RpoS and Salmonella virulence
The alternative sigma factor RpoS (σ38) plays a key role in Salmonella infection and is required for full virulence of S. Typhimurium.68,69 Specifically, RpoS is important for persistence in lymphoid organs, such as the spleen and liver, and for the initial stages of infection in murine Peyer's patches.69 RpoS also activates the plasmid-borne spvR and spvABCD genes, which are required for intracellular growth and systemic infection in mice and humans.70
In E. coli, the translation of RpoS is repressed by OxyS68 and the sigma factor is positively regulated by 3 Hfq-dependent sRNAs, namely DsrA, ArcZ and RprA, which act by relieving the inhibitory secondary structure that prevents rpoS translation.71-73 This type of regulation is conserved, but is less pronounced in Salmonella, questioning the significance of DsrA, ArcZ and RprA for Salmonella virulence.74,75 Further study of the function of the RpoS sigma factor in S. Typhimurium is required, and may lead to the identification of more links with sRNA biology.
Hfq as a mediator of sRNA regulation
The Hfq protein is a key player in the global post-transcriptional regulatory network that facilitates the interactions of Salmonella sRNAs with target mRNAs.18 Deletion of hfq gene in Salmonella gives rise to a non-motile strain which is highly attenuated in its ability to infect mice, invade epithelial cells, secrete virulence factors and to survive and proliferate within macrophages. These significant phenotypes suggest that Hfq interacts with a number of sRNAs which are involved in virulence.54,76,77 As most trans-acting sRNAs depend upon Hfq to stabilize their binding to target mRNAs, the chaperone can facilitate the binding of an sRNA to its target mRNA and thereby prevent translation or induce target degradation. Hfq can also bring about positive regulation by recruiting an sRNA to its target binding site and thereby de-stabilizing secondary structures which inhibit target translation.78,79 Additionally, Hfq can regulate sRNA levels independently from their mRNA targets by protecting sRNAs from endonucleolytic decay.80 There are several suggestions as to how Hfq regulates sRNAs by controlling the base-pairing interaction between the sRNA and its target mRNA. The protein may act as a catalyst which increases the rate of complex formation between the trans-acting sRNA and an mRNA to stabilize the imperfect base-pairing between the two RNAs, as duplex formation in the absence of Hfq is relatively poor.81,82
In E. coli, Hfq has been demonstrated to interact with other RNA-associated proteins, such as PNPase, an exoribonuclease, and PAP, a poly(A) polymerase which may add an additional level of sRNA regulation by Hfq.83 Recently, it was also suggested that Hfq plays a role in transcription termination in E. coli by associating with the transcription termination factor Rho.84 Furthermore, limiting concentrations of Hfq can regulate sRNAs as the abundance of Hfq per cell remains fairly constant while the amount of its target sRNAs can increase under certain conditions.85 Sequestration of Hfq can therefore serve to modulate sRNA function by creating competition for binding between different sRNAs. This was demonstrated in E. coli when overexpression of one sRNA led to a decrease in the accumulation of other sRNAs, as Hfq protein levels become limiting.86 This method of regulation by Hfq was also suggested by the transcriptomic profile of a strain overexpressing ArcZ which showed some similarities to that of an ∆hfq mutant, indicating that an individual sRNA may displace other sRNAs from Hfq.74
Future prospects: Next-Generation Sequencing
RNA-seq is now the tool of choice for the discovery of novel small RNAs in bacteria.77,87,88 The rapid reduction in the costs of RNA-seq will lead to increasing numbers of new sRNAs being identified in the near future. The addition of small RNA genes to existing genome annotations will help to shed light on the complex nature of the bacterial transcriptome.
The recent publication of the transcriptional landscape of S. Typhimurium represents an important advance. An RNA-seq-based approach was used to identify the major transcriptional start sites and to define the motif of σ70-dependent promoters.44 About 140 sRNAs were found to be expressed at one stage of growth. The fact that 60 novel sRNAs were discovered in a single set of experiments suggests that next-generation sequencing-based methods will make a big impact upon the RNA world.
In future, technical advances promise to extend the applicability of RNA-seq for the monitoring of transcriptional changes in complex environments or from the very low (femtomolar) amounts of RNA obtained from infected animals.89,90 Another challenge will be to simplify the identification of mRNA targets. Until now, pulse overexpression of a small RNA and subsequent monitoring of transcript levels using a microarray has been widely used to identify many mRNA targets,91 but this is a labor-intensive approach. Sequence-based target prediction tools are available on the web, and as they become more effective they will be a valuable and cost-effective alternative to experimental approaches.92
Although many un-answered questions remain about the precise role of sRNAs during the infection process, it is likely that the burgeoning field of sRNA biology will have a great impact on our understanding of Salmonella pathogenicity.
Acknowledgements
We are grateful to anonymous reviewers for their insightful comments, to Karsten Hokamp for assistance with Figure 2 and to Science Foundation Ireland for financial support under Grant Number 08/IN.1/B2104.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/20480
References
- 1.Frenzen PD, Riggs TL, Buzby JC, Breuer T, Roberts T, Voetsch D, et al. Salmonella cost estimate updated using FoodNet data. Food Review. 1999;22:10–5. [Google Scholar]
- 2.Hindley J. Fractionation of 32P-labelled ribonucleic acids on polyacrylamide gels and their characterization by fingerprinting. J Mol Biol. 1967;30:125–36. doi: 10.1016/0022-2836(67)90248-3. [DOI] [PubMed] [Google Scholar]
- 3.Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, et al. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11:941–50. doi: 10.1016/S0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang A, Wassarman KM, Rosenow C, Tjaden BC, Storz G, Gottesman S. Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol. 2003;50:1111–24. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- 5.Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001;15:1637–51. doi: 10.1101/gad.901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivas E, Klein RJ, Jones TA, Eddy SR. Computational identification of noncoding RNAs in E. coli by comparative genomics. Curr Biol. 2001;11:1369–73. doi: 10.1016/S0960-9822(01)00401-8. [DOI] [PubMed] [Google Scholar]
- 7.Sorek R, Cossart P. Prokaryotic transcriptomics: a new view on regulation, physiology and pathogenicity. Nat Rev Genet. 2010;11:9–16. doi: 10.1038/nrg2695. [DOI] [PubMed] [Google Scholar]
- 8.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–75. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 1995;14:4569–77. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, et al. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 2007;21:1353–66. doi: 10.1101/gad.423507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papenfort K, Vogel J. Regulatory RNA in bacterial pathogens. Cell Host Microbe. 2010;8:116–27. doi: 10.1016/j.chom.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Majdalani N, Vanderpool CK, Gottesman S. Bacterial small RNA regulators. Crit Rev Biochem Mol Biol. 2005;40:93–113. doi: 10.1080/10409230590918702. [DOI] [PubMed] [Google Scholar]
- 13.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell. 2011;43:880–91. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pichon C, Felden B. Proteins that interact with bacterial small RNA regulators. FEMS Microbiol Rev. 2007;31:614–25. doi: 10.1111/j.1574-6976.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- 15.Vogel J. A rough guide to the non-coding RNA world of Salmonella. Mol Microbiol. 2009;71:1–11. doi: 10.1111/j.1365-2958.2008.06505.x. [DOI] [PubMed] [Google Scholar]
- 16.Georg J, Hess WR. cis-antisense RNA, another level of gene regulation in bacteria. Microbiol Mol Biol Rev. 2011;75:286–300. doi: 10.1128/MMBR.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomason MK, Storz G. Bacterial antisense RNAs: how many are there, and what are they doing? Annu Rev Genet. 2010;44:167–88. doi: 10.1146/annurev-genet-102209-163523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9:578–89. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polayes DA, Rice PW, Dahlberg JE. DNA polymerase I activity in Escherichia coli is influenced by spot 42 RNA. J Bacteriol. 1988;170:2083–8. doi: 10.1128/jb.170.5.2083-2088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell. 1997;90:43–53. doi: 10.1016/S0092-8674(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 21.Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:4620–5. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boysen A, Møller-Jensen J, Kallipolitis B, Valentin-Hansen P, Overgaard M. Translational regulation of gene expression by an anaerobically induced small non-coding RNA in Escherichia coli. J Biol Chem. 2010;285:10690–702. doi: 10.1074/jbc.M109.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durand S, Storz G. Reprogramming of anaerobic metabolism by the FnrS small RNA. Mol Microbiol. 2010;75:1215–31. doi: 10.1111/j.1365-2958.2010.07044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma CM, Darfeuille F, Plantinga TH, Vogel J. A small RNA regulates multiple ABC transporter mRNAs by targeting C/A-rich elements inside and upstream of ribosome-binding sites. Genes Dev. 2007;21:2804–17. doi: 10.1101/gad.447207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma CM, Papenfort K, Pernitzsch SR, Mollenkopf HJ, Hinton JC, Vogel J. Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol Microbiol. 2011;81:1144–65. doi: 10.1111/j.1365-2958.2011.07751.x. [DOI] [PubMed] [Google Scholar]
- 26.Papenfort K, Bouvier M, Mika F, Sharma CM, Vogel J. Evidence for an autonomous 5′ target recognition domain in an Hfq-associated small RNA. Proc Natl Acad Sci U S A. 2010;107:20435–40. doi: 10.1073/pnas.1009784107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillier M, Gottesman S. The 5′ end of two redundant sRNAs is involved in the regulation of multiple targets, including their own regulator. Nucleic Acids Res. 2008;36:6781–94. doi: 10.1093/nar/gkn742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balbontín R, Fiorini F, Figueroa-Bossi N, Casadesús J, Bossi L. Recognition of heptameric seed sequence underlies multi-target regulation by RybB small RNA in Salmonella enterica. Mol Microbiol. 2010;78:380–94. doi: 10.1111/j.1365-2958.2010.07342.x. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer V, Papenfort K, Lucchini S, Hinton JC, Vogel J. Coding sequence targeting by MicC RNA reveals bacterial mRNA silencing downstream of translational initiation. Nat Struct Mol Biol. 2009;16:840–6. doi: 10.1038/nsmb.1631. [DOI] [PubMed] [Google Scholar]
- 30.Tjaden B. TargetRNA: a tool for predicting targets of small RNA action in bacteria. Nucleic Acids Res. 2008;36(Web Server issue):W109-13. doi: 10.1093/nar/gkn264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busch A, Richter AS, Backofen R. IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24:2849–56. doi: 10.1093/bioinformatics/btn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padalon-Brauch G, Hershberg R, Elgrably-Weiss M, Baruch K, Rosenshine I, Margalit H, et al. Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–27. doi: 10.1093/nar/gkn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JC, Vogel J. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol. 2006;62:1674–88. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massé E, Vanderpool CK, Gottesman S. Effect of RyhB small RNA on global iron use in Escherichia coli. J Bacteriol. 2005;187:6962–71. doi: 10.1128/JB.187.20.6962-6971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urban JH, Vogel J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 2007;35:1018–37. doi: 10.1093/nar/gkl1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frohlich KS, Papenfort K, Berger AA, Vogel J. A conserved RpoS-dependent small RNA controls the synthesis of major porin OmpD. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeiffer V, Sittka A, Tomer R, Tedin K, Brinkmann V, Vogel J. A small non-coding RNA of the invasion gene island (SPI-1) represses outer membrane protein synthesis from the Salmonella core genome. Mol Microbiol. 2007;66:1174–91. doi: 10.1111/j.1365-2958.2007.05991.x. [DOI] [PubMed] [Google Scholar]
- 38.Martínez LC, Yakhnin H, Camacho MI, Georgellis D, Babitzke P, Puente JL, et al. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. Mol Microbiol. 2011;80:1637–56. doi: 10.1111/j.1365-2958.2011.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timmermans J, Van Melderen L. Post-transcriptional global regulation by CsrA in bacteria. Cell Mol Life Sci. 2010;67:2897–908. doi: 10.1007/s00018-010-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fortune DR, Suyemoto M, Altier C. Identification of CsrC and characterization of its role in epithelial cell invasion in Salmonella enterica serovar Typhimurium. Infect Immun. 2006;74:331–9. doi: 10.1128/IAI.74.1.331-339.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershberg R, Altuvia S, Margalit H. A survey of small RNA-encoding genes in Escherichia coli. Nucleic Acids Res. 2003;31:1813–20. doi: 10.1093/nar/gkg297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang HY, Chang HY, Chou CH, Tseng CP, Ho SY, Yang CD, et al. sRNAMap: genomic maps for small non-coding RNAs, their regulators and their targets in microbial genomes. Nucleic Acids Res. 2009;37(Database issue):D150–4. doi: 10.1093/nar/gkn852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinni SV, Raabe CA, Zakaria R, Randau G, Hoe CH, Zemann A, et al. Experimental identification and characterization of 97 novel npcRNA candidates in Salmonella enterica serovar Typhi. Nucleic Acids Res. 2010;38:5893–908. doi: 10.1093/nar/gkq281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kröger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, Hokamp K, et al. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1201061109. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papenfort K, Podkaminski D, Hinton JC, Vogel J. The ancestral SgrS RNA discriminates horizontally acquired Salmonella mRNAs through a single G-U wobble pair. Proc Natl Acad Sci U S A. 2012;109:E757–64. doi: 10.1073/pnas.1119414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–6. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 47.Doolittle RF, Feng DF, Tsang S, Cho G, Little E. Determining divergence times of the major kingdoms of living organisms with a protein clock. Science. 1996;271:470–7. doi: 10.1126/science.271.5248.470. [DOI] [PubMed] [Google Scholar]
- 48.Achtman M. Evolution, population structure, and phylogeography of genetically monomorphic bacterial pathogens. Annu Rev Microbiol. 2008;62:53–70. doi: 10.1146/annurev.micro.62.081307.162832. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-Ordóñez A, Begley M, Prieto M, Messens W, López M, Bernardo A, et al. Salmonella spp. survival strategies within the host gastrointestinal tract. Microbiology. 2011;157:3268–81. doi: 10.1099/mic.0.050351-0. [DOI] [PubMed] [Google Scholar]
- 50.Shimoni Y, Friedlander G, Hetzroni G, Niv G, Altuvia S, Biham O, et al. Regulation of gene expression by small non-coding RNAs: a quantitative view. Mol Syst Biol. 2007;3:138. doi: 10.1038/msb4100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Julio SM, Heithoff DM, Mahan MJ. ssrA (tmRNA) plays a role in Salmonella enterica serovar Typhimurium pathogenesis. J Bacteriol. 2000;182:1558–63. doi: 10.1128/JB.182.6.1558-1563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem. 2007;76:101–24. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- 53.Bäumler AJ, Kusters JG, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–30. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ansong C, Yoon H, Porwollik S, Mottaz-Brewer H, Petritis BO, Jaitly N, et al. Global systems-level analysis of Hfq and SmpB deletion mutants in Salmonella: implications for virulence and global protein translation. PLoS One. 2009;4:e4809. doi: 10.1371/journal.pone.0004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlosser-Silverman E, Elgrably-Weiss M, Rosenshine I, Kohen R, Altuvia S. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J Bacteriol. 2000;182:5225–30. doi: 10.1128/JB.182.18.5225-5230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortega A, Gonzalo-Asensio J, García-Del Portillo F. Dynamics of Salmonella small RNA expression in non-growing bacteria located inside eukaryotic cells. RNA Biol. 2012;9:9. doi: 10.4161/rna.19317. [DOI] [PubMed] [Google Scholar]
- 57.Gong H, Vu GP, Bai Y, Chan E, Wu R, Yang E, et al. A Salmonella small non-coding RNA facilitates bacterial invasion and intracellular replication by modulating the expression of virulence factors. PLoS Pathog. 2011;7:e1002120. doi: 10.1371/journal.ppat.1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu Y, Galán JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–7. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 59.Zhang S, Santos RL, Tsolis RM, Stender S, Hardt WD, Bäumler AJ, et al. The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect Immun. 2002;70:3843–55. doi: 10.1128/IAI.70.7.3843-3855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baxter MA, Fahlen TF, Wilson RL, Jones BD. HilE interacts with HilD and negatively regulates hilA transcription and expression of the Salmonella enterica serovar Typhimurium invasive phenotype. Infect Immun. 2003;71:1295–305. doi: 10.1128/IAI.71.3.1295-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santiviago CA, Reynolds MM, Porwollik S, Choi SH, Long F, Andrews-Polymenis HL, et al. Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog. 2009;5:e1000477. doi: 10.1371/journal.ppat.1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogel J, Argaman L, Wagner EG, Altuvia S. The small RNA IstR inhibits synthesis of an SOS-induced toxic peptide. Curr Biol. 2004;14:2271–6. doi: 10.1016/j.cub.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Vogel J, Bartels V, Tang TH, Churakov G, Slagter-Jäger JG, Hüttenhofer A, et al. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 2003;31:6435–43. doi: 10.1093/nar/gkg867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb E, Claas K, Downs D. thiBPQ encodes an ABC transporter required for transport of thiamine and thiamine pyrophosphate in Salmonella typhimurium. J Biol Chem. 1998;273:8946–50. doi: 10.1074/jbc.273.15.8946. [DOI] [PubMed] [Google Scholar]
- 65.Zhang A, Altuvia S, Storz G. The novel oxyS RNA regulates expression of the sigma s subunit of Escherichia coli RNA polymerase. Nucleic Acids Symp Ser. 1997;36:27–8. [PubMed] [Google Scholar]
- 66.Retamal P, Castillo-Ruiz M, Mora GC. Characterization of MgtC, a virulence factor of Salmonella enterica Serovar Typhi. PLoS One. 2009;4:e5551. doi: 10.1371/journal.pone.0005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee EJ, Groisman EA. An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol Microbiol. 2010;76:1020–33. doi: 10.1111/j.1365-2958.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang A, Altuvia S, Tiwari A, Argaman L, Hengge-Aronis R, Storz G. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 1998;17:6061–8. doi: 10.1093/emboj/17.20.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilmes-Riesenberg MR, Foster JW, Curtiss R., 3rd An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect Immun. 1997;65:203–10. doi: 10.1128/iai.65.1.203-210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guiney DG, Fierer J. The Role of the spv Genes in Salmonella Pathogenesis. Front Microbiol. 2011;2:129. doi: 10.3389/fmicb.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci U S A. 1998;95:12462–7. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Majdalani N, Chen S, Murrow J, St John K, Gottesman S. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol Microbiol. 2001;39:1382–94. doi: 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- 73.Mandin P, Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29:3094–107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papenfort K, Said N, Welsink T, Lucchini S, Hinton JC, Vogel J. Specific and pleiotropic patterns of mRNA regulation by ArcZ, a conserved, Hfq-dependent small RNA. Mol Microbiol. 2009;74:139–58. doi: 10.1111/j.1365-2958.2009.06857.x. [DOI] [PubMed] [Google Scholar]
- 75.Jones AM, Goodwill A, Elliott T. Limited role for the DsrA and RprA regulatory RNAs in rpoS regulation in Salmonella enterica. J Bacteriol. 2006;188:5077–88. doi: 10.1128/JB.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sittka A, Lucchini S, Papenfort K, Sharma CM, Rolle K, Binnewies TT, et al. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 2008;4:e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fröhlich KS, Vogel J. Activation of gene expression by small RNA. Curr Opin Microbiol. 2009;12:674–82. doi: 10.1016/j.mib.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Soper TJ, Woodson SA. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. 2008;14:1907–17. doi: 10.1261/rna.1110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Bläsi U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 2003;9:1308–14. doi: 10.1261/rna.5850703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci U S A. 2010;107:9602–7. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawamoto H, Koide Y, Morita T, Aiba H. Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol Microbiol. 2006;61:1013–22. doi: 10.1111/j.1365-2958.2006.05288.x. [DOI] [PubMed] [Google Scholar]
- 83.Mohanty BK, Maples VF, Kushner SR. The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Mol Microbiol. 2004;54:905–20. doi: 10.1111/j.1365-2958.2004.04337.x. [DOI] [PubMed] [Google Scholar]
- 84.Rabhi M, Espéli O, Schwartz A, Cayrol B, Rahmouni AR, Arluison V, et al. The Sm-like RNA chaperone Hfq mediates transcription antitermination at Rho-dependent terminators. EMBO J. 2011;30:2805–16. doi: 10.1038/emboj.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussein R, Lim HN. Disruption of small RNA signaling caused by competition for Hfq. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1010082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moon K, Gottesman S. Competition among Hfq-binding small RNAs in Escherichia coli. Mol Microbiol. 2011;82:1545–62. doi: 10.1111/j.1365-2958.2011.07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Croucher NJ, Thomson NR. Studying bacterial transcriptomes using RNA-seq. Curr Opin Microbiol. 2010;13:619–24. doi: 10.1016/j.mib.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perkins TT, Kingsley RA, Fookes MC, Gardner PP, James KD, Yu L, et al. A strand-specific RNA-Seq analysis of the transcriptome of the typhoid bacillus Salmonella typhi. PLoS Genet. 2009;5:e1000569. doi: 10.1371/journal.pgen.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ozsolak F, Milos PM. Transcriptome profiling using single-molecule direct RNA sequencing. Methods Mol Biol. 2011;733:51–61. doi: 10.1007/978-1-61779-089-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mandlik A, Livny J, Robins WP, Ritchie JM, Mekalanos JJ, Waldor MK. RNA-Seq-based monitoring of infection-linked changes in Vibrio cholerae gene expression. Cell Host Microbe. 2011;10:165–74. doi: 10.1016/j.chom.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vogel J, Wagner EG. Target identification of small noncoding RNAs in bacteria. Curr Opin Microbiol. 2007;10:262–70. doi: 10.1016/j.mib.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Tjaden B, Goodwin SS, Opdyke JA, Guillier M, Fu DX, Gottesman S, et al. Target prediction for small, noncoding RNAs in bacteria. Nucleic Acids Res. 2006;34:2791–802. doi: 10.1093/nar/gkl356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papenfort K, Pfeiffer V, Lucchini S, Sonawane A, Hinton JC, Vogel J. Systematic deletion of Salmonella small RNA genes identifies CyaR, a conserved CRP-dependent riboregulator of OmpX synthesis. Mol Microbiol. 2008;68:890–906. doi: 10.1111/j.1365-2958.2008.06189.x. [DOI] [PubMed] [Google Scholar]
- 94.Wadler CS, Vanderpool CK. Characterization of homologs of the small RNA SgrS reveals diversity in function. Nucleic Acids Res. 2009;37:5477–85. doi: 10.1093/nar/gkp591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corcoran CP, Podkaminski D, Papenfort K, Urban JH, Hinton JC, Vogel J. Superfolder GFP reporters validate diverse new mRNA targets of the classic porin regulator, MicF RNA. Mol Microbiol. 2012;84:428–45. doi: 10.1111/j.1365-2958.2012.08031.x. [DOI] [PubMed] [Google Scholar]
- 96.Bossi L, Figueroa-Bossi N. A small RNA downregulates LamB maltoporin in Salmonella. Mol Microbiol. 2007;65:799–810. doi: 10.1111/j.1365-2958.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- 97.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 98.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monteiro C, Papenfort K, Hentrich K, Ahmad I, Le Guyon S, Reimann R, et al. Hfq and Hfq-dependent small RNAs are major contributors to multicellular development in Salmonella enterica serovar Typhimurium. RNA Biol. 2012;9:9. doi: 10.4161/rna.19682. [DOI] [PubMed] [Google Scholar]
- 100.Altier C, Suyemoto M, Lawhon SD. Regulation of Salmonella enterica serovar typhimurium invasion genes by csrA. Infect Immun. 2000;68:6790–7. doi: 10.1128/IAI.68.12.6790-6797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Urbanowski ML, Stauffer LT, Stauffer GV. The gcvB gene encodes a small untranslated RNA involved in expression of the dipeptide and oligopeptide transport systems in Escherichia coli. Mol Microbiol. 2000;37:856–68. doi: 10.1046/j.1365-2958.2000.02051.x. [DOI] [PubMed] [Google Scholar]
- 102.Urban JH, Vogel J. Two seemingly homologous noncoding RNAs act hierarchically to activate glmS mRNA translation. PLoS Biol. 2008;6:e64. doi: 10.1371/journal.pbio.0060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Göpel Y, Lüttmann D, Heroven AK, Reichenbach B, Dersch P, Görke B. Common and divergent features in transcriptional control of the homologous small RNAs GlmY and GlmZ in Enterobacteriaceae. Nucleic Acids Res. 2011;39:1294–309. doi: 10.1093/nar/gkq986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, et al. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol Microbiol. 2005;58:1421–9. doi: 10.1111/j.1365-2958.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- 105.Kint G, De Coster D, Marchal K, Vanderleyden J, De Keersmaecker SC. The small regulatory RNA molecule MicA is involved in Salmonella enterica serovar Typhimurium biofilm formation. BMC Microbiol. 2010;10:276. doi: 10.1186/1471-2180-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mizuno T, Chou MY, Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA) Proc Natl Acad Sci U S A. 1984;81:1966–70. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Esterling L, Delihas N. The regulatory RNA gene micF is present in several species of gram-negative bacteria and is phylogenetically conserved. Mol Microbiol. 1994;12:639–46. doi: 10.1111/j.1365-2958.1994.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 108.Figueroa-Bossi N, Valentini M, Malleret L, Fiorini F, Bossi L. Caught at its own game: regulatory small RNA inactivated by an inducible transcript mimicking its target. Genes Dev. 2009;23:2004–15. doi: 10.1101/gad.541609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bouvier M, Sharma CM, Mika F, Nierhaus KH, Vogel J. Small RNA binding to 5′ mRNA coding region inhibits translational initiation. Mol Cell. 2008;32:827–37. doi: 10.1016/j.molcel.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 110.Antal M, Bordeau V, Douchin V, Felden B. A small bacterial RNA regulates a putative ABC transporter. J Biol Chem. 2005;280:7901–8. doi: 10.1074/jbc.M413071200. [DOI] [PubMed] [Google Scholar]
- 111.Balbontín R, Figueroa-Bossi N, Casadesús J, Bossi L. Insertion hot spot for horizontally acquired DNA within a bidirectional small-RNA locus in Salmonella enterica. J Bacteriol. 2008;190:4075–8. doi: 10.1128/JB.00220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanderpool CK, Gottesman S. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol Microbiol. 2004;54:1076–89. doi: 10.1111/j.1365-2958.2004.04348.x. [DOI] [PubMed] [Google Scholar]
- 113.Fozo EM, Kawano M, Fontaine F, Kaya Y, Mendieta KS, Jones KL, et al. Repression of small toxic protein synthesis by the Sib and OhsC small RNAs. Mol Microbiol. 2008;70:1076–93. doi: 10.1111/j.1365-2958.2008.06394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han K, Kim KS, Bak G, Park H, Lee Y. Recognition and discrimination of target mRNAs by Sib RNAs, a cis-encoded sRNA family. Nucleic Acids Res. 2010;38:5851–66. doi: 10.1093/nar/gkq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Møller T, Franch T, Udesen C, Gerdes K, Valentin-Hansen P. Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 2002;16:1696–706. doi: 10.1101/gad.231702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beisel CL, Storz G. Discriminating tastes: Physiological contributions of the Hfq-binding small RNA Spot 42 to catabolite repression. RNA Biol. 2011;8:8. doi: 10.4161/rna.8.5.16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S. A pH-responsive riboregulator. Genes Dev. 2009;23:2650–62. doi: 10.1101/gad.552209. [DOI] [PMC free article] [PubMed] [Google Scholar]