Abstract
We used multimodal measurement to evaluate whether (a) nicotine dependence is associated with baseline and postquit negative affect and craving, (b) smoking relapse is associated with greater negative affect and craving than abstinence, and (c) craving is associated with negative affect. Treatment-seeking smokers were randomly assigned to either a brief behaviorally based smoking-cessation treatment condition or to a delayed treatment control condition. Participants in the treatment condition attended four assessment sessions, 4 –5 days prequit (baseline), 1–2 days postquit, 3–5 days postquit, and 10 –14 days postquit, while controls attended four sessions spaced over the same intervals. Retrospective questionnaires were collected at the beginning of each session, and corrugator EMG and in-session ratings were collected during viewing of affective and cigarette-related slides. The multimodal measures indicated that more dependent smokers experienced greater negative affect and craving at baseline and postquit, regardless of abstinence status. The self-report measures indicated that both relapsed and abstinent smokers reported greater negative affect and craving than control smokers. Craving was associated with negative affect across measurement modalities. These results highlight the benefits of using multimodal measures to study the impact of nicotine dependence and withdrawal on negative affect and craving.
Keywords: smoking cessation, withdrawal, negative affect, craving, corrugator EMG
The relationships between nicotine dependence, negative affect, craving, and smoking behaviors are remarkably complex. Smokers commonly express that the desire to relieve negative affect is a major trigger for smoking (Piper et al., 2004; Wetter et al., 1994). Negative affect following a quit attempt has been related to treatment failure and relapse across a variety of treatment modalities (Borrelli et al., 1996; Kenford et al., 2002; Burgess et al., 2002), characterizing over 50% of all smoking lapses (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). However, several naturalistic studies in which smokers record self-report of affect and behavior in real time have reported little to no correlation between negative affect and smoking behavior (Shapiro, Jamner, Davydov, & James, 2002; Shiffman et al., 2002). Additionally, relapse can occur during positive or negative affect states (Brandon, Tiffany, Obremski, & Baker, 1990; Shiffman et al., 1996). The equivocal findings among studies examining the associations among smoking, negative affect, and craving has obscured the causal mechanisms linking these concepts. In an effort to tease apart some of the complexity in this area, the current study addresses three questions regarding the relationship between negative affect, smoking dependence, smoking abstinence, and craving using several measurement modalities.
Do More Dependent Smokers Experience Greater Postquit Negative Affect and Craving?
Evidence suggests that smokers with greater nicotine dependence self-report worse withdrawal symptoms, including negative affect, compared to less dependent smokers. Heavier smokers have greater withdrawal symptoms than lighter smokers (Killen, Fortmann, Telch, & Newman, 1988) and regular smokers report greater negative affect and craving during withdrawal than less dependent “chippers” (Shiffman, 1989; Shiffman, Paty, Kassel, Gnys, & Zettler-Segal, 1994). However, the assumption that greater nicotine dependence produces higher levels of negative affect during withdrawal has not consistently been supported, as other studies have found little relationship between nicotine dependence and withdrawal symptoms (Gritz, Carr, & Marcus, 1991; Pomerleau et al., 2005).
Strategies can be employed to better address the relationship between nicotine dependence and withdrawal severity. One strategy is to control for preexisting (baseline) differences in negative affect and craving in order to examine their postcessation relationships to dependence. Unfortunately, the use of such baselines in analyses of postquit negative affect and craving are uncommon (Shiffman, West, & Gilbert, 2004). Another way to address this is to include nonquitting smokers, high and low in nicotine dependence, as a control condition. This would allow for the effects of time to be disentangled from those of quitting on negative affect, effects that are often confounded due to the tendency of negative affect to decrease over repeated measurement, independent of intervention (Gilbert et al., 1998).
Do Relapsers Experience Greater Negative Affect and Craving Than Abstainers Following a Smoking Cessation Attempt?
Postcessation negative affect has been found to predict smoking relapse in retrospective (Piasecki, Kenford, Smith, Fiore, & Baker, 1997; Wetter et al., 1999) and prospective studies (Shiffman & Waters, 2004). However, do smokers who relapse experience an improvement in their affect once they resume smoking? Unfortunately, negative affect following smoking relapse has not received much scrutiny and has produced equivocal results. Some studies, involving unaided quitters, have found that relapsers experience greater negative affect than abstainers over time (Carey, Kalra, Carey, Halperin, & Richards, 1993; Cohen & Lichtenstein, 1990). In a previous study, we provided an intensive counseling intervention to smokers with current threshold and subthreshold depressive disorders who were seeking to quit smoking and found that relapsers experienced decreased positive affect and increased depressive symptoms and craving over time compared to abstainers (Blalock, Robinson, Wetter, Schreindorfer, & Cinciripini, 2008). However, Gilbert and colleagues found that, compared to a nonquitting control group, unaided male (Gilbert et al., 1998) and female (Gilbert et al., 2002) quitters experienced increased negative affect that was unabated for up to a month postquit.
One explanation for these disparate results is that there could be both pharmacological and nonpharmacological effects on affect and craving, effects that are dependent on measurement modality. “Hot” processing, which is conceptualized as a reflexive response to classical conditioning (Metcalfe & Mischel, 1999) that suppresses regions of the brain associated with reasoning (Goel & Dolan, 2003), has been postulated to be associated with withdrawal-induced negative affect (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). On the other hand, “cold” processing involves cognitive mediation of an emotional response and is thus more deliberative. Smoking abstinence might lead to increased negative affect compared to relapse, due to withdrawal-induced hot processing, but the self-report of negative affect might more clearly register the negative affect resulting from cognitively mediated cold processing in relapsers. Examples of cognitively mediated evaluations include the Abstinence Violation Effect, which postulates that smokers may experience negative affect such as guilt following relapse, particularly if the slip is attributed to stable internal rather than to situational determinants (Curry, Marlatt, & Gordon, 1987; Marlatt, 1985). Thus, both relapsers and abstainers might experience negative affect, but as the result of different levels of emotional processing, which may only be evident depending on the measurement modality. One possible solution is to use multimodal measures of negative affect, including psychophysiological measures that might be more sensitive to hot processing and not as susceptible to the cognitively mediated evaluations presumably captured by self-report.
Must Craving and Negative Affect Co-Occur?
One unanswered question in the literature is whether smokers in a state of craving experience it as an unpleasant or pleasant state. Craving can be considered a motivation state to use a substance that has been previously associated with a pleasant emotional state (Baker, Morse, & Sherman, 1987; Franken, 2003). Negative reinforcement models such as Baker’s (Baker et al., 2004) postulate that individuals experience abstinence-associated craving secondary to withdrawal, meaning that craving is part of the withdrawal syndrome and should co-occur with negative affect. This idea of craving as a withdrawal symptom is supported by studies that found significant inverse relationships between craving and blood nicotine level over time (Guthrie, Ni, Zubieta, Teter, & Domino, 2004; Jarvik et al., 2000). Additionally, in what is known as the cue reactivity paradigm, stimuli that have been paired with smoking can become conditioned stimuli that elicit conditioned withdrawal responses, including craving (Dols, Willems, van den Hout, & Bittoun, 2000; Mucha, Pauli, & Angrilli, 1998). Laboratory induction of negative affect has also been shown to increase craving in smokers (Conklin & Perkins, 2005; Maude-Griffin & Tiffany, 1996).
However, craving can be experienced during conditions of positive affect. Baker and colleagues (Baker et al., 1987) theorized that, in the absence of withdrawal and limitation in drug access, craving can occur in dependent users during drug use and during conditions of positive affect and cue exposure. For example, there is evidence that some drug-dependent individuals report greater craving during or immediately after drug use compared to during drug deprivation, presumably when the drug’s hedonic effects are at their greatest (Meyer, 1988). Anticipation or availability of a drug use opportunity can increase both craving and positive affect in dependent users (Carter & Tiffany, 2001; Zinser, Fiore, Davidson, & Baker, 1999). Despite eliciting craving, smoking cues themselves have generally been found to evoke self-report ratings of positive affect compared to neutral cues (Muñoz et al., 2010). Psychophysiological assessment of the motivational significance of smoking cues has been mixed, with some studies finding them to produce acoustic startle response magnitudes similar to pleasant cues (Cinciripini et al., 2006; Geier, Mucha, & Pauli, 2000), suggesting an activation of appetitive motivation, while other studies suggest the opposite (Muñoz et al., 2010; Orain-Pelissolo, Grillon, Perez-Diaz, & Jouvent, 2004).
A variety of factors could account for the inconsistent relationship between craving and affect. First, self-report may not be the most sensitive measure of negative affect and craving. The negative affect (Baker et al., 2004) and craving (Berridge & Robinson, 1995) that result from nicotine withdrawal and/or cue elicitation may operate preconsciously, and self-report measures, which require extensive cognitive processing, may not be sensitive to these subtle changes. Self-report may also be influenced by the expectancy that smoking deprivation leads to increased negative mood and craving. Additionally, it is likely that craving and the cues that prompt craving coactivate appetitive and aversive motivation (Breiner, Stritzke, & Lang, 1999). For instance, a smoker early in the quitting process may have previously associated smoking cues with the pleasures of smoking, but now views such cues as something to be avoided. Such motivational conflict and ambivalence have been reported among food cravers (Rogers & Smit, 2000). Using a multimodal measurement approach, with both self-report and psychophysiology, may allow for a more sophisticated analysis of these subtle and potentially conflicting motivation states, while avoiding the potential biases inherent to the measurement of cognitively mediated self-reported affect (Waldron, 1983).
The Current Study
In the present study, we used a multimodal measurement battery, including retrospective self-report, current self-report, and psychophysiology, to address important questions concerning the relationship between nicotine dependence, nicotine withdrawal, craving, and negative affect. Retrospective questionnaires were included to provide baseline and presession measures of negative affect and craving and to facilitate comparison with previous research. Current in-session ratings during the viewing of affective and smoking-related slides were used to obtain immediate ratings about brief and subtle changes in motivational predisposition that are less subject to recall bias. We measured electromyography (EMG) from the corrugator supercillii, a muscle located on the brow that is associated with frowning, which produces greater activation to unpleasant compared to pleasant or neutral slides (Robinson, Cinciripini, Carter, Lam, & Wetter, 2007; Witvliet & Vrana, 1995). A psychophysiological index of affect, such as corrugator EMG, is immediate, sensitive to subtle motivational changes (Cacioppo, Petty, Losch, & Kim, 1986), and less subject to cognitively mediated biases inherent to self-report measures.
We evaluated three hypotheses: (1) more dependent smokers would experience greater negative affect and craving at baseline and after quitting than less dependent smokers; (2) smoking relapsers would experience greater postquit negative affect and craving than abstainers and controls; (3) smokers reporting greater craving would have greater negative affect than those reporting less craving. To evaluate these hypotheses, we assessed retrospective self-report, current self-report, and psychophysiology of negative affect and craving in smokers assigned to either a brief behaviorally based smoking-cessation treatment condition or to a delayed treatment control condition. Participants in the treatment condition attended four sessions, 4 –5 days prequit (baseline), 1–2 days postquit, 3–5 days postquit, and 10 –14 days postquit, while participants in the control condition attended four sessions spaced over the same intervals.
Method
Participants
The analyses included 111 treatment-seeking smokers (52 women) who were recruited using newspaper ads from the Houston metropolitan area and who were paid $125 for attending one screening and four laboratory sessions. Smokers were included if they were willing to quit smoking within 30 days of screening, were between the ages of 18 and 59, smoked 10 or more cigarettes per day, produced an expired carbon monoxide level greater than 8 ppm (or produced saliva cotinine <30 ng/ml), were fluent in English, and had no uncontrolled medical illness. Individuals were excluded if they were taking psychotropic or narcotic medication, met criteria for a current psychiatric disorder, reported hearing loss, or were involved in current smoking-cessation activity.
In terms of participant flow, 497 individuals called and completed the phone screen, 186 were eligible on the phone screen and attended the in-person baseline screening session, and 132 of these met all screening criteria and were randomized to either an immediate treatment (n = 80) or delayed treatment control condition (n = 52). Of those randomized, 111 participants provided usable physiological data (66 in the treatment group, 45 in the control group), with the remainder excluded due to equipment failure (11), to dropping out after the orientation session (7), or to experimental noncompliance (3). Participants provided informed consent and the protocol was approved by the University of Texas MD Anderson Cancer Center’s Institutional Review Board.
Procedures
Telephone screen and screening session
All smokers were initially screened in a 40-min telephone interview to establish their initial eligibility for the study. Potential participants were administered a telephone version of the PRIME-MD semistructured interview (Spitzer et al., 1994), which screened for major mental disorders and substance use. Participants who were deemed initially eligible after the telephone screening attended a screening session where biochemical verification of smoking status was assessed and questionnaires completed concerning their demographic, medical, mood, and smoking history.
Retrospective questionnaires
At the in-person baseline screening session, participants completed the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), the Center for Epidemiologic Studies’ Depression Scale (CES-D; Radloff, 1977), the Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988), and the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999). The FTND is a 6-item questionnaire that measures nicotine dependence by assessing various components of smoking behavior such as daily intake, difficulty in refraining from smoking, and time to first cigarette. The CES-D is a 20-item self-report measure developed to assess depressive symptoms in community (nonclinical) populations. The PANAS is a 20-item scale that obtained measures of positive and negative affect over the past week. The WSWS is a self-report measure of nicotine withdrawal that consists of seven subscales: Anger, Anxiety, Concentration, Craving, Hunger, Sadness, and Sleep. Each of these questionnaires, except for the FTND, was also administered at the beginning of the four laboratory sessions, and are referred to collectively as the retrospective questionnaires.
Laboratory sessions
Participants in the treatment condition completed four laboratory sessions: Session 1 (baseline; 4 –5 days prequit), Session 2 (1–2 days postquit), Session 3 (3–5 days postquit), and Session 4 (10 –14 days postquit). Participants in the delayed treatment control condition also attended four sessions spaced over the same intervals, though they did not receive treatment or quit smoking. In addition to the tasks described below, participants completed the PANAS and WSWS questionnaires immediately following smoking assessment.
Treatment regimen and smoking-cessation therapy
Participants were informed of their random group assignment (treatment vs. delayed treatment) following their first laboratory (baseline) session. Participants in the treatment regimen were assigned to a doctorate-level smoking-cessation counselor, who provided 30 min of behaviorally based smoking cessation counseling immediately following each of the four laboratory sessions. Smoking quit dates were assigned to occur 4 –5 days after the baseline session. The cessation intervention was cognitive– behavioral in nature and adapted from smoking cessation guideline-based treatment used in our previous studies (Cinciripini et al., 2005). However, no nicotine replacement or other pharmacotherapy was provided. Smokers in the treatment group who were not abstinent at the end of the last laboratory session were offered a free 10-week supply of nicotine patches. Participants in the control group were offered delayed treatment, which consisted of self-help materials based on the counseling program described above, and were provided with a free 10-week supply of nicotine patches. Both were provided after the last laboratory session.
Smoking assessment
Smoking status was measured at the beginning of each laboratory session using self-reported smoking, in cigarettes per day since last session, and biochemical verification, in expired carbon monoxide (CO ppm). To ensure similar conditions of nicotine nondeprivation at their baseline (prequit) session, participants smoked one cigarette of their usual brand before completing the session questionnaires and viewing the affective slides. All participants were asked to limit their intake of caffeinated beverages to no more than two cups before 8:00 a.m. on the day of each laboratory session.
Affective slide viewing
Each laboratory session involved the presentation of 48 slides, 12 each from the categories of pleasant, unpleasant, neutral, and cigarette. Each slide was presented for 6 s followed by a randomly determined inter-slide interval that varied from 10 to 20 s. Four separate slide orders, one for each session, were pseudorandomized such that no slide valence was shown more than twice consecutively. The pleasant, unpleasant, and neutral slides were selected from the International Affective Picture System (IAPS; Center for the Study of Emotion & Attention, 1999),1 with the unpleasant slides selected from the negative valence, high arousal dimensions; the pleasant slides from the positive valence, high arousal dimensions; and the neutral slides from the neutral valence, low arousal dimensions. The cigarette slides, consisting of smoking cues such as slides of burning cigarettes and people smoking in a social context, were created for this experiment and are validated elsewhere (Carter et al., 2006). A PC using Psychology Tools’ E-Prime software (v1.1; Pittsburgh, PA) presented a 91.5 cm × 122 cm image of the slides using a digital projector, on a screen approximately 1.5 m from the participant. Participants were instructed to sit quietly and keep their eyes on the slides during the slide viewing task. A future publication will report the results from acoustic startle probe assessments, presented during 32 of the 48 slides (8 per valence) at a random time of 2.5 to 5 s after slide onset.
Physiological measurement
After completing the retrospective questionnaires (CES-D, PANAS, and WSWS), EMG electrodes (Ag-AgCl) filled with saline gel were attached in the participant’s face to the right corrugator supercilii region using a bipolar configuration (Fridlund & Cacioppo, 1986). These COR EMG signals were acquired and amplified with a BIOPAC Systems’ (Goleta, CA) EMG100A Electromyogram Amplifier module attached to their MP100 system. COR EMG was filtered offline using 10 –500 Hz bandpass (Tassinary & Cacioppo, 2000) and 60 Hz notch. COR EMG was recorded at 1000 Hz and displayed using BIOPAC Systems’ AcqKnowledge III data acquisition software (version 3.5.3) installed on a Pentium III computer. Following sensor attachment, participants sat quietly for 5 minutes to allow for initial habituation to the environment. Zygomaticus major EMG, heart rate, and skin conductance were also collected, but were unresponsive to our smoking-related predictors and were not included in this manuscript.
In-session ratings
After every third slide, participants were asked to rate their negative mood, positive mood, and craving to smoke on screen using a computer mouse. The positive mood state question asked “I am feeling happy, joyful, or pleased,” the negative mood state question asked “I am depressed, angry, worried, or frustrated,” and the craving question asked “I am craving a cigarette right now.” Each question presented choices on a five-point scale: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, and (5) strongly agree. Within each session, four sets of ratings were collected for each slide valence. Participants were instructed to make the ratings based on how they felt at that moment.
Data Reduction and Analysis
Scoring was conducted offline using AcqKnowledge 3.5.3 software. Data were first visually inspected and edited for movement artifacts and excessive noise. COR EMG was scored in microvolts (μV) after being rectified and integrated using a 20-ms time constant. To minimize the impact of outliers, 1% of observations were trimmed from both sides of the overall distribution for each of the psychophysiological measures (Winer, 1971). The 6 s after the onset of each slide was scored in 1-s epochs for COR EMG. The largest value was selected from among these six epochs to represent the peak response to each slide. We next subtracted the mean of the 2-s baseline prior to the slide onset from these values to get a measure of reactivity to each slide, and then calculated Subject by Session by Slide Valence means of this reactivity score. To obtain a measure of standardized reactivity for each subject, we calculated the standard deviation across all valences, within subject for each session, and divided each reactivity score within session by this standard deviation. This approach produces an effect size statistic, called a D score (Greenwald, Nosek, & Banaji, 2003), tailored to repeated measures with a scaling similar to a Cohen’s d (Cohen, 1988). The resulting standardized difference score takes into account individual variability in physiological response while scaling individual reactivity differences over the four slide valences at each session.
Mixed model regression (SAS Proc Mixed v9.2; SAS Institute Inc., Cary, NC) was used to assess all models where physiology or in-session ratings were the dependent variables. The mixed model approach is a form of the generalized linear model (GLM) that allows for more specific estimation of the correlation structure of the residuals and that will not exclude cases with missing observations, thus allowing the use of all available data (Bageilla, Sloan, & Heitjan, 2000; Kristjansson, Kircher, & Webb, 2007). All models included subject as a random effect. Models run on postquit time points included baseline dependent measures and session abstinence status (abstinent vs. smoking) as covariates. All descriptions of differences between means following a significant mixed model effect were the result of contrast comparisons of least-square means (LSM) and standard errors (SE) of fixed effects using tests of simple effects (Winer, 1971). To correct for the effects of multiple comparisons on Type I error rate, the family-wise α levels of post hoc contrasts were adjusted using the Holm-Bonferroni correction (Holm, 1979). For interactions of continuous (e.g., FTND) with categorical variables (e.g., valence), we calculated slopes for average change over the continuous variable, which are reported as point estimates (PE) and standard errors (SE). Point estimates are slopes that indicate change on a dependent measure for every 1-unit increase in the predictor, analogous to a regression beta statistic. Differences on demographic and baseline smoking behavior variables were evaluated by group using univariate ANOVAs.
Results
Demographics and Smoking Behavior
Table 1 contains mean and frequency data summarizing the demographic and smoking characteristics of the sample. The sample was compared, by assigned treatment regimen, on baseline measures of age, number of daily cigarettes smoked, expired carbon monoxide (CO), body mass index (BMI), CES-D, and the FTND using univariate ANOVA, but no significant differences were found. Additionally, no significant differences were found when the sample was compared by gender on these same characteristics. The categorical variables (gender, ethnicity/race, employment, and education) were compared using chi-square tests, but none differed by treatment regimen.
Table 1.
Participant Demographic and Baseline Smoking Data by Treatment Regimen
| Measure | Control | Treatment | Total |
|---|---|---|---|
| Gender, Female | 17 (37.8%) | 35 (53.0%) | 52 (46.8%) |
| Ethnicity/Race | |||
| African-American1 | 12 (26.7%) | 23 (34.8%) | 35 (31.5%) |
| Euro-American1 | 25 (55.5%) | 31 (47.0%) | 56 (50.5%) |
| Other2 | 8 (17.8%) | 12 (18.2%) | 20 (18.0%) |
| Currently Employed | 35 (77.8%) | 53 (80.3%) | 88 (79.3%) |
| Education | |||
| High School or less | 13 (28.9%) | 16 (24.2%) | 29 (26.1%) |
| Some College | 22 (48.9%) | 36 (54.6%) | 58 (52.3%) |
| College Degree | 10 (22.2%) | 14 (21.2%) | 24 (21.6%) |
| Mean Age (SD) | 37.39 (11.18) | 40.73 (10.41) | 39.05 (10.76) |
| Mean Cigs (SD) | 20.87 (7.27) | 20.86 (8.06) | 20.86 (7.71) |
| Mean CO (SD) | 21.57 (10.71) | 23.22 (10.95) | 22.55 (10.83) |
| Mean FTND (SD) | 4.67 (2.12) | 4.73 (2.17) | 4.70 (2.14) |
| Mean CES-D (SD) | 8.91 (7.10) | 7.68 (7.38) | 8.17 (7.26) |
| Mean BMI (SD) | 26.76 (5.21) | 27.40 (5.57) | 27.14 (5.41) |
| Total Subjects | 45 (40.5%) | 66 (59.5%) | 111 |
Non-hispanic.
Includes individuals of self-identified Hispanic, Asian-American, and multi-ethnic backgrounds. None of the comparisons differed significantly by gender.
Abstinence Outcome
Abstinence was calculated in two ways, at each postquit time point (session) and at end of treatment (EOT). Session abstinence was calculated independently for each of the three postquit time points (1–2 days, 3–5 days, and 10 –14 days postquit), with session abstinence at any given time point being defined as no reported cigarettes smoked since the last time point (since quit date for Session 2, the 1–2 day postquit time point) and an expired CO ≤10 ppm. Missing data points were coded as smoking. EOT abstinence was calculated at the final time point (Session 4; 10 –14 days postquit). A participant was considered an abstainer at EOT if he or she reported smoking no cigarettes, not even a puff, from the quit date to Session 4.2 Session abstinence was confirmed, using expired CO (≤10 ppm), at postquit Sessions 2, 3, and 4. Relapsers were those who relapsed to smoking by one of the time points subsequent to the quit date. Table 2 depicts abstinence frequencies at EOT and for each session. In terms of cigarettes smoked per day, relapsers reported smoking significantly less (M = 7.86 cigs/day, SD = 6.91) at Session 4 (10 –14 days postquit) than at baseline (M = 19.89 cigs/day, SD = 6.98), F(1, 71) = 54.93, p < .0001), while controls did not significantly alter their smoking rate at Session 4 compared to baseline ( p < .13).
Table 2.
Session Abstinence N (%) at Each Time Point Based on Cumulative Abstinence at EOT, Verified by Expired CO, for Participants Assigned to the Treatment Regimen (n = 66)
| Abstainers (n = 28) | Relapsers (n = 38) | Total abstinent | |
|---|---|---|---|
| Abstinent at Session 2 | 27 (96.4%)* | 16 (42.1%) | 43 (65.2%) |
| Abstinent at Session 3 | 28 (100.0%) | 5 (13.2%) | 33 (50.0%) |
| Abstinent at Session 4 | 28 (100.0%) | 4 (10.5%) | 32 (48.5%) |
One participant who was nonabstinent at session 2 but was abstinent at sessions 3 and 4 was classified as an Abstainer at EOT.
Baseline Manipulation Checks
Before examining our hypotheses, we conducted analyses to determine whether our physiological and in-session ratings measures were sensitive to the valence of our slide stimuli at baseline, prior to treatment regimen randomization. In-session ratings (craving, negative mood, and positive mood) were self-report measures collected after every third slide, and slide valence was coded as the slide immediately preceding the in-session ratings. We conducted mixed models analyses separately for each measure at baseline (Session 1), including all subjects, with slide valence, regimen, and their interactions terms with slide valence as the fixed effects, and subject as the random effect.
With COR EMG, we found a significant main effect of slide valence, F(3, 315) = 8.99, p < .0001, such that COR EMG was larger to unpleasant slides compared to pleasant, cigarette, and neutral slides (see Table 3). We found no effect of treatment regimen, either as a main effect or as an interaction with slide valence. With in-session ratings, we found a main effect for slide valence for craving, F(3, 324) = 3.06, p < .03; negative mood, F(3, 324) = 9.65, p < .0001; and positive mood, F(3, 324) = 27.21, p < .0001 (see Table 3). Craving was larger following smoking slides compared to unpleasant and neutral slides, negative mood was larger following unpleasant slides compare to neutral, pleasant, and smoking slides, and positive mood was larger following positive and smoking slides, neutral and unpleasant. We found no main effect of treatment regimen for any of the in-session ratings, or any interactions of those terms with slide valence. Overall, these findings indicate that the COR EMG and in-session ratings were responsive to the valence of the slides used in our experimental task.
Table 3.
The Least Square Means (and SE) of the Significant Slide Valence Main Effects on Corrugator EMG and In-Session Ratings at Baseline
| DV | Slide valence
|
Significance | |||
|---|---|---|---|---|---|
| Neutral (N) | Pleasant (P) | Unpleasant (U) | Cigarette (C) | ||
| Corrugator EMG | 0.57 (0.04) | 0.53 (0.04) | 1.05 (0.04) | 0.49 (0.04) | U-N: p < .0001* U-P: p < .0001* U-C: p < .0001* |
| Craving | 2.66 (0.10) | 2.70 (0.10) | 2.65 (0.10) | 2.74 (0.10) | C-N: p < .005* C-U: p < .0001* |
| NegMood | 2.29 (0.09) | 2.25 (0.09) | 2.41 (0.09) | 2.25 (0.09) | U-N: p < .0001* U-P: p < .0001* U-C: p < .0001* |
| PosMood | 3.20 (0.09) | 3.32 (0.09) | 2.95 (0.09) | 3.34 (0.09) | P-N: p < .02 P-U: p < .0001* C-N: p < .005* C-U: p < .0001* N-U: p < .0001* |
Note. DV = dependent measure; NegMood = negative mood; PosMood = positive mood. Significance refers to the p values of the pair-wise post hoc contrasts between slide valences.
Statistically significant after application of the Holm-Bonferroni correction to the post hoc contrasts.
Is Increased Nicotine Dependence Associated With Greater Negative Affect and Craving?
To address this question, we first analyzed the impact of FTND, a measure of nicotine dependence, on each of the multimodal measures at baseline. We then analyzed the impact of FTND on each of the multimodal measures during the postquit sessions, while covarying for baseline response.
Psychophysiology
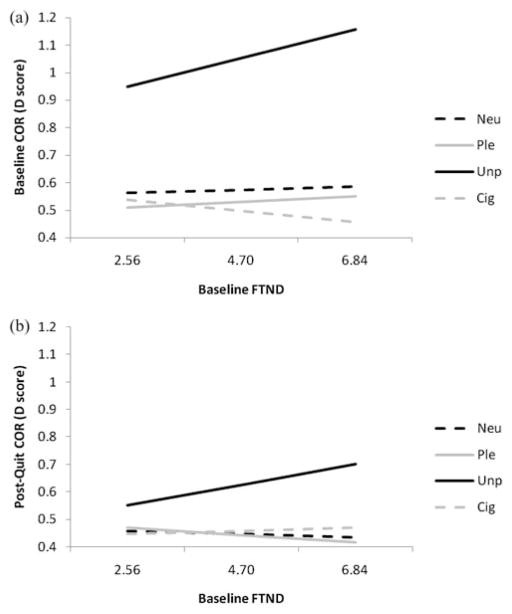
We modeled the effects of FTND,3 as a main effect and as an interaction term with valence, on baseline COR EMG. FTND was modeled as a continuous variable. Total FTND score interacted with valence in predicting COR EMG scores, F(3, 315) = 2.68, p < .05, with slopes indicating that those with higher FTND values had larger COR EMG responses to unpleasant slides than did those with lower FTND values, PE = 0.049, SE = 0.02, t(315) = 2.38, p < .02 (see Figure 1a).
Figure 1.
Slopes for significant FTND by slide valence interactions for corrugator EMG (COR) at (a) the baseline (Session 1) and (b) the postquit sessions (Sessions 2 to 4). The mean and ± 1 SD of FTND are plotted on the x-axis. Neu = Neutral; Ple = Pleasant; Unp = Unpleasant; and Cig = cigarette slides.
For postquit COR EMG, we modeled the effects of FTND, as a main effect and as an interaction term with valence, session, and EOT group status, on each of the physiological measures, covarying for baseline COR EMG. Total FTND score interacted with valence in predicting postquit COR EMG scores, F(3, 1095) = 4.59, p < .004, with slopes indicating that those with higher FTND values had larger COR EMG responses to unpleasant slides than did those with lower FTND values, PE = 0.035, SE = 0.014, t(1095) = 2.58, p < .01 (see Figure 1b). However, there was no interaction of FTND score with EOT group status or session for COR EMG. Overall, these findings suggest that increased nicotine dependence is associated with increased COR EMG response to unpleasant stimuli, regardless of nicotine abstinence status.
In-session ratings
To examine the impact of FTND on baseline in-session ratings, which were administered during slide viewing every third slide, we examined the main effects of FTND, along with its interaction with slide valence, on each of the in-session ratings at baseline. We found main effects indicating that both overall craving (PE = 0.11, SE = 0.05; F(1, 107) = 5.90, p < .02) and negative mood (PE = 0.09, SE = 0.04; F(1, 107) = 4.63, p < .03) ratings increased with each increase in FTND score. There were no interactions of FTND with slide valence for any of the in-session ratings.
To examine the impact of baseline smoking dependence on postquit in-session ratings, which were administered during slide viewing every third slide, we examined the main effects of FTND, along with its interactions with valence, session, and EOT group status, separately for each of the in-session ratings, while covarying baseline in-session ratings. We found no main effects or interactions for any of the models involving FTND for any of the in-session ratings. Overall, these results suggest that increased nicotine dependence is associated with greater overall craving and negative mood ratings, though only at baseline.
Retrospective questionnaires
We modeled the main effect of FTND on each of the retrospective questionnaires (CES-D, PANAS, and WSWS) separately, at baseline. PANAS Negative scale scores were positively associated with increases in FTND (PE = 0.76, SE = 0.32; F(1, 105) = 5.46, p < .02). There were no significant main effects of FTND on baseline CES-D, PANAS Positive, or any of the WSWS subscale scores.
We examined the main effects of FTND and its interaction with EOT group status and session on each of the retrospective questionnaires at postquit, while covarying baseline retrospective questionnaire values. None of the models predicting postquit retrospective questionnaire responses were significant. Overall, our retrospective questionnaire findings suggest that increased nicotine dependence is associated with greater negative affect, though this relationship is not found after quitting smoking.
Do Abstainers or Relapsers Experience Greater Negative Affect or Craving?
Psychophysiology
The impact of between-subjects EOT group status (abstainer, relapser, and control) and its interactions with valence and session on postquit COR EMG were examined, with baseline psychophysiology as the covariate. None of the models were significant, suggesting that COR EMG was not sensitive to EOT group status.
In-session ratings
For analysis of the postquit in-session ratings data, we ran models containing EOT group status and its interaction with slide valence (of the slide immediately preceding each in-session rating) and session as IVs, with baseline in-session ratings as covariates, separately for each of the in-session rating DVs. Only postquit in-session craving ratings were sensitive to EOT group status, F(2, 106) = 3.68, p < .03, with relapsers (LSM = 2.79, SE = 0.13; t(106) = 2.51, p < .02) having larger in-session craving ratings than controls (LSM = 2.34, SE = 0.12). The difference between abstainers (LSM = 2.72, SE = 0.15) and controls approached statistical significance, t(106) = 1.97, p < .06, while the in-session craving ratings did not differ between relapsers and abstainers. There were no significant EOT group status interactions with slide valence, or session for any of the in-session ratings. These findings indicate that only the craving in-session ratings, and not the negative or positive mood ratings, were sensitive to EOT group status, with relapsers producing higher postquit craving values than controls.
Retrospective questionnaires
We examined the impact of between-subjects EOT group status (abstainer, relapser, and control) and its interactions with session on postquit retrospective questionnaires (CES-D, PANAS, and WSWS), with baseline retrospective questionnaires as covariates. Significant main effects for EOT group status were found for PANAS NEG, F(2, 105) = 11.63, p < .0001; WSWS Anger, F(2, 104) = 5.50, p < .005; WSWS Anxiety, F(2, 104) = 7.92, p < .0006; WSWS Craving, F(2, 104) = 7.58, p < .0008; and WSWS Sadness, F(2, 104) = 11.90, p < .0001. A consistent pattern emerged for the EOT group status main effect, with relapsers producing larger scores than controls for all of these retrospective measures (see Table 4). However, relapsers did not differ from abstainers on these measures. There were no significant main effects or interactions involving EOT group status and session on postquit CES-D, PANAS POS, WSWS Concentration, WSWS Hunger, or WSWS Sleep. Overall, the analyses of the retrospective questionnaires found that only relapsers experience greater postquit negative affect than controls.
Table 4.
The Least Square Means (and SE) of the Significant Main Effect of EOT Group Status on Post-Quit Session Questionnaires
| DV | EOT group status
|
Significance | ||
|---|---|---|---|---|
| Abstainers (A) | Relapsers (R) | Controls (C) | ||
| PANAS NEG | 18.65 (1.11) | 18.26 (0.75) | 13.49 (0.76) | R-C: p < .0001* A-C: p < .0008* |
| WSWS Anger | 5.19 (0.55) | 5.87 (0.35) | 4.29 (0.35) | R-C: p < .001* |
| WSWS Anxiety | 7.64 (0.54) | 7.99 (0.37) | 5.97 (0.37) | R-C: p < .0001* A-C: p < .03 |
| WSWS Craving | 8.95 (0.69) | 9.79 (0.50) | 7.16 (0.48) | R-C: p < .0002* A-C: p < .06 |
| WSWS Sadness | 6.13 (0.51) | 6.72 (0.33) | 4.53 (0.33) | R-C: p < .0001* A-C: p < .03 |
Note. DV = dependent measure. Significance refers to the p values of the pair-wise post hoc contrasts between EOT group statuses.
Statistically significant after application of the Holm-Bonferroni correction to the post hoc contrasts.
Is Increased Craving Associated With Greater Negative Affect?
Psychophysiology
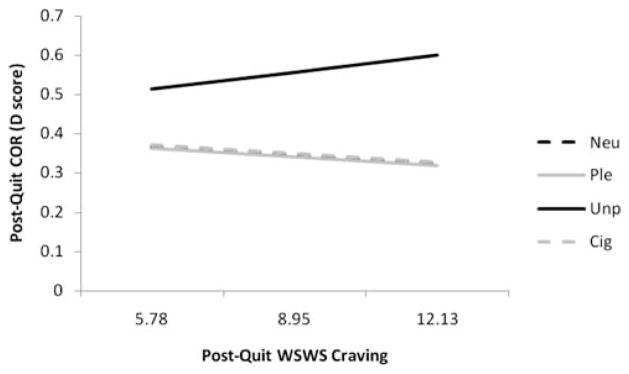
The impact of postquit WSWS Craving scores and their interactions with EOT group status, slide valence, and session on COR EMG was examined, with baseline COR EMG included as the covariate. A WSWS Craving × Slide Valence interaction for COR EMG, F(3, 985) = 3.25, p < .03 (see Figure 2), indicated that, postquit, the slope of COR EMG to unpleasant slides significantly increased (PE = 0.011, SE = 0.007) with every unit increase in WSWS Craving scale score, compared to pleasant (PE = −0.008, SE = 0.007; t(985) = 2.51, p < .01), neutral (PE = −0.009, SE = 0.007; t(985) = 2.66, p < .008), and cigarette-related slides (PE = −0.009, SE = 0.007; t(985) = 2.58, p < .01). There were no other significant main effects or interactions involving postquit WSWS Craving scores for COR EMG. These analyses suggest that increased postquit craving is associated with increased COR EMG responding to unpleasant slides.
Figure 2.
Slopes for significant postquit WSWS Craving by slide valence interactions at postquit COR EMG. The mean and ± 1 SD of WSWS Craving are plotted on the x-axis. Neu = neutral; Ple = pleasant; Unp = unpleasant; and Cig = cigarette slides.
In-session ratings
For analysis of the postquit in-session ratings data, we ran models containing postquit WSWS Craving and its interactions with EOT group status, slide valence (of the slide immediately preceding the in-session ratings), and session, with baseline in-session ratings as covariates, separately for each of the in-session rating DVs. We found significant WSWS Craving × EOT Group Status interactions for in-session craving, F(2, 1009) = 31.27, p < .0001; negative mood, (2,1009) = 6.99, p < .001; and positive mood ratings, F(2, 1009) = 9.21, p < .0001 (see Table 5). Post hoc comparisons indicated that the slope for in-session craving ratings was larger for relapsers compared to abstainers and controls, and the slope for abstainers was significantly larger than that of controls. For in-session negative mood ratings, each unit increase in WSWS Craving score led to significant slope increases in relapsers and controls compared to abstainers. Conversely, for in-session positive mood ratings, each unit increase in WSWS Craving score let to significant slope decreases in relapsers and controls compared to abstainers. There were no other significant effects involving postquit WSWS Craving scores. Overall, the in-session ratings indicated that increased craving was associated with a worsening of mood for relapsers and controls, but not for abstainers.
Table 5.
Slopes, Expressed as Point Estimates (and SE) of the Significant WSWS Craving × EOT Group Status Interactions for In-Session Craving, Negative Mood, and Positive Mood Ratings, Post-Quit
| DV | Abstainers (A) | Relapsers (R) | Controls (C) | Significance |
|---|---|---|---|---|
| Craving | 0.11 (0.016) | 0.17 (0.015) | 0.003 (0.015) | R-A: p < .005* R-C: p < .0001* A-C: p < .0001* |
| NegMood | −0.01 (0.015) | 0.07 (0.014) | 0.04 (0.014) | R-A: p < .0002* C-A: p < .02* |
| PosMood | 0.02 (0.015) | −0.06 (0.014) | −0.04 (0.015) | R-A: p < .0001* C-A: p < .003* |
Note. DV = dependent measure; NegMood = negative mood; PosMood = positive mood. Significance refers to the p values of the pair-wise post hoc contrasts between slide valences.
Statistically significant after application of the Holm-Bonferroni correction to the post hoc contrasts.
Retrospective questionnaires
We examined the impact of postquit craving, as measured by the WSWS Craving scale and its interactions with EOT group status, and session, with baseline retrospective questionnaires as covariates, separately for each of the affect-related retrospective questionnaires (CES-D, PANAS) during the postquit sessions (2 to 4). We found main effects indicating that CES-D (PE = 0.41, SE = 0.10; F(1, 178) = 17.08, p < .001) and PANAS NEG (PE = 0.47, SE = 0.09; F(1, 181) = 24.78, p < .0001) increased, and PANAS POS ratings decreased (PE = −0.27, SE = 0.10; F(1, 181) = 6.80, p < .01), with each unit increase in WSWS Craving score. There were no other significant effects involving postquit WSWS Craving scores. The retrospective questionnaires suggest that increased craving is associated with increased negative affect.
Discussion
Our results support our first hypothesis that heavier and more dependent smokers experience greater negative affect and craving at baseline with convincing evidence across modalities. Smokers with higher FTND had larger COR EMG to unpleasant slides, higher PANAS Negative scale scores, higher in-session Negative ratings, and higher in-session Craving ratings at baseline. The self-report measures did not support the second part of this hypothesis, that more dependent smokers would experience greater negative affect after quitting than less dependent smokers. However, postquit COR EMG responses to unpleasant slides were positively associated with baseline FTND scores. Overall, these multimodal findings suggest that more dependent smokers experience greater day-to-day negative affect and craving, and reactivity to unpleasant emotional cues, regardless of smoking abstinence status. Our findings do not support previous studies’ findings that greater nicotine dependence causes greater negative affect and craving during withdrawal (Killen et al., 1988; Shiffman et al., 1994). We also found no evidence that less dependent smokers are more reactive to smoking cues (Shiffman & Paty, 2006; Watson, Carpenter, Saladin, Gray, & Upadhyaya, 2010), contrary to arguments that less dependent smokers smoke in more proscribed and hence stimulus-dependent situations (Stewart, de Wit, & Eikelboom, 1984).
Contrary to our second hypothesis, the self-report retrospective questionnaire and in-session ratings results suggest that relapsers and abstainers experience comparable levels of postquit negative affect and craving. Furthermore, postquit COR EMG did not differ by EOT group status. These results fail to support previous work by our lab (Blalock et al., 2008) and others (Carey et al., 1993; Cohen & Lichtenstein, 1990) that found increased self-report negative affect for relapsers compared to abstainers. Given that the relapsers’ rates of smoking never returned to baseline levels, both relapsers and abstainers were likely experiencing some degree of withdrawal, suggesting that these self-report measures were sensitive to the pharmacological properties of nicotine (i.e., loss of negative reinforcement). However, we cannot rule out whether cognitively mediated evaluations had some effect on the self-report measures, as one might have expected differences in negative affect and craving between relapsers and abstainers, given the differences between the two groups in postquit nicotine usage.
Our third hypothesis, that craving is associated with negative affect, was supported across measurement type (Drobes & Tiffany, 1997; Carter & Tiffany, 2001). Retrospective WSWS Craving was associated with increased postquit COR EMG to unpleasant slides, CES-D, and PANAS NEG, and decreased PANAS POS, regardless of EOT group status. However, the results for in-session ratings were dependent on EOT group status, with relapsers and controls producing a significant association between WSWS Craving and in-session negative and positive mood ratings, compared to abstainers. In other words, increased craving at the beginning of the lab session was associated with a worsening in-session mood ratings for relapsers and controls, but not for abstainers. As both relapsers and controls presumably had the same opportunity, if not the same motivation, to smoke postsession, our in-session ratings findings that smokers in both groups experienced an association between craving and negative affect does not support previous findings that smoking availability leads to an association between craving and positive affect (Carter & Tiffany, 2001).
The results of this study should be interpreted in light of the following considerations. First, the sample size was small for a clinical trial, limiting the number of participants in the EOT group status between-subjects categorization and reducing our ability to detect group differences. Second, the study only evaluated relapse symptoms out to 2 weeks postquit. This allowed for an examination of short-term withdrawal, and could have magnified differences that might not be present at later time points. Third, assignment to the relapser or abstainers groups was by participant self-selection, which could have led smokers with greater withdrawal symptoms (negative affect and craving) to fall out of treatment and become relapsers. The result could be that the remaining abstainers are simply those who experience less intense withdrawal symptoms compared to relapsers (Gilbert et al., 2002).
The use of multimodal measures to assess negative affect in craving in nicotine-dependent individuals offers advantages over the typical reliance on retrospective self-report. First, when multimodal measures are in concordance, this provides additional evidence that the motivational phenomenon of interest is not simply the result of some cognitively mediated evaluation. That is, the motivational state being assessed is reflective of some biological substrate (i.e., withdrawal) and not of a frontal-lobe mediated reconstruction of how a person “should” feel. Second, when multimodal measures are in discordance, this could be reflective of the presence of co-occurring, even potentially conflicting, motivational states. Recent theoretical models acknowledge that individuals often experience conflicting motivational states (Larsen, McGraw, & Cacioppo, 2001), many of which are outside of awareness but influence behavior nonetheless (Berridge & Winkielman, 2003).
In summary, we found that more dependent smokers experienced greater negative affect and craving at baseline and postquit, regardless of abstinence status. This suggests that those with a predisposition to experience negative affect are prone to greater nicotine dependence, and not that greater nicotine dependence causes greater negative affect and craving during withdrawal. We found no support for the hypothesis that smoking relapse results in greater negative affect and craving than abstinence. Craving was associated with negative affect across measurement modalities. These results highlight the benefits of using multimodal measures to study the impact of nicotine dependence and withdrawal on negative affect and craving.
Acknowledgments
Portions of this article were presented at the annual meeting of the Society for Psychophysiological Research in Portland, OR, September, 2010. This project was supported by State of Texas Tobacco Settlement Funds and a National Cancer Institute grant (P50CA70907) awarded to Paul M. Cinciripini, a career development grant (K23DA024697) and an MD Anderson Education Program in Cancer Prevention Postdoctoral Fellowship Grant (R25CA57730) to Jason D. Robinson, and a career development grant (K07CA92209) to Brian L. Carter. We thank Cathy Sanders, Renata Benjamin, Deena Martinez, and Dr. Tracy Long for their assistance in data collection.
Footnotes
The following IAPS slides were used: Pleasant, 4220, 4652, 4658, 4659, 4660, 4670, 5621, 5629, 8030, 8370, 8490, and 8500; Neutral, 7000, 7010, 7020, 7030, 7040, 7050, 7060, 7080, 7090, 7100, 7150, and 7170; Unpleasant, 3010, 3060, 3100, 3120, 3130, 3150, 3170, 3500, 6230, 6350, 6560, and 9410. The pleasant (M = 6.54, SD = 0.59) and unpleasant pictures (M = 6.96, SD = 0.30) did not differ on arousal but did differ from neutral (M = 2.80, SD = 0.39), p < .05.
One participant who was nonabstinent at Session 2 but was abstinent at Sessions 3 and 4 was classified as an Abstainer at EOT.
We did not evaluate baseline cigarettes per day as a proxy for nicotine dependence because 42% of the sample reported smoking 20 cigarettes per day, which greatly restricted variability.
Contributor Information
Jason D. Robinson, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center
Cho Y. Lam, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center
Brian L. Carter, Graduate College of Social Work, The University of Houston
Jennifer A. Minnix, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center
Yong Cui, Graduate Program in the Department of Translational Biology and Molecular Medicine, Baylor College of Medicine.
Francesco Versace, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center.
David W. Wetter, Department of Health Disparities Research, The University of Texas MD Anderson Cancer Center
Paul M. Cinciripini, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center
References
- Bageilla E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13–20. [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. In: Rivers C, editor. The Nebraska symposium on motivation: Alcohol use and abuse. Lincoln, NE: University of Nebraska Press; 1987. pp. 257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Berridge K, Winkielman P. What is an unconscious emotion? (The case for unconscious “liking”) Cognition & Emotion. 2003;17:181–211. doi: 10.1080/02699930302289. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. The mind of an addicted brain: Neural sensitization of wanting versus liking. American Psychological Society. 1995;4:71–76. [Google Scholar]
- Blalock JA, Robinson JD, Wetter DW, Schreindorfer LS, Cinciripini PM. Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychology of Addictive Behaviors. 2008;22:122–128. doi: 10.1037/0893-164X.22.1.122. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, DePue JD, Murphy C, Abrams DB. Development of major depressive disorder during smoking-cessation treatment. Journal of Clinical Psychiatry. 1996;57:534–538. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Breiner MJ, Stritzke WGK, Lang AR. Approaching avoidance: A step essential to the understanding of craving. Alcohol Research & Health. 1999;23:197–206. [PMC free article] [PubMed] [Google Scholar]
- Burgess ES, Kahler CW, Niaura R, Abrams DB, Goldstein MG, Miller IW. Patterns of change in depressive symptoms during smoking cessation: Who’s at risk for relapse? Journal of Consulting and Clinical Psychology. 2002;70:356–361. doi: 10.1037//0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Losch ME, Kim HS. Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology. 1986;50:260–268. doi: 10.1037//0022-3514.50.2.260. [DOI] [PubMed] [Google Scholar]
- Carey MP, Kalra DL, Carey KB, Halperin S, Richards S. Stress and unaided smoking cessation: A prospective investigation. Journal of Consulting and Clinical Psychology. 1993;61:831–838. doi: 10.1037//0022-006x.61.5.831. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: NIMH-Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, Wetter DW. The Effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Tsoh JT, Wetter DW, Lam CY, De Moor CA, Cinciripini LG, Minna J. Combined effects of venlafaxine, nicotine replacement & brief counseling on smoking cessation. Experimental and Clinical Psychopharmacology. 2005;13:282–292. doi: 10.1037/1064-1297.13.4.282. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Erlbaum; 1988. [Google Scholar]
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychology. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- Curry S, Marlatt GA, Gordon JR. Abstinence violation effect: Validation of an attributional construct with smoking cessation. Journal of Consulting and Clinical Psychology. 1987;55:145–149. doi: 10.1037//0022-006x.55.2.145. [DOI] [PubMed] [Google Scholar]
- Dols M, Willems B, van den Hout M, Bittoun R. Smokers can learn to influence their urge to smoke. Addictive Behavior. 2000;25:103–108. doi: 10.1016/s0306-4603(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: Influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurements. Personality and Individual Differences. 1998;25:399–423. [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, Sly KF. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology. 2002;70:142–152. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. Neuroimage. 2003;20:2314–2321. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Carr CR, Marcus AC. The tobacco withdrawal syndrome in unaided quitters. British Journal of Addiction. 1991;86:57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Guthrie SK, Ni L, Zubieta JK, Teter CJ, Domino EF. Changes in craving for a cigarette and arterial nicotine plasma concentrations in abstinent smokers. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:617–623. doi: 10.1016/j.pnpbp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry & Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70:216–227. [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Telch MJ, Newman B. Are heavy smokers different from light smokers? A comparison after 48 hours without cigarettes. Journal of the American Medical Association. 1988;260:1581–1585. [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology. 2007;44:728–736. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? Journal of Personality and Social Psychology. 2001;81:684–696. [PubMed] [Google Scholar]
- Marlatt G. Relapse prevention: Theoretical rationale and overview of the model. In: Marlatt G, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. pp. 3–70. [Google Scholar]
- Maude-Griffin PM, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4:198–208. [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Meyer RE. Conditioning phenomena and the problem of relapse in opioid addicts and alcoholics. NIDA Research Monograph. 1988;84:161–179. [PubMed] [Google Scholar]
- Mucha RF, Pauli P, Angrilli A. Conditioned responses elicited by experimentally produced cues for smoking. Canadian Journal of Physiology and Pharmacology. 1998;76:259–268. [PubMed] [Google Scholar]
- Muñoz MÁ, Viedmadel-Jesus MI, Fernández-Santaella MC, Peralta-Ramírez MI, Cepeda-Benito A, Vila J. Assessment of tobacco craving by means of the affective image visualization paradigm. Motivation and Emotion. 2010;34:93–103. [Google Scholar]
- Orain-Pelissolo S, Grillon C, Perez-Diaz F, Jouvent R. Lack of startle modulation by smoking cues in smokers. Psychopharmacology. 2004;173:160–166. doi: 10.1007/s00213-003-1715-4. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Kenford SL, Smith SS, Fiore MC, Baker TB. Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science. 1997;8:184–189. [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Robinson JD, Cinciripini PM, Carter BL, Lam CY, Wetter DW. Facial EMG as an index of affective response to nicotine. Experimental and Clinical Psychopharmacology. 2007;15:390–399. doi: 10.1037/1064-1297.15.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PJ, Smit HJ. Food craving and food “addiction”: A critical review of the evidence from a biopsychosocial perspective. Pharmacology Biochemistry & Behavior. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Davydov DM, James P. Situations and moods associated with smoking in everyday life. Psychology of Addictive Behaviors. 2002;16:342–345. doi: 10.1037//0893-164x.16.4.342. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”-individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: Contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel J, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGruy FV, Hahn SR, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. Journal of the American Medical Association. 1994;272:1749–1756. [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. The role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- Tassinary LG, Cacioppo JT. The skeletomotor system: Surface electromyography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 163–199. [Google Scholar]
- Waldron I. Sex differences in illness incidence, prognosis and mortality. Social Science and Medicine. 1983;17:1107–1123. doi: 10.1016/0277-9536(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson NL, Carpenter MJ, Saladin ME, Gray KM, Upadhyaya HP. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addictive Behaviors. 2010;35:673–677. doi: 10.1016/j.addbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD, Baker TB. Smoking outcome expectancies: Factor structure, predictive validity, and discriminant validity. Journal of Abnormal Psychology. 1994;103:801–811. doi: 10.1037//0021-843x.103.4.801. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2. New York: McGraw-Hill; 1971. [Google Scholar]
- Witvliet CVO, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32:436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating Smoking Motivation: Impact on an Electrophysiological Index of Approach Motivation. Journal of Abnormal Psychology. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]