Abstract
Early morphogenic movements are an important feature of embryonic development in vertebrates. During zebrafish gastrulation, epiboly progression is driven by the coordinated remodeling of the YSL microtubule network and F-actin cables. We recently described the implication of Nrz, an anti-apoptotic Bcl-2 homolog, in the control of the YSL cytoskeleton dynamics. Nrz knock-down induces premature actin-myosin ring formation leading to margin constriction, epiboly arrest and embryo lethality. At the molecular level, the Nrz protein controls the actin-myosin dynamics through IP3R-dependent calcium levels variation. Here, we discuss these novel findings and propose a model in which reversible phosphorylation of the Nrz/IP3R complex modulates the permeability of the IP3R calcium channel and thus may explain the Nrz-dependent control of IP3R opening required for proper epiboly completion.
Keywords: Bcl-2, IP3R, Nrz, actin cytoskeleton, calcium, phosphorylation, zebrafish
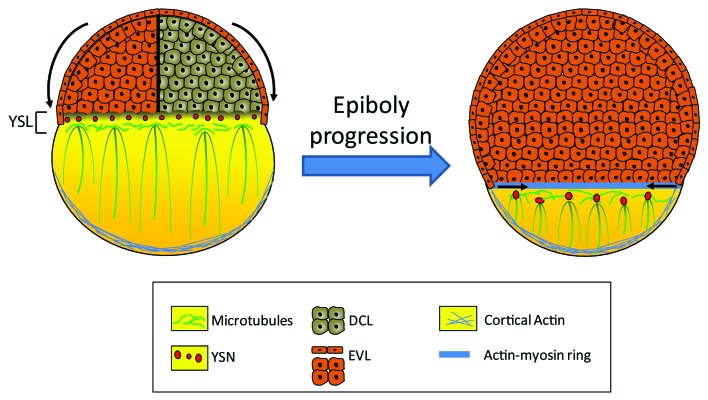
Early embryonic development is a complex process requiring highly coordinated signals to shape the embryo and set up the body plan of the future adult. During zebrafish gastrulation, a series of cell rearrangements and movements result in the formation of the three germ layers (the endoderm, the ectoderm and the mesoderm) as well as the dorsoventral and anteroposterior axes. The first morphogenetic movement of gastrulation, known as epiboly, begins just at the end of the blastula period.1 At this time, the embryo is organized into three cell layers; the enveloping cell layer (EVL) and the deep cell layer (DCL) forming the blastoderm, which is layed on top of the yolk syncitial layer (YSL) (Fig. 1). This latter layer is formed during blastula period when the blastomeres in contact with the yolk release their entire content in the yolk cell. Epiboly is characterized by the migration of the blastoderm from the animal pole down to the vegetal pole. This process can be divided into three phases: initiation, progression and completion.2 During the initiation phase, part of the YSL spreads beneath the blastoderm forming the internal-YSL (I-YSL) while the other part of the YSL, called the external-YSL (E-YSL), forms a ring under the EVL. As the yolk spreads toward the animal pole, the blastoderm thins out and the DCL moves radially outward to eventually form a cap of cells over the yolk. Once this so-called dome stage is reached, epiboly begins. During this phase, the blastoderm continues to grow thinner and all three layers migrate coordinately toward the vegetal pole until they reach the equator of the embryo (50% epiboly). At this point, the DCL migration pauses, allowing other gastrulation movements to begin while EVL and E-YSL migration continues. Finally, the EVL keeps on moving until the entire yolk cell is covered.
Figure 1. Schematic representation of the epiboly progression during early zebrafish development. During epiboly, the blastomeres, migrate toward the vegetal pole to envelope the yolk cell. This morphogenic movement is orchestrated by the coordinate changes in the yolk syncitial layer (YSL) cytoskeleton mainly composed of microtubules and F-actin cables. Parallel microtubule meshwork originating from the yolk syncitial nuclei (YSN) are extending toward the vegetal embryo pole, and participates actively in the epiboly progression. The margin actin-myosin ring formed at 75% epiboly is important for the completion of the epiboly. EVL: enveloping layer; DCL: deep cell layer.
The molecular mechanisms underlying epiboly are still under debate but it seems that cytoskeleton dynamics plays a crucial role in this process. Indeed, it was shown that the yolk cell harbors a large number of microtubules, which play a central role in epiboly initiation and progression. These microtubules are organized in a dense network in the YSL from which parallel arrays of microtubules extend toward the vegetal pole of the yolk cell (Fig. 1). This latter network grows shorter as epiboly progresses; it should be noted that when it is disrupted, YSL migration stops while EVL and DCL migration slows down, suggesting that YSL microtubules drive, at least in part, epiboly progression.
The actin cytoskeleton also seems to be implicated in epiboly movements. Indeed, during gastrulation, a distinct actin-based structure, called the actin-myosin ring, forms at the interface of the EVL and the E-YSL around 75% epiboly (Fig. 1). This actin-myosin ring was first proposed to participate in the contraction of the margin which ensures blastopore closure at the end of epiboly.3 More recently, it was shown that the actin-myosin ring can also contract earlier during epiboly. It was thus proposed that this contraction may be involved in changing the shape of the adjacent EVL cells and reducing the circumference of the margin in motion to fit the shape of the yolk cell.4
The mechanisms underlying the contraction of the actin-myosin ring are still under investigation. Köppen and colleagues showed that Msn1, a Ste20- like kinase, participates in the recruitment of actin and myosin light chain 2 (MLC2) in the YSL and promotes actin ring contraction.4Calcium may be another key regulator of actin ring contraction. Indeed, two different studies report that after 50% epiboly, high calcium levels are transiently observed at the margin of the blastoderm from which the actin ring originates.5,6 This model of calcium-induced actin contraction is corroborated by the fact that calcium chelation inhibits actin ring formation.3 Furthermore, it was shown that a mutation in MAPKAPK2 is associated with premature ring contraction and ectopic calcium levels increase in the blastoderm.7 Finally, we recently demonstrated that the knock-down of nrz, a member of the bcl-2 family of apoptosis inhibitors, induces similar premature actin ring contraction.8 Indeed, we showed that this contraction is triggered by increased calcium levels at the margin between the blastoderm and the YSL. In this particular situation, calcium presumably activates the calcium-dependent protein calmodulin which in turns may activates the myosin light chain kinase (MLCK) leading to increased myosin phosphorylation and subsequent actin-myosin contraction. Interestingly, during epiboly, Nrz expression is restricted to the YSL which contains significant amount of endoplasmic reticulum (ER) and mitochondria. In fact, Nrz is located to both organelles; of note, at the ER, Nrz interacts with the Inositol 1,4,5-triphosphate receptor type 1 (IP3R1) thus regulating calcium release through this channel. These results reveal that by interacting with IP3R1, Nrz controls calcium fluxes inside the YSL and regulates actin-myosin ring formation during epiboly. Together, the above studies point out to the critical role of calcium signaling in the dynamics of the actin cytoskeleton during epiboly progression. How calcium signaling is regulated during epiboly remains an open question. Indeed, calcium is a versatile messenger requiring to be finely tuned as excess or lack of calcium is detrimental for the developing embryo. As a matter of fact, an underlying regulatory mechanism may be based on the interaction between Nrz and IP3R1 in the YSL.
Of note, Nrz is present as soon as the YSL forms and its expression increases as epiboly progresses. We can thus assume that before 50% epiboly, Nrz strongly interacts with IP3R1 in the YSL thus preventing abnormal calcium release from the ER and inhibiting premature actin ring formation. However, around 60% epiboly, calcium transients begin originating from the margin of the migrating blastoderm. It is still unclear if these transients originate from the blastomeres or the YSL but we can assume that at least some of them come from the YSL as the actin-myosin ring is localized at the interface of the EVL and the YSL. Thus at this stage, the interactions between Nrz and IP3R are expected to be finely regulated to control calcium fluxes in the YSL.
To confirm this hypothesis it appears critical to determine how Nrz interacts with IP3R1 and regulates calcium release from the ER. Indeed, until now several studies have pointed out to a role of the Bcl-2 proteins on IP3R-dependent calcium release from the ER, but the underlying mechanism is still a matter of intense debate. The members of the Bcl-2 family, including Bcl-2 and Bcl-XL, were shown to interact with IP3R and decrease calcium release from the ER; however, while according to certain studies this might be the consequence of increased calcium leakage thus reducing ER calcium content,9,10 other reports support direct inhibition of calcium release through IP3R.11 Our data indicate that in the zebrafish embryo, Nrz seems to directly inhibit IP3R-dependent calcium release since nrz knock-down significantly increases cytosolic calcium levels whereas treatment of nrz-invalidated embryos with IP3R inhibitors restores normal development. To further understand how Nrz inhibits IP3R-dependent calcium release it is required to determine which domain of Nrz interacts with IP3R1. It should be noted that Bcl-2 interacts with IP3R1 via its BH4 (Bcl-2 homology 4) domain; moreover, this domain alone is able to inhibit calcium release through IP3R1. In contrast, the BH4 domain of Bcl-XL does not affect IP3R1 permeability,12 suggesting that additional BH domains presumably participate to the regulation of IP3R-dependent calcium release. Actually, the BH4 domain is the less conserved domain among the Bcl-2 family; thus, due to this variability, the BH4 domain of some Bcl-2 family members may be unable to bind IP3R1 or regulate channel opening. Our results indicate that Nrz binds IP3R1 via BH4 and that this domain is essential for Nrz-induced inhibition of IP3R1. However whether the Nrz BH4 domain alone can inhibit calcium release through IP3R1 is not known.
In the same line, the Nrz binding site within IP3R1 remains to be characterized. So far, the characterization of the interaction domains between the Bcl-2 family members and IP3R is still a highly controversial issue. Recently, White and colleagues reported that the Bcl-2 binding site would be located at the C-terminus of IP3R,13 while in contrast the team of Distelhorst proposed this binding site to be in the modulatory domain of IP3R, right in the middle of the protein.14 Interestingly, both groups agree that Bcl-2 members do not interact with the inositol 1,4,5-triphosphate (Ins(1,4,5)P3) binding domain suggesting that they do not compete for the interaction of the ligand with IP3R. There is no doubt that removing this kind of ambiguity is a prerequisite to understand the regulation of Nrz/IP3R interactions. Indeed, a number of regulatory binding partners of IP3R have been identified,15,16 and, depending on the location of the Nrz binding site(s) within the IP3R subunits, different proteins are expected to affect this interaction and the opening of the calcium channel. Inhibition or activation of IP3R by direct interaction have been seldom described, indeed the most common regulation of IP3R calcium permeability appears to occur via phosphorylation or dephosphorylation mechanisms.
So far numerous protein kinases or phosphatases were described to interact with IP3R and to regulate its opening via phosphorylation or dephosphorylation at specific sites which are well conserved among species including zebrafish. In general, phosphorylation of IP3R is associated with its activation and it has been shown that Bcl-2 modulates the phosphorylation status of IP3R. Indeed, it was reported that Bcl-2 can directly interact with calcineurin,17 a calcium/calmodulin-dependent phosphatase known to dephosphorylate IP3R.18 In this latter study, the authors showed that calcineurin specifically regulates the increased IP3R-dependent calcium release induced by protein kinase C (PKC) phosphorylation of IP3R. Thus, by recruiting calcineurin, Bcl-2 may trigger IP3R dephosphorylation and control the IP3R-dependent calcium release. Interestingly, it should be noted that Bcl-2 could also be phosphorylated by several kinases including PKC which phosphorylate Bcl-2 at Ser70, a residue located in an unstructured loop at the N-terminus of the protein between the BH4 and BH3 domains.19 Bcl-2 phosphorylation on serine or threonine residues in this loop is associated to its inactivation since mutation of these phosphorylated sites enhances anti-apoptotic effect of Bcl-2.20,21 More interestingly, a non-phosphorylatable form of Bcl-2 was shown to be more efficient to decrease calcium release through IP3R compared with native Bcl-2.22 In addition, as Bcl-2 interacts with calcineurin, the PKC-dependent phosphorylation should be reversibly dephosphorylate by calcineurin. Finally, it should be noted that Nrz also harbors an unstructured loop between its BH4 and BH3 domains and that this loop contains two serine residues and one threonine residue as Bcl-2, it can thus be speculated that similar phosphorylation may occur in Nrz too.
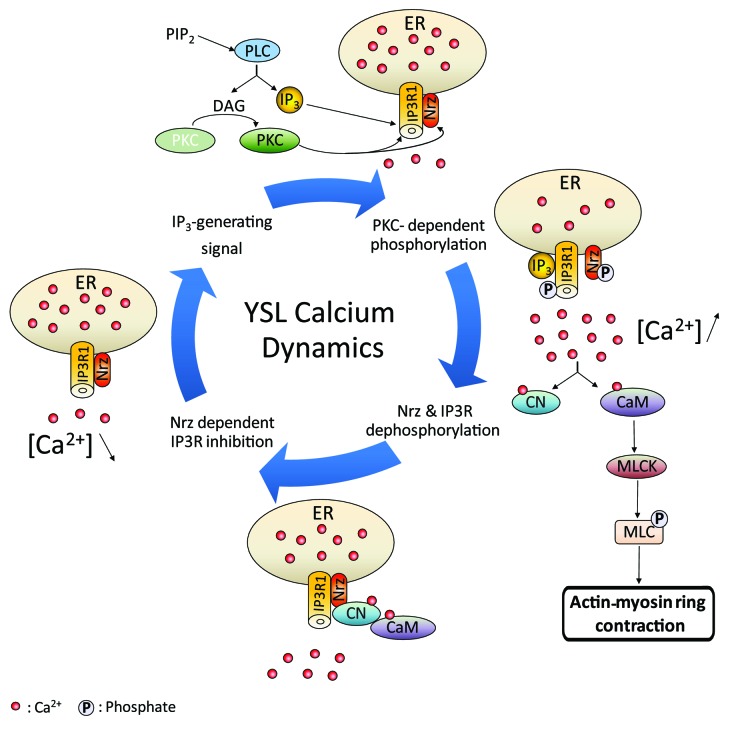
Considering these data, we built a model for the control of IP3R1/Nrz interactions by reversible phosphorylation and dephosphorylation (Fig. 2). According to this model, before the embryo reaches 50% epiboly, Nrz interacts with IP3R1 and keeps the channel in closed-state by an unknown mechanism possibly mediated by a phosphatase, such as calcineurin, or by direct inhibition via the interaction of Nrz with IP3R regulatory domain. Then, after the migrating cells crossed the equator, a signal is expected to originate from the embryo to trigger calcium transients and subsequent formation of contractible actin rings. This signal presumably activates phospholipase C (PLC), generating Ins(1,4,5)P3, the ligand of IP3R, and diacylglycerol (DAG) which in turn activates PKC.23 Activated PKC may then phosphorylate both Nrz and IP3R1, thus resulting in Nrz loss of function and subsequent activation of IP3R1 which allows calcium release from the ER upon Ins(1,4,5)P3 binding. Interestingly, the PKC-dependent phosphorylation of IP3R is inhibited by calcium,24 consequently the maintenance of low levels of calcium in the YSL due to Nrz interaction with IP3R should promote phosphorylation of the channel. Finally, calcium release may, on the one hand, activate calmodulin which is consequently prone to interact with MLCK and promote MLC2 phosphorylation, thus inducing actin-myosin ring contraction. On the other hand, calcium and calmodulin may activate calcineurin which could in turn dephosphorylate both Nrz and IP3R1 by interacting with Nrz and prevent channel opening. This feedback loop may be of major importance during epiboly since abnormal calcium levels in the YSL may induce unwanted actin ring contraction leading to the detachment of the bastoderm from the yolk and thus death of the embryo.
Figure 2. Schematic drawing of possible model of Nrz-dependent IP3R1 regulation during epiboly completion. Prior to actin-myosin ring formation Nrz interacts with IP3R1 and inhibits its opening. As epiboly progresses Ins(1,4,5)P3 inducing signals trigger Phospholipase C (PLC) molecular cascade which activates PKC. Synergic action of Ins(1,4,5)P3 and PKC dependent IP3R1/Nrz phosphorylation induces intracellular [Ca2+] increase, with subsequent actin-myosin ring formation trough (CaM) calmodulin/MLCK pathway. Calcineurin (CN) feedback dephosphorylation results in increasing of IP3R1/Nrz interaction and decreasing of intracellular Calciumlevels.
In conclusion, there is a growing body of evidence that epiboly and subsequent gastrulation stages are driven, at least in part, by calcium signaling and the activity of IP3R. Thus this channel, which is at the crossroads of a number of kinase-dependent pathways appear to be a key integrator of the various signals upstream of these pathways, ensuring precise time and space dependent progression of epiboly via the fine tuning of calcium transients throughout the embryo. Here we propose that reversible phosphorylation of the Nrz/IP3R complex is critical for proper calcium signaling and subsequent actin cytoskeleton remodeling in the developing embryo. According to this model a phosphorylation/dephosphorylation loop may be implicated in generating temporally controlled calcium transients and actin-myosin ring contraction, which drive epiboly progression. If confirmed, this hypothesis may give new insights into calcium signaling and cytoskeleton dynamics during early zebrafish development.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/18116
References
- 1.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 2.Betchaku T, Trinkaus JP. Contact relations, surface activity, and cortical microfilaments of marginal cells of the enveloping layer and of the yolk syncytial and yolk cytoplasmic layers of fundulus before and during epiboly. J Exp Zool. 1978;206:381–426. doi: 10.1002/jez.1402060310. [DOI] [PubMed] [Google Scholar]
- 3.Cheng JC, Miller AL, Webb SE. Organization and function of microfilaments during late epiboly in zebrafish embryos. Dev Dyn. 2004;231:313–23. doi: 10.1002/dvdy.20144. [DOI] [PubMed] [Google Scholar]
- 4.Köppen M, Fernandez BG, Carvalho L, Jacinto A, Heisenberg CP. Coordinated cell-shape changes control epithelial movement in zebrafish and Drosophila. Development. 2006;133:2671–81. doi: 10.1242/dev.02439. [DOI] [PubMed] [Google Scholar]
- 5.Créton R, Speksnijder JE, Jaffe LF. Patterns of free calcium in zebrafish embryos. J Cell Sci. 1998;111:1613–22. doi: 10.1242/jcs.111.12.1613. [DOI] [PubMed] [Google Scholar]
- 6.Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc Natl Acad Sci USA. 1999;96:157–61. doi: 10.1073/pnas.96.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holloway BA, Gomez de la Torre Canny S, Ye Y, Slusarski DC, Freisinger CM, Dosch R, et al. A novel role for MAPKAPK2 in morphogenesis during zebrafish development. PLoS Genet. 2009;5:e1000413. doi: 10.1371/journal.pgen.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popgeorgiev N, Bonneau B, Ferri KF, Prudent J, Thibaut J, Gillet G. The apoptotic regulator Nrz controls cytoskeletal dynamics via the regulation of Calciumtrafficking in the zebrafish blastula. Dev Cell. 2011;20:663–76. doi: 10.1016/j.devcel.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–62. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Calciumconcentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–65. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, Bultynck G. Selective regulation of IP(3)-receptor-mediated Ca(2+) signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ 2011 [DOI] [PMC free article] [PubMed]
- 13.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Calciumsignaling. J Biol Chem. 2010;285:13678–84. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci USA. 2009;106:14397–402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE 2006; 2006:re15. [DOI] [PubMed]
- 16.Vanderheyden V, Wakai T, Bultynck G, De Smedt H, Parys JB, Fissore RA. Regulation of inositol 1,4,5-trisphosphate receptor type 1 function during oocyte maturation by MPM-2 phosphorylation. Cell Calcium. 2009;46:56–64. doi: 10.1016/j.ceca.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–31. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 18.Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Calciumflux. Cell. 1995;83:463–72. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 19.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274:20296–300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–78. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shitashige M, Toi M, Yano T, Shibata M, Matsuo Y, Shibasaki F. Dissociation of Bax from a Bcl-2/Bax heterodimer triggered by phosphorylation of serine 70 of Bcl-2. J Biochem. 2001;130:741–8. doi: 10.1093/oxfordjournals.jbchem.a003044. [DOI] [PubMed] [Google Scholar]
- 22.Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Calciumhomeostasis and apoptosis. EMBO J. 2004;23:1207–16. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 24.Vermassen E, Fissore RA, Nadif Kasri N, Vanderheyden V, Callewaert G, Missiaen L, et al. Regulation of the phosphorylation of the inositol 1,4,5-trisphosphate receptor by protein kinase C. Biochem Biophys Res Commun. 2004;319:888–93. doi: 10.1016/j.bbrc.2004.05.071. [DOI] [PubMed] [Google Scholar]