Abstract
Since the first tissue-engineered vascular graft (TEVG) was implanted in a child over a decade ago, growth in the field of vascular tissue engineering has been driven by clinical demand for improved vascular prostheses with performance and durability similar to an autologous blood vessel. Great strides were made in pediatric congenital heart surgery using the classical tissue engineering paradigm, and cell seeding of scaffolds in vitro remained the cornerstone of neotissue formation. Our second-generation bone marrow cell-seeded TEVG diverged from tissue engineering dogma with a design that induces the recipient to regenerate vascular tissue in situ. New insights suggest that neovessel development is guided by cell signals derived from both seeded cells and host inflammatory cells that infiltrate the graft. The identification of these signals and the regulatory interactions that influence cell migration, phenotype and extracellular matrix deposition during TEVG remodeling are yielding a next-generation TEVG engineered to guide neotissue regeneration without the use of seeded cells. These developments represent steady progress towards our goal of an off-the-shelf tissue-engineered vascular conduit for pediatric congenital heart surgery.
Keywords: congenital heart surgery, drug-eluting scaffold, tissue-engineered vascular graft, tissue engineering, vascular graft
Single ventricle cardiac anomalies: a significant health problem
From the beginning, our efforts at translating tissue engineering technology from bench to bedside have focused on treatments for pediatric congenital heart disease (CHD). There are currently no manmade vascular conduits with growth potential, making the tissue-engineered vascular graft (TEVG) a novel technology. Great numbers of patients, including those requiring grafts for dialysis angioaccess, coronary artery bypass graft or peripheral arterial bypass surgery, stand to benefit from improvements in vascular graft technology. However, the TEVG, with its inherent capacity for somatic growth, is uniquely suited to the treatment of pediatric CHD. It is in this population that the TEVG could have the greatest impact.
According to the American Heart Association (AHA), congenital cardiovascular defects are present in approximately 1% of live births, making CHD the most common congenital malformation of newborns. CHD is the leading cause of death in the first year of life, and nearly twice as many children die from CHD in the USA each year as do from all forms of childhood cancers combined [101]. Approximately half of all infants born with CHD require surgical intervention to establish cardiovascular continuity that can support growth and prevent development of arrhythmias, developmental disorders, congestive heart failure and early death [101].
CHD can be categorized as mild, moderate or severe [1]. Between 1990 and 2000, an estimated 200,000 infants were born with severe CHD in the USA [2]. Single ventricle cardiac anomalies, a diverse group of structural anomalies that includes tricuspid atresia, pulmonary atresia and hypoplastic left heart syndrome, represent the most severe form of CHD. Surgical reconstruction of single ventricle physiology re-establishes division between pulmonary and systemic circulations, prevents premature heart failure and is essential for long-term survival [3,4]. This intervention can be accomplished in a series of staged operations referred to as the Fontan operation [5].
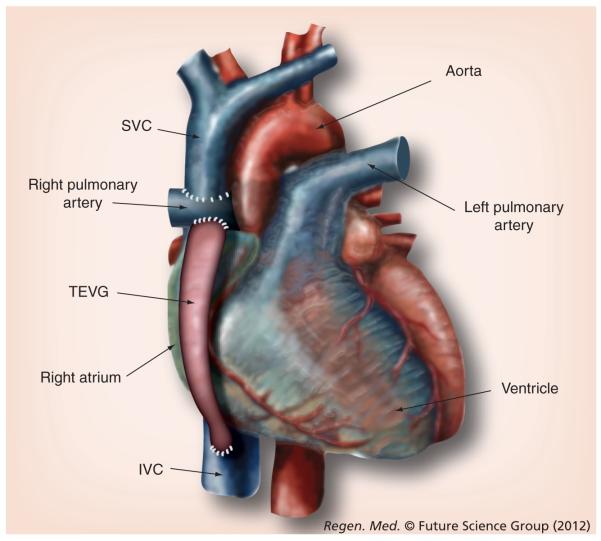
In the Fontan operation, venous return to the heart is routed directly to the pulmonary artery (PA), allowing deoxygenated blood to flow passively through the pulmonary circulation (Figure 1). Reoxygenated blood then returns to the single functional ventricle, where it is pumped through the systemic circulation and delivers oxygen. In the first stage of the Fontan operation (called the hemi-Fontan or Glenn operation), the superior vena cava (the great vein that returns deoxygenated blood from the upper half of the body) is directly connected (anastomosed) to the PA. In the second stage of the Fontan operation (completion Fontan, modified Fontan or extracardiac total cavopulmonary connection [EC TCPC]) the inferior vena cava (IVC), the great vein that returns deoxygenated blood from the lower half of the body, is connected to the PA. This is accomplished by placing a vascular conduit between the vena cavae and the pulmonary circulation (Figure 1).
Figure 1. Tissue-engineered vascular graft as an extracardiac total cavopulmonary connection in the modified Fontan operation.
In the treatment of single ventricle physiology, the two-stage modified Fontan diverges from the classic Glenn operation by isolating the right atrium from venous return. Venous blood is passively delivered to the right pulmonary artery. In the first stage of the operaton, the SVC is anastomed to the right pulmonary artery. After the second stage, a vascular conduit such as a TEVG connects the IVC to the right pulmonary artery. IVC: Inferior vena cava; SVC: Superior vena cava; TEVG: Tissue-engineered vascular graft.
Currently employed vascular conduits: a limiting factor in the treatment of severe CHD
The Fontan procedure was developed in the 1970s as a treatment for patients with single ventricle physiology. In the original operation, the atrium was directly connected to the PA [5,6]. The flow of blood from the IVC to the atrium and then to the PA resulted in loss of laminar flow and development of turbulence due to the size mismatch between the IVC and atrium. Since the pulmonary circulation is passive after Fontan surgery, loss of laminar flow significantly decreased the pulmonary circulation, thereby limiting its effectiveness. The original Fontan operation was associated with unacceptably high rates of morbidity and mortality [7], which encouraged further development and modification of the procedure. The modified Fontan that is most commonly performed uses an extracardiac conduit to channel blood from the IVC directly to the right PA. This EC TCPC bypasses the right atrium, thus maintaining laminar flow and increasing the flow of blood through the pulmonary circulation [8]. Outcomes are vastly improved compared with the original procedure; however, complications arising from the use of synthetic vascular grafts are a leading source of morbidity and mortality [9].
No existing vascular graft is an ideal conduit for the EC TCPC Fontan procedure. Polytetrafluoroethylene (PTFE or Gore-Tex™ [Gore-Tex Stretch Vascular Graft, WL Gore and Associates Inc., AZ, USA]) conduits, which have a lower failure rate than Dacron (Meadox woven double velour, Meadox Medicals Inc., NJ, USA) conduits [10], are currently the most widely used vascular grafts for this operation [11]. Autografts have also been used as EC TCPC conduits, but to a much more limited extent than PTFE [12]. Due to the anticipated growth of the child, oversized grafts are often needed to avoid outgrowing the conduit. When possible, surgery is delayed until the patient is between 2 and 4 years of age, at which time the diameter of the pediatric IVC approaches 60–80% of the adult IVC. This delay permits the placement of a near adult-sized conduit (20–22 mm) and limits the need for conduit replacement due to somatic growth alone. However, graft oversizing is associated with an increased risk of complications such as thrombus formation due to flow abnormalities [13]. Delaying surgery to minimize the number of reoperations can also precipitate cardiac dysfunction and heart failure from prolonged exposure to volume overload and chronic hypoxia [12,14]. Recent studies have demonstrated marked improvements in somatic growth in patients who undergo surgery at an earlier age, thus providing further support for early intervention with use of EC TCPC [15]. These findings illustrate the desirability of early intervention with an expansile conduit that can accommodate the somatic growth of the IVC.
Ultimately, thrombosis is the leading cause of graft failure and conduit replacement in the early postoperative period for synthetic conduits. The incidence of this mode of failure and conduit replacement operations could be reduced by matching graft diameter to the patient’s IVC [10]. Notably, there is a small group of patients that can benefit from the use of autologous tissue for EC TCPC. However, this is only possible in those patients with either sufficient vascular tissue to make a direct end-to-end connection between the vena cavae and the PA [16] or with sufficient pericardium to bridge these circuits with a pericardial roll [17]. Use of autologous tissue offers superior outcomes compared with synthetic grafts [18], but the vast majority of patients are not amenable to such reconstruction [19]. These patients would benefit from a vascular graft that is both thromboresistant and capable of growing with the patient.
Tissue engineering offers an additional strategy for constructing an autologous graft for use in patients undergoing the modified Fontan procedure. The advantages of a TEVG include decreased thrombogenicity and the ability to grow, remodel and rebuild. For more than a decade, our laboratory has been dedicated to the design, study and optimization of the TEVG. Using the classic tissue engineering paradigm of cells and scaffold [20], we have developed a TEVG for use in pediatric cardiothoracic applications that is superior to all other available synthetic vascular conduits.
Evolution of the tissue-engineered vascular conduit: animal studies demonstrating the feasibility of large-caliber TEVGs for low-pressure, high-flow applications & our first clinical trial
We were the first to describe the successful use of a TEVG as a PA interposition graft using tissue engineering methodology [21]. Using a lamb model, we seeded either autologous arterial or venous cells onto a biodegradable scaffold of tubularized polyglactin-woven mesh sealed with nonwoven polyglycolic acid (PGA) mesh. Cells were obtained by explanting segments of autologous artery or vein, expanding the resulting mixed cell population in culture and separating the cultures into endothelial-rich and endothelial-deficient fractions. The latter was seeded onto the scaffold and maintained in culture for 1 week. After this initial culture, the endothelial-rich fraction was seeded onto the inner lumen and maintained for an additional day. Seeded or unseeded constructs were implanted as interposition grafts replacing 2-cm sections of the main PA in juvenile lambs (n = 8). No anticoagulation therapy was used. The tissue engineered pulmonary conduits were serially monitored using echocardiography and angiography and were harvested 6 months after implantation. All seeded scaffolds were patent and demonstrated nonaneurysmal increases in size, while unseeded scaffolds formed thrombi and stenosed within 2 weeks of implantation. Within 11 weeks, no scaffold material remained in the TEVG. Histologically, grafts showed significant collagen content (73.9% of native IVC), medial elastic fiber production and luminal endothelial-specific factor VIII-related antigen and, in addition, showed evidence of remodeling and no macroscopic calcifications. We concluded that living vascular grafts that functioned well as a PA replacements could be engineered according to classical tissue engineering techniques that TEVGs demonstrated nonaneurysmal growth in diameter and that grafts possessed an endothelial lining and overall morphology resembling native PA [21].
In 2001, we reported our findings using a new biodegradable scaffold composed of a 50:50 copolymer of l-lactide and ε-caprolactone reinforced with nonwoven PGA fiber fabric (poly[chitosan-g-lactic acid]/PGA). Mixed cell populations obtained from explanted segments of saphenous vein were expanded in culture, seeded onto the lumen of the scaffold, and maintained in culture for 1 week before implantation as intrathoracic IVC interposition grafts in a dog model (n = 4). The TEVGs were harvested over a 6-month time course and showed no evidence of stenosis, dilatation or thromboembolic complications, even without anticoagulation therapy. Immunohistochemical staining confirmed the presence of a factor VIII antigen-positive endothelial monolayer lining the graft lumen, α-actin and desmin-expressing smooth muscle cells (SMCs) in the graft wall and a robust extracellular matrix with both collagen and elastin fibers [22].
Using this methodology, we performed the first successful clinical application of a TEVG in 1999 [23]. A 2-cm thrombosed segment of a PA was replaced with a TEVG in a 4-year-old girl who had undergone a PA angioplasty and Fontan procedure at 3 years of age. The graft was created from autologous cells obtained from a 2-cm segment of explanted peripheral vein. Vascular cells were isolated from the explant according to our established protocol [24], expanded in culture for 8 weeks and statically seeded onto a tubular scaffold fabricated from a 50:50 copolymer of ε-polycaprolactone–polylactic acid reinforced with woven PGA fibers. The biodegradable polymer conduit measured 10 mm in diameter, 20 mm in length and 1 mm in thickness and was designed to degrade over an 8-week period. A total of 10 days after seeding, the graft was implanted. The patient was maintained on intravenous heparin in the early postoperative period, followed by 5 mg/kg aspirin and warfarin for at least 3 months. No postoperative complications occurred, and on follow-up angiography, the graft was noted to be completely patent. A total of 7 months after implantation, the patient was doing well, without evidence of graft occlusion or aneurysmal changes on chest radiography. While this investigation clearly demonstrated the feasibility of using TEVGs in congenital heart surgery, the ultimate clinical utility of this technology was limited by the prolonged period of time required to expand the cells in culture. Therefore, we began to explore the potential of using alternative cell types and cell sources for constructing TEVGs.
Prior studies by other investigators had demonstrated that vascular grafts seeded with bone marrow cells (BMCs) formed an endothelial monolayer and that autocrine signals released by seeded BMCs contribute to endothelialization and vasculogenesis [25]. In particular, a subset of BMCs described as endothelial progenitor cells hone to sites of neovascularization and differentiate into endothelial cells (ECs) [26]. Rapid endothelization is desirable because healthy ECs reduce the immediate thrombogenicity and immunogenicity of the TEVG and prevent early graft occlusion [27]. Based on these observations, we investigated the use of BMCs as a potential cell source for vascular tissue engineering. In 2003, we described a large animal study evaluating the use of autologous BMCs enriched for the mononuclear fraction (BM-MNCs) to create a TEVG using our poly(chitosan-g-lactic acid)/PGA matrix [28]. Approximately 1000 autologous BM-MNCs/mm2 were statically seeded onto the luminal surface of the scaffold and maintained in culture for 2 h to allow for cell attachment. The seeded constructs were then implanted as interposition grafts replacing the intrathoracic IVC in an adult beagle model (n = 16) and harvested over a 2-year time course. All seeded grafts were patent without evidence of thrombosis (no anticoagulation was used), stenosis or aneurysm formation. Immunohistochemical analysis demonstrated that the seeded BM-MNCs adhered to the scaffold before implantation. Upon explantation, we observed expression of endothelial markers in the scaffold such as CD146, factor VIII, von Willebrand factor (vWF) and CD31, and smooth muscle cell markers such as smooth muscle cell-specific α-actin, SMemb, SM1 and SM2. VEGF and Ang-1 were also produced by the tissue engineered grafts. We concluded that autologous BM-MNCs could be used as a practical cell source for creating TEVGs, enabling rapid cell harvest without the need for cell culture, and we observed that the BM-MNCs promoted organized neotissue formation [28].
We had particular interest in using autologous BM-MNCs as our cell source because of the intrinsic difficulties associated with using cells derived from explanted venous tissue. Harvesting venous cells requires a more invasive operative procedure with its own inherent risks [29]. Explantation is not always successful and sometimes fails to produce cells for culture [30], and the ability to harvest cells can be affected by the patient’s age or underlying medical conditions [31]. The expansion of cells in culture is time-consuming (in our experience requiring 8–12 weeks), which limits the availability and clinical utility of the TEVGs. Finally, growth in culture exposes cells to various environmental factors including media, serum and environmental pathogens, which increases the risk of contamination and even cellular dedifferentiation [30]. These factors justified our investigation into the use of BM-MNCs as a cell source for our TEVG. On the basis of our animal studies, we adapted the use of autologous BM-MNCs for the creation of TEVGs for clinical use. Importantly, the abundance of BM-MNCs available from a single harvest eliminated the need for cell expansion and permitted rapid construction of an autologous TEVG that was ready for implantation on the same day as cell harvest. While not strictly ‘off-the-shelf’, the BMC-seeded graft represented a leap forward in clinical utility.
Along with our investigation into this new cell source for the TEVG, we fabricated a second scaffold designed to degrade more slowly than the PGA-based scaffold. The rationale for this modification was to design a scaffold that would provide structural support for a longer period of time and might allow greater neotissue formation and development. Although the degradation process is unchanged, a polylactic acid-based matrix degrades over a 2-year time frame compared with 2 months for PGA [28].
Based on our animal experiments using BM-MNC-seeded scaffolds, we initiated a clinical trial in 2001 evaluating the use of TEVGs and patches for congenital heart operations at the Woman’s Medical University in Tokyo, Japan [23,28,30,32]. In 2005, we presented our midterm clinical results of TEVGs seeded for these operations performed between 2001 and 2004 [30]. In these operations, we obtained 4–5 ml/kg BMCs, enriched for the mononuclear cell populations, and seeded by manual pipetting onto poly-l-lactic acid/ poly(ε-caprolactone/l-lactide) or polyglycolic acid/ poly(ε-caprolactone/l-lactide) scaffolds. Seeded scaffolds were cultured for 2–4 h intraoperatively and then implanted. Twenty-three patients had a tube graft as an EC TCPC implant, while the other 19 patients had a sheet-type patch used for repair of congenital cardiac defects. Inclusion criteria for patients for this procedure were elective surgery, age younger than 30 years and good quality of other organ function. All patients underwent a catheterization study, CT scan or both after the operation. The patients received 3–6 months of anticoagulation therapy, consisting of warfarin sodium and aspirin, with international normalized ratio maintained between 1.5 and 2.0. After 6 months and for another 12 months, patients were maintained on aspirin alone. At midterm followup after surgery (average follow-up: 1.3 years), there were no complications using TEVG such as thrombosis, stenosis or obstruction. All tube grafts were patent, and the diameter of the tube graft increased with time (110 ± 7% of the implanted size). One late death at 3 months after EC TCPC occurred in a patient with hypoplastic left heart syndrome, although this was unrelated to TEVG function. There was no evidence of aneurysm formation or calcification on cineangiography or CT at the midterm analysis.
Late-term analysis (average follow-up: 5.8 years) similarly demonstrated no cases of graft-related mortality, aneurysmal dilatation, graft infection, graft rupture or ectopic calcification. By contrast with Dacron and PTFE graft recipients who typically remain on long-term anticoagulation therapy [10,33,34], 96% of patients were able to discontinue anticoagulation therapy 6 months after the TEVG operation. Three patients with known cardiovascular anomalies died of causes unrelated to TEVG failure during the late-term analysis period. Notably, surveillance imaging in the months prior to death demonstrated patent TEVGs in all four deceased trial participants [35].
Further translation of this work was limited by our understanding of the processes involved in transforming a TEVG scaffold into a functional neovessel. We returned to animal models to characterize these processes and to validate the TEVG in an expanded range of applications. Early efforts to optimize BM-MNC seeding methods yielded standardized protocols for cell concentration and culture time [36], as well as an operator-independent vacuum seeding technique that replaces manual pipette-based seeding and achieves a far more consistent seeding pattern [37]. These standardizations and the validation thereof in large animal models [36] paved the way for the first US FDA-sanctioned clinical trial of a TEVG for a pediatric cardiothoracic application in the USA (Figure 2)[102].
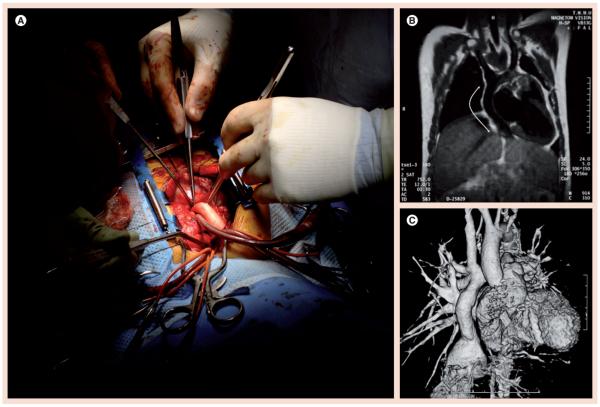
Figure 2. Modified Fontan operation with tissue-engineered vascular graft as an extracardiac total cavopulmonary connection graft in pediatric patients with congenital heart defects.
(A) The tissue-engineered vascular graft was seeded with bone marrow-derived mononuclear cells harvested from the patient at the start of the operation. (B) MRI 9 months after implantation showing patent tissue-engineered vascular graft (arrows). (C) Computed tomography angiogram of the graft shown in (B) 1 year after implantation. (B & C) Reproduced with permission from [30].
Next-generation TEVG: investigating the molecular mechanisms of neotissue formation
In the Japanese clinical trial and consistently in our animal models, the primary graft-related complication is late asymptomatic stenosis observed 6 months after implantation [35,36]. Graft stenosis in our human trials has been discovered on surveillance imaging in 16% of patients and managed pre-emptively by percutaneous angioplasty. Mural thrombi are infrequent causes of postoperative graft failure and are reversible if managed appropriately by anticoagulation therapy. Aneurysm formation, graft rupture, graft infection or ectopic calcification have not been observed.
The observation of graft stenosis led us to investigate in detail the cellular and molecular mechanisms of neotissue formation in our TEVG, as well as the mechanisms of aberrant signaling that precipitate TEVG stenosis. In 2008, we described the development of mouse models of aortic and IVC replacement [38]. A dual-chamber molding system allowed us to reproducibly fabricate PGA-P(CL/LA) and PLLA-P(CL/LA) scaffolds with a sub-1-mm internal diameter, which is well below the lower size limit of clinical utility for Dacron and PTFE vascular prostheses with or without cell seeding [39]. We implanted unseeded TEVG scaffolds as infrarenal aortic or IVC interposition grafts in SCID/bg mice without the use of peri- or post-operative anticoagulation or antiplatelet therapy. Grafts were explanted at week 3 and later demonstrated typical foreign body innate immune reactions to the scaffold [40], with predominant macrophage infiltration and foreign body giant cell formation. Explanted grafts were completely infiltrated with cells and extracellular matrix organized in a prototypical three-layer vascular architecture with vWF+ endothelization at the luminal surface, a mural α-SMA-expressing media layer and collagenous adventitia [38].
We then implanted small-diameter PLLA-P(CL/LA) scaffolds seeded with terminally differentiated human vascular cells in immunodeficient mice to characterize the mechanisms by which stenotic failure occurs in our tissue-engineered constructs. These studies illuminated the influence of cell seeding on the development of TEVG stenosis. Human aortic smooth muscle cells (hASMCs) and human aortic ECs (hAECs) were labeled with ultrasmall superparamagnetic iron oxide nanoparticles to enable magnetic resonance cell tracking. Near-total loss of seeded hASMCs and hAECs from PLLA-P(CL/LA) scaffolds was observed over 3 weeks in a SCID/bg aortic interposition model [41]. Long-term results of PLLA-P(CL/LA) TEVG scaffolds seeded with unlabeled hASMCs and hAECs in the SCID/bg aortic interposition model demonstrated that the natural history of TEVG development occurs due to rapid mural infiltration by macrophages, late endothelialization, initial dilation of the grafts with PLLA fiber degradation and late contraction, increased collagen deposition with time in vivo and increasingly organized tissue architecture approaching that of the native vessel over the course of 1 year (Figure 3) [42]. This chimeric model began to elucidate the process of TEVG maturation as an aortic interposition graft, but required the harvest and ex vivo culture of differentiated cells that Shin’oka and colleagues sought to avoid in the first clinical trial [23]. Additionally, the course of dilation and contraction seen in the mouse aortic model has not been observed in our venous studies in mice, sheep or humans.
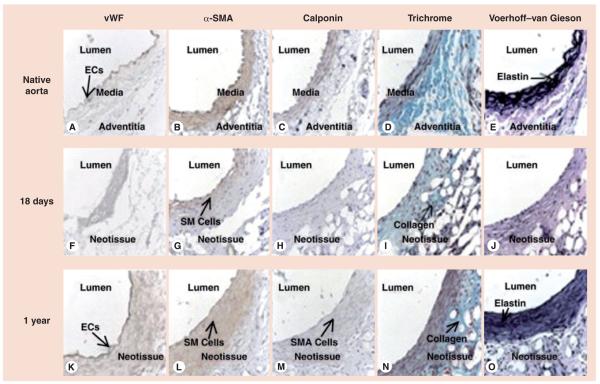
Figure 3. Natural history of tissue-engineered vascular graft remodeling by histologic evaluation of the native mouse aorta and tissue-engineered vascular graft at 18 days and 1 year postimplantation.
Native mouse aorta stained with (A) vWF, (B) α-SMA, (C) calponin, (D) Gomori one-step trichrome and (E) Voerhoff–van Gieson. Graft at 18 days postimplantation stained with (F) vWF, (G) α-SMA, (H) calponin, (I) Gomori one-step trichrome and (J) Voerhoff–van Gieson shows formation of luminal endothelial and mural SM cell layers. Graft at 1 year postimplantation stained with (K) vWF, (L) α-SMA, (M) calponin, (N) Gomori one-step trichrome and (O) Voerhoff–van Gieson shows organized trilayer architecture of native aorta (A–E). Original magnification: ×400.
EC: Endothelial cell; SM: Smooth muscle; SMA: Smooth muscle actin; vWF: von Willebrand factor.
Reproduced with permission from [42].
Meanwhile, the cellular and molecular mechanisms that underpinned the success of the human BM-MNC (hBM-MNC)-seeded scaffolds in the human trial remained unidentified and unexplained. MCP-1, a chemokine involved in monocyte recruitment and angiogenesis, was posited to play a role in graft development [43], so we adapted the small-diameter TEVG IVC mouse model to investigate the role of monocytes and monocyte chemotactic factors in TEVG maturation. In a second chimeric model, PLLA-P(CL/LA) scaffolds seeded with CD14+/CD45+ monocytes isolated from hBM-MNCs were implanted in SCID/bg mice and demonstrated less intimal hyperplasia and were less likely to stenose at 6 months than scaffolds seeded with unfractionated hBM-MNCs or hBM-MNCs excluding monocytes [44]. These results implicated a role for monocytes in maintaining long-term TEVG patency.
CD34+ hematopoietic stem cells and other potential vascular progenitor cells had been hypothesized to differentiate into the ECs and SMCs of the mature TEVG [28,45]. The monocyte fraction used in the above cell-seeding experiment excluded human CD34+ hematopoietic stem cells and other potential vascular progenitor cells, yet monocyte-seeded scaffolds gave rise to functional neovessels replete with all vascular lineages (Figure 4) [44]. Immunohistochemical analysis in the chimeric model demonstrated that the ECs and SMCs were of murine origin and that seeded hBM-MNCs did not directly contribute to TEVG neotissue. Moreover, seeded human cells were completely absent from the graft 1 week after implantation. Despite the rapid departure of these cells, hBM-MNC seeding was associated with significantly higher levels of MCP-1 production and enhanced early monocyte recruitment to the scaffold. We investigated the role of MCP-1 in neotissue formation by locally releasing this chemokine from the scaffold. Scaffolds were seeded with MCP-1-releasing alginate microspheres to mimic the presence and action of hBM-MNCs. Local MCP-1 delivery accelerated macrophage infiltration and produced organized neotissue formation similar to hBM-MNC seeding. These experiments suggested that seeded cells enhance patency by releasing cytokines that attract host monocytes to the graft and, more generally, provided the first evidence that seeded cells act in a paracrine fashion to recruit host cells to the TEVG [46].
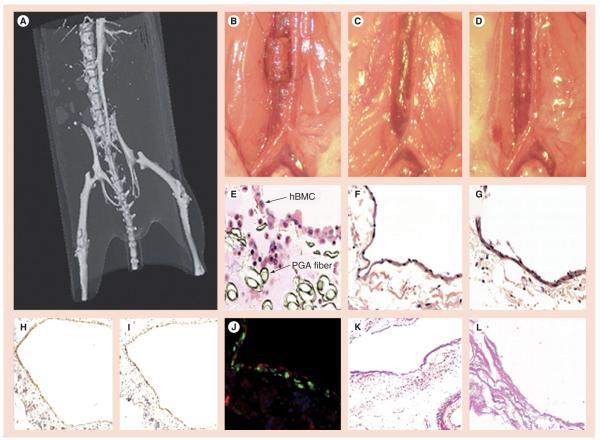
Figure 4. Human tissue-engineered vascular grafts constructed from human bone marrow cell-seeded scaffolds transform into living blood vessels in SCID/bg mice.
(A) Micro-computed tomography angiography at week 10 shows a patent tissue-engineered vascular graft (TEVG) functioning as an inferior vena cava (IVC) venous conduit. Gross images of a human TEVG interposed into the IVC of the SCID/bg mouse (B) at operative day 0 and (C) after 24 weeks in vivo. (D) Gross image of native mouse IVC for comparison. Corresponding hematoxylin and eosin images of (E) a TEVG at day 0 (demonstrating hBMCs transplanted into the scaffold wall), (F) a TEVG at 24 week (notice that scaffold has degraded) and (G) native mouse IVC. Low-magnification (×100) photomicrographs of a TEVG at 10 weeks postimplantation show scaffold materials still present, but also the development of a confluent smooth muscle cell (α-smooth muscle actin, brown) layer (H) and endothelial cell (von Willebrand factor, brown) lining (I) throughout the inner lumen. By 24 weeks, the scaffold material has degraded and the TEVG displays mature vessel architecture. (J) High-magnification (×400) photomicrograph demonstrates an organized endothelial cell-lined intima (von Willebrand factor, red) and smooth muscle cell media (α-smooth muscle actin, green). (K) Low-magnification (×100) Verhoeff–van Gieson stain shows scaffold replaced by a supportive adventitial layer composed of collagen (pink). (L) High-magnification (×400) Verhoeff–van Gieson stain demonstrates collagen fibrils but no elastin fibers (elastin, black; collagen, pink). hBMC: Human bone marrow mononuclear cell; PGA: Polyglycolic acid.
Reproduced with permission from [46].
The evident departure of seeded cells from the developing TEVG raised two more questions: what happens to the seeded cells, and what is the source of the cells that populate the scaffold? To follow seeded cells, we first demonstrated that donor BM-MNCs do not contribute to the host TEVG in immunocompetent C57BL/6 as well as SCID/bg mice. Magnetic resonance tracking of USPIO-labeled murine macrophages seeded on PGA-P(CL/LA) scaffolds showed that seeded macrophages are rapidly lost after implantation and are not incorporated into the TEVG [47]. We then obtained and harvested bone marrow from inbred transgenic CB57BL/6 mice expressing GFP under the control of the ubiquitin-C promoter. We seeded PLLA-P(CL/LA) scaffolds with GFP-labeled syngeneic mouse BM-MNCs (mBN-MNCs), implanted these grafts into wild-type CB57BL/6 mice, and observed that GFP-labeled donor mBM-MNCs leave the scaffold within 1 week of implantation.
Next, we rescued lethally irradiated wild-type CB57BL/6 female mice with tail vein injection of unfractionated bone marrow from transgeneic inbred male CB57BL/6 mice to create a sex-mismatched source of endogenous mBM-MNC. These chimeric females received PLLA-P(CL/LA) scaffolds seeded with unlabeled mBM-MNCs. FISH analysis of grafts explanted at 1 week revealed colocalization of YChr and F4/80+ in cells of the graft wall, demonstrating that host bone marrow-derived macrophages initially populate the scaffold. At 2 weeks, cells positive for vWF and SMA but negative for YChr began to appear. At 6 months, the presence of a vWF+YChr− endothelial layer and calponin+YChr− media confirmed that the TEVG is populated by vascular cells that are not descended from bone marrow-derived vascular progenitors, but from differentiated ECs and SMCs that migrate in from the adjacent anastomosed blood vessels. The few remaining Mac-3+YChr+ cells confirmed that the early macrophage infiltrate is bone marrow-derived but does not differentiate into vascular phenotypes. Next, we implanted composite vascular grafts made from anastomosed syngeneic male IVC and unseeded TEVG in female CB57BL/6 mice and observed that late ECs and SMCs inhabiting the TEVG segment closest to the male IVC segment stained strongest for YChr. These experiments confirmed that host-derived but not seeded cells contribute to the TEVG cellular population [48].
Finally, we returned to the role of the host macrophage in the development of TEVG stenosis. PLLA-P(CL/LA) scaffolds not seeded or seeded with CB57BL/6 mMB-MNCs were implanted as infrarenal IVC interposition grafts in CB57BL/6 mice. Early stenosis (>50% reduction in luminal diameter on ultrasound) was observed in 80% of unseeded TEVGs and 20% of seeded TEVGs. Critical stenosis only occurred in grafts with early stenosis, and all significant stenosis occurred within 2 weeks of implantation by inward wall thickening with constant outer graft diameter. These outcomes were similar to the observations made in the clinical trial [35] and justify our comparison of the natural history of stenosis in this model to that observed in human TEVG. The extent of macrophage infiltration in the CB57BL/6 model was inversely related to BM-MNC seeding and directly correlated with the degree of TEVG stenosis at the critical 2-week time point. Macrophage phenotype was also related to BM-MNC seeding and the development of stenosis, with M1 expression predominant in seeded scaffolds and M1 to M2 transition evident in unseeded scaffolds, but absent in seeded scaffolds. However, TEVG implants in CD11b-diptheria toxin receptor-transgenic mice, which were depleted of macrophages by intraperitoneal administration of clodronate liposomes, also demonstrated reduced macrophage infiltration, poor endothelialization and stenosis. These results underscore the importance of macrophage infiltration and activation to the in vivo development of our TEVG into a functional vascular conduit. Furthermore, the macrophage activation state observed in this model defies the contested M1/M2 classification of macrophage activation and may represent an as-yet uncharacterized activation phenotype that promotes remodeling of the TEVG [49].
In summary, our clinical and laboratory results thus far demonstrate that the BMC-seeded TEVG has clear clinical potential as a conduit for pediatric CHD and warrants investigation as a conduit for other cardiac and vascular procedures. Limitations of our approach to TEVG fabrication include the necessities of adequate recipient bone marrow and a functional host inflammatory foreign body response, as well as the inefficiencies and poor shelf-life associated with ex vivo cell seeding. Furthermore, bone marrow extraction and seeding significantly increases intraoperative time, and BM-MNC purification requires advanced facilities and techniques. Further study of the cellular and molecular mechanisms of TEVG neotissue formation may lead to the development of a new generation of cell-free, off-the-shelf vascular conduits that could broaden the scope and clinical applications of cardiovascular tissue engineering.
Future perspective
Cytokine release from unseeded TEVG scaffolds can modulate the host response to an implanted TEVG to achieve similar graft phenotypes and patency rate as those attained with cell-seeded grafts [48]. These results have opened a new frontier for cell-free tissue engineering of living vascular grafts. Integration of biomedically engineered drug delivery systems with proven and novel scaffold technologies may allow us to fabricate vascular grafts that elute cytokines and other therapeutics and thereby direct the cellular and molecular processes responsible for neovessel formation. Cell-free TEVGs would eliminate the need for biopsy, ex vivo culture and seeding of recipient cells – existing barriers to the clinical use of TEVGs – and will render autologous vessel harvest unnecessary. Thus, cell-free TEVGs will reduce the number of invasive procedures required to treat a patient with a vascular graft. Further characterization of the natural history of TEVG remodeling will inform the selection of cytokines with which to functionalize TEVG scaffolds. Advances in drug delivery will permit the stable encapsulation of cytokines and facilitate the development of grafts with clinically useful shelf-lives. The era of an off-the-shelf vascular graft that grows with the patient and behaves like a native vessel is clearly in sight.
Executive summary.
Single ventricle cardiac anomalies: a significant pediatric health problem
▪ Congenital heart disease causes significant morbidity and mortality worldwide.
▪ Single ventricle anomalies are among the most severe of congenital heart diseases, often requiring surgical intervention very early in life.
Currently employed vascular conduits: a limiting factor in the treatment of severe congenital heart disease
▪ Synthetic conduits used for extracardiac total cavopulmonary connection in the modified Fontan operation are associated with significant morbidity and mortality.
▪ Tissue engineering promises a nonimmunogenic and thromboresistant vascular graft that is capable of growing with patients.
Evolution of the tissue-engineered vascular conduit: animal studies demonstrating the feasibility of large-caliber tissue-engineered vascular grafts for low-pressure, high-flow applications & our first clinical trials
▪ Tissue-engineered vascular grafts (TEVGs) created by seeding mature venous cells onto a biodegradable matrix mature in vivo into functional neovessels resembling native vein.
▪ Autologous human bone marrow cells enriched for the mononuclear fraction (hBM-MNCs) can replace mature vascular cells as a cell source for the TEVG and increase its clinical utility.
Next-generation TEVG: investigating the molecular mechanisms of neotissue formation
▪ Histological characterization of small-diameter TEVGs in mouse models illuminated the natural history of TEVG maturation.
▪ Cell seeding with hBM-MNCs accelerates macrophage infiltration and enhances graft cellularity and patency through paracrine signaling.
▪ Cell-free tissue engineering: local release of MCP-1 from grafts achieves similar outcomes as seeding hBM-MNCs.
▪ A neovessel is not derived from seeded hBM-MNCs, which rapidly leave the scaffold; host bone marrow-derived macrophages and vascular cells from adjacent vessels migrate into and populate the graft.
Acknowledgments
T Shinoka receives partial funding from Gunze, Ltd, a company that makes scaffolds for clinical trials and animal studies. CK Breuer receives partial funding from Pall, Corp. None of the funding for the work described in this manuscript was provided by Gunze, Ltd, or Pall, Corp. The research described was funded by NIH grants (5K08HL083980-05 and 5401HL098228-03).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Connelly M, Webb G, Somerville J, et al. Canadian consensus conference on adult congenital heart disease 1996. Can. J. Cardiol. 1998;14(3):395–452. [PubMed] [Google Scholar]
- 2.Webb CL, Jenkins KJ, Karpawich PP, et al. Collaborative care for adults with congenital heart disease. Circulation. 2002;105(19):2318–2323. doi: 10.1161/01.cir.0000017557.24261.a7. [DOI] [PubMed] [Google Scholar]
- 3.Hager A, Kaemmerer H, Eicken A, Fratz S, Hess J. Long-term survival of patients with univentricular heart not treated surgically. J. Thorac. Cardiovasc. Surg. 2002;123(6):1214–1217. doi: 10.1067/mtc.2002.122535. [DOI] [PubMed] [Google Scholar]
- 4.Samánek M. Children with congenital heart disease: probability of natural survival. Pediatr. Cardiol. 1992;13(3):152–158. doi: 10.1007/BF00793947. [DOI] [PubMed] [Google Scholar]
- 5.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontan F. Atrio–pulmonary conduit operations for tricuspid atresia and single ventricle. Nihon Kyobu Geka Gakkai Zasshi. 1978;26(3):276–284. [PubMed] [Google Scholar]
- 7.Ohuchi H, Kagisaki K, Miyazaki A, et al. Impact of the evolution of the Fontan operation on early and late mortality: a single-center experience of 405 patients over 3 decades. Ann. Thorac. Surg. 2011;92(4):1457–1466. doi: 10.1016/j.athoracsur.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 8.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava–pulmonary artery extracardiac conduit. A new form of right heart bypass. J. Thorac. Cardiovasc. Surg. 1990;100(2):228–232. [PubMed] [Google Scholar]
- 9.Mayer J, Bridges N, Lock J, Hanley F, Jonas R, Castaneda A. Factors associated with marked reduction in mortality for Fontan operations in patients with single ventricle. J. Thorac. Cardiovasc. Surg. 1992;103(3):444–451. [PubMed] [Google Scholar]
- 10.Giannico S, Hammad F, Amodeo A, et al. Clinical outcome of 193 extracardiac fontan patients: the first 15 years. J. Am. Coll. Cardiol. 2006;47(10):2065–2073. doi: 10.1016/j.jacc.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 11.Wells W, Malas M, Baker CJ, Quardt SM, Barr ML. Depopulated vena caval homograft: a new venous conduit. J. Thorac. Cardiovasc. Surg. 2003;126(2):498–503. doi: 10.1016/s0022-5223(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 12.Petrossian E, Reddy VM, McElhinney DB, et al. Early results of the extracardiac conduit Fontan operation. J. Thorac. Cardiovasc. Surg. 1999;117(4):688–696. doi: 10.1016/S0022-5223(99)70288-6. [DOI] [PubMed] [Google Scholar]
- 13.Alexi-Meskishvili V, Ovroutski S, Ewert P, et al. Optimal conduit size for extracardiac Fontan operation. Eur. J. Cardio-Thorac. Surg. 2000;18(6):690–695. doi: 10.1016/s1010-7940(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 14.Pearl J, Laks H, Drinkwater D, Capouya E, George B, Williams R. Modified Fontan procedure in patients less than 4 years of age. Circulation. 1992;86(Suppl. 5):II100–II105. [PubMed] [Google Scholar]
- 15.Ovroutski S, Ewert P, Alexi-Meskishvili V, et al. Comparison of somatic development and status of conduit after extracardiac Fontan operation in young and older children. Eur. J. Cardiothorac. Surg. 2004;26(6):1073–1079. doi: 10.1016/j.ejcts.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Van Son J, Reddy M, Hanley F. Extracardiac modification of the Fontan operation without use of prosthetic material. J. Cardiothorac. Surg. 1997;110(6):1766–1768. doi: 10.1016/s0022-5223(95)70043-9. [DOI] [PubMed] [Google Scholar]
- 17.Hvass U, Pansard Y, Böhm G, Depoix JP, Enguerrand D, Worms AM. Bicaval pulmonary connection in tricuspid atresia using an extracardiac tube of autologous pediculated pericardium to bridge inferior vena cava. Eur. J. Cardiothorac. Surg. 1992;6(1):49–51. doi: 10.1016/1010-7940(92)90099-j. [DOI] [PubMed] [Google Scholar]
- 18.Woods RK, Dyamenahalli U, Duncan BW, Rosenthal GL, Lupinetti FM. Comparison of extracardiac Fontan techniques: pedicled pericardial tunnel versus conduit reconstruction. J. Thorac. Cardiovasc. Surg. 2003;125(3):465–471. doi: 10.1067/mtc.2003.153. [DOI] [PubMed] [Google Scholar]
- 19.Adachi I, Yagihara T, Kagisaki K, et al. Fontan operation with a viable and growing conduit using pedicled autologous pericardial roll: serial changes in conduit geometry. J. Thorac. Cardiovasc. Surg. 2005;130(6):1517–1522. doi: 10.1016/j.jtcvs.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 20.Bell E. Tissue engineering in perspective. In: Lanza RP, Vacanti J, editors. Principles of Tissue Engineering. Elsevier Academic Press; MA, USA: 2007. pp. XXXV–XII. [Google Scholar]
- 21.Shinoka T, Shum-Tim D, Ma PX, et al. Creation of viable pulmonary artery autografts through tissue engineering. J. Thorac. Cardiovasc. Surg. 1998;115(3):536–546. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Shin’oka T, Tohyama S, et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–439. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 23.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N. Engl. J. Med. 2001;344(7):532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 24.Breuer CK, Shin’oka T, Tanel RE, et al. Tissue engineering lamb heart valve leaflets. Biotechnol. Bioeng. 1996;50(5):562–567. doi: 10.1002/(SICI)1097-0290(19960605)50:5<562::AID-BIT11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Noishiki Y, Tomizawa Y, Yamane Y, Matsumoto A. Autocrine angiogenic vascular prosthesis with bone marrow transplantation. Nat. Med. 1996;2(1):90–93. doi: 10.1038/nm0196-90. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 27.Kaushal S, Amiel GE, Guleserian KJ, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 2001;7(9):1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108(14):1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 29.Bitondo JM, Daggett WM, Torchiana DF, et al. Endoscopic versus open saphenous vein harvest: a comparison of postoperative wound complications. Ann. Thorac. Surg. 2002;73(2):523–528. doi: 10.1016/s0003-4975(01)03334-3. [DOI] [PubMed] [Google Scholar]
- 30.Shin’oka T, Matsumura G, Hibino N, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005;129(6):1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. ▪ Describes the first clinical trial of a tissue-engineered vascular graft (TEVG) for pediatric congenital heart defects.
- 31.Poh M, Boyer M, Solan A, et al. Blood vessels engineered from human cells. Lancet. 2005;365(9477):2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 32.Naito Y, Imai Y, Shin’oka T, et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J. Thorac. Cardiovasc. Surg. 2003;125(2):419–420. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury UK, Airan B, Kothari SS, et al. Specific issues after extracardiac fontan operation: ventricular function, growth potential, arrhythmia, and thromboembolism. Ann. Thorac. Surg. 2005;80(2):665–672. doi: 10.1016/j.athoracsur.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Kim SJ, Kim WH, Lim HG, Lee JY. Outcome of 200 patients after an extracardiac Fontan procedure. J. Thorac. Cardiovasc. Surg. 2008;136(1):108–116. doi: 10.1016/j.jtcvs.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010;139(2):431–436. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 36.Brennan MP, Dardik A, Hibino N, et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann. Surg. 2008;248(3):370–377. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udelsman B, Hibino N, Villalona GA, et al. Development of an operator-independent method for seeding tissue-engineered vascular grafts. Tissue Eng. Part C Methods. 2011;17(7):731–736. doi: 10.1089/ten.tec.2010.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roh JD, Nelson GN, Brennan MP, et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials. 2008;29(10):1454–1463. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Lin P, Yao Q, Chen C. Development of small-diameter vascular grafts. World J. Surg. 2007;31(4):682–689. doi: 10.1007/s00268-006-0731-z. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. ▪ Excellent review of the inflammatory and healing processes that influence the development of a TEVG scaffold into a neovessel.
- 41.Nelson GN, Roh JD, Mirensky TL, et al. Initial evaluation of the use of USPIO cell labeling and noninvasive MR monitoring of human tissue-engineered vascular grafts in vivo. FASEB J. 2008;22(11):3888–3895. doi: 10.1096/fj.08-107367. ▪ Early evidence that TEVG neotissue does not derive from seeded cells.
- 42.Mirensky TL, Nelson GN, Brennan MP, et al. Tissue-engineered arterial grafts: long-term results after implantation in a small animal model. J. Pediatr. Surg. 2009;44(6):1127–1133. doi: 10.1016/j.jpedsurg.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 43.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96(1):34–40. [PubMed] [Google Scholar]
- 44.Mirensky TL, Hibino N, Sawh-Martinez RF, et al. Tissue-engineered vascular grafts: does cell seeding matter? J. Pediatr. Surg. 2010;45(6):1299–1305. doi: 10.1016/j.jpedsurg.2010.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 46.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl Acad. Sci. USA. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. ▪▪ Important contribution to the understanding of vascular scaffold remodeling, as well as a proof-of-concept for cytokine release as an alternative to cell seeding.
- 47.Harrington JK, Chahboune H, Criscione JM, et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J. 2011;25(12):4150–4161. doi: 10.1096/fj.11-185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hibino N, Villalona G, Pietris N, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25(8):2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hibino N, Yi T, Duncan DR, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011;25(12):4253–4263. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.The Children’s Heart Foundation fact sheets. http://childrensheartfoundation.org.
- 102.Breuer C. NCT01034007: a pilot study investigating the clinical use of tissue engineered vascular grafts in congenital heart surgery. http://clinicaltrials.gov/ct2/show/NCT01034007.