Abstract
Recent studies indicate that beside digestion and absorption of nutrients and water and electrolytes homeostasis, another key function of the intestine is to regulate the trafficking of environmental antigens across the host mucosal barrier. Intestinal tight junctions (TJ) create gradients for the optimal absorption and transport of nutrients and control the balance between tolerance and immunity to non-self antigens. To meet diverse physiological challenges, intestinal epithelial TJ must be modified rapidly and in a coordinated fashion by regulatory systems that orchestrate the state of assembly of the TJ multi-protein network. While considerable knowledge exists about TJ ultrastructure, relatively little is known about their physiological and pathophysiological regulation. Our discovery of zonulin, the only known physiologic modulator of intercellular TJ described so far, increased understanding of the intricate mechanisms that regulate the intestinal epithelial paracellular pathway and led us appreciate that its up-regulation in genetically susceptible individuals leads to autoimmune diseases.
Introduction
Improved hygiene leading to a reduced exposure to microorganisms have been implicated as one possible cause for the ‘epidemic’ of immune-mediated diseases, particularly autoimmune diseases, in industrialized countries during the past 3–4 decades (1). Collectively, autoimmune diseases are highly prevalent in the U.S., affecting between 14.7 and 23.5 million people — up to 8 percent of the population (2). The social and financial burdens imposed by these chronic, debilitating diseases include poor quality of life, high health care costs, and substantial loss of productivity. For example, in 2002 the average annual medical costs for treating type 1 diabetes (T1D) in the U.S. were estimated $6.7 billion (3). In less than a decade these costs jumped to $14.4 billion (4). Apart from genetic makeup and exposure to environmental triggers, a third key element, i.e., increased intestinal permeability, which may be influenced by the composition of the gut microbiota, has been proposed in the pathogenesis of these diseases (5–8). Intestinal permeability, together with antigen (Ag) sampling by enterocytes and luminal dendritic cells, regulates molecular trafficking between the intestinal lumen and the submucosa, leading to either tolerance or immunity to non-self Ag (9–11). However, the dimensions of the paracellular space (10 to 15 Å) suggest that solutes with a molecular radius exceeding 15 Å (~3.5 kDa) (including proteins) are normally excluded and taken up by the transcellular pathway. Intercellular tight junctions (TJ) tightly regulate paracellular Ag trafficking. TJ are extremely dynamic structures that operate in several key functions of the intestinal epithelium under both physiological and pathological circumstances (6). However, despite major progress in our knowledge on the composition and function of the intercellular TJ, the mechanism(s) by which they are regulated is(are) still incompletely understood.
In the past decade we have focused our research effort on the discovery and characterization of zonulin as the only human protein discovered to date that is known to reversibly regulate intestinal permeability by modulating intercellular TJs (12–14). Zonulin expression is augmented in autoimmune conditions associated with TJ dysfunction, including celiac disease (CD) and T1D (13,15). Both animal studies (16) and human trials (17) using the zonulin synthetic peptide inhibitor AT1001 (now named Larazotide acetate) established that zonulin is integrally involved in the pathogenesis of autoimmune diseases. Zonulin can be used as a biomarker of impaired gut barrier function for several autoimmune, neurodegenerative, and tumoral diseases (18), and can be a potential therapeutic target for the treatment of these devastating conditions.
The zonulin system
Identification of zonulin as pre-haptoglobin 2
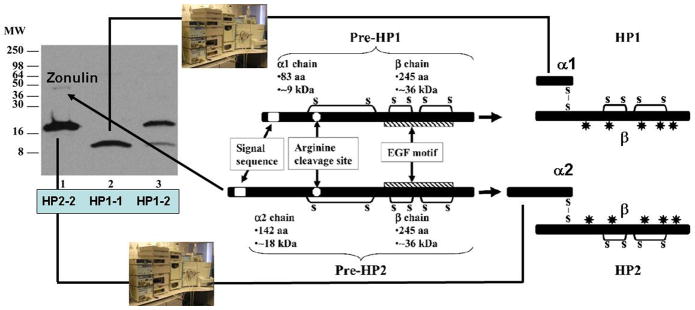
Through proteomic analysis of human sera, we have recently identified zonulin as pre-haptoglobin (HP)2 (19)), a molecule that, to date, has only been regarded as the inactive precursor for HP2, one of the two genetic variants (together with HP1) of human HPs. Mature human HPs are heterodimeric plasma glycoproteins composed of α and β polypeptide chains that are covalently associated by disulfide bonds and in which only the β chain is glycosylated (20) (Fig. 1). While the β chain (36 kDa) is constant, the α chain exists in two forms, i.e., 1 (~9 kDa) and 2 (~18 kDa). The presence of one or both of the chains results in the three human HP phenotypes, i.e., HP1-1 homozygote, HP2-1 heterozygote, and HP2-2 homozygote.
Figure 1.
Western blotting using zonulin cross-reacting anti-Zot polyclonal antibodies on CD patient sera showed three main patterns: sera showing a 18 kDa immunoreactive band and a fainter _45 kDa band (lane 1), sera showing only a 9 kDa band (lane 2), and sera showing both the 18 and 9 kDa bands (lane 3). The cartoon shows the structure of both pre-haptoglobin (HP) 1 and pre-HP2 and their mature proteins. HPs evolved from a complement-associated protein (mannose-binding lectin-associated serine protease, MASP), with their α-chain containing a complement control protein (CCP), while the β chain is related to chymotrypsin-like serine proteases (SP domain) containing an epidermal growth factor-like motif. The gene encoding the α2-chain of pre-HP2 originated in India almost 2 million years ago through a chromosomal aberration (unequal crossing over) of HP1. Pre-HPs are translated as single-chain precursor proteins. Pre-HPs may be proteolytically cleaved intracellularly into α - and β-chains that remain disulfide linked, referred to as cleaved, two-chain mature HPs.
Despite this multidomain structure, the only function assigned to HPs, to date, is to bind Hb to form stable HP-Hb complexes thereby preventing Hb-induced oxidative tissue damage (21). In contrast, no function has ever been described for their precursor forms. The primary translation product of mammalian Hp mRNA is a polypeptide that dimerizes contranslationally and is proteolitically cleaved while still in the endoplasmic reticulum (22). Conversely, zonulin is detectable in an uncleaved form in human serum, adding an extremely intriguing aspect of the multifunctional characteristics of HPs. HPs are unusual secretory proteins in that they are proteolytically processed in the endoplasmic reticulum, the subcellular fraction of which we detected the highest zonulin concentration (23) . Wicher and Fries (22) found that Cr1LP mediates this cleavage in a specific manner, since the enzyme did not cleave the proform of complement C1s, a protein similar to Pre-HP2, particularly around the cleavage site. Therefore, it is conceivable to hypothesize that the activity of Cr1LP modulates the zonulin pool.
Zonulin functional characterization
Since we have reported previously that the key biological effect of zonulin is to affect the integrity of intercellular TJ, we specifically focused our efforts on demonstrating that the recombinant pre-HP2 alters intestinal permeability. Indeed, ex vivo experiments showed that recombinant pre-HP2 induced a time- and dose-dependent reduction in TEER when added to murine small intestinal mucosa (19). These results were validated independently in an in vivo intestinal permeability assay in which zonulin, but not its cleaved form, induced a significant and reversible increase in both gastro-duodenal and small intestinal permeability (19). The evidence that zonulin cleaved in its α2 and β subunits lost the permeability activity further supports the notion that pre-HP2 (alias, zonulin) and mature HP2 exerts two different biological functions most likely related to the different folding of the protein in its cleaved or uncleaved form.
Structural analysis of zonulin revealed similarities with several growth factors. Like zonulin, growth factors affect intercellular TJ integrity (24,25). Our data showing that zonulin but not its cleaved subunits activate EGFR (19) and that its effect on TEER was prevented by the EGFR tyrosine kinase inhibitor AG-1478 (19) suggest that zonulin is properly folded to activate EGFR and, therefore, to cause TJ disassembly only in its uncleaved form. Several G protein coupled receptors (GPCR), including PAR2, transactivate EGFR (26). Zonulin prokaryotic counterpart Zot active peptide FCIGRL (AT1002) has structural similarities with PAR2-Activating Peptide (AP), SLIGRL, and causes PAR2–dependent changes in TEER, a finding that we have demonstrated in WT, but not PAR2−/−mice (27). Therefore, it was not totally unexpected that experiments in Caco2 cell in which PAR2 was silenced showed decreased EGFR Y1068 phosphorylation in response to recombinant zonulin compatible with PAR2–dependent transactivation of EGFR (19). To further establish a role for PAR2 in EGFR activation in response to zonulin, we conducted small intestinal barrier function studies using segments isolated from either C57BL/6 WT or PAR2−/−mice. As expected, zonulin decreased TEER in intestinal segments from C57BL/6 WT mice, while it failed to reduce TEER in small intestinal segments from PAR2−/−mice (19), so linking zonulin-induced PAR2–dependent transactivation of EGFR with barrier function modulation.
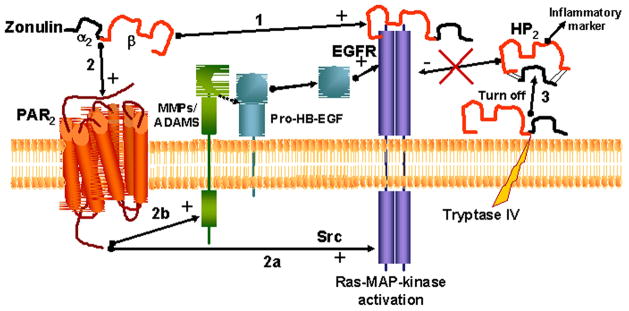
To summarize, we have reported for the first time the novel characterization of zonulin as pre-HP2, a multifunctional protein that, in its intact single chain form, regulates intestinal permeability caused by EGFR transactivation through PAR2, while in its cleaved two-chain form acts as a Hb scavenger (Fig. 2).
Figure 2.
Zonulin can activate EGFR through direct binding (1) and/or through PAR2 transactivation (2). This second mechanism can be mediated by either Src signaling (2a) or by the release of MMPs and/or ADAMS that in turn will activate Pro-HB-EGF. When cell tryptase IV cleaves zonulin in its two subunits (so eliminating one of the three required disulfide bridges necessary for EGF activity), the molecule is not able to bind to EGFR (3), while will acquire a different function (Hb binding) and becomes an inflammatory marker.
Stimuli that cause zonulin release in the gut
Among the several potential intestinal luminal stimuli that can trigger zonulin release, we identified small intestinal exposure to bacteria and gluten as the two more powerful triggers (18,23,28). Enteric infections have been implicated in the pathogenesis of several pathological conditions, including allergic, autoimmune, and inflammatory diseases, by causing impairment of the intestinal barrier. We have generated evidence that small intestines exposed to enteric bacteria secrete zonulin (28). This secretion was independent either of the animal species from which the small intestines were isolated or the virulence of the microorganisms tested, occurred only on the luminal aspect of the bacteria-exposed small intestinal mucosa, and was followed by an increase in intestinal permeability coincident with the disengagement of the protein ZO-1 from the tight junctional complex (28). This zonulin-driven opening of the paracellular pathway may represent a defensive mechanism, which flushes out microorganisms so contributing to the innate immune response of the host against bacterial colonization of the small intestine.
Beside bacterial exposure, we have shown that gliadin, a storage protein present in wheat and that triggers celiac disease in genetically susceptible individuals, also affect the intestinal barrier function by releasing zonulin (29). This effect of gliadin is polarized, i.e., gliadin increases intestinal permeability only when administered on the luminal side of the intestinal tissue (29). This observation led us to the identification of the chemokine receptor CXCR3 as the target intestinal receptor for gliadin (30). Our data demonstrate that in the intestinal epithelium, CXCR3 is expressed at the luminal level, is over-expressed in CD patients, co-localizes with gliadin, and that this interaction coincides with recruitment of the adapter protein, MyD88, to the receptor (28). We also demonstrated that binding of gliadin to CXCR3 is crucial for the release of zonulin and subsequent increase of intestinal permeability, since CXCR3-deficient mice failed to respond to gliadin challenge in terms of zonulin release and TJ disassembly (30). Using an α-gliadin synthetic peptide library, we identified two α-gliadin 20mers (QVLQQSTYQLLQELCCQHLW and QQQQQQQQQQQQILQQILQQ) that bind to CXCR3 and release zonulin (30).
Role of zonulin in autoimmune, inflammatory, and tumor diseases
Celiac disease
Celiac disease (CD) is an immune mediated chronic enteropathy with a wide range of presenting manifestations of variable severity. It is triggered by the ingestion of gliadin fraction of wheat gluten and similar alcohol-soluble proteins (prolamines) of barley and rye in genetically susceptible subjects with subsequent immune reaction leading to small bowel inflammation and normalization of the villous architecture in response to a gluten-free diet (31). CD is a unique model of autoimmunity in which, in contrast to most other autoimmune diseases, a close genetic association with HLA genes, a highly specific humoral autoimmune response against tissue transglutaminase auto-antigen, and, most importantly, the triggering environmental factor (gliadin), are all known. It is the interplay between genes (both HLA and non-HLA associated) and environment (i.e. gluten) that leads to the intestinal damage typical of the disease (32). Under physiological circumstances this interplay is prevented by competent intercellular TJ. Early in CD, TJs are opened (33–36) and severe intestinal damage ensues.
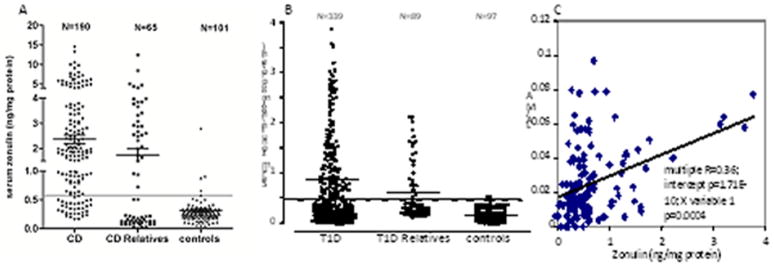
The repertoire of gluten peptides involved in the disease pathogenesis is greater than appreciated previously and may differ between children and adult patients (37). There are at least 50 toxic epitopes in gluten peptides exerting cytotoxic, immunomodulatory, and gut permeating activities (38). The effect of the permeating gliadin peptides in vivo was confirmed by the analysis of intestinal tissues from patients with active CD and non-CD controls probed for zonulin expression (23). Quantitative immunoblotting of intestinal tissue lysates from active CD patients confirmed the increase in zonulin protein compared to control tissues (23). Zonulin upregulation during the acute phase of CD was confirmed by measuring zonulin concentration in sera of 189 CD patients using a sandwich ELISA (Fig. 3A) (13).
Figure 3.
(A). CD patients showed higher serum zonulin levels compared to both their relatives and controls. (B). Similar results were obtained in T1D patients. (C). Serum zonulin correlated with intestinal permeability evaluated by the LA/MA test. The percentage of CD patients (81%) and their relatives (50%) with elevated serum zonulin levels was higher compared to T1D patients (42%) and their relatives (29%), respectively.
Type 1 diabetes
The trigger of the autoimmune destruction of pancreatic beta cells in type 1 diabetes (T1D) is unknown. T1D has the same pathogenic challenges as other autoimmune diseases: what are the environmental triggers, and how do these triggers cross the intestinal barrier to interact with the immune system (39,40). Certain HLA class II alleles account for 40% of the genetic susceptibility to T1D in Caucasians (41); however, the majority of individuals with these HLA alleles do not develop T1D. This supports the concept that reaction to some environmental products triggers autoimmune destruction of beta cells and leads to T1D. Gastrointestinal symptoms in T1D have been generally ascribed to altered intestinal motility secondary to autonomic neuropathy (42). However, more recent studies have shown that altered intestinal permeability occurs in T1D prior to the onset of complications (43), which is not the case in type 2 diabetes. This has led to the suggestion that an increased intestinal permeability due to alteration in intestinal TJ is responsible for the onset of T1D (43,44). This hypothesis is supported by studies performed in an animal model that develops T1D spontaneously that showed an increased permeability of the small intestine (but not of the colon) of the BioBreeding diabetic prone (BBDP) rats that preceded the onset of diabetes by at least a month (45). Further, histological evidence of pancreatic islet destruction was absent at the time of increased permeability but clearly present at a later time (45). Therefore, these studies provided evidence that increased permeability occurred before either histological or overt manifestation of diabetes in this animal model. We confirmed these data by reporting in the same rat model that zonulin-dependent increase in intestinal permeability precedes the onset of T1D by 2–3 weeks (16).
Several reports have linked gliadin (the environmental trigger of CD autoimmunity that also causes zonulin release from the gut, see ref 23 and 29) to T1D autoimmunity both in animal models and in human studies. Findings from studies using non-obese diabetic (NOD) mice and BBDP rats have implicated wheat gliadin as a dietary diabetogen (46,47). We have recently reported a direct link between antibodies to Glo-3a (a wheat-related protein), zonulin upregulation, and islet autoimmunity (IA) in children at increased risk for T1D (48).
Association of HP genotypes (HP1-1, HP1-2, and HP2-2) with serum zonulin expression and clinical severity in human disease states
We have recently discovered that human zonulin is identical to pre-Hp2 (see above). Two co-dominant allele variants, termed HP1 and HP2, the latter unique to the human species, are variously distributed in the general population, resulting in three phenotypes, Hp1-1 (~20% in western populations), Hp2-1 (~50%), and Hp2-2 (~30%) (49). Several studies have suggested that the presence of the HP2 allele correlates with higher risk to develop immune-mediated diseases (50,51) and HP2 homozygosis is associated with poor prognosis (51) and decreased longevity (52).
Zonulin phenotype in CD and T1D and its correlation with intestinal permeability
Using a serum zonulin ELISA, we measured serum zonulin levels in both CD and T1D patients, their relatives, and age and sex-matched healthy controls. CD patients showed statistically higher serum zonulin levels (2.37 ± 0.17 ng/mg protein) as compared to both their relatives (1.75 ± 0.27 ng/mg protein, P = 0.05) and control subjects (0.31 ± 0.03 ng/mg protein, P < 0.00001) (Fig. 3A). Eighty-one percent (154/190) of CD patients and 50% of their first-degree relatives (33/65) had serum zonulin levels that were 2 SD above the mean zonulin levels detected in age-matched healthy controls. Only 4.9% (5/101) of controls had zonulin levels 2 SD above the mean (P < 0.01). Serum zonulin was higher in CD as compared to their relatives (P < 0.00001). Similar results were obtained in T1D patients in which we detected increased serum zonulin levels (0.83 ± 0.05 ng/mg protein) as compared to both their relatives (0.62 ± 0.07 ng/mg protein, P = 0.011) and control subjects (0.21 ± 0.02 ng/mg protein, P < 0.00001) (15) (Fig. 3B). Forty-two percent (141/339) of T1D patients and 29% of their first-degree relatives (26/89) had serum zonulin levels that were 2 SD above the mean zonulin levels detected in age-matched healthy controls. Only 4% (4/97) of controls had zonulin levels 2 SD above the mean (P < 0.01). Serum zonulin was higher in T1D as compared to their relatives (P = 0.01). To establish whether serum zonulin levels correlated with intestinal permeability, lactulose (LA)/mannitol (MA) urine ratio was determined in both a subset of T1D subjects with documented zonulin up-regulation (N = 36) and their relatives (N = 56). Intestinal permeability correlated with serum zonulin (Fig. 3C) (15).
HP2 (alias zonulin) allele is over-represented in immune-mediated diseases
To establish the distribution of HP1 and HP2 genes among CD patients and matched controls, we developed a single step RT-PCR protocol using specific primers in exon 2 and exon 5 of HP1 corresponding to exons 2 and 7 of HP2. After PCR the amplicons were run on a 1% agarose gel and read under a UV bulb. The HP1 genotype ran at the predicted size of 2.5 kb while the HP2 ran at 4.3 kb. Our results showed that in CD patients HP1-1 genotype (0 copies of zonulin gene) was decreased, while the HP2-2 genotype (2 copies of zonulin gene)was increased as compared to healthy controls (Table 1). Interestingly, the percentage of HP 1-1 CD patients (0 copies of zonulin gene) was in the same range of the percentage of CD patients that tested negative by zonulin ELISA see above). Similar distribution of the HP genes have been reported by other investigators in other immune-mediated diseases, including Crohn’s disease (58), schizophrenia (59), and chronic kidney disease (CKD)(60) (Table 1).
Table 1.
HP genotype distribution in several immune-mediated diseases
Proof of concept for the pathogenic role of zonulin-dependent intestinal barrier defect in celiac disease and type 1 diabetes
CD and T1D autoimmune models suggest that when the finely tuned trafficking of macromolecules is deregulated in genetically susceptible individuals, autoimmune disorders can occur (53). This new paradigm subverts traditional theories underlying the development of autoimmunity, which are based on molecular mimicry and/or the bystander effect, and suggests that the autoimmune process can be arrested if the interplay between genes and environmental triggers is prevented by re-establishing the intestinal barrier function. To challenge this hypothesis, zonulin inhibitor AT1001 was used with encouraging results in the BBDP rat model of autoimmunity (16). Beside preventing the loss of intestinal barrier function, the appearance of auto-antibodies, and the onset of disease, pre-treatment with AT1001 protected against the insult of pancreatic islets and, therefore, of the insulitis responsible for the onset of type 1 diabetes.
This prove-of-concept in an animal model of autoimmunity provided the rationale to design human clinical trials in which AT1001 was initially tested in an inpatient, double-blind, randomized placebo controlled trial to determine its safety, tolerability, and preliminary efficacy (54). No increase in adverse events was recorded among patients exposed to AT-1001 as compared to placebo. Following acute gluten exposure, a 70% increase in intestinal permeability was detected in the placebo group, while no changes were seen in the AT-1001 group (54). After gluten exposure, IFN-γ levels increased in 4 out of 7 patients (57.1%) of the placebo-group, but only in 4 out of 14 patients (28.6%) of the AT-1001-group. Gastrointestinal symptoms were significantly more frequent among patients of the placebo group as compared to the AT-1001 group (54). Combined, these data suggest that AT-1001 is well tolerated and appears to reduce pro-inflammatory cytokine production and gastrointestinal symptoms in CD patients. AT1001 has now been tested in approximately 500 subjects with excellent safety profile and promising efficacy as concern protection against symptoms caused by gluten exposure in CD patients (55).
Concluding remarks
The GI tract has been extensively studied for its digestive and absorptive functions. A more attentive analysis of its anatomo-functional characteristics, however, clearly indicates that its functions go well beyond the handling of nutrients and electrolytes. The exquisite regional-specific anatomical arrangements of cell subtypes and the finely-regulated cross talk between epithelial, neuroendocrine and immune cells highlights other less-studied, yet extremely important functions of the GI tract. Of particular interest is the regulation of antigen trafficking by the zonulin-dependent paracellular pathway and its activation by intestinal mucosa-microbiota/gluten interactions. These functions dictate the switch from tolerance to immunity, and are likely integral mechanisms involved in the pathogenesis of inflammatory and autoimmune processes.
The classical paradigm of inflammatory pathogenesis involving specific genetic makeup and exposure to environmental triggers has been challenged recently by the addition of a third element, the loss of intestinal barrier function. Genetic predisposition, mis-communication between innate and adaptive immunity, exposure to environmental triggers, and loss of intestinal barrier function secondary to the activation of the zonulin pathway by food-derived environmental triggers or changes in gut microbiota, all seem to be key ingredients involved in the pathogenesis of inflammation, autoimmunity, and cancer. This new theory implies that once the pathologic process is activated, it is not auto-perpetuating. Rather it can be modulated or even reversed by preventing the continuous interplay between genes and the environment. Since zonulin-dependent TJ dysfunction allows such interactions, new therapeutic strategies aimed at re-establishing the intestinal barrier function by downregulating the zonulin pathway offer innovative and not yet-explored approaches for the management of these debilitating chronic diseases.
Acknowledgments
Work presented in this review was supported in parts by grants from the National Institutes of Health Grants DK-48373 and DK-078699 to AF.
References
- 1.Okada H, Kuhn C, Feillet H. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Progress in Autoimmune Diseases Research. Report to Congress, National Institutes of Health, The Autoimmune Diseases Coordinating Committee; March 2005. [Google Scholar]
- 3.National Institutes of Health. Plan. 2002. Autoimmune Diseases research. [Google Scholar]
- 4.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the Cost of Type 1 Diabetes in the U.S: A Propensity Score Matching Method. Plus One. 2010;5:1–11. doi: 10.1371/journal.pone.0011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- 7.Wapenaar MC, Monsuur AJ, van Bodegraven AA, Weersma RK, Bevova MR, Linskens RK, Howdle P, Holmes G, Mulder CJ, Dijkstra G, van Heel DA, Wijmenga C. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- 8.Monsuur AJ, de Bakker PI, Alizadeh BZ, Zhernakova A, Bevova MR, Strengman E, Franke L, van't SR, van Belzen MJ, Lavrijsen IC, Diosdado B, Daly MJ, Mulder CJ, Mearin ML, Meijer JW, Meijer GA, van OE, Wapenaar MC, Koeleman BP, Wijmenga C. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–1344. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- 9.Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247–59. doi: 10.1038/mi.2010.5. [DOI] [PubMed] [Google Scholar]
- 10.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mowat AM, Millington OR, Chirdo FG. Anatomical and cellular basis of immunity and tolerance in the intestine. J Pediatr Gastroenterol Nutr. 2004;39(Suppl 3):S723–S724. doi: 10.1097/00005176-200406003-00003. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(Pt 24):4435–4440. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 13.Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214–222. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 15.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 16.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005;102:2916–21. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 18.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799–804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjorkman PJ, Saper MA, Samraoui B, et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 21.Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, Cohen O, Levy NS, Levy AP. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 22.Wicher KB, Fries E. Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the complement C1r-like protein. Proc Natl Acad Sci U S A. 2004;101:14390–14395. doi: 10.1073/pnas.0405692101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drago S, El AR, Di PM, Grazia CM, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D'Agate C, Not T, Zampini L, Catassi C, Fasano A. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–419. doi: 10.1080/00365520500235334. [DOI] [PubMed] [Google Scholar]
- 24.Hollande F, Blanc EM, Bali JP, Whitehead RH, Pelegrin A, Baldwin GS, Choquet A. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol. 2001;280:G910–G921. doi: 10.1152/ajpgi.2001.280.5.G910. [DOI] [PubMed] [Google Scholar]
- 25.Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 26.van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G441–G451. doi: 10.1152/ajpgi.00303.2007. [DOI] [PubMed] [Google Scholar]
- 27.Goldblum SE, Rai U, Tripathi A, Thakar M, De Leo L, Di Toro N, Not T, Ramachandran R, Puche AC, Hollenberg MD, Fasano A. The active Zot domain (aa 288-293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011;25:144–58. doi: 10.1096/fj.10-158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-Dependent Activation of the Zonulin System is Involved in the Impairment of the Gut Barrier Function Following Bacterial Colonization. Gastroenterol. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 29.Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signaling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branski D, Fasano A, Troncone R. Latest developments in the pathogenesis and treatment of celiac disease. J Pediatr. 2006;149:295–300. doi: 10.1016/j.jpeds.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Plenge RM. Unlocking the pathogenesis of celiac disease. Nat Genet. 2010;42:281–2. doi: 10.1038/ng0410-281. [DOI] [PubMed] [Google Scholar]
- 33.Madara JL, Trier JS. Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab Inves. 1980;43:254–261. [PubMed] [Google Scholar]
- 34.Wolters VM, Alizadeh BZ, Weijerman ME, Zhernakova A, van Hoogstraten IM, Mearin ML, Wapenaar MC, Wijmenga C, Schreurs MW. Intestinal barrier gene variants may not explain the increased levels of antigliadin antibodies, suggesting other mechanisms than altered permeability. Hum Immunol. 2010;71:392–6. doi: 10.1016/j.humimm.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Szakál DN, Gyorffy H, Arató A, Cseh A, Molnár K, Papp M, Dezsofi A, Veres G. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch. 2010;456:245–50. doi: 10.1007/s00428-009-0879-7. [DOI] [PubMed] [Google Scholar]
- 36.Schumann M, Günzel D, Buergel N, Richter JF, Troeger H, May C, Fromm A, Sorgenfrei D, Daum S, Bojarski C, Heyman M, Zeitz M, Fromm M, Schulzke JD. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–8. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- 37.Arentz-Hansen H, McAdam S, Molberg O, Fleckenstein B, Lundin K, Jorgensen T, et al. Celiac lesion T cells recognized epitopes that cluster in regions of gliadin rich in proline residues. Gastroenterology. 2003;123:803–9. doi: 10.1053/gast.2002.35381. [DOI] [PubMed] [Google Scholar]
- 38.Nikulina M, et al. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol. 2004;173:1925–1933. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- 39.Fasano A. Tight Junctions. Boca Raton, FL: CRC Press, Inc; 2001. Pathological and therapeutical implications of macromolecule passage through the tight junction; pp. 697–722. [Google Scholar]
- 40.Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol. 2008;173:1243–52. doi: 10.2353/ajpath.2008.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brorsson C, Tue Hansen N, Bergholdt R, Brunak S, Pociot F. The type 1 diabetes - HLA susceptibility interactome--identification of HLA genotype-specific disease genes for type 1 diabetes. PLoS One. 2010;5:e9576. doi: 10.1371/journal.pone.0009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 43.De Magistris L, Secondulfo M, Iafusco D, Carbone AG, Urio A, Pontoni G, Carratu R. Altered mannitol absorption in diabetic children. Ital J Gastroenterol. 1996;28:367. [PubMed] [Google Scholar]
- 44.Mooradian AD, Morley JE, Levine AS, Prigge WF, Gebhard RL. Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia. 1996;29:221–224. doi: 10.1007/BF00454879. [DOI] [PubMed] [Google Scholar]
- 45.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–7. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 46.Funda DP, Kaas A, Tlaskalová-Hogenová H, Buschard K. Gluten-free but also gluten-enriched (gluten+) diet prevent diabetes in NOD mice; the gluten enigma in type 1 diabetes. Diabetes Metab Res Rev. 2008;24:59–63. doi: 10.1002/dmrr.748. [DOI] [PubMed] [Google Scholar]
- 47.Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, Zandvoort A, Harmsen H, Welling G, Stellaard F, Bos NA, Fasano A, Rozing J. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010;53:2621–8. doi: 10.1007/s00125-010-1903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson M, Mojibian M, Barriga K, Scott F, Fasano A, Rewers M, Norris J. An exploration of Glo-3A antibody levels in children at increased risk for type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:563–72. doi: 10.1111/j.1399-5448.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, Levy AP. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 50.Blum S, Milman U, Shapira C, Levy AP. Pharmacogenomic application of the haptoglobin genotype in the prevention of diabetic cardiovascular disease. Pharmacogenomics. 2008;9:989–91. doi: 10.2217/14622416.9.8.989. [DOI] [PubMed] [Google Scholar]
- 51.Papp M, Foldi I, Nemes E, Udvardy M, Harsfalvi J, Altorjay I, Mate I, Dinya T, Varvolgyi C, Barta Z, Veres G, Lakatos PL, Tumpek J, Toth L, Szathmari E, Kapitany A, Gyetvai A, Korponay-Szabo IR. Haptoglobin polymorphism: a novel genetic risk factor for celiac disease development and its clinical manifestations. Clin Chem. 2008;54:697–704. doi: 10.1373/clinchem.2007.098780. [DOI] [PubMed] [Google Scholar]
- 52.Napolioni V, Giannì P, Carpi FM, Concetti F, Lucarini N. Haptoglobin (HP) polymorphisms and human longevity: A cross-sectional association study in a Central Italy population. Clin Chim Acta. 2011;412:574–7. doi: 10.1016/j.cca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Fasano A. Surprises from celiac disease. Sci Am. 2009;301:54–61. doi: 10.1038/scientificamerican0809-54. [DOI] [PubMed] [Google Scholar]
- 54.Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The Safety, Tolerance, Pharmacokinetic and Pharmacodynamic Effects of Single Doses of AT-1001 in Celiac Disease Subjects: A Proof of Concept Study. Aliment Pharmacol Ther. 2007;26:757–66. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 55.Kelly CP, Green PH, Murray JA, DiMarino AJ, Arsenescu RI, Colatrella AM, Leffler DA, Alexander TJ, Jacobstein D, Leon F, Jiang J, Fedorak RN. Safety, Tolerability and Effects On Intestinal Permeability of Larazotide Acetate in Celiac Disease: Results of a Phase IIB 6-Week Gluten-Challenge Clinical Trial. Gastro. 2009;136,5(Supplement 1):A-474. [Google Scholar]
- 56.Márquez L, et al. Effects of haptoglobin polymorphisms and deficiency on susceptibility to inflammatory bowel disease and on severity of murine colitis. Gut. 2012;61:528–34. doi: 10.1136/gut.2011.240978. [DOI] [PubMed] [Google Scholar]
- 57.Márquez L, et al. Effects of haptoglobin polymorphisms and deficiency on susceptibility to inflammatory bowel disease and on severity of murine colitis. Gut. 2012;61:528–34. doi: 10.1136/gut.2011.240978. [DOI] [PubMed] [Google Scholar]
- 58.Wan C, et al. Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids. 2007;32:101-8. doi: 10.1007/s00726-005-0292-8. [DOI] [PubMed] [Google Scholar]; Chen YC, et al. Haptoglobin polymorphism as a risk factor for chronic kidney disease: a case-control study. Am J Nephrol. 2011;33:510–4. doi: 10.1159/000327822. [DOI] [PubMed] [Google Scholar]