Abstract
Dynamic regulation of paracellular permeability is essential for physiological epithelial function, while dysregulated permeability is common in disease. The recent elucidation of the molecular composition of the epithelial tight junction complex has been accompanied by characterization of diverse intracellular mediators of paracellular permeabiltiy. Myosin light chain kinase, which induces contraction of the perijunctional actomyosin ring through myosin II regulatory light chain phosphorylation, has emerged as a key regulator of tight junction permeability. Examination of the regulation and role of MLCK in tight junction dysfunction has helped to define pathological processes, characterize the role of barrier loss in disease pathogenesis, and may provide future therapeutic targets to treat intestinal disease.
Keywords: tight junction, myosin light chain kinase, TNF-α, IBD
Introduction
Epithelial cells serve an essential role in homeostasis by providing and regulating the physical barrier between tissue compartments.1 In the intestine, the epithelium forms a barrier between the sterile tissues and the harsh environment of the lumen. This barrier must be selectively permeable to allow the absorption and secretion of large volumes of solutes and fluids while maintaining a barrier to pathogens and xenobiotic materials. In addition, it is now clear that the barrier function is dynamic, and can be regulated by a variety of physiological and pathophysiological stimuli.2 The apical and basal plasma membranes of mechanically-linked epithelial cells form the majority of the barrier surface, and constitute the transcellular barrier, but the space between adjacent cells, that is, the paracellular space, must also be sealed to maintain the continuity of the barrier. Throughout the gastrointestinal tract, the transcellular and paracellular routes are used for transport of solutes and fluids through and between epithelial cells. Specific pumps, transporters, and channels on the apical and basolateral plasma membranes facilitate transcellular transport. In contrast, paracellular transport is passive, occurring down electrochemical gradients established either by the activity of the transcellular transporters or by external events, such as ingestion and luminal digestion of nutrients.
Paracellular transport is regulated by an intercellular seal, formed by two main protein complexes located at the apical-most part of the lateral membrane, collectively termed the apical junctional complex (Fig. 1).3 The adherens junction, which contains cadherin and catenin proteins, is linked to the dense ring of perijunctional actin and myosin that underlies the apical junction complex. While critical for structural integrity, the adherens junction does not contribute to sealing of the paracellular space. The tight junction, which is located just apical to the adherens junction, is the essential determinant of paracellular flux.4, 5 Tight junction-associated proteins, including the claudin family and occludin,6 and the specialized lipid composition of local membranes7 form a seal that limits paracellular flux. As with the adherens junction, the tight junction protein complex is attached to perijunctional actomyosin ring8 by direct and indirect protein–protein interactions.9, 10 As discussed below, these interactions are critical to tight junction structure and function.11–13
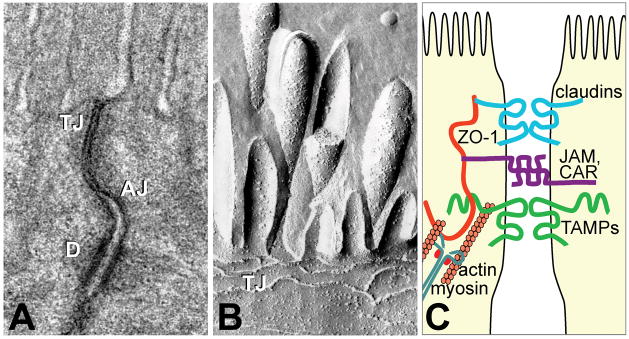
Figure 1.
The apical junctional complex. (A) Transmission electron micrograph shows the tight junction, TJ, located most apically, the adherens junction, AJ, located below, and desmosomes, D, located basolaterally. (B) Freeze-fracture electron microscopy shows that the TJ is composed of particulate intramembranous strands. Diagrammatic representation of the apical junctional complex. Myosin and actin interact with the tight junction through plaque proteins, such as ZO-1. Integral membrane proteins, including junctional adhesion molecules (JAM), Coxsackie adenovirus receptor (CAR), and tight junction-associated MARVEL proteins (TAMPs), such as occludin, bridge the intercellular space. Figure reproduced from Shen et al.72 with permission.
Myosin light chain kinase
Original studies assumed that the tight junction was a static barrier. However, demonstration of rapid modulation of structure and barrier function by plant cytokinins, compounds derived from purines, suggested the possibility of physiological tight junction regulation. This was subsequently described as a consequence of Na+-nutrient cotransport in mammalian small intestine.14–19 However, the contribution of the paracellular pathway to overall nutrient and water absorption was considered controversial.20–25 Many reasons exist for the failure of some studies to demonstrate paracellular water and nutrient absorption, most of which reflect technical issues and, potentially, the species studied.24, 25 The clearest in vivo example of the role of physiologic tight junction regulation in paracellular glucose transport is provided by a perfusion study of rat jejunum.26 This work showed that D-glucose absorption over a wide range of perfusate concentrations could only be explained by the sum of active (i.e., transcellular) and passive (i.e., paracellular) transport.26 As discussed below, in vivo perfusion studies of mouse jejunum have confirmed the critical contribution of tight junction regulation and paracellular transport to overall nutrient and water absorption in health and disease.
While study of intact tissue, either in vivo or ex vivo, was critical to the initial demonstration of tight junction regulation, it was not suitable for investigation of the underlying mechanisms. However, study of intact tissue did provide one essential clue. Ultrastructural studies showed that Na+-glucose cotransport-induced tight junction regulation was associated with perijunctional actomyosin condensation.15, 18, 27 This led to the hypothesis that perijunctional actomyosin contraction might regulate the tight junction barrier function. Development of a model for study of Na+-glucose cotransport-induced tight junction regulation in cultured epithelial monolayers demonstrated that permeability was affected in a size-selective manner, that is, permeability to small, but not large, molecules was increased.28 This model also allowed biochemical analysis, which revealed increased myosin II regulatory light chain (MLC) phosphorylation, a biochemical marker of actomyosin contraction, following Na+-glucose cotransport activation.28 Further, pharmacological inhibition of myosin light chain kinase (MLCK) prevented Na+-glucose cotransport-induced tight junction regulation, both in cultured monolayers and isolated rodent mucosae.28 Subsequent ex vivo analyses confirmed Na+-glucose cotransport-induced tight junction regulation in human jejunum, and quantitative fluorescence microscopy demonstrated MLC phosphorylation within the perijunctional actomyosin ring of absorptive enterocytes within these tissues. Thus, MLCK-dependent MLC phosphorylation is an essential intermediate in physiological tight junction regulation.
MLCK is necessary for TNF-α–induced barrier loss
A role for the cytoskeleton in the pathophysiological tight junction regulation was first suggested by the observation that MLC phosphorylation is markedly increased following infection with enteropathogenic E. coli.29 Shortly thereafter, a role for MLCK in tight junction dysfunction induced by tumor necrosis factor (TNF-α) was examined.30 Analyses of cultured monolayers demonstrated TNF-α–induced barrier loss could be corrected acutely using a highly-specific pseudosubstrate peptide MLCK inhibitor.31 Further study demonstrated that TNF-α activated MLCK by at least two separate mechanisms: increased transcription and increased enzymatic function.32
While some details of TNF-α–induced barrier loss are best identified using highly manipulable in vitro models, determination of the effect on transepithelial transport requires use of ex vivo or in vivo models. Thus, an in vivo perfusion system was developed to allow quantitative analysis of barrier function and fluid transport in neurovascularly intact mouse jejunum.33 Mice were treated with anti-CD3 to activate T cells systemically.34 This induced jejunal barrier loss and reversal of net fluid movement, from absorption to secretion, in a TNF-α–dependent manner.33, 35 Ultrastructural examination revealed that this was associated with perijunctional actomyosin contraction (Fig. 2) similar to that induced by Na+-glucose cotransport, and phosphorylation of perijunctional MLC.33 Furthermore, mice lacking long MLCK, the intestinal epithelial MLCK isoform,36 or mice treated with the peptide inhibitor of MLCK failed to phosphorylate intestinal epithelial MLCK and were protected from both barrier loss and fluid secretion.33 Thus, MLCK is critical effector of pathological barrier dysfunction in vivo.
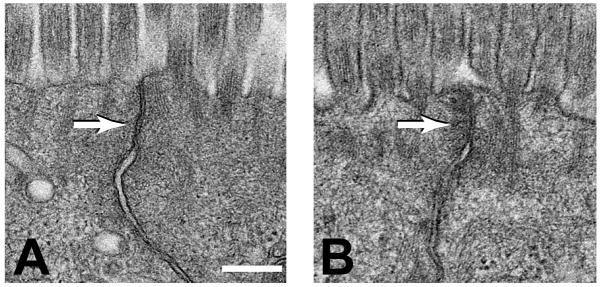
Figure 2. T cell activation induces perijunctional actomyosin condensation.
The tight junctions (arrows) of jejunal villous enterocytes within control (A) and anti-CD3–induced T cell activation (B) mice. Note the perijunctional cytoskeletal condensation induced by T cell activation. Figure reproduced from Clayburgh et al.33 with permission.
MLCK regulation in the gastrointestinal tract
The emergence of MLCK as a critical regulator of epithelial paracellular permeability has provided an opportunity to prevent intestinal barrier dysfunction in experimental models and examine the potential therapeutic benefit of this intervention. As noted above, TNF regulates MLCK transcription and enzymatic activity.31, 32, 37 Moreover, MLCK is expressed in intestinal epithelia as two splice variants. Short, or smooth muscle, MLCK38 is not expressed in intestinal epithelia.36 Long MLCK is derived from the same gene as short MLCK, but uses an upstream promoter that gives rise to 5′ transcriptional and translational start sites and additional amino terminal protein sequence.38 Two long MLCK isoforms, MLCK1, or full-length long MLCK, and MLCK2, which lacks a single exon within the unique, long MLCK upstream sequences, are expressed in intestinal epithelia. These splice variants have distinct subcellular localizations and functions,36 and their expression is differentially regulated during epithelial differentiation. MLCK1 is predominantly expressed in villous epithelium, while MLCK2 is expressed throughout the crypt villus axis. Moreover, MLCK1 is concentrated at the perijunctional actomyosin ring and specific MLCK1 knockdown increases barrier function.36, 39 Finally, the ability of cultured intestinal epithelial monolayers to regulate barrier function after initiation of Na+-glucose cotransport, which develops during enterocyte differentiation, coincides with the onset of MLCK1 expression.36 Given that MLCK participates in multiple cellular processes, MLCK1 may be the preferred molecular target for therapeutic MLCK inhibition.
Despite the unique role of MLCK1 in tight junction regulation, MLCK1 and MLCK2 transcription appear to be activated similarly by TNF-α.37 There has been debate regarding the signaling events that lead to increased MLCK transcription, The first study examining this found that inhibitors of NF-κB could block TNF-α–induced MLCK upregulation at extremely low concentrations.32 In contrast, NF-κB inhibition required use of these agents at significantly greater doses that actually enhanced TNF-α–induced MLCK upregulation.32 A subsequent study suggested that NF-κB was critical to TNF-α–induced MLCK upregulation.40, 41 Although both of these studies used the Caco-2 intestinal epithelial cell line, which is derived from a human colonic adenocarcinoma, the first used the well-differentiated, absorptive (surface) enterocyte-like BBe subclone,31, 32, 42 while the second study used the less well-differentiated parent line.40, 41 A detailed analysis of the human long MLCK promoter showed that this discrepancy likely explains the difference in mechanism of transcriptional regulation.37 While the promoter was responsive to both AP-1 and NF-κB, the data show that TNF preferentially activates NF-κB in poorly-differentiated monolayers and AP-1 in well-differentiated monolayers.37 While activation of MLCK transcription by TNF has been demonstrated in vivo,37, 39 the mechanism of transcriptional regulation has not been defined. However, transgenic mice expressing an epithelial-specific IκBα mutant, which functions as an NF-κB super repressor, were protected from anti-CD3–induced tight junction regulation.43
A plethora of studies have identified distinct mechanisms to control MLCK expression and activity, which appear to be altered in disease states. While numerous groups have shown MLCK increases MLC activity by phosphorylation at Ser19, the regulation of MLCK expression is less well defined. Perhaps with greatest relevance to inflammatory bowel disease (IBD), numerous studies have established the ability of inflammatory cytokines TNF-α and interferon (IFN)-γ, which are elevated in Crohn’s disease, to induce barrier loss in vitro at relatively high doses. Critically, TNF-α and IFN-γ inhibition can reverse barrier loss and substantially reduce inflammation in patients and animal models. At low doses, TNF-α and IFN-γ act synergistically to decrease barrier function in vitro 31, 32, 44, which may be more relevant to the pathogenesis of human disease where both cytokines are elevated. In monolayers primed with IFN-γ, TNF-α decreased barrier function and increased MLC phosphorylation. Barrier function was restored by specifically inhibiting MLCK, suggesting that MLCK activity was responsible for the loss of barrier function 31. The molecular mechanism leading to increased MLCK activity was subsequently studied in this model and it was found that MLCK protein 32 and gene 37 expression was also increased by IFN-γ/TNF-α, and corresponded with increased MLC phosphorylation. Thus, MLCK was found to be inducible by TNF-α, uncovering a novel mechanism of epithelial barrier regulation by the cytokine. Crucially, this observation is supported by patient data. In intestinal resections and biopsies, MLCK expression was slightly increased in ileal epithelia of patients with inactive Crohn’s disease, and this increased further in active disease, correlating with histological disease activity 39. MLC phosphorylation was also increased in the colon tissues of patients with active disease (Fig. 3). Taken together, these studies provide a key insight into the regulation of MLCK by inflammatory cytokines, and the role of MLCK activity in the pathogenesis leading to epithelial barrier loss in IBD.
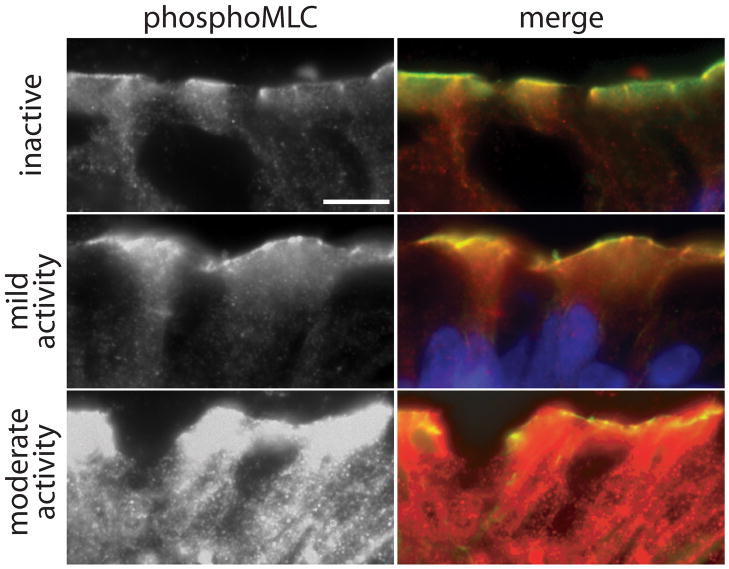
Figure 3.
Myosin light chain (MLC) phosphorylation is correlated with inflammatory activity in inflammatory bowel disease. Phosphorylated MLC (red) is primarily detected at the perijunctional actomyosin ring (green) in biopsies without active disease. While still predominantly within the perijunctional actomyosin ring, the intensity of phosphorylated MLC detection is markedly enhanced with increasing disease activity. Matched exposures are shown. Bar = 5 μm. Figure reproduced from Blair et al.39 with permission.
Mechanism of MLCK-dependent barrier regulation
While the data above demonstrate that MLCK is a critical mediator of tight junction barrier function, the downstream events activated by MLCK are only beginning to be defined. First, while Na+-glucose cotransport and TNF-α both regulate tight junctions by MLCK-dependent processes, the impact each has a distinct impact on barrier function. Na+-glucose cotransport induces a size-selective increase in permeability that is limited to small molecules.25, 27, 28 In contrast, TNF-α–induced increases in paracellular flux of small and large molecules.32, 33, 45
Inducible expression of constitutively-active MLCK in cultured intestinal epithelia caused a size-selective increase in permeability similar to that following initiation of Na+-glucose cotransport.46 Detailed analysis of tight junction structure in these monolayers demonstrated MLCK-dependent reorganization of perijunctional actin, occludin, and ZO-1.46 The normally smooth, arc-like tight junction profiles viewed en face were modified to irregularly undulating profiles after MLCK activation.46 This resulted in a nearly 20% increase in tight junction length which, by virtue of an increase in potential paracellular channels, could partially explain the MLCK-induced increase in paracellular flux. This may have also been associated with a change in lipid composition of tight junction membrane microdomains, as occludin was redistributed to a higher density population of glycoplipid- and cholesterol-rich membranes.46 However, given the critical role of claudins in defining paracellular permeability,47–51 it is notable that neither the en face profiles nor density of membranes containing claudin-1 and claudin-2 were affected by MLCK activation.
To better understand the mechanism of physiological MLCK-dependent barrier regulation, the dynamic behaviors of occludin, ZO-1, claudin-1, and actin were examined in monolayers with active Na+-glucose cotransport.52, 53 MLCK inhibition markedly reduced ZO-1 exchange between tight junction and cytosolic pools, but did not affect dynamic behavior of other tight junction proteins or perijunctional actin.53 Exchange of a ZO-1 mutant lacking the actin binding region was not affected by MLCK inhibition, thereby demonstrating that this domain mediated the observed increased in ZO-1 anchoring.53 Further, either ZO-1 knockdown or expression of the free actin binding region, as a dominant negative inhibitor, prevented MLCK-dependent barrier regulation.53 Thus, physiological MLCK-dependent barrier regulation occurs via a ZO-1–dependent process. The role of occludin in this form of tight junction barrier regulation has not been established.
Similar to physiological tight junction regulation, pathophysiological MLCK-dependent barrier loss is associated with increased undulation of en face ZO-1 profiles.33 However, in vivo TNF-α–induced tight junction regulation is also accompanied by occludin internalization.33 This caveolin-1–dependent endocytosis is prevented by MLCK inhibition.33, 54 Further, in vivo occludin overexpression reduced TNF-α–induced barrier dysfunction by ~50%.54 Similar data have demonstrated a critical role for occludin during in vitro TNF-α–induced barrier loss.55 Thus, occludin endocytosis is a critical intermediate in pathophysiological tight junction regulation, both in vitro and in vivo. While not reported, it may be that this occludin endocytosis is responsible for the lack of size selectivity in pathophysiological, relative to physiological, MLCK-dependent barrier loss.
The role of MLCK-dependent barrier regulation in disease initiation and progression
Identification of MLCK as a central mediator of intestinal epithelial tight junctions has enabled further characterization of its role in disease pathogenesis as well as the wider role of barrier loss in disease initiation and progression. Patient data supporting the critical role of barrier function include the increased risk of relapse from remission in Crohn’s disease patients with increased intestinal permeability.56 The observed permeability increases in a subset of healthy first degree relatives of Crohn’s disease patients57, 58 also suggests a role for tight junction dysregulation in disease initiation, while, simultaneously, demonstrating that barrier dysfunction alone is insufficient to cause disease.
To determine the contribution of MLCK to initiation and development of disease, transgenic mice that express constitutively-active MLCK within the intestinal epithelium were developed.59 As expected, these mice displayed increased intestinal epithelial MLC phosphorylation and paracellular permeability. While mucosal immune activation, including increased TNF-α, IFN-γ, IL-10, and IL-13, as well as increased numbers of lamina propria T cells were observed,47, 59 these mice did not develop spontaneous disease. Thus, the mice may be similar to healthy first degree relatives of Crohn’s disease patients. However, when immunodeficient mice expressing constitutively-active MLCK were challenged with adoptive transfer of naive T cells, they developed colitis more rapidly than non-transgenic littermates.59 In addition, disease in the transgenic mice was more severe in terms of cytokine production, histopathology, and overall survival.59 Thus, while insufficient to initiate disease, epithelial tight junction dysregulation can accelerate disease progression and enhance barrier function. Conversely, delayed disease onset and reduced severity have been reported after adoptive transfer of naive T cells into long MLCK knockout mice.60 Thus, targeted MLCK inhibition could be of therapeutic benefit.
Potential of MLCK inhibition as a therapeutic approach
The prospect of preventing initial development of Crohn’s disease, maintaining remission, and reducing severity of active flares by inhibition of epithelial long MLCK is compelling. The safety of such an approach is supported by the observation that long MLCK knockout mice are healthy61 and are at least partially protected from many stressors.33, 61–64 However, established pharmacological inhibitors such as ML-7 and ML-9, are not useful, as they inhibit many kinases at concentrations necessary to block MLCK.65 Although inhibitors with greater specificity are available,61, 66, 67 these are also unsuitable, as they cannot distinguish between long and short MLCK, whose catalytic domains are derived from a single gene and are, therefore, identical.38 Thus, a pharmacological approach targeting epithelial long MLCK enzymatic activity will also inhibit the short MLCK expressed in smooth muscle. Toxicities that would follow are demonstrated by the perinatal death of genetically-modified mice lacking the MLCK catalytic domain,68 and the hypotension, gut dysmotility, and viscus obstruction of mice with smooth muscle specific MLCK deletion.69, 70 Thus, targeting of MLCK enzymatic activity may not be a viable approach to therapy when the risk of adverse effects is considered. One possible future direction may involve direct targeting of specific MLCK isoforms. Due to its expression within well-differentiated intestinal epithelia, direct targeting of long MLCK1 might prevent tight junction dysfunction without systemic toxicities. The presence of a unique IgCAM domain that contains src phosphorylation sites could represent an alternative targets to inhibit MLCK1.71 However, these studies are limited and further investigation is needed.
Conclusions
In summary, understanding the contribution of epithelial paracellular permeability to physiological processes in the intestine, and its dysregulation in disease, has provided invaluable insight into disease mechanisms. While not yet practical, isoform-specific long MLCK inhibition may ultimately provide a viable approach to restoring tight junction barrier function and preventing or treating intestinal disease.
Acknowledgments
We are grateful to past and present members of our research group, our collaborators, and many others in the field for the insights they have provided into tight junction structure and function. We also apologize to colleagues whose outstanding work was not cited due to length restrictions. Our work is supported by the National Institutes of Health (R01DK61931, R01DK68271, P01DK067887), the Department of Defense (W81XWH-09-1-0341), the Broad Medical Research Foundation (IBD-022), the University of Chicago Cancer Center (P30CA14599), and the University of Chicago Institute for Translational Medicine (UL1RR024999).
References
- 1.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 2.Nusrat A, Turner JR, Madara JL. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am J Physiol -Gastrointest Liver Physiol. 2000;279:G851–857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar M, Palade G. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Palomo A, Erlij D. Structure of tight junctions in epithelia with different permeability. Proc Natl Acad Sci USA. 1975;72:4487–4491. doi: 10.1073/pnas.72.11.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzel CJ, Hainau B, Edelman A, Anagnostopoulos T, Benedetti EL. Effect of plant cytokinins on microfilaments and tight junction permeability. Nature. 1976;264:666–668. doi: 10.1038/264666a0. [DOI] [PubMed] [Google Scholar]
- 6.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: Leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 7.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 8.Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. AJP - Cell Physiology. 1987;253:C171–175. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- 9.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein zo-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 10.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein zo-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 11.Bentzel CJ, Hainau B, Ho S, Hui SW, Edelman A, Anagnostopoulos T, Benedetti EL. Cytoplasmic regulation of tight-junction permeability: Effect of plant cytokinins. Am J Physiol. 1980;239:C75–89. doi: 10.1152/ajpcell.1980.239.3.C75. [DOI] [PubMed] [Google Scholar]
- 12.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin d on occluding junctions of intestinal absorptive cells: Further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madara JL, Carlson S. Supraphysiologic l-tryptophan elicits cytoskeletal and macromolecular permeability alterations in hamster small intestinal epithelium in vitro. J Clin Invest. 1991;87:454–462. doi: 10.1172/JCI115017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 16.Pappenheimer JR. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J Membr Biol. 1987;100:137–148. doi: 10.1007/BF02209146. [DOI] [PubMed] [Google Scholar]
- 17.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 18.Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am J Physiol. 1990;258:C77–C85. doi: 10.1152/ajpcell.1990.258.1.C77. [DOI] [PubMed] [Google Scholar]
- 19.Turner JR, Cohen DE, Mrsny RJ, Madara JL. Noninvasive in vivo analysis of human small intestinal paracellular absorption: Regulation by na+-glucose cotransport. Dig Dis Sci. 2000;45:2122–2126. doi: 10.1023/a:1026682900586. [DOI] [PubMed] [Google Scholar]
- 20.Madara JL. Sodium-glucose cotransport and epithelial permeability. Gastroenterol. 1994;107:319–320. doi: 10.1016/0016-5085(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 21.Turner JR, Madara JL. Physiological regulation of intestinal epithelial tight junctions as a consequence of na+-coupled nutrient transport. Gastroenterol. 1995;109:1391–1396. doi: 10.1016/0016-5085(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 22.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of d-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterol. 1993;105:1117–1125. doi: 10.1016/0016-5085(93)90957-e. [DOI] [PubMed] [Google Scholar]
- 23.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Mechanism by which glucose stimulates the passive absorption of small solutes by the human jejunum in vivo. Gastroenterol. 1994;107:389–395. doi: 10.1016/0016-5085(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 24.Lane JS, Whang EE, Rigberg DA, Hines OJ, Kwan D, Zinner MJ, McFadden DW, Diamond J, Ashley SW. Paracellular glucose transport plays a minor role in the unanesthetized dog. Am J Physiol. 1999;276:G789–794. doi: 10.1152/ajpgi.1999.276.3.G789. [DOI] [PubMed] [Google Scholar]
- 25.Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus- crypt axis: Effects of glucose transport. Gastroenterol. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 26.Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: Model analysis and derivation of corrected kinetic constants. Clin Sci (Lond) 1989;76:403–413. doi: 10.1042/cs0760403. [DOI] [PubMed] [Google Scholar]
- 27.Atisook K, Madara JL. An oligopeptide permeates intestinal tight junctions at glucose-elicited dilatations. Gastroenterol. 1991;100:719–724. doi: 10.1016/0016-5085(91)80016-3. [DOI] [PubMed] [Google Scholar]
- 28.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 29.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterol. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 30.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: Role for polarized release of tumor necrosis factor alpha. Gastroenterol. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- 31.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits mlc kinase restores barrier function in in vitro models of intestinal disease. Gastroenterol. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates t cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferran C, Dy M, Sheehan K, Merite S, Schreiber R, Landais P, Grau G, Bluestone J, Bach JF, Chatenoud L. Inter-mouse strain differences in the in vivo anti-cd3 induced cytokine release. Clin Exp Immunol. 1991;86:537–543. doi: 10.1111/j.1365-2249.1991.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial na+/k+-atpase. J Clin Invest. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clayburgh DR, Rosen S, Witkowski ED, Wang F, Blair S, Dudek S, Garcia JG, Alverdy JC, Turner JR. A differentiation-dependent splice variant of myosin light chain kinase, mlck1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 38.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 39.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 40.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of tnf-alpha modulation of caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am J Physiol - Gastrointest Liver Physiol. 2005;288:G422–430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 41.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. Tnf-alpha-induced increase in intestinal epithelial tight junction permeability requires nf-kappa b activation. Am J Physiol - Gastrointest Liver Physiol. 2004;286:G367–376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 42.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the c2bbe clones of the human intestinal cell line, caco-2. J Cell Sci. 1992;102(Pt 3):581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Clayburgh DR, Mittal N, Goretsky T, Dirisina R, Zhang Z, Kron M, Ivancic D, Katzman RB, Grimm G, Lee G, Fryer J, Nusrat A, Turner JR, Barrett TA. Epithelial nf-kappab enhances transmucosal fluid movement by altering tight junction protein composition after t cell activation. Am J Pathol. 2010;176:158–167. doi: 10.2353/ajpath.2010.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 45.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial nhe3 inhibition and barrier dysfunction are required for tnf-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 47.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 50.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 52.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. Mlck-dependent exchange and actin binding region-dependent anchoring of zo-1 regulate tight junction barrier function. Proc Natl Acad Sci USA. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for tnf-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 57.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 58.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with crohn’s disease: Role of card15 3020insc mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterol. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su L, Nalle SC, Sullivan EA, Fu Y-X, Turner JR. Genetic ablation of myosin light chain kinase limits epithelial barrier dysfunction and attenuates experimental inflammatory bowel disease. Gastroenterol. 2009;136:A81 (abstract). [Google Scholar]
- 61.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: Evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reynoso R, Perrin RM, Breslin JW, Daines DA, Watson KD, Watterson DM, Wu MH, Yuan S. A role for long chain myosin light chain kinase (mlck-210) in microvascular hyperpermeability during severe burns. Shock. 2007;28:589–595. doi: 10.1097/SHK.0b013e31804d415f. [DOI] [PubMed] [Google Scholar]
- 63.Rossi JL, Velentza AV, Steinhorn DM, Watterson DM, Wainwright MS. Mlck210 gene knockout or kinase inhibition preserves lung function following endotoxin-induced lung injury in mice. Am J Physiol - Lung Cell Mol Physiol. 2007;292:L1327–1334. doi: 10.1152/ajplung.00380.2006. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Gao XP, Ramchandran R, Zhao YY, Vogel SM, Malik AB. Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins. Nat Immunol. 2008;9:880–886. doi: 10.1038/ni.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: An update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Behanna HA, Watterson DM, Ranaivo HR. Development of a novel bioavailable inhibitor of the calmodulin-regulated protein kinase mlck: A lead compound that attenuates vascular leak. Biochim Biophys Acta. 2006;1763:1266–1274. doi: 10.1016/j.bbamcr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Owens SE, Graham WV, Siccardi D, Turner JR, Mrsny RJ. A strategy to identify stable membrane-permeant peptide inhibitors of myosin light chain kinase. Pharm Res. 2005;22:703–709. doi: 10.1007/s11095-005-2584-9. [DOI] [PubMed] [Google Scholar]
- 68.Somlyo AV, Wang H, Choudhury N, Khromov AS, Majesky M, Owens GK, Somlyo AP. Myosin light chain kinase knockout. J Muscle Res Cell Motil. 2004;25:241–242. doi: 10.1023/b:jure.0000038362.84697.c0. [DOI] [PubMed] [Google Scholar]
- 69.He WQ, Qiao YN, Zhang CH, Peng YJ, Chen C, Wang P, Gao YQ, Chen X, Tao T, Su XH, Li CJ, Kamm KE, Stull JT, Zhu MS. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am J Physiol - Heart Circ Physiol. 2011;301:H584–591. doi: 10.1152/ajpheart.01212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterol. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60src. J Biol Chem. 2000;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 72.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: A dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]