Abstract
microRNAs (miRNAs), defined as 21–24 nucleotide non-coding RNAs, are important regulators of gene expression. Initially, the functions of miRNAs were recognized as post-transcriptional regulators on mRNAs that result in mRNA degradation and/or translational repression. It is becoming evident that miRNAs are not only restricted to function in the cytoplasm, they can also regulate gene expression in other cellular compartments by a spectrum of targeting mechanisms via coding regions, 5′ and 3′untransalated regions (UTRs), promoters, and gene termini. In this point-of-view, we will specifically focus on the nuclear functions of miRNAs and discuss examples of miRNA-directed transcriptional gene regulation identified in recent years.
Keywords: Argonaute proteins, RNA activation, chromatin remodeling, gene regulation, promoter-targeting miRNAs, transcriptional gene silencing
Introduction
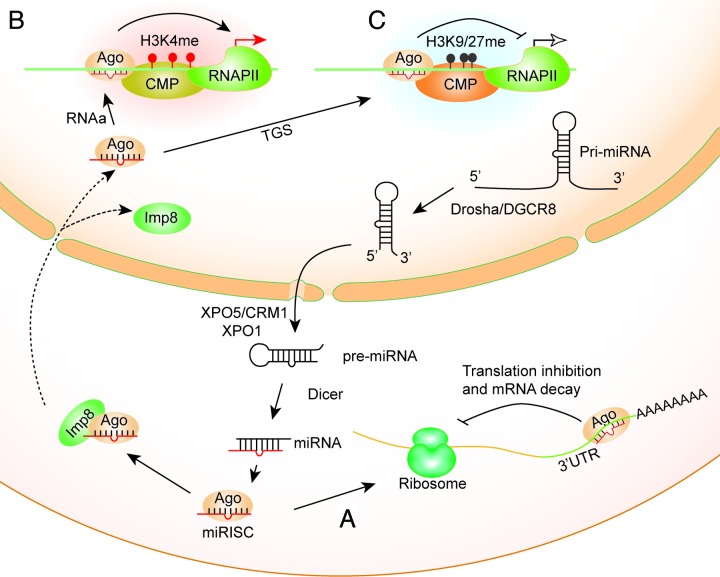
microRNAs (miRNAs), a class of non-coding small RNAs, play important roles in gene regulation and impact a myriad of biological processes and diseases.1 Most miRNAs are generated by the canonical biogenesis pathway2 (Fig. 1). miRNA genes are transcribed by RNA polymerase II (RNAP II) into primary miRNA transcripts (pri-miRNAs), which are further processed into miRNA precursors (pre-miRNAs) in the nucleus by the microprocessor complex Drosha/Dgcr8. Pre-miRNAs are then exported to the cytoplasm by Exportin 5 and converted into ~22-nt mature miRNAs by Dicer. Based on the thermodynamic properties, one of the strands is preferentially incorporated into the Argonaute (Ago) protein, a key component of the RNA-induced silencing complex (RISC) complex, and guides it to target(s). miRNAs are conventionally regarded as negative regulators of gene expression, mostly through post-transcriptional events taking place in the cytoplasm. They are known to target complementary sequence on the mRNA at different sites or on many different mRNAs through base-pairing between the miRNA seed region and the 3′ untranslated region (UTR) in the target mRNA. It has been reported that miRNAs can also regulate gene expression by targeting the 5′ UTR,3 coding regions,4 promoters,5-8 and gene termini.9 In addition, miRNAs are predicted by several genome-wide computational analyses to target gene promoters because potential targets for miRNAs are commonly found based on sequence homology in promoter sequences as in 3′ UTRs,10,11 with some targets highly complementary to miRNA sequences.8 Intriguingly, functional RISC activity and RNAi components were detected in the nucleus12-14 and the mitochondria,15 suggesting that miRNAs also regulate gene expression in cellular compartments other than the cytoplasm. A well-illustrated example of small-RNA dependent transcription gene silencing through heterochromatin formation came from fission yeast and plants.16 Deep sequencing analysis revealed a subset of miRNAs predominantly localized in the nucleus of human cells and most miRNAs are imported into the nucleus.17 It has also been shown that nuclear-cytoplasmic shuttling of miRNAs and RNAi components involve CRM1/Exportin 1 and Importin 818,19 (Fig. 1). Therefore, cytoplasmically processed miRNAs can be imported into the nucleus to regulate gene expression. In support, a number of miRNAs have been recently identified to regulate gene expression in the nucleus by binding to the promoter of targeted genes.5-8 In our recent work, by using a combination of computational prediction and experimental validation, we identified miRNAs highly complementary to promoter sequences (also known as promoter-targeting miRNAs) which can activate gene expression in both human and mouse cells.6,7
Figure 1. Actions of miRNAs in the nucleus. Canonical miRNAs are transcribed by RNA polymerase II (RNAP II) into primary miRNA transcripts (pri-miRNAs), which are further processed into miRNA precursors (pre-miRNAs) in the nucleus by Drosha/Dgcr8. Pre-miRNAs are then exported to the cytoplasm by Exportin 5 (XPO5)/CRM1 and are processed into mature miRNAs by Dicer. One of the strands is preferentially incorporated into the Argonaute (Ago) protein, a component of the miRISC complex. (A) Classical function of miRNA-mediated post-transcriptional inhibition by 3 ‘UTR targeting. (B and C) In order for miRNAs to function in the nucleus, Ago-miRNA complex is imported into the nucleus by binding to Importin 8 (Imp8). Promoter-targeted miRNA complexed with Ago binds to chromosomal DNA sequences or nascent cognate transcripts derived from promoters. During RNAa, recruitment of chromatin modifying proteins (CMPs) leads to increased H3K4 methylation thereby activates transcription at the targeted promoter (B). In TGS, recruitment of CMPs leads to increased H3K9/K27 methylation thereby inhibits transcription at the targeted promoter (C). RNAa: RNA activation; TGS: transcriptional gene silencing; CMPs: chromatin modifying proteins; miRISC: miRNA-containing RNA induced silencing complex
Promoter targeting miRNAs
We and others showed that synthetic double-stranded RNA (dsRNA) targeting gene promoters, also known as small activating RNA (saRNA), activate gene expression via a process known as RNA activation (RNAa) (see recent review by Portnoy et al.11). Mutation analysis showed that dsRNAs with mismatches between dsRNA and targeted promoter sequences retained the ability to induce gene expression,20,21 indicating that RNAa does not require perfect complementarity between the guide RNA and target sequences, a feature reminiscent of miRNA targeting mRNA sequence. This observation led to the hypothesis that endogenously expressed miRNAs might also trigger RNAa. We supported this notion by identifying miR-373 as the first example of a promoter-targeting miRNA in human cells7 (Table 1). We showed that miR-373 can readily activate E-Cadherin (CDH1) and cold-shock domain-containing protein C2 (CSDC2), which contain putative miR-373 target sites with at least 80% sequence complementarity in their promoters. Furthermore, gene activation by miR-373 is Dicer dependent and involves recruitment of RNAP II at the target promoter.
Table 1. Promoter-targeting miRNAs.
Kim et al.8 subsequently reported another promoter-targeting miRNA, miR-320. Computational analysis revealed that miR-320 is among one of the 9 mature miRNAs which exhibited perfect sequence complementarity with gene promoters and is transcribed from the promoter of POLR3D gene. miR-320 levels correlated inversely with POLR3D expression in different tissues examined and transfection of miR-320 mimics induced gene silencing of POLR3D, implying that miR-320 targets the promoter of POLR3D and directs transcriptional gene silencing (TGS) of POLR3D in cis (Table 1). Following transfection of miR-320, enrichment of Ago1 and H3K27me3 was observed at the POLR3D promoter. mR-320 also induced enrichment of EZH2, a histone methyltransferase, suggesting miR-320 mediated TGS of POLR3D associated with epigenetic changes.
Very recently, Younger et al.5 identified multiple exogenous miRNA mimics (miR-423-5p, miR-372, miR-373, miR-520c-3p) that inhibit the expression of progesterone receptor (PR), a locus well-characterized for small RNA mediated gene regulation (Table 1). Consistent with their previous reports using perfectly matched dsRNAs, they showed that TGS at the PR promoter mediated by miR-423-5p in trans is associated with recruitment of Ago2 to a non-coding RNA (ncRNA) transcript transcribed from the PR promoter. Similar to miR-373 which targets multiple promoters for transcriptional regulation,7 miR-423-5p can target additional genes which bear its targets within their promoters (Table 1). An increase in H3K9me2 was detected at the PR promoter, again, suggesting that epigenetic changes were associated with miRNA-induced TGS (Fig. 1).
The authors also evaluated the endogenous functions of miR-423-5p in PR regulation. However, they were unable to detect changes in PR gene expression following the expression of antisense RNAs against miR-423-5p in the two cell lines used in this study. The use of exogenous miRNA mimics allowed for proof-of-principle demonstration for small RNA-mediated gene regulation studies at the well-characterized PR locus. However, due to the lack of functional evidence of miR-423-5p, the endogenous functions of this miRNA in mediating TGS still needs to be further examined in other cell types and/or other physiological conditions.
Ccnb1 Promoter-targeting miRNAs
In our recent work by Huang et al.,6 we identified miRNAs (miR-744, miR-1186, miR-466d-3p) which are highly complementary to sites in the mouse Cyclin B1 (Ccnb1) promoter and can activate Ccnb1 expression (Table 1). In an attempt to identify miRNAs that may have gene activating roles in an endogenous context, Ccnb1 came out of the initial screen as one of the genes that were downregulated by depletion of Drosha and Dicer. In silico miRNA target prediction conducted on a 1-kb promoter region of the mouse Ccnb1 gene identified 21 potential targets for 20 miRNAs. Among the top candidate miRNAs, miR-744 and miR-1186 possess over 90% complementarity with the Ccnb1 promoter and consistently activate Ccnb1 expression. Depletion of miR-744 resulted in the downregulation of Ccnb1 expression, suggesting the basal expression of Ccnb1 is in part miR-744 dependent. Upregulation of Ccnb1 by the miRNAs involves recruitment of Ago1 and RNAP II and accompanied by an increase in histone mark H3K4me3 at the Ccnb1 promoter. Based on these findings, it is suggested that Ccnb1 activating miRNAs activate Ccnb1 expression by binding to the Ccnb1 promoter in an Ago1 dependent manner although the exact molecular targets (promoter transcript vs. chromosomal DNA) remain to be determined. Upon binding to the Ccnb1 promoter, it is likely that Ago1 further recruits chromatin modifying proteins to activate transcription (Fig. 1).
Given the observation that mouse physiological Ccnb1 expression depends on the miRNA pathway and the fact that Ccnb1 is an essential protein that drives mitotic cell cycle entry, it is expected that perturbation of such intricate relationship may have profound functional consequences. Indeed, transient overexpression of Ccnb1 promoter targeting miRNAs enhanced in vitro cell proliferation and promoted mitosis in the short-term. Surprisingly, stable expression of these miRNAs in mouse prostate cancer cells disrupted global chromosome stability and suppressed in vivo tumorigenecity. Collectively, this work provides the first example of physiologically relevant RNAa and demonstrated that miRNAs have nuclear function to positively impact gene transcription. What is more, cancer cells may exploit such mechanism to gain a growth advantage. Identifying additional examples will provide insights into contextual requirement and mechanism for miRNA-mediated gene regulation.
Roles of Ago Proteins in miRNA-Mediated Gene Regulation
Members of the Ago proteins belong to a highly evolutionarily conserved protein family. There are four Ago family members expressed in mammals. It has been reported that all four human Ago1–4 exhibit similar biochemical preferences for binding to duplex RNA, although only Ago2 uniquely exhibits cleavage activity.22,23 Ago 1–4, especially Ago1 and Ago2, have been implicated in small RNA-mediated gene regulation.5,6,8,20,24 Subcellular localization studies in human cells have shown that Ago1 and Ago2 are localized in the nucleus,13,25,26 suggesting their possible interactions with the chromatin.
Several recent studies reflect functional segregation between Ago1 and Ago2 in miRNA-mediated gene regulation. For example, Kim et al.8 showed that Ago1 is enriched at the POLR3D promoter following transient overexpression of miR-320, suggesting that Ago1 may be one of the effector proteins for initiating TGS. A recent report by Younger et al.5 showed that miRNAs can robustly inhibit PR transcription despite their low degree of complementarity with the target ncRNA and suggested that miRNAs with incomplete complementarity to their target require Ago2 as opposed to Ago1 as shown by Kim et al.8 Recruitment of Ago2 to their respective target ncRNAs results in alteration in the level of RNAP II at the PR promoter. Therefore, the authors concluded that Ago2 is required for recognition of promoter-overlapping ncRNAs by miRNA mimics.5 Consistent with Kim et al.,8 our recent work suggested that Ago1 seemed to play a major role in Ccnb1 gene activation mediated by miRNA. We found Ago1 but not Ago2 is selectively enriched at the Ccnb1 promoter in vicinity to the miR-744 target site on mouse Ccnb1 promoter. Moreover, depletion of Ago1 by RNAi had a stronger effect on Ccnb1 down regulation in mouse cells than depletion of Ago2. Mescalchin et al.27 has shown that while Ago2 is primarily involved in siRNA-mediated silencing pathways, Ago1 and other family members are primarily involved in miRNA-mediated gene regulation. Ago1 and Ago2 also showed differential distribution in the nucleus in response to promoter-targeted siRNAs during TGS.26 Taken together, these findings suggest that the promoter targeting mechanism mediated by miRNAs may be different from those utilized by perfectly matched dsRNAs in terms of requirement for Ago proteins. Moreover, chromatin environment, sequence complementarity, and the type of promoter targets for miRNAs may account for the differential requirement for Ago proteins in miRNA mediated gene regulation.
Future Perspectives
Recent discoveries of the noncanonical functions of miRNAs in gene regulation present a paradigm shift—miRNAs possess the ability to fine-tune gene expression at different levels of gene regulation. The nuclear functions of miRNAs have begun to emerge in recent years, although the mechanism of miRNA-mediated transcriptional regulation remains to be fully elucidated. Studies have shown that endogenously expressed miRNAs have similar gene regulatory effects on target gene promoters compared with promoter-targeting short interfering RNAs (siRNAs) or saRNAs.5,6,28,29 Thus, rules that govern gene regulation utilized by promoter-targeting dsRNAs can be partly applied to the understanding of the targeting mechanism mediated by promoter-targeting miRNAs. It has been reported, at least for the PR gene, ncRNAs overlapping the promoter serve as targets for both promoter-targeting miRNAs and siRNAs.5,30 Hansen et al.31 reported circular noncoding antisense transcripts as direct targets of miR-671, a nuclear enriched miRNA. Identification of other types of nuclear targets of miRNAs needs to be determined. Long ncRNAs have been shown to interact with chromatin remodeling factors in mammalian cells,32 suggesting that ncRNAs have the potential to regulate gene transcription through epigenetic reprogramming. Indeed, Drosophila Ago2 and other RNAi components have been recently shown to be directly involved in chromatin regulation.14,33 Other than transcriptional regulation, synthetic dsRNAs have also been shown to redirect splicing in the nucleus of mammalian cells.34 Deciphering the roles of miRNAs and other small RNA species in regulation of chromatin dynamics and additional nuclear processes in mammalian systems is a fascinating area of investigation in the years to come.
Acknowledgments
We thank members of the Li lab for insightful discussions. This work was supported by grants from the National Institutes of Health (1R01GM090293-0109 to L.-C.L.) and the Department of Defense (W81XWH-08-1-0260 to L.-C.L.). V.H. is supported by a Prostate Cancer Postdoctoral Training Award from the Department of Defense (W81XWH-10-1-0505).
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/19354
References
- 1.Czech B, Hannon GJ. Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet. 2011;12:19–31. doi: 10.1038/nrg2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci U S A. 2007;104:9667–72. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 5.Younger ST, Corey DR. Transcriptional gene silencing in mammalian cells by miRNA mimics that target gene promoters. Nucleic Acids Res. 2011;39:5682–91. doi: 10.1093/nar/gkr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang V, Place RF, Portnoy V, Wang J, Qi Z, Jia Z, et al. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr934. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–13. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DH, Saetrom P, Snøve O, Jr., Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci U S A. 2008;105:16230–5. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younger ST, Corey DR. Transcriptional regulation by miRNA mimics that target sequences downstream of gene termini. Mol Biosyst. 2011;7:2383–8. doi: 10.1039/c1mb05090g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younger ST, Pertsemlidis A, Corey DR. Predicting potential miRNA target sites within gene promoters. Bioorg Med Chem Lett. 2009;19:3791–4. doi: 10.1016/j.bmcl.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portnoy V, Huang V, Place RF, Li LC. Small RNA and transcriptional upregulation. Wiley Interdiscip Rev RNA. 2011;2:748–60. doi: 10.1002/wrna.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat Struct Mol Biol. 2005;12:133–7. doi: 10.1038/nsmb886. [DOI] [PubMed] [Google Scholar]
- 13.Tan GS, Garchow BG, Liu X, Yeung J, Morris JP, 4th, Cuellar TL, et al. Expanded RNA-binding activities of mammalian Argonaute 2. Nucleic Acids Res. 2009;37:7533–45. doi: 10.1093/nar/gkp812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cernilogar FM, Onorati MC, Kothe GO, Burroughs AM, Parsi KM, Breiling A, et al. Chromatin-associated RNA interference components contribute to transcriptional regulation in Drosophila. Nature. 2011;480:391–5. doi: 10.1038/nature10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandiera S, Rüberg S, Girard M, Cagnard N, Hanein S, Chrétien D, et al. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One. 2011;6:e20746. doi: 10.1371/journal.pone.0020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdel A, Vavasseur A, Le Gorrec M, Touat-Todeschini L. Common themes in siRNA-mediated epigenetic silencing pathways. Int J Dev Biol. 2009;53:245–57. doi: 10.1387/ijdb.082691av. [DOI] [PubMed] [Google Scholar]
- 17.Liao JY, Ma LM, Guo YH, Zhang YC, Zhou H, Shao P, et al. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS One. 2010;5:e10563. doi: 10.1371/journal.pone.0010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castanotto D, Lingeman R, Riggs AD, Rossi JJ. CRM1 mediates nuclear-cytoplasmic shuttling of mature microRNAs. Proc Natl Acad Sci U S A. 2009;106:21655–9. doi: 10.1073/pnas.0912384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinmann L, Höck J, Ivacevic T, Ohrt T, Mütze J, Schwille P, et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103:17337–42. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui M, Sakurai F, Elbashir S, Foster DJ, Manoharan M, Corey DR. Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter. Chem Biol. 2010;17:1344–55. doi: 10.1016/j.chembiol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, et al. ATP-dependent human RISC assembly pathways. Nat Struct Mol Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–41. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 24.Janowski BA, Huffman KE, Schwartz JC, Ram R, Nordsell R, Shames DS, et al. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat Struct Mol Biol. 2006;13:787–92. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- 25.Ohrt T, Mütze J, Staroske W, Weinmann L, Höck J, Crell K, et al. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res. 2008;36:6439–49. doi: 10.1093/nar/gkn693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlenstiel CL, Lim HG, Cooper DA, Ishida T, Kelleher AD, Suzuki K. Direct evidence of nuclear Argonaute distribution during transcriptional silencing links the actin cytoskeleton to nuclear RNAi machinery in human cells. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr891. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mescalchin A, Detzer A, Weirauch U, Hahnel MJ, Engel C, Sczakiel G. Antisense tools for functional studies of human Argonaute proteins. RNA. 2010;16:2529–36. doi: 10.1261/rna.2204610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang V, Qin Y, Wang J, Wang X, Place RF, Lin G, et al. RNAa is conserved in mammalian cells. PLoS One. 2010;5:e8848. doi: 10.1371/journal.pone.0008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janowski BA, Huffman KE, Schwartz JC, Ram R, Hardy D, Shames DS, et al. Inhibiting gene expression at transcription start sites in chromosomal DNA with antigene RNAs. Nat Chem Biol. 2005;1:216–22. doi: 10.1038/nchembio725. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz JC, Younger ST, Nguyen NB, Hardy DB, Monia BP, Corey DR, et al. Antisense transcripts are targets for activating small RNAs. Nat Struct Mol Biol. 2008;15:842–8. doi: 10.1038/nsmb.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, et al. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–22. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7:582–5. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moshkovich N, Nisha P, Boyle PJ, Thompson BA, Dale RK, Lei EP. RNAi-independent role for Argonaute2 in CTCF/CP190 chromatin insulator function. Genes Dev. 2011;25:1686–701. doi: 10.1101/gad.16651211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Hu J, Corey DR. Expanding the action of duplex RNAs into the nucleus: redirecting alternative splicing. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr780. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]