Summary
Background
Psychological distress and metabolic dysregulation are associated with markers of accelerated cellular aging, including reduced telomerase activity and shortened telomere length. We examined whether participation in a mindfulness-based intervention, and, secondarily, improvements in psychological distress, eating behavior, and metabolic factors are associated with increases in telomerase activity in peripheral blood mononuclear cells (PBMCs).
Methods
We enrolled 47 overweight/obese women in a randomized waitlist-controlled pilot trial (n = 47) of a mindfulness-based intervention for stress eating and examined changes in telomerase activity from pre- to post-intervention. In secondary analyses, changes in telomerase activity across the sample were examined in relation to pre- to post-intervention changes in psychological distress, eating behavior, and metabolic factors (weight, serum cortisol, fasting glucose and insulin, and insulin resistance).
Results
Both groups increased in mean telomerase activity over 4 months in intent-to-treat and treatment efficacy analyses (p < 0.001). Nonsignificant trends showed that greater attendance was associated with increases in telomerase, and telomerase increases were 18% higher among ‘as treated’ participants compared to controls. Across groups, changes in chronic stress, anxiety, dietary restraint, dietary fat intake, cortisol, and glucose were negatively correlated with changes in telomerase activity. In exploratory analyses, decreases in dietary fat intake partially mediated the association between dietary restraint and telomerase activity with marginal significance.
Conclusions
While there was no clear effect of the intervention on telomerase activity, there was a striking pattern of correlations between improvements in psychological distress, eating behavior, and metabolic health and increases in telomerase activity. These findings suggest that telomerase activity may be in part regulated by levels of both psychological and metabolic stress.
Keywords: Stress, Anxiety, Mindfulness, Dietary restraint, Telomerase, Cell aging, Cortisol
1. Introduction
Chronic stress and overeating are prevalent in modern societies and can lead to metabolic dysregulation. Chronic stress can promote overeating, which, in turn, can elevate cortisol, glucose, and insulin levels, cause weight gain, and increase inflammatory and oxidative stress processes (Epel, 2009). Growing evidence suggests that these biological factors work together to accelerate cellular aging by inhibiting the telomere maintenance system.
Telomere length in immune cells provide a window into the aging of the immune system (Andrews et al., 2010). Telomeres are DNA—protein complexes at the end of linear chromosomes, required for the complete replication of DNA and chromosome stability. Intact telomeres protect chromosomes from nuclease degradation, end-to-end fusion, and cellular senescence. The cellular enzyme, telomerase, adds telomeric repeat sequences to the chromosomal DNA ends, preserving not only telomere length but also healthy cell function and long-term immune function (Blackburn, 2000). An aged immune system, as indicated by shorter telomere length (TL) and lower telomerase activity, secretes proinflammatory cytokines (Effros, 2007) and is predictive of earlier cell mortality and mortality in people (Bakaysa et al., 2007; Cawthon et al., 2003; Honig et al., 2006; Kimura et al., 2008; Martin-Ruiz et al., 2006). However, cells containing chromosomes with shortened telomeres can remain genetically stable if telomerase activity is high (Blackburn, 2000). Recently, telomerase was found to be expressed at low levels in PBMCs and to be a dynamic enzyme capable of immediate and short term changes (Broccoli et al., 1995; Weng et al., 1996). Thus, PBMC telomerase can be measured over short time periods (hours or weeks), unlike telomere length, which is thought to take months to years for changes to be detectable (Epel et al., 2010). Studying telomerase activity provides a unique opportunity to examine how lifestyle and metabolic factors affect the aging process, and further, if modulating lifestyle retards cellular aging processes.
Emerging research suggests that an unhealthy lifestyle, including psychological distress, poor nutrition, and physical inactivity, is associated with either lower telomerase or shorter TL. Chronic psychological stress and mood disorders are linked to shorter telomere length (Damjanovic et al., 2007; Epel et al., 2004; Lung et al., 2007; Puterman et al., 2010; Simon et al., 2006) and dampened telomerase activity (Epel et al., 2004). Conversely, longer leukocyte telomeres are related to a more healthy diet, including greater intake of antioxidants (multivitamins, vitamins C, E, D) (Richards et al., 2007; Xu et al., 2009); less processed meat consumption (Nettleton et al., 2008); greater frequency or intensity of exercise (Cherkas et al., 2008; Puterman et al., 2010; Werner et al., 2009); and a healthy lifestyle index consisting of greater intake of fruits and vegetables, less dietary fat and cigarette smoking, and greater exercise (Mirabello et al., 2009). Further, dietary restraint, a set of attitudes and behaviors reflecting a preoccupation with weight and unsuccessful attempts to restrict calorie intake that can result in episodic overeating, has been related to shorter TL (Kiefer et al., 2008).
Metabolic factors, including a greater body mass index (BMI), abdominal fat, and increased circulating glucose levels, have been related to shorter TL and lower telomerase activity (Epel et al., 2006; Valdes et al., 2005). Increases in body mass index, and in particular, insulin resistance, predict telomere shortening over a 10—13 year period (Gardner et al., 2005). Further, higher levels of nocturnal cortisol excretion, an indicator of chronic stress, are related to shorter TL (Epel et al., 2006). In vitro, exposure to high levels of cortisol dampens telomerase activity (Choi et al., 2008).
These studies suggest that lifestyle and metabolic factors are related to the telomerase/telomere length maintenance system. However, it is not clear whether reducing psychological distress and improving health behaviors can improve cell aging. No controlled studies have yet examined the effects of lifestyle interventions on TL; however, two studies have examined associations of behavioral interventions with telomerase activity. In one study, men diagnosed with early stage prostate cancer participating in a 3-month intensive lifestyle change program involving diet, exercise, stress management, and group support had increased telomerase levels in PBMCs from pre- to post-intervention (Ornish et al., 2008). However, this study lacked a randomized control group. In a second study, telomerase was examined at the end of a randomized controlled trial of a 3-month residential meditation program. The meditation group had higher post-intervention telomerase than the waitlist control group, and increases in psychological well-being (increased perceived control and purpose in life and decreased negative affectivity) were related to higher post-intervention telomerase in the treatment but not control group (Jacobs et al., 2011). No studies, to our knowledge, have examined the effects of lifestyle interventions on pre- to post-intervention changes in telomerase activity within a randomized controlled study design.
The current randomized, waitlist-controlled pilot study explores the effects of a mindfulness-based intervention for stress eating on telomerase activity among overweight to obese women. The present report is a substudy of a parent study which aimed to explore the effects of the mindfulness intervention on abdominal fat and whether improvements in chronic stress, cortisol, and dysregulated eating mediated the effects. The design and results of this parent study are reported elsewhere (Daubenmier et al., 2011).
Telomerase activity appears to be regulated both by stress and behavioral pathways linked to metabolic health. Given the findings from cross sectional studies, we expected that improvements in stress, dietary behaviors (including reductions in dietary restraint) and metabolic factors, would all be associated with improvements in telomerase activity. Thus, we explored whether a mindfulness-based intervention for stress eating increases telomerase activity in PBMCs. Secondarily, we assessed whether changes in psychological distress, cortisol levels, eating behavior, and metabolic factors are associated with changes in telomerase activity from pre- to post-intervention across treatment groups.
2. Materials and methods
2.1. Study design
The study design was a randomized waitlist-controlled pilot trial examining the effects of a mindfulness-based stress reduction and eating awareness intervention compared to a waitlist control group on telomerase activity in PMBCs in overweight and obese women. The Institutional Review Board of the University of California, San Francisco approved this study and all participants provided informed consent. Detailed methods adhering to the CONSORT guidelines are described in the parent study (Daubenmier et al., 2011). Briefly, adult female participants were recruited through media outlets with key eligibility criteria as follows: a body mass index (BMI) between 25 and 40; pre-menopausal; no history of diabetes or cardiovascular disease, or active endocrinologic disorder; not pregnant or less than 1 year postpartum; no prior or current meditation or yoga practice; not currently on a diet plan; no current self-reported eating disorder or alcohol or drug addiction; not taking opiate pain medication, steroids, or antipsychotic medications; and ability to speak and read English. Eligible participants completed two baseline assessment visits, one post-intervention assessment, and an on-line questionnaire battery at each timepoint. The intervention was provided free of charge and participants were compensated for the pre/post testing sessions.
2.2. Randomization
Participants were randomized to the treatment or waitlist control group in a 1:1 ratio and stratified on BMI category (overweight: BMI 25—29.99 vs. obese: 30—39.99), age (< and ≥ 40 years) and current anti-depressant medication use (n = 7) as these factors may influence weight change and telomerase activity.
2.3. Intervention groups
2.3.1. Treatment condition
A novel intervention was developed by integrating components from two programs, Mindfulness-Based Stress Reduction (MBSR) (Kabat-Zinn, 1990) and Mindfulness-Based Eating Awareness Training (MB-EAT) (Kristeller and Hallett, 1999; Kristeller and Wolever, 2011). Mindfulness meditation is the systematic training of a focused state of awareness through repeated attendance to sensations of breath, other sensory experiences, thoughts, and emotions. Mindfulness is characterized by an open, impartial stance towards present moment experience as a way to interrupt habitual patterns of thoughts, emotions, and behaviors to allow for more adaptive responses. MB-EAT, in particular, promotes awareness of physiological cues related to hunger, satiety, and taste satisfaction, and of emotional triggers for overeating.
In the current study, the intervention program consisted of nine 2.5-h classes and one 7-h silent day of guided meditation practice during the sixth week of the 4-month program. Participants were instructed in sitting meditation, body scan and mindful yoga stretches as taught in MBSR. Participants were also instructed in mindful eating practices, which included paying attention to physical sensations of hunger, stomach fullness, taste satisfaction, and responding mindfully to food cravings and other eating triggers. Meditations that cultivate feelings of loving kindness and forgiveness towards self and others were included as supplemental meditations. Participants were encouraged to engage in daily home assignments that included up to 30 min per day of formal mindfulness practices and mindful eating practices during meals.
2.3.2. Control condition
Participants randomly assigned to the waitlist group were offered an expanded version of the mindfulness intervention after completion of all post-intervention assessments. To provide guidelines for healthy eating and exercise during the intervention and to control for the effects of such information on study outcomes, both groups participated in a 2-h nutrition and exercise information session aimed at moderate weight loss mid-way through the intervention, in which mindfulness was not discussed.
2.4. Measures
2.4.1. Self-report measures
Mindfulness
Mindfulness was assessed using the 39-item Kentucky Inventory of Mindfulness Skills questionnaire (Baer et al., 2004) which assesses 4 aspects of mindfulness: Observing, which involves the ability to pay attention to internal and external sensory stimuli; Describing, which involves the ability to verbally express one’s experience; Acting with Awareness, which involves engaging in current activities with undivided attention; and Accepting without Judgment, which assesses the ability to accept one’s experience, particularly if it is unpleasant or unwanted. Participants rated each item on a 5-point Likert type scale ranging from 1 (never or very rarely true) to 5 (almost always or always true). A factor analysis indicated that a single factor accounted for 48% of the variance in the subscales, with factor loadings ranging from 0.37 to 0.79. Cronbach’s alpha of the single scale was .80, indicating good internal consistency. Thus, all items were combined and a single mean score was used in analyses.
Psychological stress
Stress was measured with two scales. First, the 10-item Perceived Stress Scale was used to evaluate perception of stressful events over the past month by using a 5-point Likert scale (0 = never to 4 = very often) (Cohen et al., 1983). The 51-item Wheaton Chronic Stress Inventory was used to measure the presence of chronic stressors related to work, relationship, and financial difficulties, and ratings of impact (Wheaton, 1994). Statements were rated according to a 5-point scale (0 = not at all true to 4 = extremely true).
Anxiety
The 20-item State-Trait Anxiety Scale-Trait Form (STAI) was used to measure general feelings of anxiety (Spielberger et al., 1970). Participants rated statements using a 5-point scale. Responses ranged from almost never = 1 to almost always = 4. Positive items were reverse-coded and averaged to create a mean score.
Dietary restraint
The 10-item Restrained Eating subscale of the Dutch Eating Behavior Questionnaire (Van Strien et al., 1986) was used to assess dietary restraint. This scale assesses intentions and behaviors to restrict food intake due to concerns about weight. It taps into eating less than desired rather than less than actually needed (relative deprivation, not actual deprivation of calories). Responses were made on a 5-point scale from 0 = never to 4 = very often. Examples of items are: “Do you try to eat less at mealtimes than you would like to eat?” and “How often do you try not to eat between meals because you are watching your weight?”
Dietary intake
The Block 2005 Food Frequency Questionnaire, a semi-quantitative food frequency questionnaire, was used to assess food consumption of 110 food items over the past year at baseline and over the past 3 months at post-intervention (Block, 2005). Total calories and percent calories from fat, carbohydrates, and protein were calculated according to standard scoring performed by NutritionQuest.
2.4.2. Metabolic factors
A standard stadiometer (Perspective Enterprises, Portage, MI) was used to measure height to the nearest 1/8 in. A digital scale (Wheelchair Scale 6002, Scale-Tronix, Carol Stream, IL) was used to measure weight to the nearest 0.10 kg. Fasting morning blood samples were obtained from an indwelling forearm venous catheter. Serum cortisol concentrations were estimated in duplicate using commercial radioimmunoassay kits (Coat-A-Count Cortisol kit, Siemens Medical Solutions Diagnostics, Los Angeles, CA). Glucose was measured enzymatically (glucose oxidase) with an automated YSI 2300 Analyzer from YSI Life Sciences (Yellow Springs, OH). Instrument precision is within 2%. Insulin was assayed with a radioimmunoassay kit using an I125-Iodinated insulin tracer, anti-Human Insulin Specific antibody, and human insulin standards from Linco Research, Inc. (St. Charles, MO). Insulin resistance was determined by homeostatic model assessment (HOMA-IR) based on fasting glucose and insulin values (Wallace et al., 2004).
2.4.3. Telomerase activity
Telomerase activity was measured in PBMC samples as previously described (Lin et al., 2010). Briefly, cryopreserved PBMCs were thawed and live cells counted using a hemocytometer by the Trypan blue exclusion method. The viability of the PBMC cell samples in this cohort fell within the normal range for samples we have previously used for telomerase assays. A paired sample t-test showed no significant difference between the percentage of viable cells available for pre- and post-intervention assays (p = 0.71). For each PBMC sample, an extract of 5000 cells per microliter was made and two concentrations, corresponding to 5000 and 10,000 cells, were assayed for each sample to ensure the assay was in the linear range. Telomerase activity was assayed by the Telomerase Repeat Amplification Protocol (TRAP) using a commercial kit (TRAPeze, Telomerase Detection kit, Upstate/ CHEMICON, Temecula, CA).
Baseline and post-intervention samples for the same participant were assayed in the same batch and run on the same gel to eliminate any differences caused by reaction or procedural batch-to-batch variations. Technicians were blind to group assignment. In addition, the same reagent batch number (lot) of the TRAPeze telomerase detection kit was used for all samples in this study to eliminate measurement shift due to different reagent batch numbers.
Cell viability was determined after thawing and telomerase activity was calculated on a per viable cell basis. Telomerase activity is defined as 1 unit = the amount of product from one 293T cell/10,000 PBMCs, and was quantified using the software ImageQuant 5.2 (GE Healthcare, Piscataway, NJ). The viability of the PBMC cell samples in this cohort fell within the normal range for samples we have previously used for telomerase assays. Inter-assay variability was determined to be 7%.
2.5. Statistical analyses
Primary analyses on groups
We performed “intention to treat” analyses, which included all subjects randomized, regardless of how much they attended the intervention. We also performed ’treatment efficacy” analyses to test the effect of the intervention on the subset of subjects who attended the minimum dose of treatment thought to be effective. Thus, the treatment efficacy analyses were performed using data from treatment participants who attended a minimum of 4 of the 10 classes. To test the primary hypothesis, both the intention-to-treat and treatment efficacy analysis were conducted using ANOVA for repeated measures to examine main effects of time and group, and independent-samples t-tests were used to test for treatment effects.
Outliers
To normalize distributions, variables underwent natural log transformation in the case of a skewed distribution. Statistical outliers (≥ than 3 standard deviations from the mean) of telomerase activity were winsorized and set to equal the next highest or lowest value. Specifically, one statistical outlier in the natural log transformed values of telomerase activity was observed at each timepoint. These two values were winsorized to reduce a disproportionate influence on analyses. The baseline value was 0.47 and set to equal the next lowest value, 1.06; the post-intervention outlier was 3.4 and was set to equal the next highest value, 2.7. Results did not differ appreciably from those of nonwinsorized data. To simplify presentation, only winsorized results are presented.
Secondary analyses across groups
For secondary analyses, multiple linear regression models were performed across groups using available data from all participants to predict changes in telomerase activity, controlling for baseline telomerase activity. Predictors included pre—post changes in psychological distress, eating behavior, and metabolic factors. Exploratory post hoc multiple linear regressions and mediation models using the Baron and Kenny method (Baron and Kenny, 1986) were conducted to further understand results. All analyses reported were conducted using p < .05.
3. Results
3.1. Sample characteristics
Sample characteristics are described in detail elsewhere (Daubenmier et al., 2011). Briefly, 322 potential female participants were screened for eligibility from November 2006 to March 2007. A total of 47 participants were randomized: twenty-four participants were randomized to the treatment group and 23 participants to the control group. Baseline telomerase activity was available for 43 participants (reasons for missing data are detailed in Fig. 1). Groups did not differ in overall ethnic composition, with 63% of the treatment and 61% of the control group identifying as White ( p = .91). No statistically significant differences between treatment and control groups were found on psychological, eating behavior, or metabolic factors, suggesting that randomization was successful. We present the combined means and SDs of baseline characteristics across groups (see Table 1). Scale means are reported. The groups did not differ in telomerase activity [p = .39; M = 1.74 ± 0.3 in the treatment group (n = 23) and M = 1.84 ± 0.4 in the control group (n = 20)]. We also tested whether there were any differences in any variables at baseline between participants who did not have any telomerase values versus those who did. We conducted t-tests and found no statistically significant differences between groups.
Figure 1.
Consort flowchart.
Table 1.
Baseline and change scores of variables across groups.
| Variable | N | Baseline (M ± SD) | N | Pre to post change (M ± SD) | p value a |
|---|---|---|---|---|---|
| Telomerase activity | 43 | 6.36 ± 2.6 | 37 | 1.36 ± 2.8 | .005 |
| Telomerase activity (ln) | 1.78 ± 0.37 | 37 | 0.20 ± 0.4 | .004 | |
| Psychological factors | |||||
| Mindfulness | 43 | 3.11 ± 0.4 | 37 | 0.15 ± 0.37 | .017 |
| Chronic Stress | 43 | 1.97 ± 0.47 | 37 | 0.10 ± 0.49 | .22 |
| Perceived Stress | 43 | 1.89 ± 0.61 | 37 | −0.12 ± 0.58 | .23 |
| Anxiety | 43 | 2.20 ± 0.49 | 37 | −0.12 ± 0.36 | .048 |
| Eating behavior | |||||
| Restrained eating | 43 | 2.80 ± 0.57 | 37 | 0.00 ± 0.49 | 1.00 |
| Total calories | 42 | 1912 ± 731 | 33 | −293 ± 442 | .001 |
| % Fat | 42 | 38 ± 5 | 33 | −1.0 ± 4.5 | .20 |
| % Carbohydrate | 42 | 46 ± 7 | 33 | 1.6 ± 6.0 | .15 |
| % Protein | 42 | 16 ± 3 | 33 | .09 ± 2.0 | .80 |
| Metabolic factors | |||||
| BMI | 43 | 31.2 ± 4.8 | 37 | 0.1 ± 0.9 | .51 |
| Weight (kg) | 43 | 84.9 ± 14.9 | 37 | 0.3 ± 2.6 | .48 |
| Cortisol (ug/dl) | 43 | 10.6 ± 4.6 | 36 | −0.22 ± 5.6 | .82 |
| Glucose (mg/dl) | 43 | 92 ± 8 | 37 | 4.9 ± 7.5 | .0001 |
| Insulin (μU/ml) | 43 | 13.9 ± 8.3 | 37 | 2.9 ± 5.1 | .001 |
| HOMA-IR | 43 | 3.2 ± 2.1 | 37 | 0.95 ± 1.6 | .001 |
p values from one sample t-tests for mean changes differing from 0.0.
3.2. Participants lost to follow-up
As shown in Fig. 1, four participants in the treatment group failed to attend the minimum 4 out of 10 classes. Two participants in each group were lost to follow up for the primary analysis examining effect of treatment on telomerase activity (i.e., did not have post telomerase values). Three participants in each group had either a failed blood draw attempt or not enough cells were collected to permit analysis of telomerase activity. Therefore, telomerase activity data were available at both time points for 19 treatment and 18 control participants for the intention-to-treat analysis and 17 participants in each group for the treatment efficacy analysis, which excluded the two intervention participants with insufficient attendance and one control participant who received a weight loss treatment during the study (liposuction).
3.3. Treatment effects
As described elsewhere in the parent study (Daubenmier et al., 2011), groups did not change or differ substantially over time in levels of chronic stress or perceived stress; however, the treatment compared to the control group had statistically significant decreases in reported anxiety and increases in mindfulness. Groups did not change or differ substantially over time in restrained eating, and both groups maintained weight over time.
The groups also did not differ over time on any metabolic factor or in macronutrient content (p’s > 0.05). Because there were so few group differences in predictors, Table 1 shows means and standard deviations of changes in variables across groups. Both groups had significant decreases in total caloric intake but did not differ substantially from each other over time ( p = 0.98). In regards to macronutrient intake, both groups, on average, maintained levels of percentage of calories from fat, protein, and carbohydrates. Both groups showed statistically significant increases in glucose, insulin, and HOMA-IR values, but not cortisol.
3.4. Effect of treatment on telomerase activity
In the intent-to-treat analyses, both groups increased in telomerase activity from pre- to post-intervention (see Table 2). A non-significant treatment effect of 0.10 on the natural log scale was observed, implying that increases in telomerase activity averaged 11% greater in the intervention group compared to controls (95% CI: −15% to 43%, p = 0.45). In the treatment efficacy analysis, both groups also increased in telomerase activity from pre- to post-intervention ( p < 0.001). A non-significant treatment effect of 0.18 on the natural log scale was observed, implying that increases in telomerase activity averaged 18% greater in the treatment group compared to controls (95% CI: −10% to 54%, p = 0.21).
Table 2.
Treatment outcomes of telomerase activity with intention to treat and treatment efficacy analyses.
| Treatment |
Control | T – C |
p values |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
N (t, c) |
Pre (M ± SD) |
Post (M ± SD) |
Pre (M ± SD) |
Post (M ± SD) |
Mean diff. (95% CI) |
Time | Group | Time× group |
|
| Intent-to-treat (ln) | 19 | 1.73 ± 0.4 | 1.97 ± 0.4 | 1.82 ± 0.4 | 1.97 ± 0.3 | 0.10 (−0.2 to 0.4) | .004 | .64 | .45 |
| Raw values | 18 | 5.99 ± 2.2 | 7.67 ± 2.8 | 6.49 ± 2.7 | 7.51 ± 2.5 | 0.66 (−1.2 to 2.5) | .006 | .81 | .47 |
| As-treated (ln) | 17 | 1.67 ± 0.3 | 1.98 ± 0.4 | 1.84 ± 0.4 | 1.98 ± 0.3 | 0.16 (−0.1 to 0.4) | .001 | .43 | .21 |
| Raw values | 17 | 5.62 ± 2.0 | 7.81 ± 3.0 | 6.59 ± 2.8 | 7.63 ± 2.5 | 1.15 (−0.7 to 3.0) | .001 | .60 | .22 |
Consistent with this finding, higher attendance was related to greater increases in telomerase (at a marginal significance level, see Table 3). Interestingly, the 3 participants who attended fewer than half the classes actually decreased (31% decrease) in telomerase activity, on average, compared to a 43% mean increase among participants who attended over half the classes.
Table 3.
Estimated effects of predictors on changes in telomerase when controlling for baseline telomerase.
| Predictor | N | Estimated effect |
Standard error |
95% CI lower bound |
95% CI upper bound |
Standardized coefficient |
p value |
|---|---|---|---|---|---|---|---|
| Attendance (treatment group) | 19 | .057 | .033 | −.012 | .127 | .33 | .10 |
| Psychological factors | |||||||
| Mindfulness | 37 | −.044 | .14 | −.337 | .249 | −.04 | .76 |
| Chronic stress | 37 | −.215 | .102 | −.422 | −.007 | −.27 | .04 |
| Perceived stress | 37 | −.061 | .089 | −.242 | .120 | −.09 | .50 |
| Anxiety | 37 | −.356 | .130 | −.619 | −.093 | −.33 | .009 |
| Eating behavior | |||||||
| Restrained eating | 37 | −.266 | .097 | −.463 | −.069 | −.34 | .01 |
| Total calories a | 33 | −.109 | .124 | −.362 | .145 | −.13 | .39 |
| % Fat b | 33 | −.032 | .011 | −.054 | −.010 | −.38 | .007 |
| % Carbohydrate b | 33 | .017 | .009 | .000 | .035 | .27 | .06 |
| % Protein | 33 | −.025 | .027 | −.081 | .031 | −.13 | .37 |
| Metabolic factors | |||||||
| Weight (kg) | 37 | .000 | .021 | −.044 | .043 | −.00 | .98 |
| Cortisol (ln) | 36 | −.224 | .107 | −.441 | −.007 | −.27 | .04 |
| Glucose (mg/dl) | 37 | −.013 | .007 | −.027 | .001 | −.25 | .06 |
| Insulin (μU/ml) | 37 | −.011 | .011 | −.032 | 0.11 | −.14 | .32 |
| HOMA-IR (ln) | 37 | −.133 | .17 | −.468 | .202 | −.11 | .43 |
Changes in total calories were divided by 1000 to facilitate interpretation of the estimated effect.
When both % fat and % carbohydrate intake were entered simultaneously into the model, % fat remained statistically significant (B = −0.041, p = 0.044) while % carbohydrate became non-significant (B = −0.008, p = 0.57).
3.5. Predictors of change in telomerase activity across groups
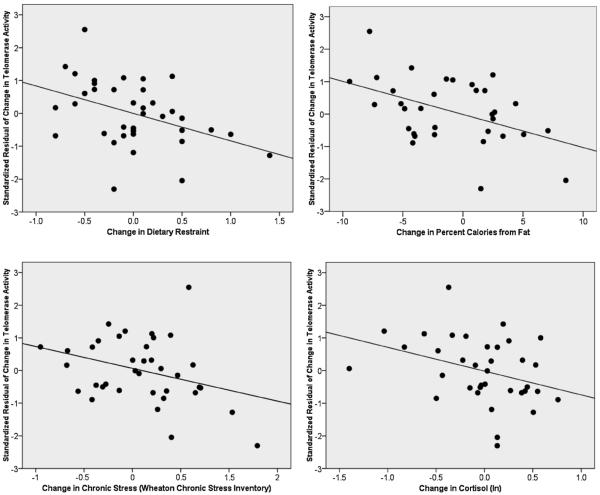
Results of multiple linear regressions are shown in Table 3. Fig. 2 displays scatter plots of selective results. Changes in mindfulness were not statistically significantly related to changes in telomerase activity.
Figure 2.
Scatter plots of associations between standardized residuals of changes in telomerase activity, natural log transformed (partialing out baseline telomerase activity) with changes in dietary restraint (upper left); changes in percent calories from fat (upper right); changes in chronic stress (bottom left); and changes in cortisol (bottom right). See Table 3 for statistical approach and significance.
As predicted, changes in chronic stress, trait anxiety, and restrained eating were negatively related to changes in telomerase activity; however, changes in perceived stress were not strongly related to changes in telomerase activity. Changes in total caloric intake were not strongly related, although changes in % calories from fat and increases in % carbohydrate intake were significantly negatively related to changes in telomerase activity. When both macronutrients were entered simultaneously into the model, however, only % fat remained statistically significant (B = −0.041, p = 0.042) while % carbohydrate became non-significant (B = −0.007, p = 0.63).
In terms of metabolic factors, we examined changes in weight, cortisol, glucose, insulin, and HOMA-IR as predictors of change in telomerase activity. Changes in telomerase activity were negatively associated with changes in morning serum cortisol levels and tended to be negatively correlated with changes in glucose levels (p = .06), but were not correlated significantly with changes in body weight, insulin, or HOMA-IR.
Post hoc exploration of mediators of the relationship between changes in dietary restraint and telomerase activity
Prior cross-sectional research found that greater dietary restraint is related to shorter leukocyte telomeres across two samples of women (Kiefer et al., 2008), but those studies did not find any mediators of this seemingly indirect relationship. Therefore, analyses were conducted to explore potential mediators of the relationship between decreases in dietary restraint and increased telomerase activity. In the present study, we considered psychological distress, cortisol, glucose, and dietary fat intake as potential mediators, given their prior associations to both lower telomerase activity and dietary restraint and significant relation to telomerase activity change in the current study.
The Baron and Kenny model of mediation (Baron and Kenny, 1986) was used, which first requires that the proposed mediator is significantly correlated with both the predictor and predicted variables. If these criteria are met, multiple linear regressions are conducted. In the present study, baseline telomerase activity and change in dietary restraint were entered on step 1 with change in telomerase activity set as the predicted variable. The proposed mediator was entered on step 2 and the resulting change in the dietary restraint coefficient was examined. A decrease in the predictive value of the coefficient indicates evidence of possible mediation.
The bivariate correlations between the proposed mediators and changes in dietary restraint were first examined. Only change in % fat intake was related to change in dietary restraint, indicating greater decreases in dietary restraint were associated with decreased dietary fat intake (r = .28, p = 0.10). Thus, only dietary fat intake was further explored as a mediator in the multiple regression model. As shown in Table 4, when change in dietary fat intake was added to the model, the unstandardized coefficient for change in dietary restraint was reduced by 37% and became nonsignificant whereas the coefficient for dietary fat was significant, suggesting that decreases in dietary fat may partially explain the relation between decreased dietary restraint and increased telomerase activity.
Table 4.
Linear regression model testing mediation of dietary fat intake of the relationship between change in dietary restraint and telomerase activity (n = 32).
| Model | Estimated effect |
Standard error |
95% CI lower bound |
95% CI upper bound |
Standardized coefficient |
p value |
|---|---|---|---|---|---|---|
| Step 1 | ||||||
| Baseline telomerase | −.656 | .141 | −9.43 | −.369 | −.685 | .000 |
| Change in dietary restraint | −.262 | .128 | −.523 | −.001 | −.301 | .049 |
| Step 2 | ||||||
| Baseline telomerase | −.600 | .133 | −.872 | −.328 | −.627 | .000 |
| Change in dietary restraint | −.165 | .126 | −.423 | .092 | −.190 | .200 |
| Change in % fat | −.027 | .011 | −.050 | −.004 | −.324 | .025 |
4. Discussion
To our knowledge, this is the first randomized controlled study to examine effects of a lifestyle intervention on pre- to post-intervention changes in telomerase activity. Mindfulness-based treatment group members who received a minimal treatment “dose” demonstrated a 39% increase in telomerase activity and 18% greater increase in telomerase activity compared to the waitlist control group over the course of the intervention; however, this analysis was underpowered and the finding was not statistically significant. Nevertheless, attendance data support the idea that greater exposure to the intervention was associated with increases in telomerase. The most striking findings were the patterns of association between psychological, eating, and metabolic factors and changes in telomerase activity, supporting the hypothesis that improvements in stress, eating, and metabolic regulation may increase telomerase activity over time. Specifically, decreased levels of chronic stress, anxiety, cortisol, dietary restraint, dietary fat, and glucose were related to increases in telomerase activity across treatment groups over the course of the intervention.
4.1. Stress and biochemical stress pathways
Previous cross-sectional studies determined that chronically stressed individuals and those with mood disorders have shorter leukocyte telomeres compared to controls (Damjanovic et al., 2007; Epel et al., 2004; Lung et al., 2007; Simon et al., 2006). However, it is unknown whether improvements in psychological well-being could improve the telomere maintenance system. The present results suggest that enhancing psychological well-being may increase telomerase activity, which may be a key mechanism to account for the association between psychological well-being and telomere length.
Two other studies provide evidence that improvements in psychological well-being may increase telomerase activity. In an uncontrolled study, Ornish et al. (2008) conducted an intensive lifestyle change intervention for men diagnosed with early prostate cancer and found that those who reported greater decreases in intrusive thoughts about their cancer had greater increases in telomerase activity in PBMCs from pre- to post-intervention. In a second study,Jacobs et al. (2011) found higher levels of telomerase activity post-intervention in participants assigned to a residential 3-month meditation training program compared to a waitlist control group (pre-intervention telomerase levels were not assessed). Mindfulness was one of several meditation methods taught in the intervention. Higher telomerase activity in the meditation group was accounted for by greater increases in perceived control, decreases in negative emotionality, and increases in purpose of life. Increases in self-reported mindfulness accounted for improvements in perceived control and negative emotionality but did not directly account for group differences in telomerase activity post-treatment. Similarly, in the present study, we found little direct association between improvements in mindfulness and telomerase activity. Limitations of using self-report measures to assess mindfulness may account for these null findings and future research could incorporate behavioral measures to assess aspects of mindfulness, such as attention tasks. Alternatively, as suggested by Jacobs et al. (2011), meditation practices may lead to a shift in personal values or meaning in life not captured by mindfulness measures. Future research could examine the role of values and purpose in life as pathways linking meditation interventions to biological and health outcomes. Overall, a growing literature suggests that enhancement of psychological well-being may regulate the telomere maintenance system. Future studies should more strongly examine the impact of psychological interventions on both telomerase activity and telomere length in psychologically vulnerable populations and explore mediating psychological mechanisms.
What biological mediators may link improvements in psychological well-being and telomerase activity? So far, experimental studies have pointed to several stress related biochemical factors that affect telomerase activity. Oxidative stress can dampen telomerase, whereas antioxidant activity appears to increase telomerase (Makino et al., 2011). Studies have also linked inflammation (O’Donovan et al., 2011), insulin resistance (Gardner et al., 2005), and stress hormones, including catecholamines (Parks et al., 2009) and cortisol (Epel et al., 2006), to shorter telomere length. Most of these biochemical mediators have not been studied mechanistically, in animal models or in vitro studies, so causal relations are unknown. In many instances, there could be bidirectional relationships, which can be best assessed by experimental studies.
Cortisol
In terms of the above mentioned biological stress pathways, the stress hormone cortisol has been studied more often than other stress pathways, in both humans and in vitro. Chronically elevated or excessively high cortisol appears to have a suppressive effect on telomerase. For example, high levels of excretion of urinary cortisol, taken on a random evening, is associated with shorter PBMC telomeres in both younger (Epel et al., 2006) and older women (Tomiyama et al., 2011) and lower PBMC telomerase activity (Epel et al., 2006). Consistent with these observations, high levels of cortisol exposure in vitro suppresses PBMC telomerase activity (Choi et al., 2008). In the present study, decreases in morning cortisol concentrations were associated with increases in telomerase activity. This is the first study to show a longitudinal association between co-occurring changes in cortisol and telomerase activity in unstimulated PBMCs. These results support the model that changes in stress related cortisol might be one of the signals regulating telomerase levels in humans.
It should be noted the effects of cortisol on telomerase are complex and may depend on the dose and duration of exposure. Shorter doses and duration appear stimulatory rather than suppressive. Acute effects of spikes in stress or cortisol appear stimulatory to telomerase within the next hour (Epel et al., 2010), but these are less relevant in the current study looking at naturalistic levels of cortisol and basal telomerase. Further, although acute spikes in cortisol are associated with short term increases in PBMC telomerase, they are also associated with the longer term measure of shorter PBMC telomere length (Tomiyama et al., 2011), suggesting that over time, stress and cortisol reactivity promote telomere shortening.
4.2. Dietary/metabolic pathways
Other relevant behavioral pathway include changes in nutrition and eating related attitudes, particularly dietary restraint. We found that changes in dietary restraint, fat intake, and glucose levels may all be important in regulating telomerase activity.
Dietary restraint and fat intake
Prior research has found that women who report greater dietary restraint (preoccupation with dieting and attempts to eat less) have shorter telomeres than women less concerned with dietary restraint (Kiefer et al., 2008). In the present study, decreases in dietary restraint were associated with increases in telomerase activity. These results support the possibility that unhealthy dietary restraint may be a risk factor for accelerated cell aging. The precise mechanisms are unknown, but dietary restraint is associated with chronic stress, cortisol, and weight gain, all which could impact cellular aging. These two behavioral pathways, high stress and high dietary restraint, appear to be independent pathways, as they were both statistically significant predictors of change in telomerase activity when entered simultaneously in a regression model controlling for baseline telomerase levels. Specifically, a reduction in restrained eating predicted higher telomerase (beta = −.27, p = .03) and a decreasein an indexof psychological distress (mean z-scoresof changes in chronic stress and anxiety) predicted an increase in telomerase (beta = −.29, p = .02).
In order to further examine how dietary restraint might be operating, we conducted post hoc analyses to test potential mediators and found that decreases in dietary fat intake showed the strongest evidence of mediation between decreased dietary restraint and increased telomerase activity. High dietary restraint may impact telomerase activity through metabolic pathways. Unsuccessful dieting attempts may result in increased dietary fat intake, which leads to greater oxidative stress (Sies et al., 2005). Alternatively, dietary fat intake promotes higher lipid accumulation, which triggers certain PBMCs to secrete more inflammatory molecules (Libby, 2006). Either of these changes could contribute to impairments of the telomere maintenance system (Paul, 2011). The present results point to the need to encourage flexible and balanced weight loss strategies to avoid a sense of deprivation that may lead to chronic consumption of highfat foods and eventual accelerated cellular aging.
Glucose
We also found that decreases in fasting blood glucose were related to higher levels of telomerase activity, an effect that approached statistical significance (p = .06). Previous work has found that Type 2 diabetes and glucose intolerance are associated with shortened telomere length (Adaikalakoteswari et al., 2007; Zee et al., 2010). Impaired telomerase function in the pancreatic beta cells of a mouse model, caused by genetic mutation of telomerase component genes, leads to glucose intolerance (Kuhlow et al., 2010). Also, mice with genetically determined short telomeres have impaired glucose tolerance and compromised beta cell signaling despite intact beta cell mass (Guo et al., 2011). The links between telomerase, telomere length and glucose regulation in humans warrant further study.
4.3. Implications and limitations
Telomerase preserves telomere length, and telomere length predicts disease and mortality in humans. However, the significance of an increase in telomerase in humans remains to be determined. Increases in telomerase activity have been related to reductions in LDL cholesterol (Ornish et al., 2008) and in animal studies appears to play a role in the development of cardiovascular disease (Serrano and Andres, 2004). Further, telomerase is an increasingly important outcome independent of its effects on telomere maintenance. We now know it serves to protect the cell, including mitochondria, from oxidative stress (Majerska et al., 2011), and mice without telomerase have short TL, mitochondrial dysfunction, and oxidative stress (Sahin et al., 2011).
Unexpectedly, telomerase activity increased across both groups by an average of 26%. It is not clear how to account for the increase among the control group. Waitlist participants may have become more health conscious after enrolling in the study and made other lifestyle changes not measured that in turn enhanced telomerase activity. Alternatively, natural variation in telomerase levels may exist that may be attributable to seasonal effects or other factors and future research could examine these possibilities. This finding emphasizes the importance of a control group when examining telomerase activity.
While these findings are provocative, there are many limitations to this study. Many analyses were conducted, and many findings were not statistically significant. It is unclear if results would generalize to men. Confidence intervals were wide when comparing groups over time. However, we found an expected pattern of relationships between psychological, eating, and metabolic factors with telomerase activity across time in participants across group condition. These findings point to the potential promise of mindfulness-based stress reduction and eating awareness strategies to improve telomerase activity. A combined intervention incorporating active weight loss with mindfulness-based strategies in a larger sample may have a significant impact on telomerase and telomere length.
Acknowledgements
We thank Deanna Sheeley and the staff of the UCSF CCRC for their assistance in conducting this study and study volunteers for their dedicated efforts, especially Kinnari Jhaveri, B.A., Daniel Purnell, B.A., Gina Polke, PhD, Dara Hayden, MA, Loren Yglecias, BA, and Susan Moore, PhD.
Role of funding source
This research was supported by the Mt Zion Health Fund of the Jewish Community Center; The William Bowes, Jr., Fund; the Robert Deidrick Fund; Bernard and Barbro Fund; Mind and Life Institute; Robert Wood Johnson Foundation; and NIH Grants K01AT004199 and P01AT005013 awarded to Dr. Daubenmier and Dr. Hecht, respectively, from the National Center For Complementary & Alternative Medicine, and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.The content is solely the responsibility of theauthors and does not necessarily represent the official viewsof the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest
Elizabeth Blackburn, Jue Lin, and Elissa Epel are co-founders of a telomere measurement company Telome Health, Inc., and own stock in the company.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.psyneuen. 2011.10.008.
References
- Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195(1):83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56(4):390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Allen KM. Assessment of mindfulness by self-report: The Kentucky Inventory of Mindfulness Skills. Assessment. 2004;11(3):191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strate-gic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Block G. Block 2005 Food Frequency Questionnaire. NutritionQuest/Block Dietary Data Systems; Berkeley, CA: 2005. [Google Scholar]
- Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl. Acad. Sci. U. S. A. 1995;92(20):9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 2008;22(4):600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J. Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, et al. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. J. Obes. 2011 doi: 10.1155/2011/651936. , doi:10.1155/2011/651936 Article ID 651936, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp. Gerontol. 2007;42(5):416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U. S. A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 2010;24(4):531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- Guo N, Parry EM, Luo-Sheng L, Kembou F, Lauder N, Hussain MA, et al. Short telomeres compromise B-Cell Signaling and Survival. PloSONE. 2011;6(3):e17858. doi: 10.1371/journal.pone.0017858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann. Neurol. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living. Dell Publishing; New York: 1990. [Google Scholar]
- Kiefer A, Lin J, Blackburn E, Epel E. Dietary restraint and telomere length in pre- and postmenopausal women. Psychosom. Med. 2008;70(8):845–849. doi: 10.1097/PSY.0b013e318187d05e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am. J. Epidemiol. 2008;167(7):799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeller J, Hallett C. An exploratory study of a meditationbased intervention for binge eating disorder. J. Health Psychol. 1999;4:357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat. Disord. 2011;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- Kuhlow D, Florian S, von Figura G, Weimer S, Schulz N, Petzke KJ, et al. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging. 2010;10:650–658. doi: 10.18632/aging.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J. Immunol. Methods. 2010;352(1-2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr. Genet. 2007;17(3):195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- Majerska J, Sykorova E, Fajkus J. Non-telomeric activities of telomerase. Mol. Biosyst. 2011;7(4):1013–1023. doi: 10.1039/c0mb00268b. [DOI] [PubMed] [Google Scholar]
- Makino N, Maeda T, Oyama J, Sasaki M, Higuchi Y, Mimori K, et al. Antioxidant therapy attenuates myocardial telomerase activity reduction in superoxide dismutase-deficient mice. J. Mol. Cell. Cardiol. 2011;50(4):670–677. doi: 10.1016/j.yjmcc.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR., Jr. Dietary patterns, food groups, and telomerelength in the Multi-Ethnic Study of Atherosclerosis (MESA) Am. J. Clin. Nutr. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol. Biomarkers Prev. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L. Diet, nutrition and telomere length. J. Nutr. Biochem. 2011;22:895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5(5):e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am. J. Clin. Nutr. 2007;86(5):1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470(7334):359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circ. Res. 2004;94(5):575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005;135(5):969–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. State Trait Anxiety Inventory Manual. Consulting Psychologists Press, Inc.; Palo Alto, CA: 1970. [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, et al. Does cellular aging relate to profiles of allostatic load? An examination of HPA axis regulation during reactivity and rest and telomere length in postmenopausal women. Physiol. Behav. 2011 in press. [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Rookus MA, Bergers GP, Frijters JE, Defares PB. Life events, emotional eating and change in body mass index. Int. J. Obes. 1986;10(1):29–35. [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- Weng NP, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J. Exp. Med. 1996;183(6):2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- Wheaton B. Sampling the stress universe. In: Avison WR, Gotlib IH, editors. Stress and Mental Health: Contemporary Issues and Prospects for the Future. Plenum; New York: 1994. pp. 77–114. [Google Scholar]
- Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 2009;89(6):1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl. Res. 2010;155(4):166–169. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]