Abstract
Many aspects of female reproduction often require intricate timing, ranging from temporal regulation of reproductive hormone secretion to the precise timing of sexual behavior. In particular, in rodents and other species, ovulation is triggered by a surge in pituitary luteinizing hormone (LH) secretion that is governed by a complex interaction between circadian signals arising in the hypothalamus and ovarian-derived estradiol signals acting on multiple brain circuitries. These circadian and hormonal pathways converge to stimulate a precisely-timed surge in gonadotropin-releasing hormone (GnRH) release (i.e., positive feedback), thereby triggering the preovulatory LH surge. Reflecting its control by afferent circadian signals, the preovulatory LH surge occurs at a specific time of day, typically late afternoon in nocturnal rodents. Although the specific mechanisms mediating the hormonal and circadian regulation of GnRH/LH release have remained poorly understood, recent findings now suggest that estradiol and circadian signals govern specific reproductive neuropeptide circuits in the hypothalamus, including the newly-identified kisspeptin and RFamide-related peptide-3 (RFRP-3) neuronal populations. Neurons producing kisspeptin, the protein product of the Kiss1 gene, and RFRP-3 have been shown to provide excitatory and inhibitory input to GnRH neurons, respectively, and are also influenced by sex steroid and circadian signals. Here, we integrate classic and recent findings to form a new working model for the neuroendocrine regulation of the circadian-timed preovulatory LH surge in rodents. This model proposes kisspeptin and RFRP-3 neuronal populations as key nodal points for integrating and transducing circadian and hormonal signals to the reproductive axis, thereby governing the precisely-timed LH surge.
Keywords: kisspeptin, Kiss1, GnIH, RFRP-3, GnRH, SCN, clock, ovulation
Introduction
Gonadotropin hormones secreted by the pituitary govern gonadal physiology, including ovulation. Gonadotropins themselves are regulated by forebrain neurons that secrete gonadotropin-releasing hormone (GnRH). In rodents, at most times during the estrous cycle, low levels of estradiol (E2) secreted from the ovaries provide negative feedback on the upstream brain circuits that regulate GnRH release, thereby regulating tonic pulsatile GnRH secretion. However, in females during proestrus, rising levels of E2 paradoxically provide positive feedback signals to the GnRH system, resulting in substantially increased GnRH secretion (1-3). This positive feedback-induced GnRH surge in turn causes the pituitary to generate a surge in luteinizing hormone (LH) secretion, which induces ovulation (4, 5).
As with most physiological processes, reproduction—and ovulation in particular—requires exquisite timing and regulation. In female rodents, ovulation is timed to occur in the hours just prior to or coincident with female sexual behavior, which occurs primarily on late proestrus and early estrus, when E2 levels are elevated. This coordinated timing of ovulation and subsequent sexual behavior ensures that the two processes are temporally synchronized with each other, thereby enhancing the probability of fertilization after mating (6). The preovulatory LH surge is therefore optimally timed; in nocturnal rodents, it reliably occurs in the late afternoon or early evening of proestrus, just prior to mating. Ovariectomized (OVX) female rodents completely lacking E2 do not exhibit an LH surge, highlighting the essential requirement for elevated E2 in the generation of the LH surge (7-9). However, similar to gonadally-intact proestrus females, OVX female rodents that are supplemented with elevated levels of E2 (OVX+E2) display robust LH surges that occur once daily at a specific time, exclusively around the onset of darkness (7-9). This precisely-timed nature of preovulatory LH release suggests involvement of the circadian system, specifically the suprachiasmatic nucleus (SCN), the circadian pacemaker located in the mammalian hypothalamus. Here we evaluate the current knowledge of the neuroanatomical, cellular, and molecular mechanisms underlying the generation and timing of the preovulatory LH surge in rodents, focusing on recently-identified signaling factors and circuits in the hypothalamus.
Circadian Component of the LH Surge
Brief overview and functional anatomy of the SCN, the mammalian circadian clock
In mammals, the circadian pacemaker located in the SCN of the hypothalamus governs the circadian rhythms of most biological processes, from gene expression to overt behavior. Several lines of evidence accumulated over the past four decades, primarily from rodent models, demonstrate the importance of the SCN as a central circadian pacemaker. First, in both light-dark cycles and static environmental conditions (such as constant darkness), the SCN demonstrates near-24 h oscillations in neuronal activity, glucose consumption, and gene expression (10-16). Second, in vivo ablation or surgical isolation of the SCN leads to arrhythmia in brain electrical activity, physiology, and overt behavior (17-20). Third, transplanting fetal SCN tissue into an arrhythmic host animal with an ablated SCN restores circadian rhythmicity in the host animal with the donor animal’s period (21-24). Fourth, SCN tissue explants retain near 24-h rhythmicity in electrical activity and neuropeptide release in vitro, demonstrating that SCN cells are themselves autonomous clocks that do not rely on external neural or hormonal input to generate rhythmicity (25-27). Lastly, the external environment—and in particular, light—can modulate and synchronize the period and phase of circadian rhythms, and supporting its role as the circadian clock, the SCN receives direct light input from melanopsin-containing photosensitive cells in the retina (via the retinohypothalamic tract) (28, 29).
The SCN is functionally divided into two parts: the ventrolateral “core” and the dorsomedial “shell.” The core neurons receive direct light input from the retinohypothalamic tract, and then transmit this information to the shell neurons via extensive neural projections (30). Although less common, some neural connections also project back from the shell to the core (31). Intriguingly, in both the core and the shell, each SCN neuron is rhythmic on its own (32); however, by synchronizing themselves as an interactive network, SCN neurons produce rhythmic output that is precise, robust, and more immune to disruptions (33). Neural fibers from cells in the SCN project to numerous brain areas, including other hypothalamic regions, such as (but not limited to) the subparaventricular zone, paraventricular nucleus (PVN), preoptic area, the periventricular nucleus, and the dorsomedial nucleus (DMN) (30, 34).
Communication of temporal information within and from the SCN relies on the activity of different neurotransmitters and neuropeptides within the SCN. The SCN produces several neuropeptides such as gastrin-releasing peptide, substance P, and prokineticin 2, as well as GABA (35, 36). However, two other SCN neuropeptides, vasoactive intestinal polypeptide (VIP) and arginine vasopressin (AVP), have been most strongly implicated in circadian rhythm biology. These two neuropeptides are synthesized in different regions of the SCN, with core and shell SCN neurons expressing and releasing VIP and AVP, respectively (37). In rodents, the SCN as a whole highly expresses VPAC2R, a VIP receptor subtype (38, 39), and both VIP and VPAC2R mRNA expression levels, as well as VIP release, demonstrate in vitro circadian rhythmicity in SCN slices (40-42). Within the SCN, VIP neurons receive and communicate photic information from the core to shell neurons, as well as to other core neurons, and may also communicate circadian information to downstream tissues, including reproductive circuits (see below) (43). Indeed, evidence from transgenic mice lacking the gene for either VIP or VPAC2R implicates VIP as an important communicator to extra-SCN areas because these mice display disrupted or altered circadian rhythms, and sometimes arrhythmicity (44, 45).
AVP, highly expressed in the SCN shell, demonstrates circadian rhythmicity in vivo, with highest levels during the subjective day (46). However, AVP is unlikely to contribute to endogenous rhythm generation within the SCN since the AVP-deficient Brattleboro rat displays intact circadian rhythmicity for several behaviors (47, 48). Yet, AVP may enhance the SCN’s firing rate since several studies demonstrate an excitatory response of SCN neurons to AVP (49-51). Moreover, AVP serves as an important output signal of the SCN and has been implicated in regulating several circadian rhythms, including circadian stress responses (52-54) and perhaps reproductive function (discussed below). Supporting this role, SCN neurons containing AVP project to several hypothalamic areas, including the PVN, subPVN, preoptic area, AVPV, and the DMN (55, 56).
Relation between the circadian clock and the LH surge
There is good evidence that the circadian clock in the SCN governs the timing of the preovulatory LH surge in rodents. First, a series of groundbreaking experiments performed over half a century ago in female rodents demonstrated that not only does the LH surge reliably occur in the late afternoon of proestrus, but treatment with dibenamine, atropine, or barbiturate delays the LH surge approximately 24 h until the late afternoon of the next day (57-61), suggesting a circadian regulation of the events generating the LH surge. Second, OVX rodents treated with constant levels of elevated E2 demonstrate a reliable late afternoon LH surge which repeats daily at the same exact time, also hinting at an underlying circadian component (9, 62, 63). Third, SCN neurons are activated immediately before the LH surge, as demonstrated by increased c-Fos expression (a marker of general cell activation) (64). Fourth, experimentally-induced phase shifts in the circadian rhythmicity of behaviors known to be driven by the SCN result in congruent shifts in the timing of the LH surge. For example, in nocturnal rodents, locomotor activity is timed by the SCN and, like the LH surge, begins around the onset of darkness (19, 65-67). Light cycle manipulations that change the period or phase of the circadian locomotor rhythm also modify the timing of the LH surge; however, the new onset of the LH surge is still tightly coupled to the new onset of locomotor activity (68-70). For example, in Syrian hamsters with the circadian τ mutation (which leads to a much shortened locomotor activity rhythm), the onset of the LH surge is still perfectly synchronized with earlier onset of locomotor activity (71). Because the timing of the LH surge is tightly tethered to the onset of locomotor activity, which itself is known to be timed by the SCN, it is likely that the LH surge is also timed by the SCN. Lastly, physical ablation of the SCN in female Syrian hamsters and rats (72-74), as well as genetic disabling of circadian clock genes in mice (75), abolishes the LH surge and ovulation, even in the presence of E2 supplementation, suggesting that the SCN clockwork is critical for the occurrence of the LH surge in rodents.
Connectivity of the SCN to GnRH neurons
If, as the evidence above suggests, a circadian pacemaker in the SCN times the LH surge, then how does the SCN communicate with the reproductive axis? Several anatomical connections have been identified from the SCN (both core and shell) to areas potentially involved in generating the LH surge (76-80). At least two physical pathways (direct and indirect) link the SCN to GnRH neurons. The direct pathway includes VIPergic core neurons directly targeting GnRH neurons that express the VIP receptor VPAC2R (79, 81). There is some evidence, albeit limited, that this VIP connection plays a role in the preovulatory LH surge. First, VIP-targeted GnRH neurons express c-Fos at the time of the preovulatory LH surge, and blocking VIP signaling via in vivo antisense antagonism in E2-treated rats delays the surge (82-84). Second, in line with E2’s requisite role in positive feedback, stimulatory effects of VIP on GnRH neurons are E2-dependent, evidenced by the finding that VIP treatment promotes electrical activity of GnRH neurons in brain slices of OVX mice only when E2 is present (85). Third, GnRH neuron electrical responses to exogenous VIP are circadian-timed, with peak responses around the predicted onset of the LH surge (85). Lastly, vip-null mice present some degree of infertility (45, 86); however, mice lacking a functional gene for VPAC2R (vipr2-null) show no impairment in fertility (44). On the whole, these data suggest that VIP may facilitate some aspects of the circadian release of GnRH that leads to the preovulatory LH surge, though additional evidence supporting this conjecture is needed.
The indirect pathway linking the SCN and GnRH axis begins with AVPergic shell neurons innervating the hypothalamic anteroventral periventricular nucleus (AVPV), which then projects to GnRH neurons (76-78, 80, 87). Several pieces of evidence strongly suggest that AVP may convey circadian information to the AVPV as part of the LH surge mechanism. First, the AVPV expresses V1a (an AVP receptor subtype), and this V1a expression increases with E2 treatment in rats and mice (88, 89). Second, the approximate time of peak AVP expression in SCN shell neurons in mice occurs in the late afternoon/early evening, similar to when the LH surge occurs (90). Third, experimentally increasing extracellular AVP levels in the AVPV area with reverse microdialysis produces an LH surge in SCN-lesioned OVX+E2 rats that do not normally display LH surges (91). Fourth, in mice with a mutation in the core circadian gene Clock that leads to a lack of preovulatory LH surges, central administration of AVP stimulates LH secretion when given during the afternoon (92). Conversely, central injection of a V1a antagonist in proestrus rats prevents the late afternoon LH surge (93). Although no genetic mouse studies have directly tested the requirement of AVP signaling for the LH surge, homozygous female Brattleboro rats (which have a spontaneous mutation in the AVP gene) have several reproductive impairments, including abnormal estrous cycles and reduced conception rates (94).
The above findings implicate an interconnected neuronal network that guides the circadian regulation of the preovulatory LH surge, with VIP and AVP neurons providing direct and indirect circadian input to GnRH neurons, respectively. However, this picture is complicated by conflicting findings on the exact nature and timing of the actions of VIP and AVP. For instance, while VIP may have an activational effect on GnRH neurons, other evidence suggests that VIP may be inhibitory during the LH surge (84, 95). Likewise, AVP treatment is effective at inducing the LH surge in the late afternoon, but not at other times of the day (96). In addition, the SCN sends axonal fibers to other targets regions besides GnRH neurons and the AVPV, opening the possibility that additional indirect pathways may be involved in gating the timing of GnRH secretion.
Potential Neural Populations Mediating Circadian Control of GnRH
The kisspeptin system: function and neuroanatomy
A key neuropeptide evidenced to regulate GnRH neurons is the recently-identified kisspeptin. Humans and mice lacking either the kisspeptin receptor, Kiss1R (formerly termed GPR54), or Kiss1, the gene that encodes kisspeptin, demonstrate hypogonadotropic hypogonadism, a condition where pubertal maturation and reproductive function are severely impaired due to a deficiency in GnRH secretion (97-100). Kiss1R is expressed in GnRH neurons, indicating the potential for kisspeptin to directly signal to these cells (101, 102). Numerous studies in rodents and other species have shown that kisspeptin directly stimulates GnRH neurons, leading to enhanced GnRH secretion and subsequent gonadotropin secretion (101-106). Likewise, kisspeptin treatment potently activates electrical firing of GnRH neurons in hypothalamic slices (107, 108). These findings indicate that kisspeptin might play a role in generating the LH surge via activation of GnRH neurons. Indeed, blocking kisspeptin signaling with Kiss1R antagonists or kisspeptin antibody treatments alleviates, or in many cases, abolishes the LH surge (109-111). Moreover, Kiss1R- and Kiss1-null mice do not exhibit LH surges, even after appropriate E2 treatment (112), suggesting that kisspeptin signaling is indeed a critical component of the LH surge mechanism. However, it should be noted that at least one study is at odds with these findings. Dungan et al. reported that OVX+E2 Kiss1R-null mice are surprisingly able to display small LH surges (113). The reasons for these discrepant findings are not known, but may relate to differences in the specific mouse transgenic model or surge paradigm used, both of which were slightly different between the two studies (112, 113).
In rodents, Kiss1 is expressed in two discrete regions of the hypothalamus, the anatomical continuum comprising the AVPV and the rostral periventricular nucleus (PeN) and, more caudally, the arcuate nucleus (ARC) (103, 114-116), as well as some extra-hypothalamic sites such as the amygdala (117). The source of kisspeptin signaling necessary for specifically promoting the LH surge is likely within the AVPV/PeN, as this region both communicates with GnRH neurons and is critical for generating the LH surge (87). Unlike GnRH neurons, which lack estrogen receptor α (ERα) (118, 119), the ER subtype known to mediate positive feedback, the AVPV/PeN does contain ERα-expressing neurons (120, 121). While the other main ER subtype, ERβ, is also present in the AVPV, antagonism or genetic knockout of this receptor does not prevent LH surges, indicating that ERβ is not critical for the LH surge process (122, 123). Functionally, lesioning the AVPV leads to acyclicity and a lack of E2-induced LH surges (74, 124, 125). Discrete implantation of E2 in or near the AVPV induces an LH surge, while microimplants of antiserum against E2 in the same area inhibits the surge (126-129). The AVPV region as a whole also expresses increased c-Fos at the time of the LH surge, supporting a role for the AVPV in the E2–mediated positive feedback process (130). Importantly, SCN neurons exhibit increased c-Fos expression just prior to the LH surge, and this expression precedes the subsequent peaks in c-Fos expression in the AVPV and GnRH neurons (64), illustrating the sequential link of activation between the SCN, AVPV, and GnRH neurons.
Emerging evidence now suggests that the c-Fos- and ERα-expressing neurons in the AVPV that participate in the LH surge are kisspeptin neurons. First, Kiss1-expressing neurons located in the AVPV/PeN express ERα, and Kiss1 gene expression in the AVPV/PeN of female rodents increases in response to E2 (115, 116, 131, 132). Second, Kiss1 neurons in the AVPV/PeN display c-Fos at the time of the LH surge in proestrous or OVX+E2-treated females but not in diestrus females or OVX females (112, 131-133). Third, both the LH surge event and Kiss1 neurons in the AVPV/PeN are sexually dimorphic (favoring females), providing further correlative evidence for the role of AVPV/PeN Kiss1 system in controlling the LH surge (115, 134). Specifically, unlike females, adult male rodents do not demonstrate LH surges even when treated with E2 (135, 136). Correspondingly, males also have fewer Kiss1 neurons and lower Kiss1 expression levels in the AVPV/PeN than do females (114, 115). These findings suggest that higher numbers of AVPV/PeN Kiss1 neurons in female rodents may comprise the underlying neural sex difference that generates the LH surge in females, but not males. In contrast, ARC Kiss1 neurons, which are inhibited by E2 treatment (111, 116, 131), are unlikely to play a significant role in generating the LH surge in rodents, though this may not be the case for some species such as sheep (137, 138).
Influence of the circadian system on kisspeptin neurons
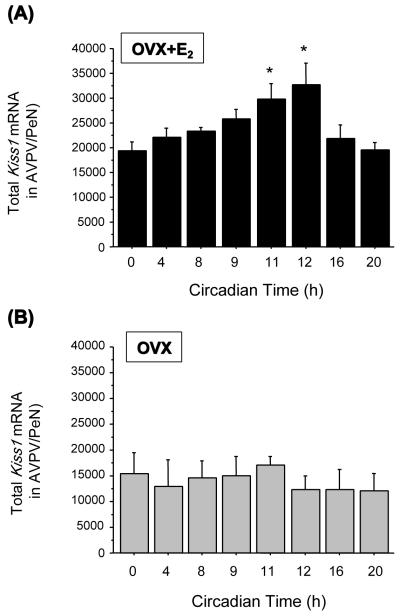
If the LH surge is governed in part by Kiss1 neurons in the AVPV/PeN, do these neurons also participate in the circadian component of the LH surge? Anatomically, Kiss1 neurons in the AVPV/PeN are poised to mediate indirect circadian signaling to GnRH neurons, as the SCN sends axonal projections to the AVPV/PeN (76, 80), which itself projects to GnRH neurons (139). The most compelling evidence for an involvement of kisspeptin signaling in the circadian gating of the LH surge comes from recent findings that AVPV/PeN Kiss1 neurons demonstrate robust circadian patterns of gene expression and neuronal activation, coincident with the circadian pattern of LH (133). Specifically, in a study by Robertson et al. (2009), female mice were entrained to a 14-10 light-dark cycle for several weeks, then OVX and implanted with an E2 implant that produces constant, steady-state E2 levels. This E2 paradigm had previously been shown to produce a reliable circadian-timed LH surge each day around the time of lights off (140). On the day of the surgery, OVX+E2 mice were then permanently transferred into constant darkness to remove changes in external light cues, which could potentially mask or induce circadian rhythmicity of Kiss1 circuits. All mice were then sacrificed 2 days later at one of eight different circadian times throughout the day. Under these constant conditions, OVX+E2 mice sacrificed an hour before or at the onset of subjective night [defined as circadian time (CT) 12] showed a robust surge of LH whereas low levels of LH were detected in the morning (CT 0, 4), early afternoon (CT 8), and late night (CT 20) (133). In synchrony with the circadian pattern of LH secretion, OVX+E2 mice demonstrated a strong circadian pattern of Kiss1 gene expression, with higher Kiss1 mRNA levels in the AVPV/PeN (determined via in situ hybridization) at CT 11 and CT 12 than at earlier or later time points (e.g., CT 0, CT 4, and CT 20) (Figure 1) (133). In addition to circadian changes in Kiss1 gene expression, AVPV/PeN Kiss1 neurons also displayed a robust circadian pattern of c-fos co-expression, with peak c-fos-Kiss1 co-expression occurring in synchrony with peak LH secretion, around CT 11 and 12 (133). These findings suggest that circadian-timed neuronal activation of Kiss1 neurons in the AVPV/PeN is involved in the circadian induction of the preovulatory LH surge. However, this conclusion awaits definitive support from future experiments in which selective blockade of Kiss1 neuronal activity in the AVPV/PeN at the presumptive time of the GnRH/LH surge prevents the surge from occurring.
Figure 1.
Total Kiss1 mRNA in the AVPV/PeN of OVX mice with (a) and without (b) E2 treatment that were sacrificed at different circadian times. In the OVX+E2 mice, an LH surge was detected at CT 11 and CT 12 (see Robertson et al., 2009). (*) indicates a statistical significance of p<0.05 relative to CT 0, CT 4, CT 8, and CT 20.
Given the robust circadian pattern of activation of Kiss1 neurons in OVX+E2 mice, Robertson et al. (2009) also asked whether the typical absence of an LH surge in OVX females could reflect a lack of circadian activation of Kiss1 neurons in the AVPV/PeN. The authors repeated their experiment in OVX mice that were not treated with E2. Interestingly, no circadian changes in LH, Kiss1 gene expression, or Kiss1/c-fos coexpression were detected in these OVX mice, with each of these parameters being low at all time-points (Figure 1) (133). This intriguing data suggests that the circadian activation of Kiss1 neurons in the AVPV/PeN is dependent on the presence of elevated E2, perhaps explaining why OVX females and diestrus females (with only low E2) do not display late afternoon LH surges. Similar findings have recently been reported in female hamsters, in which kisspeptin-Fos-immunoreactive co-expression in the AVPV/PeN displays circadian changes which are much weaker (though not eliminated) in OVX than OVX+E2 hamsters (141). The reason for the slightly different results between OVX hamsters (dampened kisspeptin-Fos circadian rhythm) and OVX mice (eliminated Kiss1-Fos rhythm) is unknown, and may reflect a true species difference and/or technical differences (for example, in mice, Kiss1 mRNA was measured whereas in hamsters, kisspeptin immunoreactivity was measured). Unfortunately, circulating E2 levels were not measured in the OVX hamsters to verify that they were completely without E2 at the time of analysis.
Because AVPV/PeN Kiss1 neurons exhibit circadian patterns of gene expression and neuronal activation, it suggests these neurons receive circadian input from the SCN (though the possibility that Kiss1 neurons are themselves autonomous circadian clocks has not been tested). Circadian input to Kiss1 neurons in the AVPV/PeN may come from AVP neurons in the shell of the SCN. This conjecture is supported by recent immunohistochemical studies in female mice showing that AVP axonal projections arising from the SCN come in close proximity to AVPV/PeN Kiss1 neurons, a finding confirmed at the electron microscopy level (142). Similar data has come from a recent hamster study in which AVP-containing fibers were found to appose AVPV/PeN kisspeptin cells (141). Importantly, in that study, lesioning the SCN abolished virtually all AVP appositions on kisspeptin neurons, suggesting that the source of the AVP fibers was in fact the SCN (141). Interestingly, in mice, the percentage of kisspeptin neurons apposed by SCN-derived AVP axons increases with E2 treatment (~47% in OVX+E2 vs. ~31% in OVX) (142), perhaps providing some explanation for the observed E2-dependence of circadian regulation of the Kiss1 system. Thus, AVPV/PeN Kiss1 neurons may be gated by the SCN via AVP signaling. In support of this, the AVP receptor subtype V1a has been detected in AVPV/PeN kisspeptin neurons of female hamsters (141). In comparison, VIP connections to AVPV/PeN Kiss1 neurons are far fewer in number and almost non-existent (< 1%, regardless of E2 treatment), at least in mice (142).
Interestingly, the stimulatory effects of kisspeptin on GnRH neurons may also be circadian-gated (141). In hamsters, AVP administration increases c-Fos expression in AVPV/PeN kisspeptin neurons in both the morning and late afternoon, but c-Fos expression in GnRH neurons of the same animals is only upregulated by afternoon AVP treatment (141). This suggests that GnRH cells possess an intrinsic or extrinsic gating mechanism that temporally modulates their responsiveness to kisspeptin input, such that GnRH neurons are more sensitive to kisspeptin signaling in the late afternoon. What allows for this GnRH gating mechanism is unknown, but one possibility is a circadian regulation of the availability or signaling of Kiss1R in GnRH neurons. In support of this, GT1-7 cells (immortalized GnRH cells) show daily changes in the responsiveness to exogenous kisspeptin treatment (143). Furthermore, circadian clock proteins PER2 and BMAL1 demonstrate circadian rhythmicity within GnRH neurons in vivo (144), indicating that GnRH neurons possess the proper circadian machinery, at least in theory, to generate intrinsic circadian signals. VIP signals coming directly from the SCN may also play a role in timing the sensitivity of GnRH cells to incoming kisspeptin signals, though this remains to be tested. Additional support for changes in responsiveness to kisspeptin input comes from studies in rats and humans in which LH responses to kisspeptin administration differ with cycle stage, being more robust during the preovulatory phase (145, 146).
While the data above demonstrate the importance of the AVPV/PeN Kiss1 system in regulating the LH surge, recordings of mouse brain slices curiously demonstrate a lack of response of AVPV kisppeptin neurons to AVP, VIP, or either antagonist, regardless of the stage of the mouse’s estrous cycle (147). It must be determined if such insensitivity to SCN neuropeptides is contingent on specific conditions, or if this is a difference between slice preparations and what occurs in vivo.
The RFRP-3 system: function and anatomy
It has been proposed that to fine-tune the excitability of GnRH neurons and the precise timing of the preovulatory LH surge, an inhibitory component complements the kisspeptin-driven stimulatory component. RFamide-related peptide (RFRP), the mammalian ortholog of the avian gonadotropin-inhibitory hormone (GnIH), modulates the reproductive axis by inhibiting GnRH neurons. Specifically, in mammals, the variant RFRP-3 has been implicated in GnRH neuronal inhibition. Administration of RFRP-3, both centrally and peripherally, leads to a rapid and significant inhibition of LH secretion, as first shown in rodents and later other species (148-150). Although there is some evidence suggesting that RFRP-3 acts in the pituitary, including the presence of the RFRP-3 receptor GPR147 in the pituitary (151), the inhibitory effects of RFRP-3 are likely achieved at the level of GnRH neurons. RFRP-3 treatment strongly decreases the electrical activity of GnRH cells in cultured brain slices, and RFRP-3 administration in vivo leads to suppressed c-Fos expression in GnRH neurons (152-154). Moreover, administration of RF9, an antagonist of the RFRP-3 receptor, leads to enhanced LH secretion in rats, further demonstrating the importance of RFRP-3 in regulating LH release (155) [though it should be noted that RF9 also antagonizes the actions of neuropeptide FF, in addition to RFRP-3, necessitating caution when interpreting these results].
RFRP-3-expressing neurons, found exclusively in the hypothalamic DMN of rodents, send numerous neural projections to GnRH cell bodies in the forebrain and GnRH axonal fibers in the median eminence; more than 40% of GnRH neurons receive RFRP-3 projections (148, 151). RFRP-3 neurons may mediate some aspect of estrogen negative feedback, as these cells express ERα and, in at least one study, showed increased c-Fos expression following E2 administration (148). Since RFRP-3 is inhibitory to GnRH neurons, increased activity of RFRP-3 neurons might be predicted to result in increased inhibition of the reproductive axis, thereby mediating negative feedback. Alternatively, other evidence suggests that estrogen-mediated positive feedback may also involve changes in RFRP-3 neuronal system. Specifically, it has been hypothesized that RFRP-3 neurons are “turned off” around the time of the LH surge, thereby removing inhibition from GnRH neurons. This possibility is supported by recent findings of decreased RFRP-3 mRNA expression in the DMN of OVX+E2 mice as well as decreased RFRP-3 cell numbers in proestrous versus diestrous hamsters, suggesting that elevated estrogen inhibits the RFRP-3 system (151, 156). In addition, the hamster study reported decreased Fos-RFRP-3 co-expression in proestrous females exhibiting an LH surge versus diestrous females. Such an E2-mediated decrease in RFRP-3 signaling might allow for reduced inhibition of GnRH neurons, thereby magnifying the LH surge. However, this issue is controversial, as a similar study in rats showed no difference in RFRP mRNA levels of females that were OVX versus OVX+E2 and P4 (157). Furthermore, in OVX ewes, E2 treatment does not significantly alter RFRP mRNA expression levels (158). Whether these discrepancies are due to species differences or other unaccounted factors has yet to be determined.
Linking the RFRP-3 system to the circadian clock
While RFRP-3 neurons send projections to GnRH neurons, they also receive projections from the SCN. However, while both AVP- and VIP-positive fibers from the SCN extend to the DMN in rodents (30, 151), it is not known which of these, if any, innervate RFRP-3 cells. Regardless, the presence of SCN-derived fibers in the DMN suggests that RFRP-3 neurons may play a role in the circadian component of positive feedback. In support of this, in female Syrian hamsters, RFRP-3 neurons display changes in protein expression levels over the course of the day. Specifically, during the afternoon of proestrus (at the time of the LH surge), RFRP-3-immunoreactive neurons are reduced in number and also show reduced c-Fos coexpression, relative to earlier or later time-points (151). This circadian-timed decrease in the RFRP-3 system suggests a reduced inhibition by RFRP-3 on the GnRH system in the late afternoon. As with Kiss1 neurons, E2 is necessary for the temporal regulation of RFRP-3 neurons; without E2, RFRP-3 neurons do not demonstrate daily changes in c-Fos expression (151). However, it remains to be determined how the SCN signals to RFRP-3 neurons and what the mechanism is underlying the E2-dependence of this signaling. Moreover, since only a minority of RFRP neurons expresses ERα, circadian signals may play a more essential role in regulating RFRP-3 cells than hormonal signals (148).
Additional evidence for a role of RFRP-3 in positive feedback comes from an experiment where the circadian system of female hamsters was experimentally “split” into two separate circadian rhythms whose phases are 180 degrees (12 h) apart [using chronic exposure to constant light (159, 160)]. This “splitting” paradigm is known to cause each half of the bilateral SCN to functionally dissociate from the other and to continue running as a circadian clock with peak activity that is 12 h out of phase with the other half of the SCN. In this split condition, an E2-treated female will exhibit 2 separate bouts of locomotor activity every day that begin ~12 h apart, along with 2 daily LH surges, with each surge occurring at the onset of one the two locomotor activity bouts (161). de la Iglesia et al. (162) showed that when one half of the SCN is active, the ipsilateral population of GnRH neurons is also active, with higher c-Fos co-expression than the contralateral GnRH population. Thus the two “split” LH surges each day are thought to be separately driven by the left and right GnRH populations which are activated ~ 12 h apart. Interestingly, Gibson et al. (2008) determined that the ipsilateral population of RFRP-3 neurons of split females shows lower activity than the contralateral RFRP population (151). Thus, the side of the brain in which GnRH neurons are being temporally activated is the same side where RFRP-3 neurons are concurrently less activated. This suggests that each side of the bilateral SCN may communicate to ipsilateral RFRP-3 neurons, which then signal to GnRH neurons.
Putting the Pieces Together: Current Model of the Circadian-Regulated Preovulatory LH Surge in Rodents
Clearly, many factors influence the rhythmic output of LH secretion, including multiple routes of circadian input to GnRH neurons as well as estrogen feedback to several areas of the hypothalamus and pituitary. While the neuroendocrine effectors kisspeptin and RFRP-3 have only recently been discovered, several studies have now implicated and expounded their roles in the circadian-timed preovulatory LH surge in rodents. At present, AVPV/PeN Kiss1 neurons, which possess ERα, are thought to be key players in the positive feedback event, providing strong stimulatory input directly to GnRH neurons. In response to SCN-derived AVP signals or other yet-to-be defined circadian input, AVPV/PeN Kiss1 neurons, which co-express the V1a receptor, exhibit robust circadian patterns of gene expression and c-fos-co-expression, peaking in the late afternoon/early evening. This circadian activation of AVPV/PeN Kiss1 neurons is dependent on the sex steroid milieu, thereby allowing Kiss1 neurons to serve as integrators of both circadian and E2 signals that are each essential for the LH surge. The SCN also projects to DMN RFRP-3 neurons, which show circadian patterns of neuronal activation that are opposite to those of Kiss1 neurons, being diminished in the late afternoon/early evening. However, unlike AVPV/PeN Kiss1 neurons, it is not known which neurotransmitters the SCN uses to communicate with RFRP-3 cells, nor is it known if RFRP-3 cells express receptors for key SCN signaling factors. Moreover, the functional role of SCN-derived VIP projections, particularly those that direct target GnRH neurons, in the circadian-timed LH surge remains unknown. GnRH neurons directly integrate multiple inputs from kisspeptin (stimulatory), RFRP-3 (inhibitory), and core VIP (likely stimulatory) neurons and may respond to some of these signals in a temporally-gated manner, resulting in a final coordinated output of high GnRH secretion that triggers the LH surge at a specific time. Table 1 summarizes changes in the expression levels of the main neuropeptides involved in regulating the timing and generation of the LH surge, and Figure 2 outlines our working model of the circadian and neuroendocrine network responsible for generating the preovulatory LH surge in rodents. This information will provide a framework for future experiments exploring the mechanism of the preovulatory LH surge in rodents and other species.
Table 1.
Summary of mRNA expression levels of neuropeptides potentially involved in the regulation of the preovulatory LH surge in female rodents, as well as corresponding data on the status of GnRH neuronal activation (Fos in GnRH) and the presence of an LH surge at different times of day (AM versus PM). Where no mRNA data is available, data for protein (immunoreactivity measures) is given instead and noted.
| Neuropeptide (region) |
OVX | OVX+E2 | Proestrus | References | |||
|---|---|---|---|---|---|---|---|
| AM | PM | AM | PM | AM | PM | ||
| AVP (SCN) | High | Low | High | Low | High | Low | (90, 163-166) |
| VIP (SCN) | Low | High/ Low* |
Low | High | Low | High | (42, 90, 164, 165, 167) |
| Kiss1 (AVPV) | Low | Low | Med | High | Med | High | (131-133) |
|
RFRP-3
(DMN) |
Med/ High |
Med/ High |
High | High/ Low* |
High (protein) |
Med (protein) |
(151, 156, 157) |
| Fos in GnRH | Low | Low | Low | High | Low | High | (64, 83, 84, 112, 168, 169) |
| LH surge | No | No | No | Yes | No | Yes | (7, 9, 62, 63, 70, 132, 133, 140, 151) |
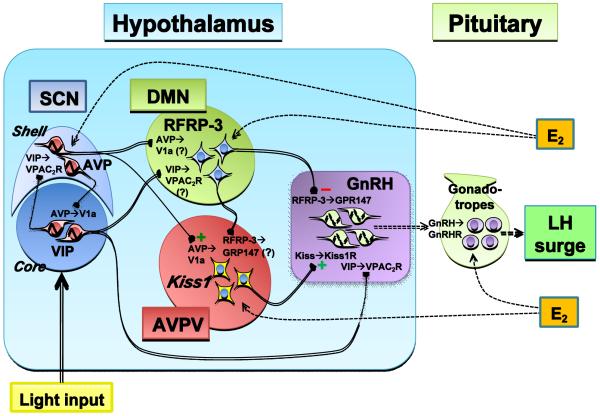
Figure 2.
Schematic summarizing the network of neurons and respective neuropeptides thought to participate in the regulation and timing of the female rodent preovulatory LH surge. This working model suggests that several levels of control are upstream of GnRH neurons: hormonal (ovarian-derived E2), circadian (SCN-derived AVP and VIP), and intermediate activators/inhibitors (kisspeptin and RFRP-3). VIP neurons in the SCN project to the DMN and GnRH neurons, and AVP neurons in the SCN project to the DMN and AVPV/PeN Kiss1 neurons, potentially providing circadian information relevant to the timing of the LH surge. Kiss1 neurons directly stimulate GnRH neurons, while RFRP-3 neurons directly inhibit them. Some RFRP-3 neurons also project to the AVPV region, but it is not known if these projections target Kiss1 neurons. GnRH neurons integrate input from Kiss1, RFRP-3, and VIP neurons directly and may respond to some of these signals in a temporally-gated manner. In addition, GnRH neurons may possess endogenous circadian oscillators, the function of which is unknown. See text for more detailed description and explanation of abbreviations.
Key: Solid lines with boutons represent neuronal connections, dotted lines with arrowhead represent hormonal pathways; Solid sinusoid and dotted sinusoid represents cell-autonomous oscillatory activity and potential cell-autonomous oscillatory activity, respectively.
Despite the recent advances in our knowledge of the LH surge mechanism, several important questions still remain to be answered. Regarding both Kiss1 and RFRP-3 neurons, does ERα oscillate in either population over the course of the day or between different stages of the estrous cycle? How does the SCN communicate with and regulate RFRP-3 neurons in the DMN? Do GnRH neurons exhibit circadian-dependent changes in sensitivity to RFRP-3 signals, as they appear to for kisspeptin, and do Kiss1R and GPR147 expression levels oscillate in a circadian manner in GnRH neurons? If these receptors do oscillate, are these temporal changes cell-autonomous or mediated by upstream SCN signaling? Lastly, do the RFRP-3 and Kiss1 populations communicate with each other? Some RFRP-3 neurons project to the AVPV and, conversely, some kisspeptin fibers have been observed in the DMN (148), but this issue needs to be examined in more detail. Answering these questions will improve our understanding of the complexities that underlie the circadian basis of GnRH/LH secretion during E2-medaited positive feedback, both in rodents and other species.
Acknowledgements
Research support provided by NICHD grants R01 HD065856 and R00 HD056157, NSF grant IOS-1025893, and a UCSD Interdisciplinary Collaboratories Fellowship.
References
- 1.Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology. 1992;130(5):2978–84. doi: 10.1210/endo.130.5.1572305. [DOI] [PubMed] [Google Scholar]
- 2.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127(3):1375–84. doi: 10.1210/endo-127-3-1375. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264(5585):461–3. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press, Ltd; New York: 1994. pp. 613–58. [Google Scholar]
- 5.Goodman RL, Inskeep EK. Neuroendocrine control of the ovarian cycle of the sheep. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press Ltd; New York: 2006. pp. 2389–447. [Google Scholar]
- 6.Kauffman AS. Mammalian Female Sexual Behavior and Hormones. In: Breed MD, Moore J, editors. Encyclopedia of Animal Behavior. Academic Press; Oxford: 2010. pp. 355–69. [Google Scholar]
- 7.Caligaris L, Astrada JJ, Taleisnik S. Release of luteinizing hormone induced by estrogen injection into ovariectomized rats. Endocrinology. 1971;88(4):810–5. doi: 10.1210/endo-88-4-810. [DOI] [PubMed] [Google Scholar]
- 8.Norman RL, Spies HG. Neural control of the estrogen-dependent twenty-four-hour periodicity of LH release in the golden hamster. Endocrinology. 1974;95(5):1367–72. doi: 10.1210/endo-95-5-1367. [DOI] [PubMed] [Google Scholar]
- 9.Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–6. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- 10.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. Oxford University Press; New York: 1991. [Google Scholar]
- 11.Schwartz WJ, Gainer H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197(4308):1089–91. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- 12.Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247(1):154–8. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- 13.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89(4):655–67. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosewell KL, Siwicki KK, Wise PM. A period (per)-like protein exhibits daily rhythmicity in the suprachiasmatic nuclei of the rat. Brain Res. 1994;659(1-2):231–6. doi: 10.1016/0006-8993(94)90884-2. [DOI] [PubMed] [Google Scholar]
- 15.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280(5369):1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 16.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389(6650):512–6. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 17.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979;76(11):5962–6. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 19.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69(6):1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- 21.Sawaki Y, Nihonmatsu I, Kawamura H. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci Res. 1984;1(1):67–72. doi: 10.1016/0168-0102(84)90031-2. [DOI] [PubMed] [Google Scholar]
- 22.Drucker-Colin R, Aguilar-Roblero R, Garcia-Hernandez F, Fernandez-Cancino F, Bermudez Rattoni F. Fetal suprachiasmatic nucleus transplants: diurnal rhythm recovery of lesioned rats. Brain Res. 1984;311(2):353–7. doi: 10.1016/0006-8993(84)90099-4. [DOI] [PubMed] [Google Scholar]
- 23.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7(6):1626–38. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 25.Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245(1):198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- 26.Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982;34(3):283–8. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- 27.Earnest DJ, Sladek CD. Circadian rhythms of vasopressin release from individual rat suprachiasmatic explants in vitro. Brain Res. 1986;382(1):129–33. doi: 10.1016/0006-8993(86)90119-8. [DOI] [PubMed] [Google Scholar]
- 28.Moore RY. The retinohypothalamic tract, suprachiasmatic hypothalamic nucleus and central neural mechanisms of circadian rhythm regulation. In: Suda M, Hayaishi O, Hakagawa H, editors. Biological Rhythms and their Central Mechanism. Elsevier/North Holland; Amsterdam: 1979. pp. 343–54. [Google Scholar]
- 29.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–49. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916(1-2):172–91. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 31.Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res. 1999;819(1-2):23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]
- 32.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 33.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130(1):165–83. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–10. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 36.Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28(3):145–51. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Card JP, Fitzpatrick-McElligott S, Gozes I, Baldino F., Jr. Localization of vasopressin-, vasoactive intestinal polypeptide-, peptide histidine isoleucine- and somatostatin-mRNA in rat suprachiasmatic nucleus. Cell Tissue Res. 1988;252(2):307–15. doi: 10.1007/BF00214373. [DOI] [PubMed] [Google Scholar]
- 38.Besson J, Sarrieau A, Vial M, Marie JC, Rosselin G, Rostene W. Characterization and autoradiographic distribution of vasoactive intestinal peptide binding sites in the rat central nervous system. Brain Res. 1986;398(2):329–36. doi: 10.1016/0006-8993(86)91493-9. [DOI] [PubMed] [Google Scholar]
- 39.Vertongen P, Schiffmann SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Ann N Y Acad Sci. 1998;865:412–5. doi: 10.1111/j.1749-6632.1998.tb11206.x. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara K, Honma S, Katsuno Y, Honma K. Circadian release of excitatory amino acids in the suprachiasmatic nucleus culture is Ca(2+)-independent. Neurosci Res. 2000;36(3):245–50. doi: 10.1016/s0168-0102(99)00131-5. [DOI] [PubMed] [Google Scholar]
- 41.Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124(2):143–51. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999;63(2):262–7. doi: 10.1016/s0169-328x(98)00289-7. [DOI] [PubMed] [Google Scholar]
- 43.Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152(2-3):165–75. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109(4):497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 45.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R939–49. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 46.Reppert SM, Artman HG, Swaminathan S, Fisher DA. Vasopressin exhibits a rhythmic daily pattern in cerebrospinal fluid but not in blood. Science. 1981;213(4513):1256–7. doi: 10.1126/science.7268432. [DOI] [PubMed] [Google Scholar]
- 47.Peterson GM, Watkins WB, Moore RY. The suprachiasmatic hypothalamic nuclei of the rat. VI. Vasopressin neurons and circadian rhythmicity. Behav Neural Biol. 1980;29(2):236–45. doi: 10.1016/s0163-1047(80)90573-7. [DOI] [PubMed] [Google Scholar]
- 48.Groblewski TA, Nunez AA, Gold RM. Circadian rhythms in vasopressin deficient rats. Brain Res Bull. 1981;6(2):125–30. doi: 10.1016/s0361-9230(81)80036-6. [DOI] [PubMed] [Google Scholar]
- 49.Shibata S, Moore RY. Electrical and metabolic activity of suprachiasmatic nucleus neurons in hamster hypothalamic slices. Brain Res. 1988;438(1-2):374–8. doi: 10.1016/0006-8993(88)91367-4. [DOI] [PubMed] [Google Scholar]
- 50.Liou SY, Albers HE. Single unit response of suprachiasmatic neurons to arginine vasopressin (AVP) is mediated by a V1-like receptor in the hamster. Brain Res. 1989;477(1-2):336–43. doi: 10.1016/0006-8993(89)91424-8. [DOI] [PubMed] [Google Scholar]
- 51.Mihai R, Coculescu M, Wakerley JB, Ingram CD. The effects of [Arg8]vasopressin and [Arg8]vasotocin on the firing rate of suprachiasmatic neurons in vitro. Neuroscience. 1994;62(3):783–92. doi: 10.1016/0306-4522(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 52.Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580(1-2):62–7. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- 53.Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16(17):5555–65. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalsbeek A, van der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J Neuroendocrinol. 1996;8(4):299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- 55.Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM, Pevet P. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci. 2008;27(4):818–27. doi: 10.1111/j.1460-9568.2008.06057.x. [DOI] [PubMed] [Google Scholar]
- 56.Dai J, Swaab DF, Van der Vliet J, Buijs RM. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J Comp Neurol. 1998;400(1):87–102. doi: 10.1002/(sici)1096-9861(19981012)400:1<87::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 57.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- 58.Everett JW, Sawyer CH, Markee JE. A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology. 1949;44(3):234–50. doi: 10.1210/endo-44-3-234. [DOI] [PubMed] [Google Scholar]
- 59.Sawyer CH, Everett JW, Markee JE. A neural factor in the mechanism by which estrogen induces the release of luteinizing hormone in the rat. Endocrinology. 1949;44(3):218–33. doi: 10.1210/endo-44-3-218. [DOI] [PubMed] [Google Scholar]
- 60.Siegel HI, Bast JD, Greenwald GS. The effects of phenobarbital and gonadal steroids on periovulatory serum levels of luteinizing hormone and follicle-stimulating hormone in the hamster. Endocrinology. 1976;98(1):48–55. doi: 10.1210/endo-98-1-48. [DOI] [PubMed] [Google Scholar]
- 61.Stetson MH, Watson-Whitmyre M. The neural clock regulating estrous cyclicity in hamsters: gonadotropin release following barbiturate blockade. Biol Reprod. 1977;16(4):536–42. [PubMed] [Google Scholar]
- 62.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- 63.Norman RL, Blake CA, Sawyer CH. Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology. 1973;93(4):965–70. doi: 10.1210/endo-93-4-965. [DOI] [PubMed] [Google Scholar]
- 64.Tsukahara S. Increased Fos immunoreactivity in suprachiasmatic nucleus before luteinizing hormone surge in estrogen-treated ovariectomized female rats. Neuroendocrinology. 2006;83(5-6):303–12. doi: 10.1159/000095341. [DOI] [PubMed] [Google Scholar]
- 65.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382(6594):810–3. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 66.Stetson MH, Anderson PJ. Circadian pacemaker times gonadotropin release in free-running female hamsters. Am J Physiol. 1980;238(1):R23–7. doi: 10.1152/ajpregu.1980.238.1.R23. [DOI] [PubMed] [Google Scholar]
- 67.Stetson MH, Gibson JT. The estrous cycle in golden hamsters: a circadian pacemaker times preovulatory gonadotropin release. J Exp Zool. 1977;201(2):289–94. doi: 10.1002/jez.1402010212. [DOI] [PubMed] [Google Scholar]
- 68.Alleva JJ, Waleski MV, Alleva FR. A biological clock controlling the estrous cycle of the hamster. Endocrinology. 1971;88(6):1368–79. doi: 10.1210/endo-88-6-1368. [DOI] [PubMed] [Google Scholar]
- 69.Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci U S A. 1976;73(8):2923–7. doi: 10.1073/pnas.73.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moline ML, Albers HE. Response of circadian locomotor activity and the proestrous luteinizing hormone surge to phase shifts of the light-dark cycle in the hamster. Physiol Behav. 1988;43(4):435–40. doi: 10.1016/0031-9384(88)90116-3. [DOI] [PubMed] [Google Scholar]
- 71.Lucas RJ, Stirland JA, Darrow JM, Menaker M, Loudon AS. Free running circadian rhythms of melatonin, luteinizing hormone, and cortisol in Syrian hamsters bearing the circadian tau mutation. Endocrinology. 1999;140(2):758–64. doi: 10.1210/endo.140.2.6538. [DOI] [PubMed] [Google Scholar]
- 72.Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci. 1977;198(1132):279–96. doi: 10.1098/rspb.1977.0098. [DOI] [PubMed] [Google Scholar]
- 73.Stetson MH, Watson-Whitmyre M. Nucleus suprachiasmaticus: the biological clock in the hamster? Science. 1976;191(4223):197–9. doi: 10.1126/science.942799. [DOI] [PubMed] [Google Scholar]
- 74.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31(2):147–57. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 75.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–73. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6(13):1715–22. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468(3):361–79. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433(3):312–34. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- 79.Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384(4):569–79. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 80.Watson RE, Jr., Langub MC, Jr., Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 1995;689(2):254–64. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- 81.Smith MJ, Jiennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology. 2000;141(11):4317–20. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- 82.Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990;87(13):5163–7. doi: 10.1073/pnas.87.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 1994;134(6):2636–44. doi: 10.1210/endo.134.6.8194489. [DOI] [PubMed] [Google Scholar]
- 84.Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137(9):3696–701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- 85.Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology. 2008;149(6):3130–6. doi: 10.1210/en.2007-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol. 2008;211(1):67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57(2):277–87. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalamatianos T, Kallo I, Goubillon ML, Coen CW. Cellular expression of V1a vasopressin receptor mRNA in the female rat preoptic area: effects of oestrogen. J Neuroendocrinol. 2004;16(6):525–33. doi: 10.1111/j.1365-2826.2004.01199.x. [DOI] [PubMed] [Google Scholar]
- 89.Funabashi T, Shinohara K, Mitsushima D, Kimura F. Estrogen increases arginine-vasopressin V1a receptor mRNA in the preoptic area of young but not of middle-aged female rats. Neurosci Lett. 2000;285(3):205–8. doi: 10.1016/s0304-3940(00)01069-7. [DOI] [PubMed] [Google Scholar]
- 90.Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998;139(10):4189–96. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- 91.Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93(2):659–66. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 92.Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75(5):778–84. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]
- 93.Funabashi T, Aiba S, Sano A, Shinohara K, Kimura F. Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci Lett. 1999;260(1):37–40. doi: 10.1016/s0304-3940(98)00940-9. [DOI] [PubMed] [Google Scholar]
- 94.Boer K, Boer GJ, Swaab DF. Reproduction in Brattleboro rats with diabetes insipidus. J Reprod Fertil. 1981;61(2):273–80. doi: 10.1530/jrf.0.0610273. [DOI] [PubMed] [Google Scholar]
- 95.Weick RF, Stobie KM. Vasoactive intestinal peptide inhibits the steroid-induced LH surge in the ovariectomized rat. J Endocrinol. 1992;133(3):433–7. doi: 10.1677/joe.0.1330433. [DOI] [PubMed] [Google Scholar]
- 96.Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res. 2001;901(1-2):109–16. doi: 10.1016/s0006-8993(01)02309-5. [DOI] [PubMed] [Google Scholar]
- 97.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714–9. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–36. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 100.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 101.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 103.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 104.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 105.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146(4):1689–97. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 106.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27(33):8826–35. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28(32):8003–13. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151(2):722–30. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- 110.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin Is Essential for the Full Preovulatory LH Surge and Stimulates GnRH Release from the Isolated Ovine Median Eminence. Endocrinology. 2011;152(3):1001–12. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 111.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 112.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–7. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27(44):12088–95. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–83. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 116.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 117.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 Expression by Sex Steroids in the Amygdala of the Rat and Mouse. Endocrinology. 2011 doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sim JA, Skynner MJ, Herbison AE. Direct regulation of postnatal GnRH neurons by the progesterone derivative allopregnanolone in the mouse. Endocrinology. 2001;142(10):4448–53. doi: 10.1210/endo.142.10.8451. [DOI] [PubMed] [Google Scholar]
- 119.Legan SJ, Tsai HW. Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J Neuroendocrinol. 2003;15(12):1164–70. doi: 10.1111/j.1365-2826.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- 120.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50(2):283–98. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 121.Bloch GJ, Kurth SM, Akesson TR, Micevych PE. Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin-immunoreactive cells. Brain Res. 1992;595(2):301–8. doi: 10.1016/0006-8993(92)91064-l. [DOI] [PubMed] [Google Scholar]
- 122.Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. Opposite roles of estrogen receptor (ER)-alpha and ERbeta in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology. 2008;149(4):1627–37. doi: 10.1210/en.2007-1540. [DOI] [PubMed] [Google Scholar]
- 123.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ronnekleiv OK, Kelly MJ. Plasma prolactin and luteinizing hormone profiles during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology. 1988;47(2):133–41. doi: 10.1159/000124903. [DOI] [PubMed] [Google Scholar]
- 125.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34(6):395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 126.Goodman RL. The site of the positive feedback action of estradiol in the rat. Endocrinology. 1978;102(1):151–9. doi: 10.1210/endo-102-1-151. [DOI] [PubMed] [Google Scholar]
- 127.Kalra PS, McCann SM. The stimulatory effect on gonadotropin release of implants of estradiol or progesterone in certain sites in the central nervous system. Neuroendocrinology. 1975;19(4):289–302. doi: 10.1159/000122450. [DOI] [PubMed] [Google Scholar]
- 128.Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989;484(1-2):279–89. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- 129.Petersen SL, Cheuk C, Hartman RD, Barraclough CA. Medial preoptic microimplants of the antiestrogen, keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized, estrogen-treated rats. J Neuroendocrinol. 1989;1(4):279–83. doi: 10.1111/j.1365-2826.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 130.Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140(1):510–9. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- 131.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–78. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 132.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–71. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Semaan SJ, Kauffman AS. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol. 2010;20(4):424–31. doi: 10.1016/j.conb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buhl AE, Norman RL, Resko JA. Sex differences in estrogen-induced gonadotropin release in hamsters. Biol Reprod. 1978;18(4):592–7. doi: 10.1095/biolreprod18.4.592. [DOI] [PubMed] [Google Scholar]
- 136.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81(6):1216–25. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 137.Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides. 2009;30(1):83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kauffman AS. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol. 2010;22(7):682–91. doi: 10.1111/j.1365-2826.2010.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de la Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-alpha-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol. 1999;11(7):481–90. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- 140.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102(43):15682–7. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152(2):595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kallo I. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 2010;22(9):1032–9. doi: 10.1111/j.1365-2826.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- 143.Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89(4):448–57. doi: 10.1159/000192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology. 2010;91(1):110–20. doi: 10.1159/000243163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958–66. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- 146.Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147(6):2864–78. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 147.Ducret E, Gaidamaka G, Herbison AE. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology. 2010;151(5):2223–32. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- 148.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–21. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 150.Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol. 2009;36(4):219–24. doi: 10.1016/j.domaniend.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–69. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 153.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–11. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–40. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 155.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenrohr M, Pinilla L, van Noort PI, Tena-Sempere M. Characterization of the potent gonadotropin-releasing activity of RF9, a selective antagonist of RF-amide-related peptides and neuropeptide FF receptors: physiological and pharmacological implications. Endocrinology. 2010;151(4):1902–13. doi: 10.1210/en.2009-1259. [DOI] [PubMed] [Google Scholar]
- 156.Molnar CS, Kallo I, Liposits Z, Hrabovszky E. Estradiol Down-Regulates RF-Amide-Related Peptide (RFRP) Expression in the Mouse Hypothalamus. Endocrinology. 2011 doi: 10.1210/en.2010-1418. [DOI] [PubMed] [Google Scholar]
- 157.Quennell JH, Rizwan MZ, Relf HL, Anderson GM. Developmental and steroidogenic effects on the gene expression of RFamide related peptides and their receptor in the rat brain and pituitary gland. J Neuroendocrinol. 2010;22(4):309–16. doi: 10.1111/j.1365-2826.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- 158.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boulos Z, Morin LP. Entrainment of split circadian activity rhythms in hamsters. J Biol Rhythms. 1985;1(1):1–15. doi: 10.1177/074873048600100103. [DOI] [PubMed] [Google Scholar]
- 160.Pickard GE, Turek FW. Splitting of the circadian rhythm of activity is abolished by unilateral lesions of the suprachiasmatic nuclei. Science. 1982;215(4536):1119–21. doi: 10.1126/science.7063843. [DOI] [PubMed] [Google Scholar]
- 161.Swann JM, Turek FW. Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science. 1985;228(4701):898–900. doi: 10.1126/science.4001926. [DOI] [PubMed] [Google Scholar]
- 162.de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: Activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci. 2003;23(19):7412–4. doi: 10.1523/JNEUROSCI.23-19-07412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maruyama T, Ohbuchi T, Fujihara H, Shibata M, Mori K, Murphy D, Dayanithi G, Ueta Y. Diurnal changes of arginine vasopressin-enhanced green fluorescent protein fusion transgene expression in the rat suprachiasmatic nucleus. Peptides. 2010;31(11):2089–93. doi: 10.1016/j.peptides.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 164.Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18(12):4767–74. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kallo I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004;16(9):758–66. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- 166.Yambe Y, Arima H, Kakiya S, Murase T, Oiso Y. Diurnal changes in arginine vasopressin gene transcription in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 2002;104(2):132–6. doi: 10.1016/s0169-328x(02)00327-3. [DOI] [PubMed] [Google Scholar]
- 167.Gozes I, Werner H, Fawzi M, Abdelatty A, Shani Y, Fridkin M, Koch Y. Estrogen regulation of vasoactive intestinal peptide mRNA in rat hypothalamus. J Mol Neurosci. 1989;1(1):55–61. doi: 10.1007/BF02896857. [DOI] [PubMed] [Google Scholar]
- 168.Doan A, Urbanski HF. Diurnal expression of Fos in luteinizing hormone-releasing hormone neurons of Syrian hamsters. Biol Reprod. 1994;50(2):301–8. doi: 10.1095/biolreprod50.2.301. [DOI] [PubMed] [Google Scholar]
- 169.Wu TJ, Segal AZ, Miller GM, Gibson MJ, Silverman AJ. FOS expression in gonadotropin-releasing hormone neurons: enhancement by steroid treatment and mating. Endocrinology. 1992;131(5):2045–50. doi: 10.1210/endo.131.5.1425409. [DOI] [PubMed] [Google Scholar]