Abstract
In exocrine organs such as the salivary glands, fluids and proteins are secreted into ductal structures by distinct mechanisms that are tightly coupled. In the acinar cells, the major secretory units of the salivary glands, fluids are secreted into the acinar canaliculi through paracellular and intracellular transport, whereas proteins are stored in large granules that undergo exocytosis and fuse with the apical plasma membranes releasing their content into the canaliculi. Both secretory processes elicit a remodeling of the apical plasma membrane that has not been fully addressed in in vitro or ex vivo models. Recently, we have studied regulated exocytosis in the salivary glands of live rodents, focusing on the role that actin and myosin plays in this process. We observed that during exocytosis both secretory granules and canaliculi are subjected to the hydrostatic pressure generated by fluid secretion. Furthermore, the absorption of the membranes of the secretory granules contributes to the expansion and deformation of the canaliculi. Here we suggest that the homeostasis of the apical plasma membranes during exocytosis is maintained by various strategies that include: (1) membrane retrieval via compensatory endocytosis, (2) increase of the surface area via membrane folds and (3) recruitment of a functional actomyosin complex. Our observations underscore the important relationship between tissue architecture and cellular response, and highlight the potential of investigating biological processes in vivo by using intravital microscopy.
Keywords: actin, cytoskeleton, exocrine glands, exocytosis, intravital microscopy, membrane tension, myosin, salivary glands, secretion
Introduction
The dynamics of several biological processes have been studied primarily in model systems, such as cell cultures, that do not recapitulate the complex architecture that tissues maintain in their physiological environment.1-4 As a consequence, the roles played by tissue components, such as surrounding cells, extracellular matrix, and signaling molecules or by other physiological events that may occur in parallel to the process of interest have not been fully appreciated. This issue can be overcome by using intravital microscopy (IVM). IVM encompasses a series of techniques based on light microscopy and has enabled imaging biological processes in live animals.5-9 Although IVM has been used for the last 10–15 y to study tissue and cell behavior, only recently has been applied to study subcellular events in vivo.3,9-11
In a recent study, we have investigated the modality and the machinery of exocytosis in the salivary glands (SGs) of live rodents that are an established model for exocrine secretion.3 In SGs, proteins destined to secretion are sorted at the trans-Golgi network into large secretory granules (SCGs), that undergo a maturation process, and are delivered to the apical plasma membrane (APM).12,13 Upon β-adrenergic stimulation, SCGs dock at the acinar canaliculi and fuse with APM, releasing their content into the extracellular space. We found that after the opening of the fusion pore SCGs gradually collapse and their membranes are completely absorbed into the acinar canaliculi.3 Usually, protein secretion is accompanied by fluid secretion. Fluids are secreted paracellularly, by osmotic-driven processes, and/or intracellularly, through the activation of water channels, such as aquaporin 5, that are both controlled by the activation of muscarinic receptors.14,15 However, smaller amount of fluids are also secreted during β-adrenergic stimulation.3
The role of the actin cytoskeleton in exocytosis has been controversial, most likely due to the fact that its function(s) may change depending on the specific architecture of the exocytic model under investigation.16,17 Furthermore, different approaches and experimental systems have been used to study the same exocytic pathway, often reaching contradicting results.18 In SGs, actin is localized primarily at the plasma membrane with a particular enrichment at the apical pole.3,19 Recently, we found that two of the isoforms of the actin-based motor myosin II (IIa and IIb) are localized at the APM.3 The actomyosin complex seems to serve multiple functions, such as facilitating the collapse of the SCGs through its contractile activity, preventing homotypic fusion between the secretory granules, and, as suggested here, counteracting the hydrostatic pressure built in the ductal system that may disrupt exocytosis and affect both the architecture and the physiology of the SGs.
Expansion of the Acinar Canaliculi during Exocytosis: Hydrostatic Pressure and Membrane Absorption
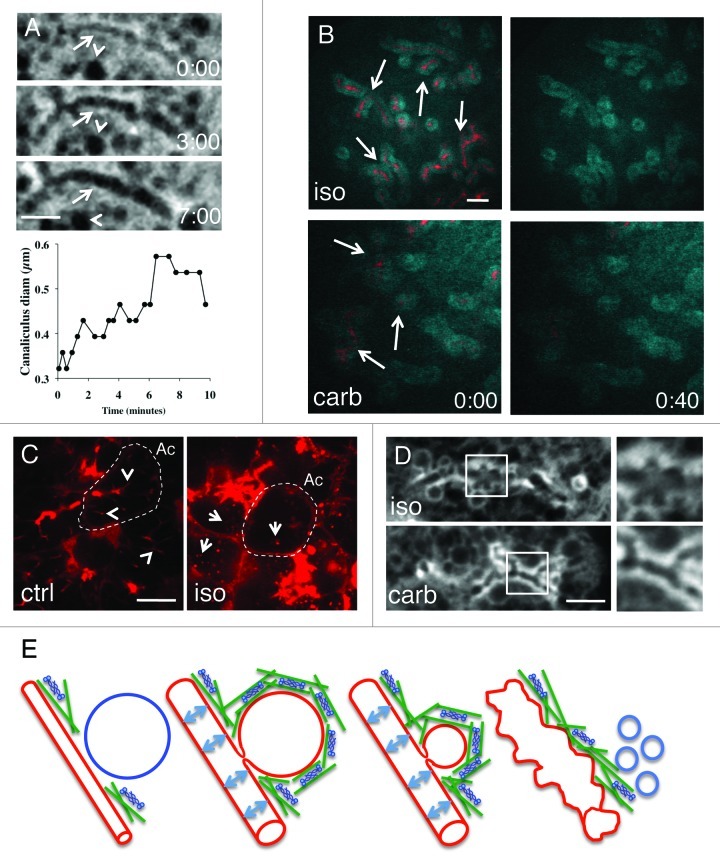
To image the exocytosis of SCGs in SGs we used a transgenic mouse model, which expresses cytosolic GFP.3,20 GFP is localized in the cytoplasm and the nuclei, is excluded from the SCGs, which appear as dark vesicles, and is enriched at the APM (Fig. 1A, arrows). When exocytosis was stimulated by isoproterenol (iso), an agonist of the β-adrenergic receptors, we observed the expansion of the canaliculi that increased significantly in diameter (Fig. 1A, arrows). This expansion may be attributed to at least two causes: (1) the hydrostatic pressure generated by the fluids that are secreted during regulated exocytosis, and (2) the membranes of the SCGs that are absorbed into the canaliculi after their fusion with the APM.3
Figure 1. Expansion of the acinar canaliculi during regulated exocytosis. (A)The APM of the SG acini were imaged upon subcutaneous (SC) injection of iso by intravital confocal microscopy, as previously described.3 A canaliculus (arrows) expanded over time as shown in the micrographs. Arrowheads point to a SCGs in the cytoplasm. Scale bar, 2µm. Lower graph shows the quantification of the diameter of the canaliculi. (B) Fluid expulsion during regulated exocytosis. A fluorescently labeled dextran (red) was retro-infused by gravity into the ducts of the SGs of a live rat. Iso (upper panels) or carb (lower panels) were injected SC and the SGs were imaged by intravital two-photon microscopy.10 The parenchyma of the glands is revealed by the excitation of endogenous fluorescence (cyan). After 40 sec from the injection of the agonists, the dextran was completely cleared from the ductal system (arrows). Scale bar, 20 µm. (C) Compensatory endocytosis. A fluorescently labeled dextran (red) was retro-infused in the ducts of the SGs of a live rat. The acinar canaliculi (arrowheads) are rapidly filled with the dye. The dotted line delimits an acinus (Ac). Iso was injected SC and the glands were imaged by intravital two-photon microscopy.11 As shown in (B), the dextran is cleared from the canaliculi. However, a fraction of it is internalized in small vesicles (arrows) generated by compensatory endocytosis from the APM. Scale bar, 10 µm. (D) Membrane folds in the acinar canaliculi. GFP-mice were injected SC with either iso (upper panels) or carb (lower panel). Acinar canaliculi were imaged by intravital confocal microscopy after 30 min from the injection. When SCGs exocytosis is elicited with iso, membrane folds appear in the canaliculi (upper panel and enlargement). Scale bar, 2 µm. (E) Model for the expansion of the canaliculi. After injection of iso, the SCG (blue) fuse with the APM (red). The hydrostatic pressure generated by fluids secretion (light blue arrows) contributes to the initial expansion of the canaliculi. SCGs are absorbed at the APM and compensatory endocytosis is stimulated (light blue vesicles). The excess of membranes that are not retrieved by compensatory endocytosis is accommodated by expanding the diameter of the canaliculi and by generating membrane folds. Actin (green) and myosin II (blue) are localized to the SCGs to counteract the hydrostatic pressure.
To show that hydrostatic pressure is built up into the ductal system during regulated exocytosis, we used an indirect method aimed at imaging the movement of fluids into ducts and canaliculi in the SGs of live rodents. As previously described, we imaged the clearance of a fluorescent dye that was retro-infused by gravity into the ductal system through a fine cannula inserted in the major excretory duct of a live rat (Fig. 1B).3,10,11 Upon injection of iso, we observed the rapid expulsion of the dye, consistent with the release of fluids from the acini. The ducts were almost completely cleared from the dye within 30–40 sec, suggesting that a high hydrostatic pressure built up into the ductal system. Although we could not image fluid expulsion beyond this time point, secretion continued for at least 30 min, as determined by collecting saliva from the cannula.3 We want to emphasize that since biological membranes are not elastic, the expansion of the canaliculi driven by the hydrostatic pressure requires membrane remodeling. Given that in the first minute after the injection of iso we did not observe any exocytosis of large SCGs, one possibility is that the extra membranes required for the expansion may be provided by the APM microvilli that were described in EM studies.21 Notably, the generation of hydrostatic pressure requires intact ductal structures, as shown by the fact that similar dilations were observed in freshly explanted lobules, but never described in isolated acinar preparations.22
As for the contribution of exocytic membranes, we estimated that approximately 200 SCGs are absorbed into a single canaliculus in 30 min. How the cell deals with this excess of membranes is a fascinating question. Indeed, a rigid canaliculus that is confined between two adjacent cells would not be able to accommodate a large excess of membranes. We hypothesized that the excess of membranes is retrieved by the activation of a process termed compensatory endocytosis, as reported for other exocytic events.11,23,24 Indeed, by retro-infusing fluorescent dextrans into the canaliculi we have shown that small endocytic vesicles are internalized from the APM during exocytosis (Fig. 1C, right panel, arrows).11 Although, we could not establish the exact size of these endocytic vesicles, they did not exceed 0.2 µm in diameter, suggesting that the membranes delivered by a single SCG (1–1.5 µm in diameter) may be retrieved by approximately 25–50 endocytic vesicles. However, in addition to compensatory endocytosis acinar cells may employ other strategies to deal with the excess of membranes, such as increasing the surface area of the APM. This can be accomplished by uniformly increasing the diameter of the canaliculi, and/or through membrane folding. Although we did not accurately measure the relative contribution of these two processes, we observed that stimulation of water secretion by carbachol (carb), an agonist of muscarinic receptors, induced a smaller expansion of the canaliculi when compared with β-adrenergic stimulation (Fig. 1D). Since muscarinic stimulation does not elicit exocytosis of large SCGs in vivo,3 this observation suggests the uniform expansion of the canaliculi along their length may be used to accommodate the excess of membranes delivered at the APM. However, since canaliculi are rigid structures embedded between two cells that are part of a compact tissue, we argued that this process would not be effective as more exocytic membranes are added. This hypothesis was indeed supported by the fact that a series of folds were observed in the canaliculi 20–30 min after the injection of iso (Fig. 1D, upper panels). These folds were not observed upon carb injection suggesting that they are originated from exocytic membranes (Fig. 1D, lower panels). However, we cannot rule out that smaller folds that are below the resolution provided by light microscopy formed in the canaliculi at the onset of exocytosis.
Finally, we observed that the actomyosin complex may also serve as a structural scaffold to maintain the integrity of the canaliculi under both resting and stimulated conditions. When the actin cytoskeleton or the motor activity of myosin II were disrupted canaliculi increased in size. This effect was more dramatic when the motor activity of myosin II was impaired than when the actin cytoskeleton was disrupted, most likely due to the fact that cytochalasin D and latrunculin A did not completely disrupt filamentous actin at the APM.3
Our findings emphasize that in vivo the APM of the acini in the salivary glands is subjected to a continuous remodeling and that membrane homeostasis is maintained by a series of distinct processes (Fig. 1E).
The Actin Cytoskeleton Provides a Scaffold to Counteract the Effect of the Hydrostatic Pressure on the Secretory Granules
To image the fate of the SCGs after their fusion with the APM we used a transgenic mouse expressing a membrane-targeted peptide fused with the fluorescent protein Tomato (m-tomato).3 We observed that the diameter of the SCGs slowly decreases in size after their fusion with the APM (within 40–60 sec) (Fig. 2A). During this process, cargo molecules are released and secreted fluids have access to the lumen of the granules. When the dynamics of the actin cytoskeleton was disrupted by pharmacological agents such as cytochalasin D or latrunculin A, SCGs expanded in size and their gradual collapse was impaired. The analysis of the diameters of the SCGs showed that there is a linear increase in their size in the first 20–30 sec after the opening of the fusion pore (Fig. 2B). Since during this period we did not detect any large membranous vesicle fusing with the SCGs, we speculated that the expansion was driven by the flow of membranes from the APM into the granule via the fusion pore. This can be facilitated by two factors, namely the difference in membrane tension (as discussed elsewhere25) and the hydrostatic pressure. After this initial step, the increase in size of the enlarged SCGs is due to membranes delivered by other SCGs that fuse with the enlarged SCGs that have acquired the characteristics of the APM, as previously shown.3 Similarly, when the activity of myosin II was impaired with blebbistatin, SCGs initially expanded in size, and later resumed their gradual collapse. This suggests that the myosin II contractile activity is also required to counteract the hydrostatic pressure after the opening of the fusion pore. Under this condition, the expanded granules did not further increase in size since the fusion with the other SCGs was prevented by the actin coat that recruited around the expanded granules (Fig. 2D).3
Figure 2. The actomyosin complex provides a scaffold to counteract the effect of the hydrostatic pressure on the secretory granules. (A)Mice expressing the m-Tomato probe were pre-treated with cytochalasin D, blebbistatin or the vehicle (DMSO, ctrl) and injected with iso to stimulate SCGs exocytosis.3 When the actin cytoskeleton was disrupted with cytochalasin D or the motor activity of myosin II was inhibited with blebbistatin the SCGs expanded in size due to the hydrostatic pressure generated by fluids secretion (light blue arrows). Scale bar, 2 µm. Actin (green) and myosin II (blue) are recruited to the SCGs to counteract the hydrostatic pressure and to facilitate the gradual collapse of the SCGs.3,24(B) SCGs were imaged after their fusion with the APM by using time-lapse intravital confocal microscopy. The diameter of the SCGs was measured in animals treated with cytochalasin D (red circles), blebbistatin (blue circles) or DMSO (black circles). (C) Fluorescently labeled dextran (red) was retro-infused in the ducts of the SGs of a live rat by using a cannula introduced in the major excretory duct as described in legend to Figure 1. The same acinus was imaged before (upper panel) and after the SC injection of iso and the artificial increase of the hydrostatic pressure achieved with a syringe connected to the cannula (lower panel). Acinar canaliculi (arrows) were filled with the dye (upper panel). Enlarged SCGs (arrowheads) appeared from the APM (lower panel). Scale bar, 10 µm.
Overall our data suggests that another function of the actomyosin complex is to be recruited on the surface of the fusing granules to counteract the effect of the hydrostatic pressure. This implies that fused SCGs represent a weak spot at the APM that needs to be “patched” to prevent disruptions in the cell architecture. This hypothesis was also supported by the fact that an artificial increase of the intraductal pressure during exocytosis induced the expansion of the SCGs even when the actin cytoskeleton was not perturbed (Fig. 2C).
Conclusions
Our observations suggest that protein and fluid secretion induce extensive remodeling of the APM in the SGs. The acinar canaliculi undergo significant expansions that may be due in part to the hydrostatic pressure generated by fluid secretion and in part to the large amount of membranes delivered to the APM during exocytosis. The homeostasis of the APM is maintained by a series of different processes that include compensatory endocytosis and modulations of the surface area of the canaliculi, whereas the structural integrity of the secretory apparatus may be maintained by the recruitment of a functional actomyosin complex on the membranes. Notably, this hypothesis can be extended to other exocrine glands where protein and fluid secretion are coupled, such as lacrimal glands and exocrine pancreas, and in which exocytosis requires more than few seconds to be completed.
Several questions still need to be addressed: first, how the contractile activity of myosin II regulates the gradual collapse of the SCGs and the homeostasis of the APM, and second, what is the role played by each of the two isoforms of myosin II during secretion. As for the exocytosis of SCGs, we would like to speculate that the forces generated by the contractile activity of myosin II may either push the membranes of the SCGs toward the APM or act at the neck of the SCGs by pulling and enlarging the fusion pore. One interesting possibility is that both these processes may contribute to this step and that each of them may be controlled by one of the myosin II isoforms. As for the homeostasis of the APM, we envision that the myosin II contractile activity around the APM may work similarly to the contractile ring during cytokinesis, maintaining a continuous tension in the membranes that opposes expansive forces, such as the hydrostatic pressure.
Our approach based on IVM in live rodents has highlighted that some biological processes occur with the coordination of several cellular events and are strongly influenced by the architecture of the experimental model. Reductionist approaches based on the use of simplified models offer the advantages of a better control of the experimental conditions and the opportunity to study processes at a molecular level. However, certain aspects of the physiology of an organ, that may have unanticipated effects on cellular functions cannot be recapitulated in vitro systems and require the use of in vivo models.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research.
Glossary
Abbreviations:
- IVM
intravital microscopy
- SGs
salivary glands
- SCGs
secretory granules
- APM
apical plasma membrane
- iso
isoproterenol
- carb
carbachol
- SC
subcutaneous
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/18405
References
- 1.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 2.Green JA, Yamada KM. Three-dimensional microenvironments modulate fibroblast signaling responses. Adv Drug Deliv Rev. 2007;59:1293–8. doi: 10.1016/j.addr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, et al. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci USA. 2011;108:13552–7. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amornphimoltham P, Masedunskas A, Weigert R. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv Drug Deliv Rev. 2011;63:119–28. doi: 10.1016/j.addr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci. 2011;124:299–310. doi: 10.1242/jcs.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–39. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A. Intravital microscopy: a novel tool to study cell biology in living animals. Histochem Cell Biol. 2010;133:481–91. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9:1801–10. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sramkova M, Masedunskas A, Parente L, Molinolo A, Weigert R. Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals. Am J Physiol Cell Physiol. 2009;297:C1347–57. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle JD. Protein secretion by rat parotid acinar cells. Pathways and regulation. Ann N Y Acad Sci. 1998;842:115–24. doi: 10.1111/j.1749-6632.1998.tb09639.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005;84:500–9. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–69. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 15.Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8:3–11. doi: 10.1034/j.1601-0825.2002.10815.x. [DOI] [PubMed] [Google Scholar]
- 16.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–81. doi: 10.1016/S0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 17.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–98. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warner JD, Peters CG, Saunders R, Won JH, Betzenhauser MJ, Gunning WT, 3rd, et al. Visualizing form and function in organotypic slices of the adult mouse parotid gland. Am J Physiol Gastrointest Liver Physiol. 2008;295:G629–40. doi: 10.1152/ajpgi.90217.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/S0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 21.Segawa A, Loffredo F, Puxeddu R, Yamashina S, Testa Riva F, Riva A. Exocytosis in human salivary glands visualized by high-resolution scanning electron microscopy. Cell Tissue Res. 1998;291:325–36. doi: 10.1007/s004410051002. [DOI] [PubMed] [Google Scholar]
- 22.Huang AY, Castle AM, Hinton BT, Castle JD. Resting (basal) secretion of proteins is provided by the minor regulated and constitutive-like pathways and not granule exocytosis in parotid acinar cells. J Biol Chem. 2001;276:22296–306. doi: 10.1074/jbc.M100211200. [DOI] [PubMed] [Google Scholar]
- 23.Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 2010;29:1961–75. doi: 10.1038/emboj.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5:727–32. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 25.Masedunskas A, Porat-Shliom N, Weigert R. Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Commun Integr Biol. 2011 doi: 10.4161/cib.18258. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]