Abstract
Osteopontin (Opn) is important for T helper type 1 (TH1) immunity and autoimmunity. However, the role of this cytokine in TH2-mediated allergic disease as well as its effects on primary versus secondary antigenic encounters remain unclear. Here we demonstrate that OPN is expressed in the lungs of asthmatic individuals and that Opn-s, the secreted form of Opn, exerts opposing effects on mouse TH2 effector responses and subsequent allergic airway disease: pro-inflammatory at primary systemic sensitization, and anti-inflammatory during secondary pulmonary antigenic challenge. These effects of Opn-s are mainly mediated by the regulation of TH2-suppressing plasmacytoid dendritic cells (DCs) during primary sensitization and TH2-promoting conventional DCs during secondary antigenic challenge. Therapeutic administration of recombinant Opn during pulmonary secondary antigenic challenge decreased established TH2 responses and protected mice from allergic disease. These effects on TH2 allergic responses suggest that Opn-s is an important therapeutic target and provide new insight into its role in immunity.
Immunity against pathogens is mediated through the induction of antigen-specific T helper (TH) type 1 and type 2 lymphocytes. TH1 immunity confers protection against intracellular pathogens and, when excessive, can lead to autoimmunity1,2. Aberrant TH2 cell activation against environmental antigens may induce allergy and asthma3. Activation and differentiation of TH immunity depends on interactions of TH cells with antigen-presenting cells, such as dendritic cells (DCs), and cytokines play a crucial role in this process.
Opn is a cytokine originally identified as the predominant transcript expressed by activated T cells4,5. Opn-deficient (Spp1−/−, also known as Opn−/−) mice exhibit reduced immunity to viruses6 and other microorganisms7, develop milder experimental autoimmune encephalomyelitis8–10 and are resistant to the development of autoimmune keratitis6, all TH1-linked responses. Increased OPN expression has also been shown in affected tissues from individuals with rheumatoid arthritis, Crohn disease and multiple sclerosis10–12. Also, polymorphisms in the gene encoding OPN have been linked to the development of systemic lupus erythematosus and multiple sclerosis13,14, suggesting a role in autoimmunity.
An important recent study has demonstrated that the intracellular form of Opn (Opn-i) is essential for interferon (IFN)-α production by plasmacytoid DCs (pDCs) upon viral infection or CpG oligonucleotide administration15. Additionally, recombinant OPN (rOPN) induces maturation of TH1-polarizing human DCs in vitro16, and blockade of Opn-s reduces costimulatory molecule and class II molecule expression on human monocyte–derived DCs17. Moreover, Spp1−/− mice exhibit reduced trinitrochlorobenzene–induced migration of DCs to draining lymph nodes (DLNs)18. In contrast, rOpn administration inhibits bacterially induced DC migration19. Opn-i and Opn-s can therefore affect DC functions, which are crucial in determining the outcome of adaptive immunity.
Previous studies have focused on the role of Opn during TH1 viral and autoimmune processes in which responses were ongoing by means of repetitive antigenic encounter6,8. However, the effect of this cytokine during primary versus secondary antigenic encounters remains unclear. Moreover, the role of Opn in TH2-mediated allergic responses, a rising health issue in industrialized countries20, has not been elucidated. Therefore, we investigated the in vivo effects of Opn-s in distinct phases of a TH2 immune response and subsequent disease development, using an established mouse model of ovalbumin (OVA)-induced allergic airway inflammation21. We also examined whether the role of Opn-s was mediated by effects on DC subsets. By comparing the results obtained upon neutralization of Opn-s with those from Spp1−/− mice, we studied the immunoregulatory activity of the Opn isoforms in TH2 allergic responses and the disease phenotype.
RESULTS
Increased lung Opn expression in allergic disease
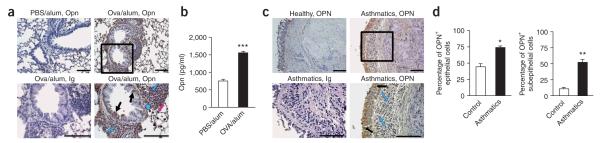
We investigated Opn expression during allergic TH2 responses, using a mouse model of airway inflammation induced by OVA/alum sensitization followed by airway OVA challenges. There was upregulation of lung Opn expression in mice sensitized with OVA/alum as compared to PBS/alum (alum controls) (Fig. 1a), localized mainly at sites of leukocytic infiltration and in bronchial and alveolar epithelial cells. Opn was also increased in lung homogenates from OVA-sensitized mice (Fig. 1b).
Figure 1.
Expression of Opn in the lung in allergic airway disease. (a) Photomicrograph of lung sections from PBS/alum-sensitized BALB/c mice (alum controls) and OVA/alum-sensitized BALB/c mice stained with Opn-specific antibody. Immunized mice also received three challenges with aerosolized OVA. In OVA/alum mice, Opn was expressed by infiltrating leukocytes (blue arrows), including macrophages (blue arrowhead), by bronchial epithelial cells (black arrows) and by alveolar epithelial cells (pink arrow). Black rectangle corresponds to magnified version shown in bottom right panel. Ig control staining of a section from OVA/alum-sensitized mice is also shown. Specific staining is depicted in brown; nuclei are stained blue with hematoxylin. (b) Opn levels in lung homogenates of OVA/alum-sensitized mice and alum controls. Data are expressed as mean ± s.e.m. n = 6–8 mice per group in two independent experiments. ***P = 0.0025. (c) Photomicrograph of OPN expression in bronchial biopsies from healthy individuals and asthmatics, stained with OPN-specific antibody. OPN expression in the asthmatics was localized in bronchial epithelial cells (black arrows) and subepithelial infiltrating leukocytes (blue arrows). Ig control staining of a biopsy from an asthmatic is shown. (d) Percentages of OPN+ epithelial and subepithelial cells from lung biopsies of asthmatics and healthy individuals. Cell counts are expressed as mean ± s.e.m. For each biopsy, data were obtained using three high-power fields (×400). *P = 0.0033, **P = 0.0071 (unpaired Student’s t-test). Scale bars, 100 μm.
In humans, lung biopsies from asthmatics had increased OPN expression in bronchial epithelial cells (ciliated epithelium) and inflammatory cells underneath the subepithelial membrane, as compared to healthy subjects (Fig. 1c). The percentage of OPN-positive epithelial and subepithelial cells was also increased in asthmatic individuals compared to controls (Fig. 1d).
Endogenous Opn-s is pro-inflammatory at sensitization
To investigate whether Opn-s participates during the induction of a TH2 response, we administered a neutralizing antibody to Opn (or an isotype (Ig) control) before OVA/alum sensitization (Fig. 2a). Following subsequent challenge through the airways with OVA, mice treated with the Opn antibody exhibited decreased numbers of bronchoalveolar lavage (BAL) eosinophils, lymphomononuclear cells (Fig. 2b) and decreased airway hyper-responsiveness (AHR), as compared to Ig-treated mice, reaching levels similar to those of the alum controls (Fig. 2c). Lung leukocytic infiltration and mucus secretion were also decreased (Fig. 2d), accompanied by a decrease in the eosinophil-specific chemokine CCL11 in the lungs (Fig. 2e).
Figure 2.
Opn-s blockade at priming reduces allergic disease. (a) Experimental protocol used to neutralize endogenous Opn-s during sensitization. BALB/c mice received two doses of anti-Opn or Ig control before each OVA/alum sensitization. (b) BAL differentials are expressed as mean ± s.e.m. n = 6–8 mice per group, three independent experiments. ***P = 0.0008, *P = 0.0209 (unpaired Student’s t-test). (c) AHR responses for enhanced pause (Penh) in mice treated with either anti-Opn or Ig. **P = 0.010 (two-way repeated-measures analysis of variance (ANOVA), and unpaired Student’s t-test). (d) Lung inflammation (top) and mucus secretion (bottom). *P = 0.0269, **P = 0.0156 (unpaired Student’s t-test). Scale bar, 100 μm. (e) Lung levels of IL-4 (**P = 0.0015), IL-13 (*P = 0.0255), IL-10 (**P = 0.007), IFN-γ (P = 0.068), IL-12 (**P = 0.0026) and CCL11 (*P = 0.0377). (f) Levels of IL-4 (***P < 0.0001), IL-13 (*P = 0.0121), IL-10 (***P = 0.0002) and IFN-γ (**P = 0.0014) in supernatants of OVA-stimulated DLNs. Serum levels of OVA-specific IgE (*P = 0.0328), IgG1 (**P = 0.0072) and IgG2a (*P = 0.0437). (g) BALB/c mice were sensitized as above and challenged with OVA on day 18. Anti-Opn or the Ig control were administered before each sensitization. Percentages of T1/ST2+ DLN cells gated on CD3+CD4+ T cells are shown, along with isotype control staining for the T1/ST2 marker. One representative experiment of three. n = 3–5 mice per group. Lung levels of CCL17 (P = 0.1749) and CCL22 (*P = 0.0115) are shown.
Lung interleukin (IL)-4, IL-13 and IL-10 levels were decreased in mice treated with Opn-specific antibody (Fig. 2e). Levels of IL-12, a TH1 cytokine produced by DCs, macrophages and airway epithelial cells22,23, were also decreased (Fig. 2e). We attribute these decreases to the overall decrease in pulmonary inflammation. Cytokine levels in BAL exhibited similar patterns (data not shown).
We examined OVA-specific TH2 responses by measuring cytokine levels in supernatants of DLN cell cultures stimulated ex vivo with OVA. Treatment with Opn-specific antibody resulted in decreased IL-4, IL-13 and IL-10 levels (Fig. 2f). Levels of OVA-specific IgG1, IgG2a and IgE were decreased in mice treated with Opn-specific antibody (Fig. 2f).
We observed decreased percentages of TH cells positive for T1/ST2, a TH2 cell marker, in lung DLNs of mice treated with Opn-specific antibody, right after the first OVA challenge (Fig. 2g) and after three challenges (data not shown). Blockade of Opn-s resulted in decreased pulmonary levels of the TH2 cell–specific chemokine CCL22 (Fig. 2g).
Thus, antibody-mediated depletion of endogenous Opn-s during antigenic sensitization resulted in a reduction of TH2 allergic responses and the consequent suppression of disease.
Endogenous Opn-s is anti-inflammatory during challenge
We investigated the role of endogenous Opn-s in secondary allergic responses by administering neutralizing antibody to Opn (or Ig control) before each OVA challenge in sensitized mice (Fig. 3a). Opn-s neutralization increased the total number of infiltrating cells and eosinophils measured in the BAL (Fig. 3b), AHR responses (Fig. 3c), pulmonary inflammation (Fig. 3d) and mucus secretion (Fig. 3d). Alum controls had lower inflammation and AHR (Fig. 3b–d).
Figure 3.
Opn-s blockade at challenge enhances allergic disease. (a) Experimental protocol used to neutralize endogenous Opn-s during challenge. BALB/c mice received three doses of anti-Opn or Ig control before challenge. (b) BAL differentials are expressed as mean ± s.e.m. n = 5–8 mice per group, five independent experiments. *P = 0.0410, **P = 0.0276 (unpaired Student’s t-test). (c) AHR responses for Penh were analyzed as in Figure 2c. *P = 0.027, **P = 0.010 (t-test and two-way ANOVA). (d) Lung inflammation and mucus secretion. **P = 0.0052, *P = 0.0355 (obtained as in Figure 2d). Scale bar, 100 μm. (e) Lung levels of IL-4 (**P = 0.0067), IL-13 (**P = 0.0022), IL-10 (*P = 0.0122), IFN-γ (*P = 0.0186), IL-12 (P = 0.4268) and CCL11 (*P = 0.0163). (f) Levels of IL-4 (P = 0.0838), IL-13 (*P = 0.0118), IL-10 (***P < 0.0001), IFN-γ (P = 0.0794) in supernatants of OVA-stimulated DLN cells. Serum levels of OVA-specific IgE (P = 0.7173), IgG1 (P = 0.1299) and IgG2a (P = 0.1012). (g) Percentages of T1/ST2+ cells gated on CD3+CD4+ T cells. Isotype control staining for the T1/ST2 marker is shown. One representative experiment of three. n = 3–5 mice per group. Top, lung levels of CCL17 (*P = 0.0396) and CCL22 (*P = 0.0126) for mice that received one OVA challenge (and antibody treatment). Bottom, lung levels of CCL17 (**P = 0.0053) and CCL22 (**P = 0.0036) for mice that received three OVA challenges (and antibody treatment).
Levels of IL-4, IL-13, IL-10, IFN-γ and CCL11 in the lung were increased in mice treated with Opn-specific antibody (Fig. 3e). It has been suggested that increased pulmonary IFN-γ levels play a pathologic role in allergic airway disease24–27. BAL cytokine levels were similarly increased in mice treated with Opn-specific antibody (data not shown).
In OVA-stimulated DLNs, blockade of Opn-s during challenge increased IL-13 and IL-10 and decreased IFN-γ (Fig. 3f). Levels of OVA-specific IgG1 were increased whereas OVA-specific IgG2a responses were decreased, indicative of a TH2 shift (Fig. 3f).
We observed increased percentages of DLN T1/ST2+ TH2 cells following Opn-s neutralization, after the first intranasal OVA challenge (Fig. 3g) as well as after three OVA challenges (percentages of T1/ST2+ cells among gated TH cells: with antibody to Opn, 23.8–34.5%; with Ig control, 4.8–13.8%). In both cases, we observed increased levels of CCL22 and CCL17 in the lungs (Fig. 3g).
Overall, and in contrast to its effect at sensitization, blockade of endogenous Opn-s during antigenic challenge enhanced TH2 allergic recall responses and exacerbated the disease phenotype.
Spp1−/− mice have enhanced TH2-mediated responses
Spp1−/− mice had increased numbers of BAL inflammatory cells and eosinophils compared to Spp1+/+ (Fig. 4a). Lung TH2 cytokine and chemokine levels were similar (data not shown). However, Spp1−/− mice have a predominantly C57BL/6 genetic background, which is thought to confer resistance to allergic inflammation, and deficiency in Opn may involve possible compensatory mechanisms.
Figure 4.
Spp1−/− mice exhibit enhanced TH2 responses. (a) Spp1+/+ and Spp1−/− mice were immunized with OVA/alum i.p. on days 0 and 12, and challenged through the airways with aerosolized OVA from day 18 to day 23. Differential cell counts in BAL from Spp1+/+ and Spp1−/− mice. **P = 0.0073 for total cell number, *P = 0.0320 for eosinophils. Data are expressed as mean ± s.e.m. n = 4–6 mice per group, three independent experiments. (b) IL-4 (***P < 0.0001), IL-13 (***P < 0.0001), IL-10 (***P < 0.0001) and IFN-γ (*P = 0.0455) levels in supernatants from OVA-stimulated DLN cells. (c) Levels of serum OVA-specific IgE (*P = 0.0415), IgG1 (*P = 0.0378) and IgG2a (P = 0.5660) from Spp1+/+ and Spp1−/− mice. (d) BALB/c mice were immunized with OVA/alum i.p. on days 0 and 12, and challenged through the airways with aerosolized OVA from day 18 to day 20. Either anti-Opn or Ig control was administered during both the OVA sensitization and challenge phases. OVA-specific IgE levels in the sera (*P = 0.0236) are shown. Values are expressed as mean ± s.e.m. n = 4–6 mice per group, two independent experiments. All P-values were obtained by unpaired Student’s t-test.
OVA-stimulated DLN cells from Spp1−/− mice produced increased IL-4, IL-13, IL-10 and IFN-γ, as compared to cells from the wild-type mice (Fig. 4b). In Spp1−/− mice, OVA-specific IgG1 levels were increased whereas OVA-specific IgG2a responses were decreased, suggestive of a TH2 shift (Fig. 4c). We also observed increased levels of OVA-specific IgE in these mice (Fig. 4c). OVA-specific IgE was increased in BALB/c mice treated with antibody to Opn during both the sensitization and challenge phases (Fig. 4d), indicating no involvement of Opn-i.
Opn-s blockade at sensitization affects pDC numbers
To explore the effect of Opn-s neutralization at sensitization on final disease outcome, we examined early TH2 responses. We treated BALB/c mice with Opn-specific antibody or Ig control before sensitization with Alexa Fluor–OVA in alum, and examined CD11c+ cell–driven responses. Cocultures of DLN CD11c+ cells from Opn-s–neutralized mice with DO11.10 responder T cells produced lower levels of IL-4, IL-13 and IFN-γ, as compared to those from Ig-treated mice (Fig. 5a), suggestive of a reduced priming effect. We obtained similar results from OVA-stimulated whole DLNs (Opn-specific antibody versus Ig: 37.33 ± 2.46 versus 96.67 ± 7.92 pg/ml of IL-4; 717.3 ± 25.00 versus 962.4 ± 38.07 pg/ml of IFN-γ).
Figure 5.
Opn-s blockade affects TH2 responses through DC recruitment. (a) IL-4 (**P = 0.0024), IL-13 (**P = 0.0017) and IFN-γ (*P = 0.0365) from OVA-stimulated cocultures of DLN CD11c+ cells with DO11.10 TH cells. Data are expressed as mean ± s.e.m. n = 4–6 mice per group, three independent experiments. (b) Percentages of DLN 7AAD−CD11c+B220−Gr-1− cDCs (left, bottom), 7AAD−CD11c+B220+Gr-1+ pDCs (left, top) and 7AAD−CD11c+PDCA-1+Gr-1+ pDCs (right). Numbers of cDCs and pDCs (*P = 0.0166), and Alexa Fluor–OVA+ cDCs and pDCs (*P = 0.0221). (c) IFN-α (*P = 0.0143) from CpG-stimulated CD11c+ cells. (d) Percentages of 7AAD−PDCA-1+ cells. (e) IL-4 (*P = 0.0461, **P = 0.0176), IL-13 (*P = 0.016, **P = 0.0014, ***P = 0.0003) and IFN-γ (*P = 0.0283, **P = 0.0365) from OVA-stimulated DLNs. [3H]thymidine incorporation (TdR) of OVA-stimulated DLNs (**P = 0.0013, ***P = 0.0004). n = 4 mice per group, three experiments. c.p.m., counts per min. (f) Numbers of cDCs (***P = 0.0001) and pDCs (*P = 0.0375), and Alexa Fluor–OVA+ cDCs (*P = 0.0202) and pDCs (*P = 0.0116). n = 5–7 mice per group, three independent experiments. (g) BAL eosinophils (*P = 0.0351) and AHR (anti-Opn, blue line; Ig + 120G8, dashed line; anti-Opn + 120G8, orange line). *P = 0.0132, **P = 0.0034. n = 5–8 mice per group, two experiments. Lung IL-4 (*P = 0.0349, **P = 0.0491), IL-13 (*P = 0.0299), IL-10 (**P = 0.0064, ***P = 0.0006) and IFN-γ. (h) Results from OVA-stimulated DLNs. IL-4 (***P = 0.0002, *P = 0.0074, **P = 0.0076), IL-13 (*P = 0.0142), **P = 0.0016, ***P < 0.0001, ****P < 0.0001), IL-10 (*P = 0.0112, **P = 0.0025, ***P < 0.0001, ****P < 0.0001) and IFN-γ. [3H]thymidine incorporation of OVA-stimulated DLNs (****P = 0.0108, *P = 0.0116, **P = 0.0016, ***P = 0.0001). Unpaired Student’s t-test for all statistical analyses.
It has been shown that two main subtypes of DCs participate in immune responses: conventional DCs (CD11c+B220− or CD11c+B220−Gr1− cDCs), considered immunogenic, and pDCs, considered mainly regulatory28–31. CD11c+PDCA-1+/120G8+Gr-1+ cells have been described as pDCs in allergic airway inflammation, exhibiting suppressive effects on TH2 responses28,32,33
Mice treated with Opn-specific antibody had increased percentages and total numbers of DLN CD11c+PDCA-1+Gr-1+ pDCs (characterized also as CD11c+Gr-1+B220+) and of Ag-loaded (Alexa Fluor–OVA+) pDCs (Fig. 5b). OVA uptake was not influenced, as the percentages of OVA+ cells among pDCs were similar (approximately 52 ± 5% for Opn-s neutralization versus 47 ± 5% for Ig). We observed no differences in the percentages and numbers of cDCs (CD11c+B220−Gr-1−) or Alexa Fluor–OVA+ cDCs (Fig. 5b). The percentages of OVA+ cells among cDCs were similar (approximately 45 ± 5% for Opn-s neutralization versus 51 ± 5% for Ig). The numbers of CD11c+ cells within DLNs were similar among groups (per mouse: 65,420 ± 2,289 cells for Opn-s neutralization versus 65,250 ± 6,284 for Ig). Purified DLN CD11c+cells from Opn-s–neutralized mice stimulated with CpG oligodeoxynucleotides produced increased levels of IFN-α, a defining characteristic of pDCs (refs. 28,34 and Fig. 5c).
Spp1−/− mice exhibited no significant enhancement of pDC recruitment in DLNs during priming, as compared to Spp1+/+ mice (8,732 ± 2,900 versus 6,518 ± 2,100 cells per mouse, P = 0.7170). We observed no differences in CD11c+ cell recruitment (39,800 ± 4,800 versus 35,800 ± 9,200 cells per mouse). A study using a substantially different sensitization protocol, involving trinitrochlorobenzene administration, has demonstrated decreased migration of CD11c+ cells to skin and DLNs in Spp1−/− mice18. The discrepancies between this report and our findings might be due to different innate mechanisms.
A recent study has shown that pDCs suppress TH2 responses28 To address whether the effects of Opn-s blockade during sensitization were mediated by the pDC population, we depleted pDCs (using the 120G8 antibody35) before OVA/alum sensitization and Opn-s blockade in naive BALB/c mice. pDC depletion was successful, as shown by flow cytometric analysis of PDCA-1+ cells (Fig. 5d). The Opn-specific antibody treatment had no effect on primary TH2 responses in pDC-depleted mice, and after treatment, these responses were similar to those in Ig-treated, pDC-depleted mice. This was indicated by the IL-4, IL-13 and IFN-γ levels and the OVA-specific proliferative responses (Fig. 5e). In both groups, pDC-depleted mice exhibited increased IL-4, IL-13 and IFN-γ levels (Fig. 5e), suggestive of a regulatory role for pDCs, as previously described28. Isolated pDCs from DLNs in cocultures with DO11.10 T cells did not induce measurable cytokine levels, whereas cDCs induced cytokine release (IL-4 levels: 92 ± 10 pg/ml; IFN-γ levels: 476 ± 20 pg/ml), suggesting that these cells might have immunogenic potential.
We therefore concluded that the decrease in TH2 priming observed after Opn-s neutralization was mediated by increased numbers of regulatory pDCs in DLNs.
Opn-s blockade at challenge affects cDC numbers
We investigated cDC and pDC recruitment when Opn-s was neutralized during challenge (Fig. 3a, protocol). There was an increase in total and Alexa Fluor–OVA+ cDCs and pDCs in DLNs of mice treated with Opn-specific antibody (Fig. 5f). OVA+ cells among cDCs and pDCs were similar (approximately 47 ± 6% and 43 ± 5% for Opn-s neutralization, versus 53 ± 3% and 49 ± 6% for Ig). Opn-s blockade increased total numbers of CD11c+ cells (data not shown). We obtained similar results on DC subsets following one, instead of three, intranasal challenges (data not shown). Of note, both triple and single challenges of mice treated with Opn-specific antibody enhanced AHR, increased the percentage of DLN T1/ST2+ TH2 cells and IL-4 in OVA-stimulated DLNs (Fig. 3g and data not shown). Overall, we observed increased recruitment of cDCs and pDCs in lung DLNs, with the increase for cDCs being greater than that for pDCs.
To examine the role of pDCs in the above settings, we used pDC-depleted mice. These exhibited increased allergic responses in comparison to their respective non–pDC-depleted mice (Fig. 5g,h), indicating a regulatory role for pDCs during secondary responses. Notably, in pDC-depleted mice, treatment with Opn-specific antibody, as compared to treatment with control Ig, increased total numbers of BAL cells (data not shown) and eosinophils as well as the levels of AHR, IL-13 and IL-10 in OVA-specific DLN responses (Fig. 5g,h), suggesting that pDCs are not involved in the proallergic effect of Opn-s neutralization during challenge.
Cocultures of cDCs with DO11.10 T cells produced increased IL-4 and IL-13 levels, showing the TH2-promoting potential of the cDCs (data not shown). Similar increases in cDC numbers have been linked to markedly enhanced inflammation32 and TH2 proliferation36. Overall, enhancement of TH2 responses due to Opn-s blockade at challenge was influenced by the increased recruitment of immunogenic cDCs.
Administration of rOpn is protective at challenge
rOpn administered along with OVA/alum during sensitization increased IL-13 and IFN-γ levels in OVA-stimulated DLNs (Fig. 6a), suggesting a pro-inflammatory role for Opn-s during TH2 priming.
Figure 6.
rOpn is protective during pulmonary challenge. BALB/c mice were treated as described in Methods. (a) Mice received rOpn before OVA/alum sensitization. Levels of IL-4 (P = 0.2266), IL-13 (*P = 0.0113) and IFN-γ (**P = 0.0079) in supernatants of OVA-stimulated DLN cells. (b–g) BALB/c mice received rOpn before OVA aerosol challenges. In b, BAL differentials (*P = 0.0430, **P = 0.0099, ***P = 0.0067). In c, AHR responses for Penh, analyzed as in Figure 2c (*P = 0.032, **P = 0.0038, ***P = 0.0083, ****P = 0.0017). In d, lung inflammation (top) and mucus secretion (bottom). Histological scores for H&E (*P = 0.03) and PAS (***P = 0.0002). Scale bar, 100 μm. In e, lung levels of IL-4 (*P = 0.0378), IL-13 (*P = 0.0141), IL-10 (*P = 0.04), IFN-γ (*P = 0.037), IL-12 (*P = 0.0271), CCL11 (**P = 0.0022), CCL17 (**P = 0.005) and CCL22 (***P γ 0.0001). In f, results from supernatants of OVA-stimulated DLN cells. Levels of IL-4 (***P < 0.0001), IL-13 (***P = 0.0003), IL-10 (P = 0.1322) and IFN-γ (*P = 0.0229). In g, serum levels of OVA-specific-IgE (P = 0.0589), IgG1 (P = 0.0703) and IgG2a (**P = 0.0045). Data are expressed as mean ± s.e.m. n = 6–8 mice per group in three independent experiments. Unpaired Student’s t-test for all statistical analyses.
Intranasal administration of rOpn before OVA challenge decreased the total numbers of BAL cells, eosinophils and mononuclear cells (Fig. 6b) and AHR responses, to the levels seen in the controls (Fig. 6c). Lung leukocytic infiltration, mucus secretion (Fig. 6d) and levels of IL-4, IL-13, IL-10, IFN-γ, CCL11, CCL17 and CCL22 were also decreased, whereas IL-12 levels were increased (Fig. 6e). BAL cytokines exhibited a similar pattern (data not shown).
OVA-stimulated DLN cells from rOpn-treated mice produced decreased IL-4, IL-13 and IFN-γ levels (Fig. 6f). OVA-specific IgG1 and IgE levels were decreased, whereas IgG2a levels were increased (Fig. 6g). These results point to a suppressive role for endogenous Opn-s during secondary allergic airway responses.
DISCUSSION
Previous studies have demonstrated the impact of Opn on TH1-associated immunity during ongoing immune responses against viral, bacterial and self antigens6,7,15. Our results point to dual and opposing effects of Opn-s on TH2-mediated allergic airway disease: pro-inflammatory at primary systemic sensitization, and anti-inflammatory during pulmonary secondary antigenic challenge. Neutralization of Opn-s during initial antigenic encounter increased the recruitment of regulatory PDCA-1+Gr-1+ pDCs in DLNs, which mediated a decrease in primary TH2 responses. In contrast, Opn-s blockade during challenge enhanced TH2 effector responses, mainly mediated by increased recruitment of TH2-promoting cDCs in DLNs. Intranasal administration of rOpn during antigenic challenge reversed established TH2 responses and conferred protection from allergic disease.
In agreement with a previous study28, our experiments revealed that pDCs were immunosuppressive for TH2 responses. pDC depletion, before Opn-s neutralization, restored OVA/alum-driven responses, revealing that the dampening effect of Opn-s neutralization during priming was mainly mediated by pDCs. This initial pDC-mediated dampening in priming provided an explanation for the subsequent decrease in TH2-mediated pathology following pulmonary challenge. Opn blockade was also accompanied by decreased IFN-γ production whereas rOpn administration enhanced TH2 priming and was accompanied by increased IFN-γ production. IFN-γ may participate in the Opn-s–mediated effect, particularly as decreased IFN-γ production during OVA/alum sensitization reduces priming24. Opn-s neutralization at sensitization resulted in increased lung IFN-γ levels following challenge. In this setting, IFN-γ may exert an immunoregulatory role, associated with the increased number of pDCs at priming. In support of this idea, adoptive transfer of pDCs during sensitization enhances IFN-γ levels and confers protection from allergic airway disease29, and induction of IFN-γ–producing regulatory T cells reduces allergic airway inflammation37.
We were surprised to note the implicit pro-inflammatory effect of Opn-s during priming, as one would expect that blocking a TH1 inducer38 at the initial point of TH differentiation would upregulate TH2 responses. However, it was rather Opn-s blockade during recall responses that resulted in enhanced allergic pulmonary inflammation and disease. We observed the same effect in mice treated with Opn-specific antibody during both the sensitization and challenge phases (data not shown and Fig. 4d) and in Spp1−/− mice, which developed increased TH2 responses. Previous studies have demonstrated that during repetitive antigenic encounters, Spp1−/− mice have decreased TH1 immunity4,6 and autoimmunity8–10. Our data imply that the previously demonstrated effect of Opn-s in TH1/TH2 balance operates predominantly during recall responses.
Opn-s neutralization during challenge increased DLN cDC and pDC numbers. In allergic airway disease, the most powerful immunogenic potential of CD11c+ cells39 stems from cDCs (refs. 28,32). For example, blockade of the C5a receptor during allergic airway inflammation increases the recruitment of cDCs, enhancing TH2 responses32. However, we found that pDCs were suppressive during antigenic challenge. In the absence of pDCs, Opn-s blockade still enhanced TH2 responses and allergic disease. Therefore, the increased induction of cDCs upon Opn-s neutralization provides an explanation for the exacerbation of TH2-mediated disease. It is also likely that Opn-s neutralization induces a stronger TH2 response, as Opn-s is known to affect antigen-presenting cells and thus influence the TH1/TH2 balance6. In support of this idea, local rOpn administration before challenge decreased TH2 responses and increased IL-12 production.
To examine whether pDCs mediate the effect of Opn-s blockade, we used the 120G8 monoclonal antibody, which has been described as pDC specific and pDC depleting28,32,35,40,41. We found by flow cytometry that 120G8 strongly bound all pDCs from naive and OVA/alum-sensitized mice (data not shown). A recent study indicated that 120G8 binds to an epitope of the bone marrow stromal antigen-2 (ref. 42). This study also showed that bone marrow stromal antigen-2 is primarily expressed on all pDCs and to a lesser degree on some immune (plasma) cells, following activation by IFN or virus42. Thus, in addition to pDCs, we cannot exclude the contribution of other cell types to the Opn-mediated effect on TH2 responses.
Comparing the results obtained from Opn-s neutralization to those from knockout of Spp1, we found that Opn-s plays a predominant role in allergic airway inflammation. However, considering the critical role of Opn-i in CpG-mediated pDC signaling15, its involvement in TH2 regulation is probable. Administration of CpG, alone or in conjunction with allergens, in the lungs of allergic mice reversed established inflammation, possibly through an effect on IFN-α production by pDCs (refs. 43,44). Notably, both isoforms affect pDCs: Opn-s regulates pDC recruitment in allergic response, as described here, whereas Opn-i is essential for functions of pDCs in viral immunity15.
Increased Opn expression in allergic airway disease may be part of an inherent protective mechanism, as suggested by the fact that the disease was exacerbated following Opn-s blockade at challenge. In fact, it was recently shown that the gene encoding OPN is critically upregulated during bee-venom immunotherapy45. In our experiments, administration of rOpn at challenge provided protection from allergic disease. This was mainly mediated through a shift toward an antiallergic TH1, as shown by increased levels of IL-12 and OVA-specific IgG2a. Intranasal administration of IL-12 during challenge suppresses airway disease46. Our data show that, as with IL-12, rOpn is an effective regulator of allergic airway disease.
The variable effect of Opn-s on TH2 immunity points once more to cytokines playing opposing roles depending on the phase and milieu of the immune response. The effects of Opn-s on pDC biology as well as their contribution to autoimmunity remain to be elucidated.
METHODS
Mice
We purchased BALB/c and OVA-specific T-cell receptor–transgenic DO11.10 (Tcr-TG-DO11.10) mice from the Jackson Laboratory. We backcrossed Spp1−/− mice onto the C57BL/6 background for seven generations. Mice were housed at the Animal Facility of the Foundation for Biomedical Research of the Academy of Athens. All procedures were in accordance with the US National Institutes of Health Statement of Compliance (Assurance) with Standards for Humane Care and Use of Laboratory Animals (#A5736–01) and with the European Union Directive 86/609/EEC for animal research.
In vivo experimental protocols
We sensitized BALB/c mice with 0.01 mg mouse OVA (Sigma-Aldrich) in 0.2 ml alum (Serva) intraperitoneally (i.p.) on days 0 and 12. Control mice received PBS/alum. We administered aerosolized OVA (5%, for 20 min) on days 18–20. Mice received 20 μg of affinity-purified neutralizing antibody to Opn (AF-808, R&D Systems) or Ig control (R&D Systems) i.p., 2–3 h before sensitization or challenge. OVA/alum-sensitized Spp1−/− and Spp1+/+ littermate mice received six OVA challenges on days 18–23. For the data depicted in Figure 5a–c, BALB/c mice received 40 μg of Opn-specific antibody or Ig control i.p.; 2–3 h later, BALB/c (or Spp1−/− and Spp1+/+ ) mice were sensitized i.p. with 0.1 mg Alexa Fluor-conjugated OVA/alum (LPS-low, Molecular Probes). We examined CD11c+ cell–driven responses and DC subsets 40 h later, which is when these cells traffic to DLNs (ref. 28). For the results shown in Figure 5e, BALB/c mice received 225 μg of 120G8 pDC-depleting antibody or Ig control (rat IgG1/κ, BD Biosciences) i.p. daily, for 4 d before sensitization. Then (day 0), mice received 40 μg of Opn-specific antibody or Ig control i.p.; 2–3 h later, they were sensitized i.p. with 0.1 mg Alexa Fluor–OVA/alum. Mice were killed 40 h following sensitization. For the data shown in Figure 5f, BALB/c mice were sensitized with OVA/alum i.p. on days 0 and 12, and, starting on day 18, were challenged intranasally with one or three doses of 0.5 mg Alexa Fluor–OVA. We administered Opn-specific antibody or Ig control (40 μg per mouse) i.p. 2–3 h before challenge. The data depicted in Figure 5g,h are from BALB/c mice sensitized with 10 μg of OVA/alum i.p. on days 0 and 12 and then given 225 μg of 120G8 pDC-depleting antibody or Ig control (i.p.) daily from days 17 to 20. Mice also received 20 μg of Opn-specific antibody or Ig control i.p. daily, 2–3 h before OVA challenge, from days 18 to 20. Mice were killed 40 h after the final challenge. In Figure 6a, the data are from BALB/c mice given 4 μg of mouse rOpn (R&D Systems) or PBS i.p., and then, 2–3 h later, sensitized i.p. with 0.1mg Alexa Fluor–OVA/alum. For the data in Figure 6b–g, we sensitized BALB/c mice with OVA/alum i.p. on days 0 and 12 and then challenged them intranasally with 0.5 mg Alexa Fluor–OVA from days 18 to 20. We administered rOpn (2.5 μg per mouse) or PBS i.p. 2–3 h before challenge. Mice were killed 40 h after the final challenge.
AHR and airway inflammation
We measured AHR, a clinical measurement of asthma, as enhanced pause (Penh) and BAL inflammatory cells, as previously described47. We stained paraffin-embedded sections with hematoxylin & eosin (H&E) or Periodic-Acid-Schiff (PAS), as previously described21.
Human subjects
We performed flexible bronchoscopy on asthmatics, classified and treated according to the Global Initiative for Asthma guidelines (one mild intermittent, one moderate and four severe), and nine healthy volunteers. We took biopsies as previously described48. The study was approved by the Sotiria Hospital Ethics Committee, and individuals signed an informed-consent form.
Immunohistochemistry
We immunostained paraffin-embedded sections as previously described47. We used antibodies to human OPN (MAB-1433, R&D Systems) and mouse Opn (AF-808, R&D Systems). For a control, we used matched isotype IgG (R&D Systems).
Cell culture, proliferation and cytokine analysis
We obtained lung homogenates as previously described47. We used a previously described method49 to isolate cells from DLNs (mediastinal, following intranasal treatment, or inguinal and axillary following i.p. treatment). We cultured DLN cells, alone or with CD4+ T cells (Dynal) from DO11.10 mice, with 125 μg/ml OVA for 48 h. We cocultured CD11c+ cells purified from DLNs (Miltenyi Biotec) with TH cells from DO11.10 mice and 125 μg/ml OVA, for 48 h. For pDC and cDC isolation, a combination of the above-described method with the pDC isolation kit (Miltenyi Biotec) was used. We performed proliferation assays as previously described49. To measure cytokines and chemokines, we used ELISA kits for IL-4, IL-10, IFN-γ and IL-12 (BD Biosciences) and IL-13, Opn, CCL11, CCL22 and CCL17 (R&D Systems). We used a newer kit for IL-13 in pDC depletion experiments (R&D Systems). We cultured CD11c+ cells with 0.2 μg/ml CpG oligodeoxynucleotides (5′-TCCATGACGTTCCTGATGCT-3′) or control GpC (5′-TCCATGAGCTTCCTGATGCT-3′) (MWG, Biotech), synthesized as described31. After 24 h, we measured IFN-α, by ELISA (PBL Biomedical Laboratories).
Serum antibody concentration
We measured OVA-specific IgE, IgG1 and IgG2a antibodies as described50.
Flow-cytometric analysis
We stained live DLN cells (7AAD−, BD Biosciences) with conjugated antibodies to CD4, CD3, CD11c, B220, CD11b, Gr-1, PDCA-1 (BD Biosciences) and T1/ST2 (MD Biosciences). To perform the FACS analysis, we used a Coulter cytometer (Cytomics, FC 500).
ACKNOWLEDGMENTS
We thank S. Spyridakis for assistance in flow-cytometry, A. Agapaki for histology preparations and S. Pagakis for assistance with final figure preparation. We thank L. Liaw (Maine Medical Center Research Institute) for permission to use the Spp1-deficient mice. We are grateful to M. Doufexis, I. Scotiniotis, C. Tsatsanis and M. Aggelakopoulou for critical reading of the manuscript, and to C. Davos, K. Karalis and A. Tsouroplis for discussions. This work was supported by the Hellenic Ministries of Health and Education (V.P. and G.X.) and by a grant award from the Hellenic Ministry of Development, General Secretariat of Research and Technology (03ED750; V.P.). C.M.L. is supported by a Senior Fellowship from the Wellcome Trust (#057704). B.N.L. is supported by a Vidi grant from the Dutch Organization for Scientific Research. D.C.M.S. is supported by the Thorax Foundation.
Footnotes
AUTHOR CONTRIBUTIONS G.X. designed experiments, performed animal studies and immunohistochemistry, generated figures, analyzed data and wrote the manuscript. T.A. performed animal studies, tissue-culture experiments, generated figures and performed flow cytometry. M.S. assisted with the animal studies and tissue-culture experiments. D.C.M.S. assisted with the animal studies and image analysis. E.E. and M.G. performed bronchoscopies, and provided human lung biopsies and clinical characteristics of the individuals. B.N.L. provided antibodies, assisted with the design of experiments and participated in discussions. C.M.L. assisted with experimental design, writing and critical editing of the manuscript. V.P. provided crucial ideas, designed experiments, analyzed data, supervised the study and wrote the manuscript with G.X.
COMPETING INTERESTS STATEMENT The authors declare no competing financial interests.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu AH. Early intervention for asthma prevention in children. Allergy Asthma Proc. 2002;23:289–293. [PubMed] [Google Scholar]
- 4.Weber GF, Cantor H. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev. 1996;7:241–248. doi: 10.1016/s1359-6101(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 5.Patarca R, et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J. Exp. Med. 1989;170:145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkar S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 7.Patarca R, Saavedra RA, Cantor H. Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit. Rev. Immunol. 1993;13:225–246. [PubMed] [Google Scholar]
- 8.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J. Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 9.Chabas D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 10.Hur EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 11.Gassler N, et al. Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum. Scand. J. Gastroenterol. 2002;37:1286–1295. doi: 10.1080/003655202761020560. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, et al. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J. Clin. Invest. 2005;115:1060–1067. doi: 10.1172/JCI23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Alfonso S, et al. Two single-nucleotide polymorphisms in the 5′ and 3′ ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2005;52:539–547. doi: 10.1002/art.20808. [DOI] [PubMed] [Google Scholar]
- 14.Niino M, Kikuchi S, Fukazawa T, Yabe I, Tashiro K. Genetic polymorphisms of osteopontin in association with multiple sclerosis in Japanese patients. J. Neuroimmunol. 2003;136:125–129. doi: 10.1016/s0165-5728(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara ML, et al. Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nat. Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renkl AC, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura K, et al. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin. Diagn. Lab. Immunol. 2005;12:206–212. doi: 10.1128/CDLI.12.1.206-212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss JM, et al. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J. Exp. Med. 2001;194:1219–1229. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begum MD, et al. Suppression of the bacterial antigen-specific T cell response and the dendritic cell migration to the lymph nodes by osteopontin. Microbiol. Immunol. 2007;51:135–147. doi: 10.1111/j.1348-0421.2007.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 20.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 21.Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 22.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J. Exp. Med. 2001;193:339–351. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimoldi M, et al. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–2826. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 24.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat. Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 25.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific TH1 cells fail to counterbalance TH2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krug N, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am. J. Respir. Cell Mol. Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 27.Magnan AO, et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Increased IFN-γ-producing CD8+ T cells in asthma. Am. J. Respir. Crit. Care Med. 2000;161:1790–1796. doi: 10.1164/ajrccm.161.6.9906130. [DOI] [PubMed] [Google Scholar]
- 28.de Heer HJ, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakkach A, et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 30.Oriss TB, et al. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J. Immunol. 2005;174:854–863. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 31.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohl J, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J. Clin. Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idzko M, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haeryfar SM. The importance of being a pDC in antiviral immunity: the IFN mission versus Ag presentation? Trends Immunol. 2005;26:311–317. doi: 10.1016/j.it.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 36.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stock P, et al. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat. Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara ML, et al. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 42.Blasius AL, et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 43.Farkas L, Kvale EO, Johansen FE, Jahnsen FL, Lund-Johansen F. Plasmacytoid dendritic cells activate allergen-specific Th2 memory cells: modulation by CpG oligodeoxynucleotides. J. Allergy Clin. Immunol. 2004;114:436–443. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 44.Hessel EM, et al. Immunostimulatory oligonucleotides block allergic airway inflammation by inhibiting Th2 cell activation and IgE-mediated cytokine induction. J. Exp. Med. 2005;202:1563–1573. doi: 10.1084/jem.20050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konno S, et al. Level of osteopontin is increased after bee venom immunotherapy. J. Allergy Clin. Immunol. 2005;115:1317–1318. doi: 10.1016/j.jaci.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Gavett SH, et al. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J. Exp. Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodelling by treatment with anti-TGF-β antibody: effect on the Smad signaling pathway. J. Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 48.Gaga M, et al. Eosinophils are a feature of upper and lower airway pathology in nonatopic asthma, irrespective of the presence of rhinitis. Clin. Exp. Allergy. 2000;30:663–669. doi: 10.1046/j.1365-2222.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 49.Panoutsakopoulou V, et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–147. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 50.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J. Exp. Med. 2002;195:51–57. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]