Abstract
Female mosquitoes gorge on vertebrate blood, a rich nutrient source for developing eggs. But gorging meals increase the risk of predation. Mosquitoes are quick to reduce the flight payload with a potent diuresis. Diuretic peptides of the insect kinin family induce a tenfold-reduction in the paracellular resistance of Malpighian tubules and increase the paracellular permeation of Cl−, the counterion of the transepithelial secretion of Na+ and K+. As a result, the transepithelial secretion of NaCl and KCl and water increases. Insect kinins signal to the opening of the paracellular pathway via G protein-coupled receptors and the elevation of intracellular [Ca2+], which leads to the reorganization of the cytoskeleton associated with the septate junction. The reorganization may affect the septate junctional proteins that control the barrier and permselectivity properties of the paracellular pathway. The proteins involved in the embryonic formation of the septate junction and in epithelial polarization are largely known for ectodermal epithelia, but the proteins that form and mediate the dynamic functions of the septate junction in Malpighian tubules remain to be determined.
Keywords: septate junction, paracellular permeability, paracellular chloride transport, leucokinin, aedeskinin, insect kinins, septa, integral membrane proteins, scaffolding proteins, actin, adducin, actin depolymerizing factor, cytoskeleton
Introduction
Only female mosquitoes take blood meals and only during the reproductive period [1]. Blood is a convenient source of salt, water, ATP, iron, and nutrients for developing eggs. However, the blood meal challenges the mosquito with excess salt and water, hemolymph dilution and hypertension, and a huge flight payload. For example, taking a blood meal, the yellow fever mosquito Aedes aegypti more than doubles her body weight in less than two minutes [2]. Such an increase in weight reduces lift and flight speed, which increases the risk of predation and shortens the flight range for finding an aqueous habitat to deposit her eggs [3]. Getting rid of the unwanted salt and water of the blood meal is therefore the immediate priority. Accordingly, the mosquito begins to urinate at high rates even before she has finished her meal [4]. The initial diuresis lasts about 20 minutes. The urine of this diuresis consists largely of Na+, Cl−,and water [2]. Thereafter, K+ starts to appear in the urine, which reflects the digestions of red blood cells.
The formation of urine in mosquitoes begins in the distal blind end of Malpighian (renal) tubules by the process of epithelial secretion. The cations Na+ and K+ take transport pathways through large principal cells of the tubule (Fig. 1). Chloride, the counterion of transepithelial Na+ and K+ secretion, can take transcellular routes through principal and stellate cells and a paracellular route between the epithelial cells (Fig. 1).
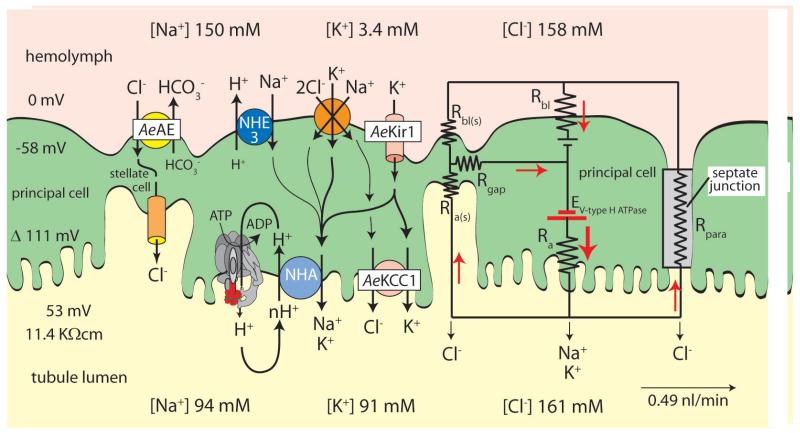
Figure 1.
Mechanisms of transepithelial NaCl and KCl secretion in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Transepithelial secretion of Na+ and K+ is active generating a lumen-positive voltage of 53 mV; transepithelial secretion of Cl− is passive. The transport of all three ions is energized by the V-type H+ ATPase located at the apical membrane of principal cells. The electrical circuit illustrates electrical coupling of transcellular cation transport and paracellular anion transport. The mosquito anion exchanger AeAE, the KCl cotransporter AeKCC1, and the inwardly rectifying K+ channel AeKir1 were recently cloned by our group [5-7]. E, electromotive force; R, electrical resistance; a, apical membrane; bl, basolateral membrane; para, paracellular pathway; (s), stellate cells. Although the tubule epithelium expresses the genes encoding subunits of the Na/K ATPase [8], we measured no significant ouabain-sensitive ATPase activity in Aedes Malpighian tubules [9].
Central to the transepithelial secretion of NaCl and KCl (and consequently water) is the V-type H+ ATPase located at the apical, brush-border membrane of principal cells (Fig. 1). It energizes both transepithelial cation and anion transport. In particular, the V-type H+ ATPase provides the electrochemical potential for driving the extrusion of Na+ and K+ across the apical membrane via presumably nH+/cation exchange mediated by the NHA family of transporters [10, 11]. By generating a lumen-positive transepithelial voltage, the V-type H+ ATPase also provides the electrochemical potential that favors the passive transport of Cl− from hemolymph to the tubule lumen via a paracellular pathway permeable to Cl− [12]. Moreover, exporting protons, the V-type H+ ATPase generates current that must return to the cytoplasmic face of the proton pump. The current is carried by Cl− passing through the paracellular pathway and/or stellate cells (Fig. 1). Current is carried across the basolateral membrane by K+ passing through an inward-rectifying K+ (Kir) channel, such as AeKir1, which we have recently cloned from Aedes Malpighian tubules [7]. Na+ can enter principal cells across the basolateral membrane via exchange transport with H+ mediated by NHE3 cloned in the laboratory of Gill [13] and via cotransport with K+ and Cl− presumably carried by NKCC [14]. Cl− can enter the epithelium via the bumetanide-sensitive NKCC of principal cells and the SLC4–like SCL4 Cl/HCO3 anion exchanger (AE) of stellate cells [5]. Cl− can exit principal cells via a SCL12-like K,Cl cotransporter (KCC) [6] and stellate cells via Cl− channels we have identified in apical membranes of these cells [15]. Under control conditions, Cl− is likely to take both transcellular and paracellular pathways for getting into the tubule lumen. However, under diuretic conditions triggered by leucokinin or aedeskinin, the paracellular pathway is the dominant route for moving Cl− across the epithelium in Aedes Malpighian tubules [16-19].
Role of diuretic hormones
Using HPLC methods our laboratory has purified a protein from mosquito heads that triggers the renal excretion of NaCl and water in the yellow fever mosquito Aedes aegypti [20]. The protein was named mosquito natriuretic peptide (MNP) because it selectively increases the transepithelial secretion of NaCl (and not KCl) by stimulating active transepithelial transport of Na+ [20]. MNP was found to be released into the hemolymph of the mosquito upon taking a blood meal [21]. MNP elevates intracellular concentrations of cyclic AMP, and cAMP mimics the effect of MNP in Malpighian tubules by stimulating transepithelial Na+ secretion [22]. Electrophysiological studies show that MNP and cAMP activate a Na+ conductance in the basolateral membrane of principal cells of Malpighian tubules [23], but the channel or electrogenic carrier providing this conductance is unknown. The Coast laboratory has gone on to identify MNP as the calcitonin-like Anoga-DH31 in Anopheles gambiae [24]. Anoga-DH31 selectively activates the transepithelial secretion of Na+ in Malpighian tubules of Anopheles and Aedes using cAMP as second messenger.
MNP, Anoga-DH31, and cAMP all have a common electrophysiological signature in isolated Malpighian tubules: they hyperpolarize the transepithelial voltage in Anopheles and Aedes Malpighian tubules as Na+ enters principal cells across the basolateral membrane [24-27]. In contrast, other HPLC fractions of mosquito (Aedes) heads depolarize the transepithelial voltage, consistent with the presence of other diuretic peptides and other mechanisms of action at the level of Malpighian tubules [20]. Leucokinin turned out to be such a depolarizing peptide of the transepithelial voltage. Leucokinin was known to stimulate the contractions of the hindgut in the cockroach Leucophaea maderae [28], which suggested excretory activity not only in the hindgut but also upstream in Malpighian tubules. Indeed, leucokinin from the cockroach was found to have diuretic activity in mosquito Malpighian tubules [29] consistent with the wide distribution of kinins in insects. Leucokinin and other insect kinins are now known to stimulate fluid secretion in the house cricket [30], locust [31], corn earworm [32], fruit fly, [33] and housefly [34]. Kinins have been found in every order of the winged insects, which includes 97% of all insects.
The mechanism of action of kinins in Malpighian tubules of Aedes aegypti
Isolated distal segments of Malpighian tubules of the yellow fever mosquito spontaneously secrete fluid in vitro for hours. Secreted fluid is approximately isosmotic with the peritubular bathing Ringer, and Na+, K+, and Cl− are the major electrolytes in secreted fluid, accounting for most of the osmotic pressure [25]. The transepithelial voltage is lumen-positive by 59 mV, reflecting the active transepithelial secretion of cations in a secretory epithelium (Fig. 2A). The transepithelial resistance is 18.5 KΩcm, indicating a moderately tight epithelium [17, 25, 26]. When leucokinin-VIII is added to the peritubular medium, the transepithelial voltage quickly drops to 5 mV, and the transepithelial resistance plummets to 3.2 KΩcm (Fig. 2B). Low transepithelial voltages and resistances are the properties of so-called “leaky epithelia” with characteristically high rates of transporting salt and water. In contrast, high transepithelial voltages and resistance are the properties of so-called “tight epithelia” with characteristically low rates of transepithelial transport that reflect largely storage functions. Thus, Malpighian tubules in the yellow fever mosquito can function as both tight and leaky epithelia depending on instructions from extracellular and intracellular signals.
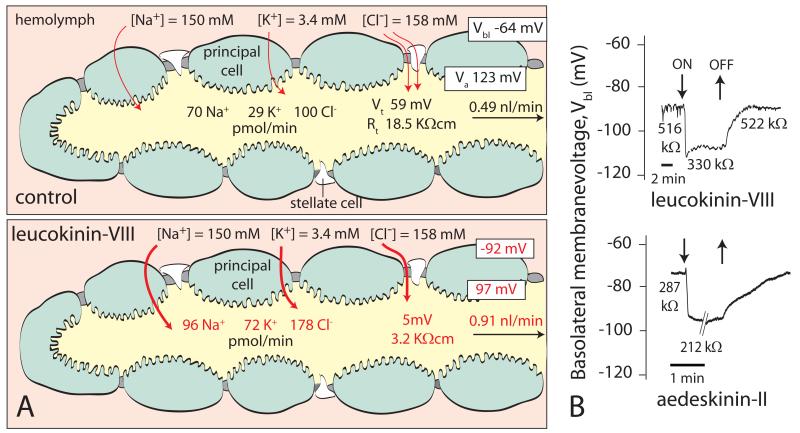
Figure 2.
The effect of insect kinins on isolated Malpighian tubules of the yellow fever mosquito Aedes aegypti. (A) Leucokinin-VIII, one of the kinins of Leucophaea, significantly increases the rate of fluid secretion by stimulating the transepithelial secretion of both NaCl and KCl. At the same time, leucokinin-VIII depolarizes the transepithelial voltage (Vt) and reduces the transepithelial resistance Rt, Vbl, and Va are respectively the basolateral membrane voltage and apical membrane voltage of a principal cell. Red letters indicate minimum statistical significance at P < 0.05. Data from [17]. (B) Time course of the on/off effects of leucokinin-VIII and aedeskinin-II, one of the kinins of Aedes aegypti. Principal cells of Malpighian tubules were studied by the methods of two-electrode-voltage-clamp [39]. Both kinins depolarize Vbl and decrease the cell-input resistance, reflecting in part the opening of Ca2+ channels in the basolateral membrane [19].
In Aedes Malpighian tubules, leucokinin-VIII significantly increases the transepithelial secretion of NaCl and KCl, and consequently water (Fig. 2A). The stimulation of both NaCl and KCl secretion together with the low values of the transepithelial voltage and resistance suggest that leucokinin-VIII opens the paracellular permeability to Cl−. Symmetrical transepithelial Cl− diffusion potentials in the presence of leucokinin-VIII confirm this hypothesis [16, 17]. Moreover, a large increase in the paracellular Cl− permeability is expected to produce an electrical short-circuit across the epithelium, which is confirmed by the drop of the transepithelial voltage and resistance to values close to zero in the presence of leucokinin (Fig. 2B).
Probing the nature of the paracellular Cl− conductance activated by insect kinins, we found that leucokinin not only increases the anion conductance but it also shifts the anion selectivity of the paracellular pathway [35]. Under control conditions, the paracellular pathway behaves like an aqueous pathway for anions with the selectivity sequence I− > Br− > Cl− > F−. In the presence of leucokinin-VIII, the paracellular pathway behaves like an anion channel with the selectivity sequence Br− > Cl− > I− > F− [35].
The presence of two natural diuretic agents in the yellow fever mosquito, MNP targeting Na+ and kinins targeting Cl− for excretion, suggests that both are released upon the enormous volume expansion of the blood meal. The potential for additive or synergistic effects is obvious. The release of both natriuretic and chloruretic agents during the blood meal is expected to lower the electrochemical potential against which Na+ must be transported across the epithelium, and reduce the resistance of the transport route taken by Cl−. At least additive effects are expected on thermodynamic grounds, but synergistic effects have been observed by others [36-38].
The discovery of insect kinins that target the paracellular pathway was all the more remarkable in that the transport pathway between epithelial cells had been considered rather stable and not subject to the rapid regulation of permeability. Moreover, rapid transitions between “tight” and “leaky” states, reflecting posttranslational mechanisms, had not been observed before in epithelia. It raised our curiosity about receptor-mediated signaling to the paracellular pathway and the molecular structure of the paracellular pathway.
Signaling to the paracellular pathway
The insect kinins, including leucokinin and aedeskinin, are considered multifunctional peptides with effects on metabolism, smooth muscle, and salt and water balance [18, 40, 41]. Their activities are attributed to a critical C-terminal pentapeptide (Fig. 3A). As shown in Figure 3B, the pentapeptide is thought to fold in order to reach binding sites in the receptor pocket [42].
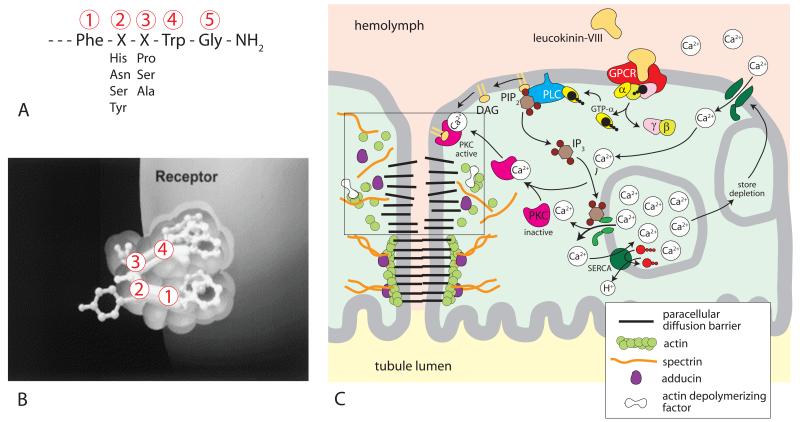
Figure 3.
Kinin signaling to the paracellular pathway of Aedes Malpighian tubules. (A) The active C-terminal amide pentapeptide sequence of insects kinins [68]. (B) Binding of insect kinin to its receptor. The critical C-terminal pentapeptide sequence is thought to fold for reaching into the receptor pocket [42, 69]. (C) G protein-coupled receptor signaling of leucokinin-VIII to septate junctions. Binding of leucokinin-VIII to its receptor increases intracellular Ca2+ concentrations. The Ca+2 activation of protein kinase C phosphorylates adducing, which destabilizes the cytoskeleton associated with the paracellular pathway. The destabilization opens paracellular diffusion barriers (box). GPCR, G protein coupled receptor; α, β, γ, subunits of G protein; GTP guanosine-5′-triphosphate; PLC, phospholipase C; PIP2, phosphatidylinositol (4,5)-triphosphate; IP3, inositol triphosphate; DAG, diacylglycerol; SERCA, sacra/endoplasmic reticulum Ca2+-ATPase.
In isolated Malpighian tubules of Aedes aegypti, leucokinin-VIII triggers diuretic activity when it is presented from the peritubular side and not the luminal side, indicating the presence of the kinin receptor on the hemolymph side of epithelial cells [17]. Physiological studies in our laboratory find the leucokinin receptor located to at least principal cells [43]. More recently, the laboratory of Pietrantonio found immunological evidence for the location of the receptor in stellate cells as well [44]. Regardless of the cellular localization of the kinin receptor, there is good agreement that insect kinin receptors are coupled to G proteins [42, 45-47]. In Aedes Malpighian tubules, high peritubular concentrations of fluoride duplicate the effects of leucokinin [35]. The effect is attributed to aluminum tetrafluoride AlF−4,which is thought to form in the presence of trace quantities of aluminum. AlF −4 is known to activate G proteins by mimicking the γ-phosphate of GTP [48-50].
Malpighian tubules of Drosophila, Aedes, and Anopheles express only one kinin receptor gene, which encodes a G protein-coupled receptor [46, 47, 51]. Stimulation of this single receptor activates more than one signaling pathway as indicated by the effects of synthetic derivatives of kinins [19]. For example, some kinin derivatives stimulate transepithelial fluid secretion in isolated Aedes Malpighian tubules but have no effect on tubule electrophysiology (voltage and resistance). Other derivatives have the opposite effect, and all three aedeskinins, the natural kinins of Aedes aegypti, significantly stimulate both fluid secretion and tubule electrophysiology [19]. The partial activation of a receptor is well known for G protein-coupled receptors as agonist-directed signaling, ligand-directed trafficking, conformation-specific agonism, or functional selectivity [52, 53].
There is good agreement that Ca2+ is one of the second messengers of insect kinins [35-38, 54-59]. Both intra- and extracellular Ca2+ are needed for leucokinin signaling in Malpighian tubules of Aedes aegypti as illustrated in the signaling model of Fig. 3C [56, 60]. In brief, the binding of leucokinin or aedeskinin [61] to the G protein-coupled receptor activates phospholipase C (Fig. 3B and 3C). One product of PLC activity is inositol triphosphate (IP3), which increases in the presence of aedeskinins, the kinins of Aedes aegypti [62]. IP3 is expected to release Ca2+ from intracellular stores. Store depletion in turn opens a nifedipine-sensitive Ca2+ channel in the basolateral membrane [56] which admits extracellular Ca2+ to the cytoplasm. Another product of PLC activity is membrane-resident diacylglycerol (DAG). Together, DAG and Ca2+ recruit cytosolic protein kinase C (PKC) to the plasma membrane, which activates the kinase (Fig. 3C). Two inhibitors of PKC, staurosporine and bisindolylmaleimide, block the effect of aedeskinin-III on tubule electrophysiology and on fluid secretion [63]. The blockade of the electrophysiological effects are significant in that they point to a role of PKC in the activation of the paracellular Cl− conductance by insect kinins.
To identify proteins that signal to the paracellular pathway, we undertook a proteomic analysis of the cytosol of Aedes Malpighian tubules. One set of tubules served as controls; the other set was treated with aedeskinin-III for only 1 min in order to observe the posttranslational effects of kinin stimulation on cytosolic proteins [64]. The amounts of actin depolymerizing factor, actin, adducing, and regucalcin significantly increase in the cytoplasm upon aedeskinin-III stimulation. The appearance of adducin in the cytoplasm as phospho-adducin is significant in that unphosphorylated adducin stabilizes actin and spectrin at sites of cell-cell contact, [65] and adducin (phosphorylated by PKC) destabilizes actin and spectrin [66, 67]. Accordingly, the appearance of phospho-adducin in the cytoplasm suggests the reorganization of the cytoskeleton associated with paracellular diffusion barriers. Figure 3C presents a hypothetical scheme where the destabilization of the cytoskeleton destabilizes unknown extracellular structures in the paracellular pathway with the effect of increasing the paracellular permeability.
Although hypothetical, the model in Figure 3 mirrors the well-known interaction of extracellular and intracellular proteins in epithelial junctions of vertebrates. Here, transmembrane proteins of the tight junction mediate cell adhesion, prevent indiscriminate paracellular permeation, and define the transport properties of the paracellular pathway. The transmembrane proteins bind to scaffolding (adapter) proteins on the cytoplasmic side of the junction. In turn, scaffolding proteins bind to actin and spectrin. The assembled protein complex stabilizes the junctions and consequently the paracellular structure and function. The junctional protein complex is assembled during embryogenesis when it plays a critical role in the development of epithelial polarity.
As links between transmembrane proteins and the cytoskeleton, scaffolding proteins of the tight junction, such as ZO-1, ZO-2, ZO-3, are the targets of regulatory proteins such as protein kinases and phosphatases, small GTPases and G proteins, cytoplasmic proteins, transcription factors, heat shock proteins, and even microRNA in vertebrate epithelia. These regulatory agents may further stabilize or destabilize the junction with obvious effects on the structure and function of the paracellular pathway. For example, the junctional complex can be disassembled entirely as in epithelial-mesenchymal transitions, or reorganized as part of the epithelial response to inflammation and infection, or radically changed in the development of epithelial tumors. In view of these dynamics observed in the vertebrate tight junctions, it seems that junctional proteins could also serve to mediate rapid, reversible, posttranslational changes in paracellular permeability [70, 71].
Tight and septate junctions
The paracellular pathway is delineated by tight junctions in vertebrate epithelia and by septate junctions in invertebrate epithelia (Fig. 4). In the past, both junctions were considered static and to serve barrier functions. Today, the dynamic nature of junctions is increasingly appreciated in: embryonic development, the maturation of epithelial cells from stem cells, the crypt-to-tip migration of epithelial cells in the mammalian intestine, epithelial-mesenchymal transitions, inflammatory responses, and the posttranslational regulation of paracellular permeability.
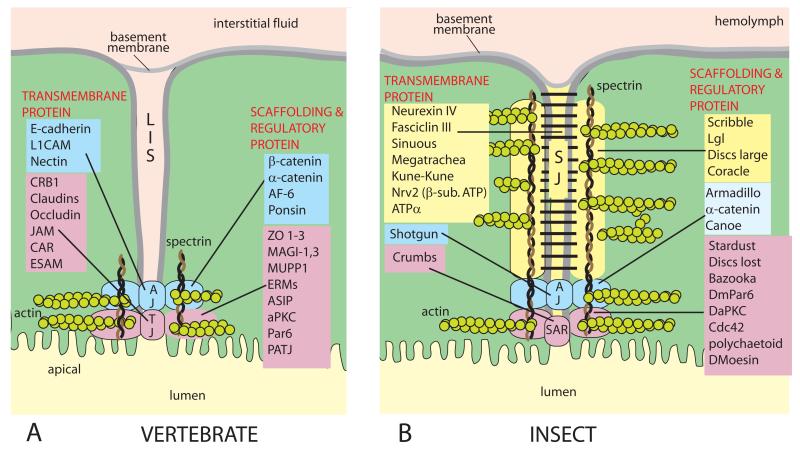
Figure 4.
The vertebrate tight junction (A) and the insect septate junction (B). A comparison between the molecular constituents of epithelial junctions in insects and vertebrates reveals a striking homology of proteins but remarkable organizational and anatomical differences [80]. The long paracellular cleft of the septa of the junction poses questions about functional roles. Gap junctions and desmosomes are omitted. Adapted from Refs. 75, 80, 88, and 89. AJ, adherens junction; LIS, lateral intercellular space; SAR, subapical region; SJ, septate junction; TJ, tight junction.
Tight junctions surround epithelial cells at their most apical region. They extend into the paracellular pathway for only a short distance, typically less than 1 μm (Fig. 4A). Tight junctions are formed by at least four integral membrane proteins claudins, occludins, tricellulins, and JAMs (junctional adhesion molecules) [72, 73]. Interactions between the extracellular loops of, for example, claudins, pull the outer membrane leaflets of adjoining cells so close to each other as to nearly fuse them [74, 75] and to form a barrier to the paracellular permeation of most solutes. Tight junctions can be quite leaky as in epithelia mediating isosmotic fluid transport, permselective to ions, and permeable to organic solutes [75-78].
In contrast to tight junctions, septate junctions of insects (and arthropods in general) occupy the more basal portion of the paracellular pathway for a considerable length (Fig. 4B). In Malpighian tubules of Aedes aegypti, the septate junction (SJ) spans the whole epithelium from apical to basal poles [18]. Rather than annealing the plasma membranes of neighboring cells, the septa of septate junctions appear to maintain a cleft of 15-20 nm between adjacent cells [79]. The septa present a ladder-like appearance in apico-basal sections where the rungs of the ladder may be regular and irregular. As illustrated in Figure 4B, adherens junctions (AJ) and/or a subapical region (SAR) have been proposed at the apical border of the insect paracellular pathway [75, 80]. The apico-basal SAR, AJ, SJ organization of the paracellular pathway stems from the observations of epithelial polarization during development [81-84], but it is not known to what extent this embryonic topology is maintained in the adult differentiated epithelium.
Septate junctions are also found in vertebrates at paranodal junctions between myelinating Schwann cells and axons [85], where they appear to function as a “fence” between excitable Na+ channels at the node (for saltatory conduction) and K+ channels in the internodes. The septal fence may preserve the axonal polarity of excitable and nonexcitable membrane stretches. In epithelia, the septate junction could play a similar role in fencing basal from apical membrane domains. In Malpighian tubules of Aedes aegypti the apical membrane forms a thick brush border, with each microvillus housing a mitochondrion [18]. The V-type H+ ATPase is exclusively and richly expressed at this membrane [9, 18]. The physiological activity of Kir channels is richly and exclusively found at the basal membrane [86]. Given such a strong separation of apical and basal membrane domains by a long septate junction, the question arises whether the septate junction maintains a third, lateral plasma membrane domain with specialized functions?
Clefts generated by septa-like structures are also observed at neuromuscular junctions and neuronal synapses in invertebrates [87]. Here the extracellular space serves the diffusion of neurotransmitters. To propose similar communicative interactions between epithelial cells baffles the imagination. A more conservative view has septa providing the structural stability for neuronal and epithelial cell contacts alike.
It is likely that the cleft in epithelial septate junctions is filled with an aqueous fluid, namely the extracellular fluid, similar to the fluid-filled lateral intercellular space of tight junctions (LIS, see Fig. 4A). However, in the case of septate junctions, a dense or regular spacing of septa would limit fluid in septal clefts and increase the electrical resistance of the paracellular pathway. Sparse and irregular septa would fill the paracellular space with conducting fluid and offer a low electrical paracellular resistance (Figs. 3C and 4). This model assigns a general barrier function to the septate junction and the selectivity filter to other domains such as AJ and SAR (Fig. 4B). Moreover, the model of variable septa density is consistent with the large reversible changes in the paracellular electrical resistance we observe in Aedes Malpighian tubules in the presence of leucokinin (Fig. 2). Measurements of specifically the paracellular electrical resistance reveal the remarkable drop from 18.6 KΩcm to 1.8 KΩcm in the presence of leucokinin [17]. Such a large resistance drop may reflect the opening of septa or a reduction in septa density. Moreover, an open septate junction would provide a low paracellular resistance from the hemolymph to the most apical junctions where the channel-like permselectivity sequence Br− > Cl− > I− > F− may be located [35].
Figure 4 illustrates the paracellular pathway between two principal cells, which may be up to 40 μm long spanning the epithelium from the tubule lumen to the hemolymph. The length of the paracellular pathway between a stellate cell and a principal cell is much shorter because stellate cells are only 1–3 μm deep [43]. It would appear that septa at these short sites of cell-to-cell contact are particularly well suited for profound and rapid changes in paracellular permeability.
In a recent study that sought to identify the proteins of the septate junction, the Furuse laboratory found a new a small tetraspan protein of 17 kDa in the midgut of Drosophila [90]. The protein called “snakeskin” is associated with the septate junction and is essential to the formation of the septate junction in the midgut of Drosophila. The lack of snakeskin is lethal during the larval stage. Importantly, in a snakeskin-deficient midgut epithelium, the septa are few, and the midgut is permeable to dextran. These studies establish a barrier function of septa in the Drosophila midgut, and the loss of this barrier when septa are lost. The expected electrical correlate of losing septa is the loss of paracellular electrical resistance. Whether snake skin also mediates rapid changes in septa density and paracellular resistance remains to be seen.
Of particular interest is that snakeskin is expressed in the Malpighian tubules as well as the midgut - namely two epithelia with a high dynamic range of epithelial transport. Here epithelial transport activity depends on the loads presented. A normal extracellular fluid volume and composition places as little work load on Malpighian tubules as an empty gut places on intestinal transport. The situation changes suddenly with the ingestion of a meal when transport mechanisms in the midgut (and Malpighian tubules) must be quickly revved up. Significantly, snakeskin is not found in epithelial cells of the hindgut [90] where transport rates are expected to be low. It would appear therefore that the expression of snakeskin in epithelia correlates well with the capacity for high rates of transport in general and with the presence of dynamic septa and paracellular functions in particular. The proteins that form the septa associated with snakeskin in Drosophila midgut are unknown as are those that mediate the dynamic resistance changes of the paracellular pathway in Aedes Malpighian tubules.
The reader who has come this far may wonder whether a dynamic paracellular pathway may be unique to gorging hematophagous insects and therefore irrelevant to other epithelia. On the contrary—the ability to commence a diuresis on short notice is also observed in phytophagous insects that gorge on the sap of plants [91]. Moreover, insects leaving their aquatic habitats with first flight undergo a so-called eclosion diuresis that lightens the load [92]. Thus, all insects appear to have a need of natural diuretic neuropeptides or hormones consistent with the wide distribution of insect kinins. Natural diuretic agents may be particularly well expressed in small animals, such as flies and bugs, where gorging meals can quickly increase body weight, up to 10 times in Rhodnius prolixus [93]. A dynamic paracellular pathway that can be activated quickly is therefore well suited for eliminating excess solute and water.
A dynamic paracellular pathway would also be well suited for extending the functional range of vertebrate epithelia. Secretory glands that produce tears, sweat, saliva and digestive secretions may operate for hours or days at modest rates, but then be suddenly called upon to produce fluids profusely. Under these conditions, a paracellular pathway responding to posttranslational modification would serve well the transport challenges in a timely manner.
Acknowledgments
The author thanks the National Science Foundation and the National Institutes of Health for supporting our work through Grants IBN 0078058 and R21 AI072102, respectively. Thank you also Peter Piermarini and Mikio Furuse for constructive comments.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Klowden MJ. Blood, sex and the mosquito. Bioscience. 1995:326–331. [Google Scholar]
- 2.Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow rate and composition of urine during the post-bloodmeal diuresis in Aedes aegypti (L.) J Comp Physiol [A] 1983;153:257–265. [Google Scholar]
- 3.Roitberg BD, Mondor EB, Tyerman JGA. Pouncing spider, flying mosquito: blood acquisition increases predation risk in mosqitoes. Behavioral Ecol. 2003;14:736–740. [Google Scholar]
- 4.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol (Oxf) 2011;202:387–407. doi: 10.1111/j.1748-1716.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piermarini PM, et al. A SCL4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): Evidence for a novel role of stellate cells in diuretic fluid secretion. Am J Physiol Regul Integr Comp Physiol. 2010;298:642–660. doi: 10.1152/ajpregu.00729.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piermarini PM, et al. Role of an apical K,Cl cotransporter in urine formation by renal tubules of the yellow fever mosquito (Aedes aegypti) Am J Physiol Regul Integr Comp Physiol. 2011;301:R1318–1337. doi: 10.1152/ajpregu.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosse C, et al. Inward rectifier K+ (Kir) channels in Malpighian tubules of the yellow fever mosquito Aedes aegypti; Presented at Ann. meeting of Soc. for Experimental Biology (SEB); Glasgow, Scotland. July 1-4, 2011.2011. [Google Scholar]
- 8.Patrick ML, et al. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol. 2006;209:4638–4651. doi: 10.1242/jeb.02551. [DOI] [PubMed] [Google Scholar]
- 9.Weng XH, et al. The V-type H+-ATPase in Malpighian tubules of Aedes aegypti: localization and activity. J. Exp. Biol. 2003;206:2211–2219. doi: 10.1242/jeb.00385. [DOI] [PubMed] [Google Scholar]
- 10.Okech BA, et al. Cationic pathway of pH regulation in larvae of Anopheles gambiae. J. Exp. Biol. 2008;211:957–968. doi: 10.1242/jeb.012021. [DOI] [PubMed] [Google Scholar]
- 11.Rheault MR, et al. Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J. Exp. Biol. 2007;210:3848–3861. doi: 10.1242/jeb.007872. [DOI] [PubMed] [Google Scholar]
- 12.Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci. 2001;16:145–151. doi: 10.1152/physiologyonline.2001.16.4.145. [DOI] [PubMed] [Google Scholar]
- 13.Pullikuth AK, et al. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J Exp Biol. 2006;209:3529–3544. doi: 10.1242/jeb.02419. [DOI] [PubMed] [Google Scholar]
- 14.Hegarty JL, et al. Dibutyryl cAMP activates bumetanide-sensitive electrolyte transport in Malpighian tubules. Am. J. Physiol. Cell Physiol. 1991;261:C521–C529. doi: 10.1152/ajpcell.1991.261.3.C521. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. J. Exp. Biol. 2001;204:367–378. doi: 10.1242/jeb.204.2.367. [DOI] [PubMed] [Google Scholar]
- 16.Beyenbach KW. Regulation of tight junction permeability with switch-like speed. Curr Opin Nephrol Hypertens. 2003;12:543–550. doi: 10.1097/00041552-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol. 1993;132:63–76. doi: 10.1007/BF00233052. [DOI] [PubMed] [Google Scholar]
- 18.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 19.Schepel SA, et al. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol. 2010;299:R612–622. doi: 10.1152/ajpregu.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petzel DH, Hagedorn HH, Beyenbach KW. Preliminary isolation of mosquito natriuretic factor. Am J Physiol. 1985;249:R379–386. doi: 10.1152/ajpregu.1985.249.4.R379. [DOI] [PubMed] [Google Scholar]
- 21.Wheelock GD, et al. Evidence for hormonal control of diuresis after a blood meal in the mosquito Aedes aegypti. Arch Insect Biochem Physiol. 1988;7:75–90. [Google Scholar]
- 22.Petzel DH, Berg MM, Beyenbach KW. Hormone-controlled cAMP-mediated fluid secretion in yellow-fever mosquito. Am J Physiol. 1987;253:R701–711. doi: 10.1152/ajpregu.1987.253.5.R701. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer DB, Beyenbach KW. Dibutyryl-cAMP increases basolateral sodium conductance of mosquito Malpighian tubules. Am. J. Physiol. 1985;248:R339–R345. doi: 10.1152/ajpregu.1985.248.3.R339. [DOI] [PubMed] [Google Scholar]
- 24.Coast GM, et al. Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles) J Exp Biol. 2005;208:3281–3291. doi: 10.1242/jeb.01760. [DOI] [PubMed] [Google Scholar]
- 25.Williams JC, Beyenbach KW. Differential effects of secretagogues on Na and K secretion in the Malpighian tubules of Aedes aegypti (L.) J. Comp. Physiol. 1983;149:511–517. [Google Scholar]
- 26.Williams JC, Beyenbach KW. Differential effects of secretagogues on the electrophysiology of the Malpighian tubules of the yellow fever mosquito. J. Comp. Physiol. [B] 1984;154:301–309. [Google Scholar]
- 27.Beyenbach KW, Petzel DH. Diuresis in mosquitoes: Role of a natriuretic factor. News Physiol. Sci. 1987;2:171–175. [Google Scholar]
- 28.Holman GM, Cook BJ, Nachman RJ. Isolation, primary structure and synthesis of leucokinin-VII and VIII: the final members of the new family of cephalomyotropic peptides isolated from head extracts of Leucophaea maderae. Comp. Biochem. Physiol. [C] 1987;88:31–34. [Google Scholar]
- 29.Hayes TK, et al. Leucokinins, a new family of ion transport stimulators and inhibitors in insect Malpighian tubules. Life Sci. 1989;44:1259–1266. doi: 10.1016/0024-3205(89)90362-7. [DOI] [PubMed] [Google Scholar]
- 30.Coast GM, Holman GM, Nachman RJ. The diuretic activity of a series of cephalomyotropic neuropeptides, the achetakinins, on isolated Malpighian tubules of the house cricket Acheta domesticus. J. Insect Physiol. 1990;36:481–488. [Google Scholar]
- 31.Thompson KS, et al. Cellular colocalization of diuretic peptides in locusts: a potent control mechanism. Peptides. 1995;16:95–104. doi: 10.1016/0196-9781(94)00158-3. [DOI] [PubMed] [Google Scholar]
- 32.Blackburn MB, et al. The isolation and identification of three diuretic kinins from the abdominal ventral nerve cord of adult Helicoverpa zea. J. Insect Physiol. 1995;41:723–730. [Google Scholar]
- 33.Terhzaz S, et al. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol. 1999;202:3667–3676. doi: 10.1242/jeb.202.24.3667. [DOI] [PubMed] [Google Scholar]
- 34.Iaboni A, et al. Immunocytochemical localisation and biological activity of diuretic peptides in the housefly, Musca domestica. Cell Tissue Res. 1998;294:549–560. doi: 10.1007/s004410051205. [DOI] [PubMed] [Google Scholar]
- 35.Yu M, Beyenbach KW. Leucokinin and the modulation of the shunt pathway in Malpighian tubules. J Insect Physiol. 2001;47:263–276. doi: 10.1016/s0022-1910(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 36.Coast GM. Synergism between diuretic peptides controlling ion and fluid transport in insect Malpighian tubules. Regul Pept. 1995;57:283–296. doi: 10.1016/0167-0115(95)00042-A. [DOI] [PubMed] [Google Scholar]
- 37.Maddrell SHP, et al. Synergism of hormones controlling epithelial fluid transport in an insect. J Exp Biol. 1993;174:65–80. [Google Scholar]
- 38.O’Donnell MJ, Spring JH. Modes of control of insect Malpighian tubules: Synergism, antagonism, cooperation and autonomous regulation. J. Insect Physiol. 2000;46:107–117. doi: 10.1016/s0022-1910(99)00119-5. [DOI] [PubMed] [Google Scholar]
- 39.Masia R, et al. Voltage clamping single cells in intact Malpighian tubules of mosquitoes. Am J Physiol. 2000;279:F747–F754. doi: 10.1152/ajprenal.2000.279.4.F747. [DOI] [PubMed] [Google Scholar]
- 40.Goldsworthy GJ, et al. In: Crampton JM, Eggleton P, editors. The structural and functional activity of neuropeptides; Royal Entomological Society Symposium on Insect Molecular Science; Academic Press. London. 1992.pp. 205–225. [Google Scholar]
- 41.Seinsche A, et al. Effect of helicokinins and ACE inhibitors on water balance and development of Heliothis virescens larvae. J. Insect Physiol. 2000;46:1423–1431. doi: 10.1016/s0022-1910(00)00065-2. [DOI] [PubMed] [Google Scholar]
- 42.Taneja-Bageshwar S, et al. Comparison of insect kinin analogs with cis-peptide bond, type VI-turn motifs identifies optimal stereochemistry for interaction with a recombinant arthropod kinin receptor from the southern cattle tick Boophilus microplus. Peptides. 2008;29:295–301. doi: 10.1016/j.peptides.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 43.Yu MJ, Beyenbach KW. Effects of leucokinin-VIII on Aedes Malpighian tubule segments lacking stellate cells. J Exp Biol. 2004;207:519–526. doi: 10.1242/jeb.00772. [DOI] [PubMed] [Google Scholar]
- 44.Lu HL, Kersch C, Pietrantonio PV. The kinin receptor is expressed in the Malpighian tubule stellate cells in the mosquito Aedes aegypti (L.): a new model needed to explain ion transport? Insect Biochem Mol Biol. 2011;41:135–140. doi: 10.1016/j.ibmb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes SP, et al. Functional analysis of a G protein-coupled receptor from the southern cattle tick Boophilus microplus (Acari: Ixodidae) identifies it as the first arthropod myokinin receptor. Insect Mol Biol. 2003;12:27–38. doi: 10.1046/j.1365-2583.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- 46.Pietrantonio PV, et al. The mosquito Aedes aegypti (L.) leucokinin receptor is a multiligand receptor for the three Aedes kinins. Insect Mol Biol. 2005;14:55–67. doi: 10.1111/j.1365-2583.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 47.Radford JC, et al. Functional characterisation of the Anopheles leucokinins and their cognate G-protein coupled receptor. J Exp Biol. 2004;207:4573–4586. doi: 10.1242/jeb.01317. [DOI] [PubMed] [Google Scholar]
- 48.Sternweis PC, Gilman AG. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982;79:4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bigay J, et al. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985;191:181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 50.Bigay J, et al. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. Embo J. 1987;6:2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radford JC, Davies SA, Dow JA. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem. 2002;277:38810–38817. doi: 10.1074/jbc.M203694200. [DOI] [PubMed] [Google Scholar]
- 52.Berg KA, Clarke WP. Development of functionally selective agonists as novel therapeutic agents. Drug Discov Today Ther Strateg. 2006;3:421–428. [Google Scholar]
- 53.Simmons MA. Functional selectivity, ligand-directed trafficking, conformation-specific agonism: what’s in a name? Mol Interv. 2005;5:154–157. doi: 10.1124/mi.5.3.4. [DOI] [PubMed] [Google Scholar]
- 54.Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol. 2003;206:3845–3856. doi: 10.1242/jeb.00639. [DOI] [PubMed] [Google Scholar]
- 55.Lu HL, et al. A calcium bioluminescence assay for functional analysis of mosquito (Aedes aegypti) and tick (Rhipicephalus microplus) G protein-coupled receptors. J Vis Exp. 2011;50:2732. doi: 10.3791/2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu MJ, Beyenbach KW. Leucokinin activates Ca2+-dependent signal pathway in principal cells of Aedes aegypti Malpighian tubules. Am J Physiol Renal Physiol. 2002;283:F499–508. doi: 10.1152/ajprenal.00041.2002. [DOI] [PubMed] [Google Scholar]
- 57.Holman GM, Nachman RJ, Coast GM. Isolation, characterization and biological activity of a diuretic myokinin neuropeptide from the housefly, Musca domestica. Peptides. 1999;20:1–10. doi: 10.1016/s0196-9781(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 58.Holtzhausen WD, Nicolson SW. Beetle diuretic peptides: the response of mealworm (Tenebrio molitor) Malpighian tubules to synthetic peptides, and cross-reactivity studies with a dung beetle (Onthophagus gazella) J Insect Physiol. 2007;53:361–369. doi: 10.1016/j.jinsphys.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Te Brugge VA, Schooley DA, Orchard I. The biological activity of diuretic factors in Rhodnius prolixus. Peptides. 2002;23:671–681. doi: 10.1016/s0196-9781(01)00661-1. [DOI] [PubMed] [Google Scholar]
- 60.Clark TM, et al. The concentration-dependence of CRF-like diuretic peptide: mechanisms of action. J Exp Biol. 1998;201:1753–1762. doi: 10.1242/jeb.201.11.1753. [DOI] [PubMed] [Google Scholar]
- 61.Schepel SA, et al. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cady C, Hagedorn HH. Effects of putative diuretic factors on intracellular second messenger levels in the Malpighian tubules of Aedes aegypti. J Insect Physiol. 1999;45:327–337. doi: 10.1016/s0022-1910(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 63.Miyauchi JT, et al. The role of adducin in the diuresis triggered by aedeskinin III in Malpighian tubules of the yellow fever mosquito; Glasgow, Scotland. July 1-4, 2011.2011. [Google Scholar]
- 64.Beyenbach KW, et al. Signaling to the apical membrane and to the paracellular pathway: changes in the cytosolic proteome of Aedes Malpighian tubules. J Exp Biol. 2009;212:329–340. doi: 10.1242/jeb.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaiser HW, et al. Localization of adducin in epidermis. J Invest Dermatol. 1993;101:783–788. doi: 10.1111/1523-1747.ep12371695. [DOI] [PubMed] [Google Scholar]
- 66.Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taneja-Bageshwar S, et al. Biostable agonists that match or exceed activity of native insect kinins on recombinant arthropod GPCRs. Gen Comp Endocrinol. 2009;162:122–128. doi: 10.1016/j.ygcen.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Nachman RJ, Pietrantonio PV. Interaction of mimetic analogs of insect kinin neuropeptides with arthropod receptors. Adv Exp Med Biol. 2010;692:27–48. doi: 10.1007/978-1-4419-6902-6_3. [DOI] [PubMed] [Google Scholar]
- 70.Duffey ME, et al. Regulation of epithelial tight junction permeability by cyclic AMP. Nature. 1981;294:451–453. doi: 10.1038/294451a0. [DOI] [PubMed] [Google Scholar]
- 71.Bentzel CJ, et al. Cytoplasmic regulation of tight-junction permeability: effect of plant cytokinins. Am J Physiol. 1980;239:C75–89. doi: 10.1152/ajpcell.1980.239.3.C75. [DOI] [PubMed] [Google Scholar]
- 72.Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diamond JM. Tight and leaky junctions of epithelia: a perspective on kisses in the dark. Fed. Proc. 1974;33:2220–2224. [PubMed] [Google Scholar]
- 75.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Diamond JM. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- 77.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 78.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology. 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 79.Lane NJ, et al. Electron microscopic structure and evolution of epithelial junctions. In: Citi S, editor. Molecular Mechanisms of Epithelial Cell Junctions: From Development to Disease. R.G. Landes; Austin, Texas: 1994. pp. 23–43. [Google Scholar]
- 80.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 81.Tepass U, et al. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 82.Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 83.Wu VM, et al. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson KS, Beitel GJ. Cell junctions: lessons from a broken heart. Curr Biol. 2009;19:R122–123. doi: 10.1016/j.cub.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Hortsch M, Margolis B. Septate and paranodal junctions: kissing cousins. Trends Cell Biol. 2003;13:557–561. doi: 10.1016/j.tcb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Beyenbach KW, Masia R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J Insect Physiol. 2002;48:375–386. doi: 10.1016/s0022-1910(02)00057-4. [DOI] [PubMed] [Google Scholar]
- 87.Prokop A. Integrating bits and pieces: synapse structure and formation in Drosophila embryos. Cell Tissue Res. 1999;297:169–186. doi: 10.1007/s004410051345. [DOI] [PubMed] [Google Scholar]
- 88.Nelson KS, Furuse M, Beitel GJ. The Drosophila claudin kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010;185:831–839. doi: 10.1534/genetics.110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wedlich D. The polarising role of cell adhesion molecules in early development. Curr Opin Cell Biol. 2002;14:563–568. doi: 10.1016/s0955-0674(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 90.Yanagihashi Y, et al. A novel smooth septate junction-associated membrane protein, Snakeskin, is required for intestinal barrier function in Drosophila. J. Cell Sci. 2010 doi: 10.1242/jcs.096800. in press. [DOI] [PubMed] [Google Scholar]
- 91.Bushman DW, Raina AK, Nelson JO. Post-eclosion diuresis in adult Heliothis zea. Physiol. Entomology. 1989;14:391–396. [Google Scholar]
- 92.Coast GM. Neuropeptides implicated in the control of diuresis in insects. Peptides. 1996;17:327–336. doi: 10.1016/0196-9781(95)02096-9. [DOI] [PubMed] [Google Scholar]
- 93.Orchard I. Serotonin: a coordinator of feeding-related physiological events in the blood-gorging bug, Rhodnius prolixus. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:316–324. doi: 10.1016/j.cbpa.2005.11.010. [DOI] [PubMed] [Google Scholar]