Abstract
Current research on analogy processing assumes that different conceptual relations are treated similarly. However, just as words and concepts are related in distinct ways, different kinds of analogies may employ distinct types of relationships. An important distinction in how words are related is the difference between associative (dog-bone) and categorical (dog-cat) relations. To test the hypothesis that analogical mapping of different types of relations would have different neural instantiations, we tested patients with left and right hemisphere lesions on their ability to understand two types of analogies, ones expressing an associative relationship and others expressing a categorical relationship. Voxel-based lesion-symptom mapping (VLSM) and behavioral analyses revealed that associative analogies relied on a large left-lateralized language network while categorical analogies relied on both left and right hemispheres. The verbal nature of the task could account for the left hemisphere findings. We argue that categorical relations additionally rely on the right hemisphere because they are more difficult, abstract, and fragile; and contain more distant relationships.
Keywords: right hemisphere, verbal analogy, semantic processing, associative relations, categorical relations, neuropsychological studies
1. Introduction
Analogy processing is an important part of cognition. For example, analogy is used to solve goal directed problems (Glick & Holyoak, 1983); reason (Gentner, 2003), understand figurative language (Gentner, Bowdle, Wolff, & Boronat, 2001); and learn both semantics and syntax in language (Gentner & Namy, 2006). In the present study we tested the hypothesis that different kinds of analogies would have different behavioral patterns and neural instantiations by investigating patients with left (left hemisphere) and right hemisphere (right hemisphere) damage.
1.1 Types of relations in analogy
Analogy involves mapping relations between two different domains. The domains are typically real-world situations (as seen below) or fields of knowledge. For example, relationships in the domain of astronomy (plants revolving around the sun) can be mapped onto relations in the domain of physics (electrons revolving around a nucleus). Processing an analogical relationship includes both understanding the abstract structure inherent in each domain and then mapping the relations common to them. The relations mapped in analogies may be abstract, spatial, or semantic. For example, in the Gick and Holyoak study (1983), participants were presented with paragraph length scenarios. They were tested on their ability to realize that a situation consisting of soldiers converging on an enemy from different directions has the same spatial structure and could be mapped onto a situation with laser beams converging on a cancerous growth from different directions. Raven's progressive matrices (Raven, Raven & Court, 2003), which tap spatial relations are also often used in studies of analogy. Many studies of analogy tap semantic relations with words (Luo, Perry, Peng, Jin, Xu, et al., 2003; Wendelken Nakhabenko, Donohue, Carter, & Bunge, 2008) or pictures (Krawczyk, Morrison, Viskontas, Holyoak, Chow, et al., 2008; Morrison, Krawczyk, Holyoak, Hummel, Chow, et al., 2004). Both word- and picture-based analogies in these studies tend to be SAT-style1 form based on a four term analogy (jeans: legs :: hat: head). In these cases, analogical processing requires the mapping of a particular relationship from one set of concepts or words to another. For example, in the analogy “Socrates: ideas :: midwife: baby”, Socrates helps his students give birth to ideas, as a midwife helps a mother give birth to a baby. There is an abstract relationship between the midwife, mother and her baby that can be mapped onto Socrates, his students, and their ideas, respectively (Bowdle & Gentner, 2005).
Current research on analogy processing implicitly assumes that different conceptual relations are treated similarly. However, words and concepts can be related in different ways (Miller & Fellbaum, 1991). An important distinction is the difference between associative (dog-bone) and categorical (dog-cat) relations (e.g. Hutchison, 2003). Associative relations are based on co-occurrence in space and time and/or spoken or written language (dog-bone), while categorical relations tend to be based on common features and membership in a taxonomic category (blue jay-robin). For example, items in the category of “birds” tend to share a set of common features, such as having two legs, wings, and being able to fly. Similar distinctions have been variously called taxonomic vs. thematic (Sachs, Weis, Krings, Huber & Kircher 2008a; Sachs, Weis, Zellagui, Huber, Zvyagintsev, et al., 2008b; Sachs, Weis, Zellagui, Sass, Huber, et al., 2011); or concrete (experience-based) versus abstract (rule-based) (e.g. Davidoff & Roberson 2004; Sass, Sachs, Krach, & Kircher, 2009).
1.2 The neural basis of analogical relations
The few studies that have examined the neural basis of verbal (four-term) analogies provide evidence for involvement of several areas. These include the left and/or right rostral prefrontal cortex (BA10): (Bunge, Wendelken, Badre & Wagner, 2005; Green, Fugelsang, Kraemer, Shamosh & Dunbar, 2006; Green, Kraemer, Fugelsang, Gray, & Dunbar, 2010; Morrison et al., 2004; Wendelken et al., 2008), the left and/or right inferior frontal gyrus (BA44, 45, 47) (Bunge et al., 2005; Green et al., 2006; Green et al., 2010; Luo et al. 2003; Wendelken et al., 2008), the left superior parietal lobule (BA7) (Green et al., 2006; Wendelken et al., 2008) and the left and/or right posterior middle temporal gyrus (BA 22) (Green et al., 2010; Luo et al. 2003).
However, this work on the neural basis of verbal four-term analogies has usually not specified the type of relationships that were mapped in the analogies. Differences have been reported in the neural instantiation of associative and categorical relations (e.g. Davidoff & Roberson, 2004). Categorical relations are more likely than associative relations to activate a larger neural network (Sachs et al., 2008a; 2011), recruit the right hemisphere (Kalenine, Peyrin, Pichat, Segebarth, Bonthoux, et al., 2009; Kotz, Cappa, von Cramon & Friederici, 2002; Sass et al., 2009) and involve regions like the precuneus (Kotz et al., 2002; Sachs et al., 2008a). We hypothesize that there must also be differences in the neural instantiation of the mapping of these relations. Typically, reports of the neural basis of analogy provide only one example of the type of stimuli being used and do not discuss the nature of the relationships in the remaining stimulus set. The sample relationships tend to be thematic: e.g. play: game :: give: party (Morrison et al., 2004); planet: sun :: electron: nucleus (Green et al., 2006; see also Green et al., 2010; Wendelken et al., 2008) but some are relationships based on part/whole relations, e.g. bouquet: flower :: chain: link (Bunge et al., 2005; Luo et al., 2003), relations not easily assigned to the associative or categorical relationship types.
1.3 The current study
To test the hypothesis that mapping different types of relations would have different neural instantiations we devised two types of analogies, ones expressing an associative relationship and others expressing a categorical relationship. Associative analogies were based on the mapping of a common relation between agents and patients of an action (e.g. cyclist: bicycle :: cowboy: stallion) or a common spatial relation between two sets of objects (e.g. fence: house :: bracelet: wrist). Categorical analogies were based on the mapping of a categorical relationship from one word pair to another; for example car: sedan :: clothing: shirt. We have previously suggested that left hemisphere language areas may be important for extracting the type of relationship between words pairs common across two domains (Schmidt, Kranjec, Cardillo, & Chatterjee, 2010; Wu, Waller, & Chatterjee, 2007). For example, the same agent-patient relationship is connoted in both the domain of a cyclist and a bicycle and the domain of a cowboy and a stallion, and the same categorical relationship is connoted in the domains of both cars and clothing. Thus we hypothesized that both associative and categorical analogies would depend on a distributed network of language areas in the left hemisphere and the fronto-polar region as previously demonstrated in other studies of verbal analogy discussed above.
While the left hemisphere may be important for extracting relationships simply because words are used, the right hemisphere may be important depending on the type of analogy relationship between the two individual words or entities (Schmidt et al., 2010), especially if they are not closely related (Jung-Beeman, 2005). The process of mapping the relationship could differ between the two analogy types if they are qualitatively different. Contrasting with the categorical analogies, associative relationships are often grounded in real events that can be directly experienced and expressed with language (although can also include abstract words such as in school-education). A dog and a leash can co-exist in an event, which is why they are associated. Categorical relations are always feature-based relationships that are not grounded in actual events. Daschunds and collies are both in the category “dog” because they share many features. However some features must be ignored (size, length of fur) while others attended to (have a tail, bark to communicate) in order to establish a categorical relationship. Understanding a categorical relationship requires selecting the relevant features in the comparison and mapping them between the two items (daschund, collie). This abstracting process does not occur for associative relations, even those incorporating abstract entities as in the school-education association. Additionally some of these featural relationships are directly experienced (have a tail, bark to communicate) but some are typically acquired by verbal learning rather than direct experience (mammal, live birth of offspring). Verbally learned relationships may have distinct neural access mechanisms compared to relationships learned by direct experience (Noppeney & Price, 2003). Since they are propositionally based (Paivio, 1991) the relationships may also be considered more abstract. Because they are more abstract, categorical relations may be more distant or coarse than associative ones in semantic space, and thus preferentially recruit the right hemisphere (Jung-Beeman, 2005).
Alternatively, the more abstract nature of categorical relations could render them more difficult to process and for that reason more likely to recruit the right hemisphere (Just, Carpenter, Keller, Eddy & Thulborn, 1996). The difficulty of processing, and right hemisphere recruitment for categorical relationships is supported by several studies that have found increased right hemisphere activation for verbal processing of categorical relations compared to thematic or associative relations (Kotz et al., 2002; Sachs et al., 2008a, Sachs et al., 2008b, Sachs et al., 2011;Sass et al., 2009). Haagort, Brown and Swaab (1996) used event-related potentials (ERPs) to show that patients with right hemisphere lesions (hereafter “right hemisphere participants”) have more difficulty with categorical relations than associative relations. We hypothesized that categorical analogies would be dependant on right hemisphere regions in addition to the left hemisphere language network (including the fronto-polar network).
1.4 Additional considerations
Several other factors were considered in the design of this study. The right hemisphere is important for certain types of linguistic tasks that include processing distant semantic relations (e.g. Joanette, 1990; Jung-Beeman, 2005) non-salient relations (Giora, 2003) or those that require extrapolating inferences (Mason & Just, 2004). Additionally, in imaging studies about a quarter of language related (Vigneau, Beaucousin, Hervé, Jobard, Peti, et al., 2010) and a third of semantic processing related (Binder, Desai, Graves & Conant, 2009) neural activations occur in the right hemisphere. Thus we included both left and right hemisphere participants to get a better picture of the neural basis of verbal analogy. We used voxel-based lesion-symptom mapping (VLSM; Bates, Wilson, Saygin, Dick, Sereno, et al., 2003) to analyze the information from the patients, providing a statistical map of lesioned areas that show differences in behavior scores across all participants. This approach provides more statistical power than lesion overlap methods (Hillis, Work, Barker, Jacobs, Breese, et al., 2004; Rorden & Karnath, 2004). Finally, using patient data allows for stronger causal inferences to be made than can be made by imaging methods which are inherently correlational (Chatterjee, 2005). The current study used verbal analogies unlike many others that use non-verbal stimuli (Baldo, Bunge, Wilson & Dronkers, 2010; Krawczyk et al., 2008; Morrison et al., 2004; Waltz, Knowlton, Holyoak, Boone, Mishkin, et al., 1999).
2. Materials and methods
2.1 Participants
Thirty-four participants with focal lesions of at least 6 months duration were recruited from the Focal Lesion Subject Database at the University of Pennsylvania (17 with lesions affecting the left hemisphere and 17 with lesions affecting the right hemisphere). Patients were not selected based on lesion locations or specific behavioral criteria, except that patients with a history of other neurological disorders, psychiatric disorders, or substance abuse are excluded from the database. Patients with left hemisphere lesions (hereafter “left hemisphere participants”) ranged in age from 22-80 years (mean = 61.1, SD = 14.9) and had an average of 14.5 years of education (SD = 3.5), and right hemisphere participants ranged in age from 42-86 years (mean = 63.5, SD = 13.0) and had an average of 13.9 years of education (SD = 2.9). Ten neurologically healthy older adults (9 female) served as an age (mean = 62.8, SD = 10.0) and education-matched (mean = 16.6, SD = 2.5) control population. The three groups did not differ significantly in terms of age or years of education (ps > .05). Left hemisphere and right hemisphere participant groups did not differ significantly in terms of lesion size or male/female ratio (p > .05). All participants were right-handed, native English speakers, gave informed consent to participate in accordance with the Institutional Review Board of the University of Pennsylvania, and were compensated $15/hour for their time. Detailed demographic and neuropsychological information about the patients can be found in Table 1. The extent of injury in both patient groups can be seen in the lesion overlays in Figure 1.
Table 1.
Patient demographic and neuropsychological data.
| Patient | Gender | Age | Lesion Side | Location | Lesion Size (# voxels) | Cause | Education (years) | WAIS - Info | AMNART | WAB - AQ |
|---|---|---|---|---|---|---|---|---|---|---|
| FC 083 | M | 68 | R | FTP | 8040 | stroke | 12 | 26 | 114 | 99.8 |
| NQ_087 | F | 69 | R | F | 10543 | stroke | 16 | 16 | 113 | 99.1 |
| MB _101 | F | 56 | R | T | 64191 | stroke | 18 | 20 | 121 | 98.4 |
| NC_112 | F | 46 | R | O | 4733 | stroke | 16 | 20 | 119 | 100.0 |
| KE_205 | F | 82 | R | F | 4228 | stroke | 18 | 23 | 115 | 99.2 |
| HX_252 | M | 76 | R | FT | 169837 | stroke | 12 | - | - | 94.6 |
| TC_312 | F | 60 | R | P | 32649 | AVM | 16 | - | - | 100.0 |
| DF_316 | F | 86 | R | P | 2981 | stroke | 12 | - | - | 97.1 |
| DC_392 | M | 54 | R | PT | 39068 | stroke | 10 | - | - | 97.6 |
| DX_444 | F | 78 | R | PT | 41172 | stroke | 12 | - | 99 | 95.5 |
| TS_474 | F | 50 | R | P | 22208 | stroke | 11 | 10 | 89 | 95.1 |
| MF_560 | M | 62 | R | FP | 3007 | stroke | 12 | 10 | 97 | 98.2 |
| NS_569 | F | 71 | R | FTP BG | 37366 | stroke | 18 | 24 | 125 | 100.0 |
| SS_590 | M | 64 | R | FPO | 64063 | stroke | 11 | 8 | 97 | 98.7 |
| DG_592 | F | 42 | R | FP | 130552 | stroke | 12 | 17 | 110 | 97.8 |
| KG_593 | F | 48 | R | FTP BG | 170128 | stroke | 12 | 10 | - | 100.0 |
| ND_640 | F | 68 | R | PT | 64603 | stroke | 18 | - | 126 | 96.8 |
| XD_003 | M | 47 | L | FT | 193601 | stroke | 12 | - | - | 87.3 |
| SL _041 | M | 67 | L | MCA | 193601 | stroke | 12 | - | - | 26.5 |
| KK_074 | F | 63 | L | PT | 34267 | stroke | 13 | 18 | 109 | 97.4 |
| BE_090 | M | 79 | L | PT | 157556 | stroke | 23 | 20 | - | - |
| CD_141 | F | 50 | L | T | 21605 | stroke | 16 | 11 | 113 | 98.8 |
| TO_221 | F | 75 | L | O | 5886 | stroke | 13 | 20 | 121 | 100.0 |
| BC_236 | M | 63 | L | FP | 155982 | stroke | 18 | 19 | 100 | 90.8 |
| XK_342 | F | 56 | L | OT | 42144 | stroke | 12 | - | - | 93.4 |
| BX_384 | M | 70 | L | F | 44467 | Hem | 12 | 15 | - | 93.1 |
| GU_412 | F | 46 | L | F | 45590 | stroke | 13 | 7 | - | 94.8 |
| MK_428 | M | 54 | L | ACC F | 3592 | stroke | 12 | - | - | 95.5 |
| CC_517 | F | 61 | L | F | 30618 | stroke | 12 | 14 | 107 | 97.2 |
| EC_587 | M | 80 | L | P | 5816 | stroke | 21 | - | - | 98.1 |
| LG_611 | F | 22 | L | T | 91594 | HE | 13 | 8 | - | 95.2 |
| UD_618 | M | 75 | L | F | 48743 | stroke | 15 | 18 | 108 | 93.6 |
| KM_642 | M | 75 | L | P | 7996 | stroke | 12 | - | - | 96.8 |
| TE_682 | F | 55 | L | FT BG | 109885 | stroke | 18 | - | - | - |
Key: T, temporal; P, parietal; F, frontal; BG, basal ganglia; ACC, anterior cingulate cortex; O, occipital; Hem, hemorrhage; HE, herpes encephalitis; AVM, arterial venous malformation. AMNART score is an estimated verbal IQ with a mean of 100 +/- 15. WAIS III – Information Subsection score indicates number correct out of 28 items. WAB AQ indicates composite language score with a maximum possible score of 100.
Figure 1.

Distribution of lesions in left (L) and right (R) hemisphere lesioned participants. The colored scale represents the number of lesions for each pixel.
Participants were also administered several neuropsychological assessments. We tested about half of the patients on the American Nelson Adult Reading Test (AMNART; Grober & Sliwinski, 1991) in order to provide an index of pre-morbid verbal IQ and the Information Subtest of the Wechsler Adult Intelligence Scale (WAIS-III; Psychological Corporation, 1999) to provide an index of pre-morbid general knowledge. All but five patients were administered the Western Aphasia Battery (WAB; Kertesz, 1982) to assess post-injury language impairment. Patients were also administered the Object and Action Naming Battery (OANB; Druks, 2000) in order to ensure that differences between noun and verb based analogies were not due to differences in underlying retrieval difficulties for nouns compared to verbs2.
Comparison of patients in right hemisphere and left hemisphere groups using these data indicated no significant differences on the AMNART (right hemisphere: mean = 116.9, SD = 11.3; left hemisphere: mean = 115.1, SD = 7.2) or WAIS (right hemisphere: mean = 10.8, SD = 3.0; left hemisphere = 9.2, SD = 2.5), suggestive of comparable pre-morbid language ability in the two populations. Left hemisphere participants did not score significantly lower than right hemisphere participants on either the WAB (left hemisphere: mean = 90.6, SD = 18.0; right hemisphere: mean = 98.1, SD = 1.8), or the OANB (left hemisphere: mean = 94.5, SD = 6.0; right hemisphere: mean = 95.9, SD = 4.5), and no significant group differences in noun and verb naming emerged.
2.2 Stimuli
Experimental trials consisted of two pairs of words that, together, either did or did not create a sensible analogy. YES trials were those trials on which upper and lower word pairs expressed the same relationship and NO trials were those trials for which the upper and lower word pairs expressed different relationships. Analogies were of two types: Associative or Categorical. Associative analogies were based on either agent-patient (thematic) or spatial relations between nouns while Categorical analogies were based on categorical relationships between either nouns or verbs. Hypothesized differences between Associative-Thematic and Associative-Spatial analogies and between Categorical-Noun and Categorical-Verb analogies did not emerge, so our analysis and discussion of the data collapses across the two Associative analogy types and the two Categorical analogy types. See Table 2 for examples of each analogy type and below for details of how each type was designed.
Table 2.
Examples of yes and no items for each analogy type.
| Analogy Type | Trial Type | Relation | Analogy Trial | |
|---|---|---|---|---|
| Associative | Yes | in | fish: sea :: nest: tree | |
| Yes | through | earring: ear :: needle: fabric | ||
| Yes | around | fence: house :: bracelet: wrist | ||
| No – foil: associative | fruit: bowl :: hiker: trail | |||
| No – foil: categorical | screen: keyboard :: drug: heroin | |||
| No – foil: unrelated | cookies: jar :: fur: radio | |||
| Yes | hit | batter: baseball :: golfer: golfball | ||
| Yes | carry | bellhop: luggage :: mover: boxes | ||
| Yes | ride | cyclist: bicycle :: cowboy: stallion | ||
| No - foil: spatial | dealer: cards :: cigarette: lips | |||
| No - foil: categorical | pianist: piano :: fuel: gas | |||
| No - foil: unrelated | hammer: nail :: clock: mask | |||
|
| ||||
| Categorical | Yes | container: box :: pattern: plaid | ||
| Yes | car: sedan :: clothing: shirt | |||
| Yes | color: green :: terrain: desert | |||
| No – foil: spatial | poem: ode :: money: wallet | |||
| No – foil: associative | home: apartment :: spatula: pancake | |||
| No – foil: unrelated | instrument: guitar :: log: bandage | |||
| Yes | to laugh: to chuckle :: to control: to manipulate | |||
| Yes | to dance: to waltz :: to walk: to stroll | |||
| Yes | to say: to exclaim :: to taste: to savor | |||
| No – foil: antonym | to jump: to leap :: to find: to lose | |||
| No – foil: associate | to break: to fracture :: to look: to leap | |||
| No – foil: unrelated | to walk: to tiptoe :: to hide: to salute | |||
Analogies were developed using an extensive norming process. The first author compiled lists of word pairs related in one of the ways described above (categorically or associatively). Next, three of the other authors (AK, EC, and PW) independently rated each word pair with the first relationship to come to mind. Only word pairs whose relationship was described the same way by all four authors were kept. Last, the word pairs were matched with each other in a way to create either sensible (YES) or non-interpretable (NO) analogies. Each word pair was used only once.
For Associative analogies, the YES trials consisted of word pairs that both implied the same action or both implied the same spatial relationship between the words constituting the pairs. For example, the action pull relates the words of each pair in the analogy CAR : TRAILER :: DONKEY : CART and the spatial relation above is common to the word pairs in the analogy FLAME : CANDLE :: STEEPLE : CHURCH. For NO trials, if the upper word pair was related associatively (i.e. by an action), then the bottom word pair was either related spatially, categorically, or unrelated with roughly equal numbers of each. If the upper word pair had a salient spatial relationship, then the bottom word pair was either related associatively, categorically, or was unrelated, with roughly equal numbers of each. All words were concrete, imageable nouns.
For Categorical analogies, the YES trials consisted of two word pairs that both expressed either noun category membership (e.g. RODENT : MOUSE :: APPLIANCE : TOASTER) or verb category membership (e.g. TO SING: TO SERENADE :: TO KILL : TO ASSASSINATE)3. All nouns described concrete, imageable objects or animals and all verbs described transitive actions with salient motion. On NO trials, if nouns constituted the first word pair, then the second word pair was related either associatively, spatially, or was unrelated with roughly equal numbers of each. If verbs constituted the first word pair, then the words in the second pair were either antonyms, weak lexical associates, or unrelated with roughly equal numbers of each.
In all, 108 Associative analogies (half with a locative relationship in the first pair and half with an associative relationship between the words in the first pair) and 122 Categorical analogies (54 involving nouns and 68 involving verbs) were pilot tested. 18 native English speakers with a mean age of 25.6 years (range 21-48) volunteered to assess whether the two pairs of words expressed the same relationship (i.e. “Is this an analogy?”). After 8 practice trials with feedback, participants received 230 experimental trials in a single 30-minute block. Each trial consisted of a 1000ms pause followed by the presentation of two pairs of words presented one above the other in the center of the screen. Participants were instructed to press F for “Yes” and J for “No”. Stimuli were presented using E-prime 1.0 on a Dell Inspiron laptop and both accuracy and reaction time were recorded.
Using the results of the pilot test, trials with the highest median reaction times or lowest mean agreement scores were eliminated, resulting in a final set of 80 Associative (40 of each type) and 80 Categorical (40 of each type) items presented to the participants. Half of the items of each analogy type were YES trials and half were NO trials. Of these, a total of 38 Associative and 40 Categorical analogies were entered into the final analyses as explained below. ANOVAs of the 78 final analogies indicated the four analogy types did not differ significantly in accuracy (Associative-Thematic: mean = .96, SD = .05; Associative-Spatial: mean = .92, SD = .07; Categorical-Noun: mean = .96, SD = .06; Categorical-Verb: mean = .95, SD = .05), or reaction times (Associative-Thematic: mean = 4330ms SD = 783ms; Associative-Spatial: mean = 4221ms SD = 1058ms Categorical-Noun: mean = 3954ms, SD = 811ms Categorical-Verb: mean = 3965ms, SD = 646ms).
Each analogy was also characterized in terms of frequency, concreteness, and semantic relatedness since these factors all strongly influence the ease with which words are read and understood. Concreteness and frequency ratings were obtained from the MRC psycholinguistic database (Wilson, 1988) for each word in the analogy. For words not found in this database, values from the Nelson norms (Nelson, McEvoy & Schreiber, 1998) were used. For the 126 words having no value in either database, we collected our own concreteness norms based on the ratings provided by 20 volunteers (mean age = 23.8, SD = 3.7) recruited in accordance with the standards of the Institutional Review Board of the University of Pennsylvania. Analogy-level frequency and concreteness values were obtained by calculating the mean of the values for the four words in each analogy. The overall word frequency values for the final set of Associative-Thematic (M= 40.1, SD = 29.6), Associative-Spatial (M= 50.8, SD = 38.1), Categorical-Noun (M= 39.4, SD = 32.1), and Categorical-Verb (M=80.1, SD = 80.0) analogies were different at a group level, F(3,74) = 2.94, p =.039, but all post hoc comparisons were nonsignificant ps > .05). Categorical-Verb analogies (M=383, SD=54) had words that were significantly less concrete than words in Categorical-Noun (M= 554, SD = 52), Associative-Thematic (M= 579, SD = 42) and Associative-Spatial (M= 580, SD = 37) analogies, F(3,74) = 80.33, p <.001, with no other differences in concreteness emerging. Latent Semantic Analysis (LSA) was used to quantify the degree of semantic relatedness between the two words in each word pair (Landauer & Dumais, 1997; http://lsa.colorado.edu). An overall LSA value for each trial was obtained by averaging the two LSA values for each word pair constituting a trial, with values closer to 1 indicating stronger relatedness. The overall LSA values for Associative-Thematic (M= .33, SD = .18), Associative-Spatial (M= .41, SD = .17), Categorical-Noun (M= .47, SD = .19), and Categorical-Verb (M=.37, SD = .12) analogies did not differ statistically.
2.3 Procedure
Participants were tested individually in their homes or in the laboratory in 2-hour testing sessions. After practice trials (minimum = 8), each participant received a unique random order of the 160 analogy trials, distributed across 5 blocks of 32 trials each. Each trial consisted of the two word pairs presented one above the other in the center of the screen (Arial 40 pt. font). Trials were presented using E-Prime 1.0 software on a Dell Latitude laptop. Participants were instructed to verbally report “Yes” if the two word pairs expressed the same relationship and “No” if they expressed two different relationships. The experimenter made key presses both to record the participants' response and to advance the trials when the participant was ready. The entire task required 40-60 minutes to complete. Most participants were subsequently tested on the WAB and completed the OANB in a separate testing session involving a different experimental language task.
2.4 Behavioral Analysis
The dependent measure used for behavioral performance was accuracy on all analogy trials (All), categorically related analogies (Categorical), and associatively related analogies (Associative). Because control participants scored less than 60% correct on 18 of the 160 trials, these items were eliminated from all subsequent analyses. Additionally the final analysis was restricted to YES trials since these are the only trials on which a single type of relation is queried. That is, because a NO trial consists of a categorically related word pair and a associatively related word pair an incorrect response could indicated difficulty with categorical, associative, or both associative and categorical relation types. Thus, only YES trials allow making inferences about specific impairments in deriving associative versus categorical relations. These two restrictions resulted in the data from 38 Associative and 40 Categorical analogies being included in all subsequent analyses.
2.5 Neuroanatomical Analysis
Clinical CT or MRI scans for all patients were rendered to a common anatomical space (Colin27; http://imaging.mrc-cbu.cam.ac.uk/downloads/Colin/) to allow statistical assessment of lesion-behavior relationships. Two senior neurologists blind to patient performance drew lesions on 1mm × 1mm × 1mm MNI templates tilted in the same axial planes of the source images using MRIcro or MRIcron software (Rorden and Brett, 2000; http://www.cabiatl.com/mricro/mricro/index.html/), resulting in lesion maps in which each voxel was labeled either 0 (intact) or 1 (lesioned). Each template was then realigned to a common axial angle using the software's realignment procedures.
VLSM analysis (Bates et al., 2003) was then conducted using Voxbo imaging-analysis software (www.voxbo.org). VLSM is a form of statistical parametric mapping that assesses the relationship between behavioral performance and brain damage on a voxel by voxel basis. One-tailed t-tests at every voxel compared behavioral scores between patients with and without lesions at that voxel, resulting in a statistical map of brain areas whose injury significantly impairs performance. In the current study, percent correct on YES trials for All, Associative, and Categorical items served as the dependent measures in the lesion analysis. To minimize the effects of outlier observations, only voxels with a minimum of two injured patients were tested. Statistical maps were thresholded at q < .05 using a false discovery rate (FDR) of .05 to correct for multiple comparisons (Genovese & Lazar, 2002).
3. Results
3.1 Behavioral Findings
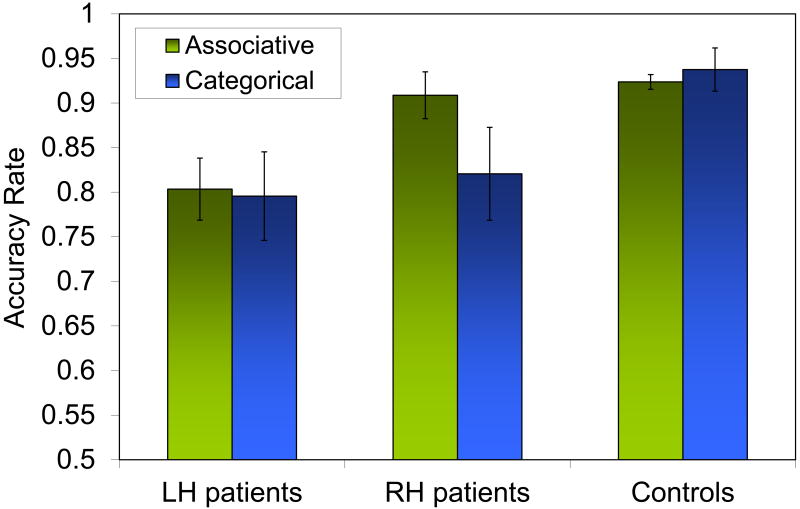
Control participants had higher accuracy scores than left hemisphere participants, t = 2.51, p = .02, but not right hemisphere participants, p= .19. Subsequent analyses of accuracy scores were completed using stimulus items rather than participants as the random variable to facilitate covariate analyses. A mixed 2×2 ANOVA yielded significant effects of the within-item factor Side of Lesion, F(1,76)= 23.4, p < .001 and the between item factor Analogy Type, F(1,76) = 4.05, p= .048; and a significant interaction, F(1,76) = 8.9, p= .004. Figure 2 shows that the performance of right hemisphere participants on Associative analogies is better than their performance on Categorical analogies, t(76)= 3.58, p= .001 and better than left hemisphere participants' performance on Associative analogies, t(37)= 5.1, p < .001. Comparison of the two types of analogies within each Analogy Type (noun, verb; thematic; spatial) revealed no differences in each pair of analogy types (ps > .35).
Figure 2.
Behavioral performance across participant groups including the 10 control participants for comparison. Error bars represent standard errors.
Concreteness was not matched across analogy conditions; however an ANCOVA incorporating it as a nuisance variable still resulted in a significant interaction between Side of Lesion and Analogy Type, F(1, 75)= 6.04, p= .016. Thus our findings were not due to confounding effects of concreteness of the stimulus items.
3.2 Neuroanatomical Findings
VLSM analyses indicated a significant lesion-behavioral relationship for both types of analogies as seen in Figure 3. There were no significant relationships between overall analogy scores (i.e. the score depicting performance across analogy types) and lesion site even at a more lenient FDR of .10. For the Associative analogies, there were significant lesion-behavior relationships in the left middle to posterior temporal gyrus (BA21, BA22, BA34), inferior frontal gyrus (BA45) and frontal white matter. For the Categorical analogies, there was a strong relationship between accuracy scores and lesions in the right posterior middle temporal gyrus (BA21).
Figure 3.

Brain regions surviving the FDR threshold of q < .05 for the two types of analogies.
4. Discussion
The current study examined the neural underpinnings of associative and categorical verbal analogies. The main question we addressed was whether all verbal analogies share a common neural substrate or whether the type of relationship used in the analogy changes its neural processing. Performance on associative and categorical analogies was associated with different behavioral and lesion patterns in patients. Associative analogy comprehension relied on a left-lateralized network while categorical analogy comprehension relied on both left and right hemispheres.
4.1 Type of analogy matters
The principal implication of these findings is that the type of analogical relationship matters. Brain damage produces different behavior patterns and neural associations. Our right-and left-lesioned participants demonstrated a clear difference in performance patterns for associative and categorical analogies. Left hemisphere participants showed similarly impaired performance understanding both types of analogy; right hemisphere participants were impaired similarly to left hemisphere participants for categorical analogies, but were normal for associative analogies (see Figure 2). Put differently, the two analogy types rely on different neural substrates to be understood. Categorical analogies require both hemispheres to be intact, whereas associative analogies can be understood with just an intact left hemisphere. When we combined the scores of the two analogy types with VLSM analysis, no effects were found, even at the more lenient FDR threshold of q = .10. This lack of finding at the general analogy level in conjunction with the significant effects at the associative and categorical levels support the hypothesis that different types of analogical relations depend on different neural substrates. Categorical analogies relied on the right posterior middle temporal gyrus. There were no significant brain-behavior relations in the left hemisphere despite the fact that these patients as a group did not do well. We do not know the reason for this lack of brain-behavior correlation in the left hemisphere. One hypothesis is that the neural underpinnings for categorical relations in the left hemisphere may have non-linear dynamics, involving multiple areas, for which VLSM might not be sensitive. Associative analogies relied on left frontal and temporal regions.
4.2 Neural basis of verbal analogy
Our findings point to the importance of the posterior middle temporal gyrus for both associative analogies (in the left hemisphere) and categorical analogies (in the right hemisphere). These findings are consistent with findings in Luo et al. (2003) and Green et al. (2010). The left inferior frontal gyrus finding for associative analogies is consistent with similar findings in many studies of verbal analogy as well (Bunge et al., 2005; Green et al., 2006; Green et al., 2010; Luo et al. 2003; Wendelken et al., 2008).
The complete lack of general analogy or mapping regions in the present data may be due to a lack of coverage in known mapping areas of the brain, such as the rostral prefrontal cortex. This area has been the most often identified region in analogical reasoning, using both verbal (e.g., Morrison et al., 2004; Wendelken et al., 2008) and non-verbal (visual, such as Raven's progressive matrices) stimuli (e.g. Christoff, Prabhakaran, Dorfman, Zhao, Kroger, et al., 2001; Volle, Gilbert, Benoit & Burgess, 2010). However, most of our participants did not have lesions in those regions, which would more typically occur with watershed infarcts. Thus, we remain agnostic about the importance for this area in mapping relationships regardless of the nature of those relationships. It is also important to point out that the analogy accuracy scores of our stroke patients are relatively high (about 80%), in contrast to studies of relational reasoning in frontal lobe patients (e.g. Morrison et al., 2004; Krawczyk et al., 2008). It remains to be determined whether the regions we have identified are essential for relational processing in the same way that prefrontal regions may be (Watson & Chatterjee, 2012).
However, our results do suggest that the semantic content of the analogy is important in determining neural resources. The collection of specific areas associated with the two analogy types could be described as classic language areas or their right homologs. Thus for verbal analogies at least, core linguistic or semantic processes seem to be important components of analogy processing.
Analogical processing also crucially involves the process of abstraction (Gentner, 2003), and this applies to the abstraction of the relationship that links the two word pairs in the analogy. Our data are consistent with the view that the general process of abstracting a relationship from two domains (which occurs in both types of analogies) must rely on the left hemisphere (Schmidt et al., 2010; Wu et al., 2007).
4.3 Categorical analogies and the right hemisphere
The specific neural differences we found between associative and categorical analogies suggest that the right mid-posterior temporal region is important for comprehension of categorical but not associative analogies. This result is consistent with the findings of Haagort et al. (1996) who report that right hemisphere participants had difficulty with categorical but not associative relations. Sachs et al. (2011) also report right frontal activation for the processing of categorical relations; and Federmeier, Wlotko and Meyer (2008) review ERP literature supporting a right hemisphere advantage for processing categorical or feature-based relations and a left hemisphere advantage for associative relationships.
Eight of our seventeen right hemisphere patients had temporal lobe lesions while others had parietal and/or frontal damage without temporal involvement. Those with temporal lobe lesions performed more poorly (accuracy = 76%) than those with other types of damage (accuracy = 88%) on the categorical analogies, although this difference was not significant probably due to power issues (p = .28). In general, other right hemisphere areas must also be important for categorical analogy understanding.
The categorical analogy reliance on the right hemisphere could be consistent with the hypothesis that the right hemisphere is adept at processing coarse or distant semantic relationships (Jung-Beeman, 2005). The associatively related words fish and sea are more highly associated than the categorically related words container and box. It may be that semantic connections in categorical relations are more distant or coarse than those in associative relations, as defined by Jung-Beeman. Although a coarse coding explanation seems reasonable, we did match strength of semantic relationship between word pairs across conditions using LSA (Landauer & Dumais, 1997). Matching the two types of analogies in this way ensures that the degree of semantic relatedness is equivalent across conditions, whereas the course coding explanation entails that the degree of semantic relatedness processed by the right hemisphere is more coarse or distant.
Although the course coding hypothesis might not apply to our findings, Jung-Beeman's (2005) concept of course semantic relationships may tap different aspects of semantic relatedness than the LSA version of semantic association strength. LSA is strongly correlated with free association measures of relatedness (Landauer & Dumais, 1997) and as such does not explicitly include feature-based (categorical) relationships. The right hemisphere is sensitive to feature-based relationships that are not associative (Deacon, Grose-Fifer, Yang, Stanick, Hewitt, et al., 2004), suggesting a dissociation between categorical and associative relationships. However, Jung-Beeman (2005) includes both associative and feature-based relations in his explanations of coarse and fine semantic relationships. Since the coarse coding hypothesis operationalizes relatedness differently than LSA we do not know if it applies to our right hemisphere finding for categorical relations.
Another possible explanation for categorical analogy reliance on the right hemisphere is that it is simply more difficult than associative analogy processing. The right hemisphere is recruited for more difficult language processing (Just et al., 1996). In a meta-analysis of right hemisphere language Vigneau et al. (2010) report right middle temporal gyrus bilateral activation for the processing of complex semantic relations across many studies (Homae, Yahata, & Sakai, 2003; Kircher, Brammer, Andreu, Williams, & McGuire, 2001; Luke, Liu, Wai, Wan, & Tan, 2002; Vogeley, Bussfeld, Newen, Herrmann, Happé, et al., 2001). Vigneau et al. distinguish between language activation in right hemisphere language homologs versus in other right hemisphere areas. Activation of language region homologs in the right hemisphere suggests cross-callosal connection, leading to recruitment of additional right hemisphere regions when language processing is difficult. Independent, non-homolog right hemisphere activation suggests processes specific to the right hemisphere. Since the region associated with categorical analogies in our study is a right hemisphere language homolog, this implies a cross-collosal connection entailing the recruitment of the right hemisphere by the left hemisphere for difficult tasks. It accounts for our finding in the right middle temporal gyrus, a homolog of a left hemisphere language area.
A number of observations support the possibility that associative relations are more robust and categorical relations are more fragile or difficult to process. Categorical relations are learned later in childhood and are weaker than associative relations for children (Hashimoto, McGregor & Graham, 2007; Scott, Greenfield, & Urbano, 1985; Scott, Serchuk & Mundy, 1982) suggesting they are more difficult to master. Verbal interference impairs adult categorical, but not associative, judgments (Lupyan, 2009). Luria reported that preliterate adults from Uzbekistan in the early twentieth century grouped items associatively and not categorically (Luria, 1979, chapter 4). The degree of difficulty of categorical versus associative analogies would also be a plausible explanation for our findings. However the difficulty explanation may not be the primary explanation for our findings. Reaction times in our pilot participants did not distinguish the two types of analogy, and there was actually a trend towards longer reaction times for associative analogies (t(76)=1.69, p=.095), suggesting that there were no differences in difficulty for this set of analogies.
We suggest that the relationships or mappings between words in categorical analogies are qualitatively different than those in associative analogies. Associative relations between two words are typically based on actual events (a fish swimming in the sea) that can be directly experienced. The two items co-occur in time and space. In this sense such relationships are concrete. Categorical relationships, even those using concrete words, are less concrete than associative ones. They are feature-based, and not all features are equally important in establishing a relationship. Boxes and bottles are both containers, but some features must be ignored (shape, material) while others attended to (function of containment) in order for a relationship to be established. Selecting the relevant features and mapping them is required to establish the relationship. This is more abstract than linking two items that co-occur (fish, sea). In addition, some of the important features can not be directly experienced but must be learned (Noppeney & Price, 2003). Some aspects of the relationship between the concepts of bottle and box can not be experienced as an event. This type of relationship can also be considered more abstract than an associative one since it is not primarily based on a concrete, experienceable event, but on a propositional concept (Paivio, 1991).
Thus categorical relationships are more abstract than associative relationships without necessarily being less associated based on co-concurrency data obtained from LSA. In other words, the right hemisphere is involved in more abstract (in this case categorical) relationships but not the more concrete associative relationships grounded in event experiences. This explanation can be profitably extended to analogies which include two-word relationships. Our right hemisphere finding for categorical analogies is consistent with right hemisphere involvement in categorical two-word relationships in other studies (Sachs et al., 2011; Federmeier et al., 2008), implying that processing the semantic link between two words or entities is a crucial part of analogy processing.
Highlights.
We investigated analogies expressing an associative or categorical relationship.
We tested patients with left and right hemisphere lesions.
Associative analogies relied on a large left-lateralized language network.
Categorical analogies relied on both left and right hemispheres.
Categorical relations are difficult, abstract, fragile; and contain distant relationships.
Acknowledgments
We thank the following individuals for their support of various aspects of this project: Bianca Bromberger, Dan Kimburg, Marianna Stark, and Christine Watson. We are also deeply grateful to the patients who participated in the study. The project was in part supported by award number T32NS007413 from the National Institutes of Neurological Disorders and Stroke (NINDS). The content is the sole responsibility of the authors and does not necessarily represent the official views of the NINDS of the National Institutes of Health. This work was also supported by the National Institutes of Health award RO1 DC004817 and the National Science Foundation [subcontract under SBE0541957].
Footnotes
The SAT Reasoning Test is a standardized test used for college and university admittance in the United States.
A nonverbal left hemisphere participant was not administered the OANB given the severity of his language impairment.
In contrast to nouns, it is less clear what constitutes a verb category. We used some of the light and dense verb pairs of Torreano, Cacciari, and Glucksberg (2005) as verb equivalents to the base and subordinate noun category levels.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldo JV, Bunge SA, Wilson SM, Dronkers NF. Is relational reasoning dependent on language? A voxel-based lesion symptom mapping study. Brain and Language. 2010;113:59–64. doi: 10.1016/j.bandl.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdle BF, Gentner D. The Career of Metaphor. Psychological Review. 2005;112:193–216. doi: 10.1037/0033-295X.112.1.193. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cerebral Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. A madness to the methods in cognitive neuroscience? Journal of Cognitive Neuroscience. 2005;17(6):847–849. doi: 10.1162/0898929054021085. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JDE. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Davidoff J, Roberson D. Preserved thematic and impaired taxonomic categorization: A case study. Language and Cognitive Processes. 2004;19:137–174. [Google Scholar]
- Deacon D, Grose-Fifer J, Yang C, Stanick V, Hewitt S, Dynowska A. Evidence for a new conceptualization of semantic representation in the left and right cerebral hemispheres. Cortex. 2004;40:467–78. doi: 10.1016/s0010-9452(08)70140-0. [DOI] [PubMed] [Google Scholar]
- Druks J. An Object and Action Naming Battery. Psychology Press; 2000. [Google Scholar]
- Federmeier KD, Wlotko EW, Meyer AM. What's “right” in language comprehension: ERPs reveal right hemisphere language capabilities. Lang Linguist Compass. 2008;2:1–17. doi: 10.1111/j.1749-818X.2007.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese NA, Lazar TN. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gentner D, Namy LL. Analogical processes in language learning. Current Directions in Psychological Science. 2006;15:297–301. [Google Scholar]
- Gentner D. Why we're so smart. In: Gentner D, Goldin-Meadow S, editors. Language in mind: Advances in the study of language and thought. Cambridge, MA: MIT Press; 2003. pp. 195–235. [Google Scholar]
- Gentner D, Bowdle B, Wolff P, Boronat C. Metaphor is like analogy. In: Gentner D, Holyoak KJ, Kokinov BN, editors. The analogical mind: Perspectives from cognitive science. Cambridge, MA: MIT Press; 2001. pp. 199–253. [Google Scholar]
- Giora R. On our mind: Salience context, and figurative language. New York: Oxford University Press; 2003. [Google Scholar]
- Glick ML, Holyoak KJ. Schema induction and analogical transfer. Cognitive Psychology. 1983;15:1–38. [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Research. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Green AE, Kraemer DJM, Fugelsang JA, Gray JR, Dunbar KN. Connecting long distance: Semantic distance in analogical reasoning modulates frontopolar cortex activity. Cerebral Cortex. 2010;20:70–76. doi: 10.1093/cercor/bhp081. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Haagort B, Brown CM, Swaab TY. Lexical-semantic event-related potential effects in patients with left hemisphere lesions and aphasia, and patients with right hemisphere lesions without aphasia. Brain. 1996;119:627–649. doi: 10.1093/brain/119.2.627. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, McGregor KK, Graham A. Conceptual organization at 6 and 8 years of age: Evidence from the semantic priming of object decisions. Journal of Speech, Language, and Hearing Research. 2007;50:161–176. doi: 10.1044/1092-4388(2007/014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Homae F, Yahata N, Sakai KL. Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. NeuroImage. 2003;20:578–586. doi: 10.1016/s1053-8119(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Hutchison KA. Is semantic priming due to association strength or feature overlap? A microanalytic review. Psychonomic Bulletin & Review. 2003;10:785–813. doi: 10.3758/bf03196544. [DOI] [PubMed] [Google Scholar]
- Joanette Y, Goulet P, Hannequin D. Right hemisphere and verbal communication. New York: Springer-Verlag; 1990. [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Science. 2005;11:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kalenine S, Peyrin C, Pichat C, Segebarth C, Bonthoux F, Baciu M. The sensory-motor specificity of taxonomic and thematic conceptual relations: A behavioral and fMRI study. NeuroImage. 2009;44:1152–1162. doi: 10.1016/j.neuroimage.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. San Antonio, TX: Psychological Corp; 1982. [Google Scholar]
- Kircher TT, Brammer M, Andreu NT, Williams SC, McGuire PK. Engagement of right temporal cortex during processing of linguistic content. Neuropsychologia. 2001;39:798–809. doi: 10.1016/s0028-3932(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical–semantic network by auditory semantic priming: An event-related functional MRI study. NeuroImage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Morrison RG, Viskontas I, Holyoak KJ, Chow TW, Mendeze MF, Miller BL, Knowlton BJ. Distraction during relational reasoning: The role of prefrontal cortex in interference control. Neuropsychologia. 2008;46:2020–2032. doi: 10.1016/j.neuropsychologia.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Landauer TK, Dumais ST. A solution to Plato's problem: The latent semantic analysis theory of the acquisition, induction, and representation of knowledge. Psychological Review. 1997;104:211–240. [Google Scholar]
- Luke KK, Liu HL, Wai YY, Wan YL, Tan LH. Functional anatomy of syntactic and semantic processing in language comprehension. Human Brain Mapping. 2002;16:133–145. doi: 10.1002/hbm.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Perry C, Peng D, Jin Z, Xu D, Ding G, Xu S. The neural substrate of analogical reasoning: An fMRI study. Cognitive Brain Research. 2003;17:527–534. doi: 10.1016/s0926-6410(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Lupyan G. Extracommunicative functions of language: Verbal interference causes selective categorization impairments. Psychonomic Bulletin & Review. 2009;16:711–718. doi: 10.3758/PBR.16.4.711. [DOI] [PubMed] [Google Scholar]
- Luria AR. The making of mind: A personal account of soviet psychology. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Mason RA, Just MA. How the brain processes causal inferences in text: A multiple process theory of the function of the language network in both hemispheres. Psychological Science. 2004;15:1–7. doi: 10.1111/j.0963-7214.2004.01501001.x. [DOI] [PubMed] [Google Scholar]
- Miller GA, Fellbaum C. Semantic networks of English. Cognition. 1991;41:197–229. doi: 10.1016/0010-0277(91)90036-4. [DOI] [PubMed] [Google Scholar]
- Morrison RG, Krawczyk DC, Holyoak KJ, Hummel JE, Chow TW, Miller BL, Knowlton BJ. A neurocomputational model of analogical reasoning and its breakdown in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience. 2004;16:260–271. doi: 10.1162/089892904322984553. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. http://www.usf.edu/FreeAssociation. [DOI] [PubMed]
- Noppeney U, Price CJ. Functional imaging of the semantic system: Retrieval of sensory-experienced and verbally learned knowledge. Brain and Language. 2003;84:120–133. doi: 10.1016/s0093-934x(02)00525-4. [DOI] [PubMed] [Google Scholar]
- Paivio A. Dual coding theory: Retrospect and current status. Canadian Journal of Psychology/Revue canadienne de psychologie. 1991;45:255–287. [Google Scholar]
- Psychological Corporation. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Brace and Company; 1999. [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. San Antonio, TX: Pearson Assessment; 2004. [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;1:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Sachs O, Weis S, Krings T, Huber W, Kircher T. Categorical and thematic knowledge representation in the brain: Neural correlates of taxonomic and thematic conceptual relations. Neuropsycholgia. 2008a;46:409–418. doi: 10.1016/j.neuropsychologia.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Sachs O, Weis S, Zellagui N, Huber W, Zvyagintsev M, Mathiak K, Kircher T. Automatic processing of semantic relations in fMRI: Neural activation during semantic priming of taxonomic and thematic categories. Brain Research. 2008b;1218:194–205. doi: 10.1016/j.brainres.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Sachs O, Weis S, Zellagui N, Sass K, Huber W, Zvyagintsev M, Mathiak K, Kircher T. How different types of conceptual relations modulate brain activation during semantic priming. Journal of Cognitive Neuroscience. 2011;23:1263–1273. doi: 10.1162/jocn.2010.21483. [DOI] [PubMed] [Google Scholar]
- Sass K, Sachs O, Krach S, Kircher T. Taxonomic and thematic categories: Neural correlates of categorization in an auditory-to-visual priming task using fMRI. Brain Research. 2009;1270:78–87. doi: 10.1016/j.brainres.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, Kranjec A, Cardillo E, Chatterjee A. Beyond laterality: a critical assessment of research on the neural basis of metaphor. Journal of the International Neuropsychological Society. 2010;16:1–5. doi: 10.1017/S1355617709990543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MS, Greenfield DB, Urbano RC. A comparison of complementary and taxonomic utilization: Significance of the dependent measure. International Journal of Behavioral Development. 1985;8:241–256. [Google Scholar]
- Scott MS, Serchuk R, Mundy P. Taxonomic and complementary picture pairs: Ability in two- to five-year-olds. International Journal of Behavioral Development. 1982;5:243–256. [Google Scholar]
- Torreano LA, Cacciari C, Glucksberg S. When dogs can fly: Level of abstraction as a cue to metaphorical use of verbs. Metaphor and Symbol. 2005;20(4):259–274. [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Jobard G, Peti L, Crivello F, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. NeuroImage. 2011;54:577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. NeuroImage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Volle E, Gilbert SJ, Benoit RG, Burgess PW. Specialization of the rostral prefrontal cortex for distinct analogy processes. Cerebral Cortex. 2010;20:2647–2659. doi: 10.1093/cercor/bhq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Mishkin FS, de Menezes Santos M, Thomas CR, Miller BL. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–125. [Google Scholar]
- Watson C, Chatterjee A. A bilateral frontoparietal network underlies visuospatial analogical reasoning. NeuroImage. 2012;59:2831–2838. doi: 10.1016/j.neuroimage.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain Is to Thought as Stomach Is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. Journal of Cognitive Neuroscience. 2008;20:1–12. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC Psycholinguistic Database: Machine Readable Dictionary, Version 2. Behavioral Research Methods, Instruments and Computers. 1988;20:6–11. http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm. [Google Scholar]
- Wu DH, Waller S, Chatterjee A. The functional neuroanatomy of thematic role and locative relational knowledge. Journal of Cognitive Neuroscience. 2007;19:1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]