Abstract
In many tissues microtubules reorganize into non-centrosomal arrays in differentiated cells. In the epidermis, proliferative basal cells have a radial array of microtubules organized around a centrosome, while differentiated cells have cortical microtubules. The desmosomal protein desmoplakin is required for the microtubules to organize around the cell cortex. Furthermore, the centrosomal and/or microtubule-associated proteins ninein, Lis1, Ndel1, and CLIP170 are recruited to the cell cortex, where they have been implicated in the cortical organization of microtubules. Recently, it has been shown that in Lis1-null epidermis, microtubules are disorganized in the differentiated layers of the epidermis. Furthermore, Lis1-null mice die perinatally due to dehydration. This is due, in part, to the unexpected desmosome phenotype observed in Lis1-null skin. Upon loss of Lis1, desmosomal proteins become less stable. Here, we propose that Lis1 may regulate desmosomal stability through its binding partners Nde1/Ndel1 and dynein.
Keywords: Lis1, desmoplakin, desmosome, epidermis, microtubule
Introduction
The epidermis provides a mechanical and chemical barrier against the environment. An integral part of maintaining this barrier is proper cell-cell adhesion. In particular, desmosomes bear the bulk of the mechanical stress to which the skin is exposed. Loss or mutation of desmosomal proteins results in blistering in the epidermis, in some cases leading to death.1-3 This occurs not only in genetic disease, but can also be caused by autoimmune disease and by bacterial infections.3,4 Traditionally, desmosomes have been thought of as static structures, strictly providing strength to tissues that undergo mechanical stress by attaching to the intermediate filament cytoskeleton. However, recent data suggest that desmosomes are dynamic structures that participate in the organization of several cytoskeletal networks.1,5,6
In many tissues, microtubules in differentiated cells adopt non-centrosomal arrays (see Fig. 1). Although their specific arrangements are thought to be essential for many cellular functions, including polarity, cell shape, and trafficking, little is known about how the microtubules adopt these non-centrosomal arrays. In both plant cells and the Drosophila trachea, microtubule reorganization involves the recruitment of microtubule-nucleating complexes including gamma-tubulin to areas at the cell cortex.7,8 Work in the epidermis highlights novel ways in which non-centrosomal microtubule arrays can form.
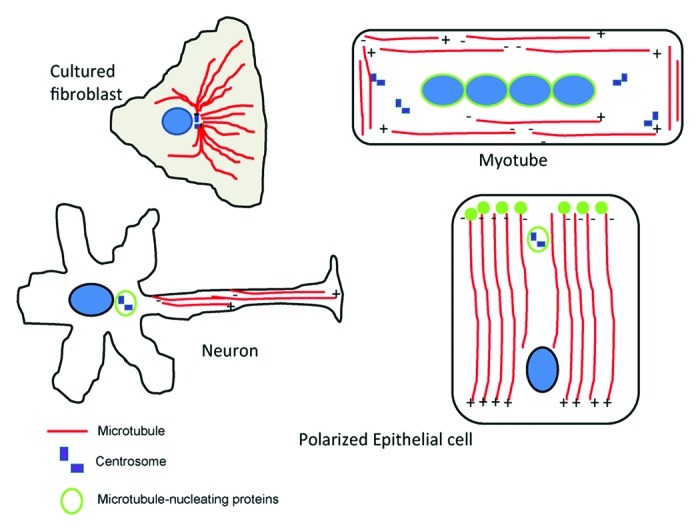
Figure 1. Microtubules adopt non-centrosomal arrays in several cell types. Cultured fibroblasts have a radial array of microtubules organized around a centrosome, where microtubule-nucleating and anchoring material is found. In myocytes the centrosomal array of microtubules is replaced by a linear array organized parallel to the long axis. Microtubule-nucleating material clusters around the nuclei. In older myotubes, centrioles completely disappear. Neurons contain linear arrays of microtubules in axons and dendrites. In axons, microtubules are long and plus ends are distal to the cell body. In dendrites, microtubules are short and have mixed polarity. In polarized epithelial cells, microtubules are organized along the apical-basal axis, with the minus ends closest to the apical surface. Microtubule-nucleating complexes localize in the apical region of the cell.
Desmoplakin Recruits a Subset of Centrosomal Proteins to the Cell Cortex
In the epidermis, microtubules are organized around an apically localized centrosome in the proliferative basal cells. Lying above the basal cells in the stratified epidermis are cells at various levels of differentiation that we will collectively call uprabasal cells. In suprabasal cells, the microtubules reorganize, ultimately to the cell cortex (Fig. 2).6 In the mouse skin, desmoplakin (DP), the linker protein that attaches keratin filaments to the desmosome, is required for cortical microtubule organization. Upon loss of DP, microtubules of the suprabasal cells are lost from the cortex and aggregate in the cytoplasm.6 Recent work has begun to uncover how DP functions in microtubule organization. At least three centrosomal proteins implicated in anchoring microtubules at the centrosome, ninein, Lis1, and Ndel1, and one plus-end binding protein, CLIP170, are recruited to the desmosome by desmoplakin.5,6 Furthermore, conditional ablation of the Lis1 gene in the epidermis led to a microtubule phenotype very similar to loss of DP.5 This is consistent with the hypothesis that centrosomal proteins are required to organize microtubules at the cortex. Unexpectedly, however, loss of Lis1 also resulted in severe defects in the desmosome itself.5 We propose two models for how loss of Lis1 may result in desmosomal defects, likely caused by the mislocalization of at least one Lis1-interacting protein.
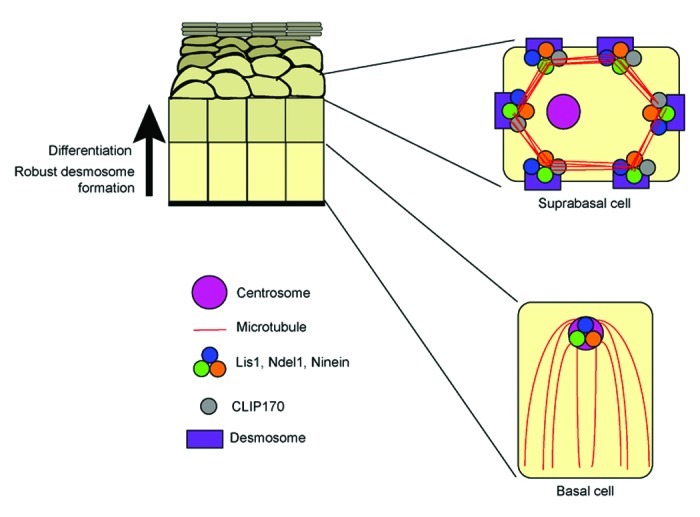
Figure 2. Centrosomal protein and microtubule organization in the epidermis. In basal cells of the epidermis, microtubules are organized in a radial array around an apically localized centrosome. Lis1, Ndel1, and ninein localize at the centrosome. In the differentiated suprabasal cells where desmosomes are robust, Lis1, Ndel1, and ninein are lost from the centrosome and are recruited to the desmosome by desmoplakin, along with CLIP170. Microtubules are organized around the cell cortex.
Notably, DP is not required for the differentiation-induced loss of these proteins from the centrosome, but only their subsequent recruitment to the desmosome. This is evident in DP-null keratinocytes where ninein, Lis1, and Ndel1 are no longer found on the centrosome, but are cytoplasmic.5 The loss of ninein from centrosomes during differentiation has been seen in a number of cell types, including myocytes, neurons and pillar cells of the inner ear.9-11 This suggests that changes in centrosome composition and function are common in many differentiated cells. The signaling pathways that are important for this change in centrosome composition during differentiation and their immediate centrosomal targets have not yet been identified.
The region of DP required for centrosomal protein recruitment is specific to one isoform of DP.5 DPI has a longer rod domain than its alternatively spliced isoform DPII. The region of the rod required for centrosomal protein recruitment is only found in DPI. This suggests an unexpected difference in functions of the DP isoforms. Previous data demonstrated that purified DPI and DPII behave as a dimer and monomer, respectively,12 but this is the first demonstration of a functional difference. Whether this difference is physiologically relevant requires further investigation, but human genetics hints that it may be. Most human mutations in DP affect both DPI and DPII, and they cause a range of disease from skin thickening and woolly hair to severe blistering diseases and cardiomyopathies.2,3 A single case has been described, however, of a mutation that affects the splicing of DP, leading essentially to complete loss of DPI, but retention of DPII. The patient presented with palmoplantar keratoderma and heart defects.13 While the heart defects may be due to the low levels of expression of DPII in that tissue, DP levels in the epidermis were not dramatically affected. The heart phenotype could therefore be due to changes in the total levels of DP, or to the specific absence of DPI. The epidermal phenotypes caused by the DPI-specific mutation may be due to DPII’s inability to recruit centrosomal proteins and/or microtubules to the cell cortex of the epidermis. That said, much work is required to define the direct protein-protein interations that allow recruitment of centrosomal proteins to the desmosome.
Human mutations also offer evidence that DP may have functions outside simply tethering intermediate filaments to the desmosome. Loss of full-length DP in mouse results in early embryonic lethality.14,15 Even after rescue of the extraembryonic defects, the mice die shortly after gastrulation with multiple defects in heart, vasculature and neuroepithelium. In humans, loss of the intermediate filament-binding region in both alleles of the DP-gene results in lethal acantholytic epidermolysis bullosa—a blistering disease causing much later lethality, just after birth.2 While this discrepancy may be ascribed to species-specific differences, it may also highlight functions for DP in addition to simply tethering desmosomes to intermediate filaments.
Lis1 is Essential for Cortical Microtubule Organization
Not only is Lis1 recruited to the desmosome by DP, but Lis1 is also essential for proper microtubule organization in differentiated epidermis. The microtubules of the suprabasal cells in Lis1 conditionally ablated epidermis were not organized at the cortex, but rather, were cytoplasmic.5 This finding that Lis1 is required for microtubule organization supports the model in which desmoplakin recruits centrosomal proteins like Lis1, which are subsequently required for the recruitment of microtubules to the cortex. Desmoplakin can recruit both plus-end binding proteins and minus-end associated proteins to the cell cortex. We hypothesize that these proteins transform the cortex into a region that is capable of binding and stabilizing microtubules.
Lis1 is Essential for Desmosome Function
In addition to microtubule defects and mitotic defects, the epidermal-specific Lis1 knockout mice (Lis1 cKO) had a compromised barrier and died at birth from dehydration. Notably, the Lis1 cKO also exhibited blistering and peeling of the skin. In general, the Lis1 cKO mouse phenocopied the DP cKO mouse.5 In both skin sections and isolated keratinocytes, desmosomal components showed lower levels of cortical localization. Furthermore, the total levels of desmosomal proteins were decreased. This effect of Lis1 was specific to desmosomal components, as loss of Lis1 did not alter adherens junction protein levels or localization. Ultrastructurally, desmosomes were smaller and had less robust attachment to the keratin filament cytoskeleton. Studies in cultured cells revealed that while the initial formation of desmosomes occurred normally in Lis1 null cells, stabilization of the desmosomal proteins at the cell cortex was compromised.5 The unexpected desmosomal defects precluded analysis of the function of cortical microtubules, as the contribution of the microtubule defect to the viability of the mouse and the epidermal barrier function could not be determined. Below, we discuss several possible reasons for the desmosomal phenotype in the Lis1 cKO mice.
Ndel1: Lis1’s Link to Intermediate Filaments
One of the most well-characterized interacting partners of Lis1 is Ndel1, initially identified in screens for nuclear distribution mutants in Aspergillus. These evolutionarily conserved proteins have been shown to form a complex with dynein to control cell migration, especially in neurons.16 The ability of Lis1 and Ndel1 to interact with dynein heavy chain may provide a mechanism for how Lis1 organizes microtubules. This will be discussed more in the next section. However, Ndel1 has also emerged as an integrator of several cytoskeletal networks. Neurofilaments, a type of intermediate filament protein, comprise part of the cytoskeletal network in the axons and dendrites of central nervous system neurons. Although intermediate filaments can self-assemble, Ndel1 has been shown to facilitate neurofilament polymerization by binding to the neurofilament light subunit. Loss of Ndel1 results in destabilized neurofilaments, and ultimately, neurodegeneration.17 Additionally, Ndel1 has been shown to bind vimentin and to be required for its transport through dynein.18 Finally, Ndel1 associates with another intermediate filament protein, lamin B, in the mitotic spindle. Lamin B is required for proper microtubule organization into a spindle. Ndel1 interacts with lamin B in the spindle, and both the lamin B matrix and spindle organization are perturbed when Ndel1 is depleted.19 This occurs in a dynein-dependent manner. Therefore, Ndel1 and dynein cooperate to organize the lamin B network, which in turn is required for proper microtubule spindle organization. Similar mechanisms for Ndel1-mediated intermediate filament organization may exist in the epidermis. We have seen that loss of Lis1 results in loss of Ndel1 from the cell cortex (unpublished data). Therefore, Lis1 is required for Ndel1 recruitment to the desmosome. Ndel1 could act locally at the desmosome to promote intermediate filament assembly or attachment. Whether attachment of keratin filaments to the desmosome is required for proper desmosome stability is unclear.20 Embryos that are null for the entire keratin superfamily had smaller, mislocalized desmosomes,20 suggesting that keratin filaments are required for the proper localization and structure of the desmosome. Furthermore, embryos that are null for desmoplakin, and therefore lack attachment of intermediate filaments, show defects in desmosome assembly and stabilization,14 again suggesting that the desmosomal connection to the intermediate filament cytoskeleton is important to maintain desmosomes. Additionally, if Ndel1 can interact with keratin filament precursors on microtubules similarly to its interactions with the lamin B subunits, the disorganization of microtubules in the Lis1 cKO may result in the inability of Ndel1 and the keratin filament precursors to be properly transported to the cell cortex. This could be dependent or independent of Ndel1’s ability to interact with dynein.
Lis1 and its Interactions with Dynein
Lis1 and Ndel1 have been shown to form a complex with dynein. Ndel1 can recruit both Lis1 and dynein to the centrosome,21 where Lis1 and Ndel1 may cooperate to regulate dynein force production.22 Lis1 is thought to prolong the interaction of dynein with microtubules, where it can cooperate with Ndel1 to allow sustained force production from dynein. In addition, the Lis1-binding protein CLIP170 can also bind to and localize dynein to microtubule plus ends. Loss of Lis1 could result in the mislocalization of CLIP170 and Ndel1, and in turn, the misregulation of dynein. Proper dynein function is required for organelle transport and has been shown to be involved in vesicle recycling.23 Misregulation of dynein upon loss of Lis1 could result in mistargeting of organelles or proteins, or in defects in the recycling and endocytosis of desmosomal proteins. Therefore, when desmosomal proteins are internalized, they may not be properly recycled back to the surface but sent to the lysosome for degradation. In fact, desmosomal components in Lis1-null keratinocytes partially colocalized with lysosomal markers. That said, we have not observed a strong enrichment of dynein at desmosomes, though dynein is present at low levels at the cell cortex.
Conclusions
Recent studies have shed light on the many roles that Lis1 plays in the epidermis, both in microtubule organization and desmosomal stability and function. Although further work is needed, our study highlights an important function for desmosomes in recruiting centrosomal proteins. Desmosomes also require Lis1, and potentially other centrosomal proteins, to stabilize and maintain proper adhesion and attachment to keratin. The mechanisms behind Lis1-mediated desmosome stability have just begun to be elucidated. While Lis1 could directly promote desmosome stability by preventing their endocytosis (similar to p120-catenin in adherens junctions),24 it may also do so indirectly by interacting with other proteins that affect intermediate filament assembly or transport of proteins to the desmosome. It is clear that the function of Nde1/Ndel1 and dynein in the epidermis must be investigated to determine the role they play in cortical microtubule organization and/or proper centrosomal protein localization.
Acknowledgments
Work on cell adhesion/cytoskeleton organization in the Lechler lab is supported by a grant from NIH/NIAMS (R01AR055926).
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/18403
References
- 1.Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–85. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 2.Jonkman MF, Pasmooij AM, Pasmans SG, van den Berg MP, Ter Horst HJ, Timmer A, et al. Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa. Am J Hum Genet. 2005;77:653–60. doi: 10.1086/496901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–33. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 4.Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. 2000;6:1275–7. doi: 10.1038/81385. [DOI] [PubMed] [Google Scholar]
- 5.Sumigray KD, Chen H, Lechler T. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J Cell Biol. 2011;194:631–42. doi: 10.1083/jcb.201104009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol. 2007;176:147–54. doi: 10.1083/jcb.200609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodu V, Baffet AD, Le Droguen PM, Casanova J, Guichet A. A developmentally regulated two-step process generates a noncentrosomal microtubule network in Drosophila tracheal cells. Dev Cell. 2010;18:790–801. doi: 10.1016/j.devcel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, et al. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol. 2005;7:961–8. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113:3013–23. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 10.Baird DH, Myers KA, Mogensen M, Moss D, Baas PW. Distribution of the microtubule-related protein ninein in developing neurons. Neuropharmacology. 2004;47:677–83. doi: 10.1016/j.neuropharm.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Bugnard E, Zaal KJ, Ralston E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil Cytoskeleton. 2005;60:1–13. doi: 10.1002/cm.20042. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe EJ, Erickson HP, Bennett V. Desmoplakin I and desmoplakin II. Purification and characterization. J Biol Chem. 1989;264:8310–8. [PubMed] [Google Scholar]
- 13.Uzumcu A, Norgett EE, Dindar A, Uyguner O, Nisli K, Kayserili H, et al. Loss of desmoplakin isoform I causes early onset cardiomyopathy and heart failure in a Naxos-like syndrome. J Med Genet. 2006;43:e5. doi: 10.1136/jmg.2005.032904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, et al. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol. 1998;143:2009–22. doi: 10.1083/jcb.143.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–41. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–96. doi: 10.1016/S0896-6273(00)00146-X. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen MD, Shu T, Sanada K, Lariviere RC, Tseng HC, Park SK, et al. A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat Cell Biol. 2004;6:595–608. doi: 10.1038/ncb1139. [DOI] [PubMed] [Google Scholar]
- 18.Shim SY, Samuels BA, Wang J, Neumayer G, Belzil C, Ayala R, et al. Ndel1 controls the dynein-mediated transport of vimentin during neurite outgrowth. J Biol Chem. 2008;283:12232–40. doi: 10.1074/jbc.M710200200. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Tsai MY, Wang S, Lu B, Chen R, Iii JR, et al. Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat Cell Biol. 2009;11:247–56. doi: 10.1038/ncb1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayaraj P, Kroger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol. 2009;187:175–84. doi: 10.1083/jcb.200906094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Yang Z, Song W, Chen Q, Wang F, Zhang Q, et al. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol Biol Cell. 2006;17:680–9. doi: 10.1091/mbc.E05-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–14. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driskell OJ, Mironov A, Allan VJ, Woodman PG. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol. 2007;9:113–20. doi: 10.1038/ncb1525. [DOI] [PubMed] [Google Scholar]
- 24.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–34. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]


