Abstract
Under inflammatory conditions (including HIV-1 encephalitis and multiple sclerosis), activated brain endothelium enhances the adhesion and transmigration of monocytes across the blood-brain barrier (BBB). Synthetic ligands that activate the peroxisome proliferator-activated receptors (PPARs) have anti-inflammatory properties, and PPARs stimulation prevents the interaction of leukocytes with cytokine stimulated-endothelium. However, the mechanism underlying these effects of PPAR ligands and their ability to intervene with leukocyte adhesion and migration across brain endothelial cells have not been explored yet. For the first time, using primary human brain endothelial cells (BMVEC), we demonstrated that monocyte adhesion and transendothelial migration across inflamed endothelium were markedly reduced by PPARγ activation. In contrast to non-brain derived endothelial cells, PPARα activation in the BMVEC had no significant effect on monocyte-endothelial interaction. Our previous work indicated a critical role of Rho GTPases (like RhoA) in BMVEC to control migration of HIV-1 infected monocytes across BBB. Here, we show that PPARγ stimulation prevented activation of two GTPases, Rac1 and RhoA, in the BMVEC that correlated with decreased monocyte adhesion to and migration across brain endothelium. Relevant to HIV-1 neuropathogenesis, enhanced adhesion and migration of HIV-1 infected monocytes across the BBB were significantly reduced when BMVEC were treated with PPARγ agonist. These findings indicate that Rac1 and RhoA inhibition by PPARγ agonists could be a new approach for treatment of neuroinflammation by preventing monocyte migration across the BBB.
Keywords: monocytes, inflammation, brain microvascular endothelial cells, blood brain barrier, tight junctions, PPAR
Introduction
Adhesion of monocytes to activated brain endothelium plays an important role in the transmigration across the blood brain barrier (BBB) during inflammation of the central nervous system (CNS) [Engelhardt, 2005 #36]. Under physiological conditions, immune surveillance by lymphocytes crossing BBB is minimal and monocyte infiltration is practically undetectable; however, during the development of autoimmune disorders (i.e. multiple sclerosis) or virus driven diseases (i.e. HIV-1 encephalitis), leukocyte migration through the BBB increases considerably [Kanwar, 2005 #38; Persidsky, 2003 #35; Stalder, 2005 #37; Eugenin, 2006 #54]. Enhanced leukocyte transendothelial migration is attributed to the activation of both the brain microvascular endothelial cells (BMVEC) and immune cells by pro-inflammatory cytokines. Cytokine activation promotes upregulation of integrins in leukocytes and adhesion molecules (e.g., ICAM-1, and VCAM-1) on the BMVEC, which strengthen leukocyte-endothelial interactions leading to increase in transendothelial migration [Dietrich, 2002 #40; Quandt, 2004 #39]. Thus, prevention of the initial leukocyte-endothelial interaction or reduction of the BMVEC controlled leukocyte passage could provide novel therapeutic avenues to lessen neuroinflammation.
Peroxisome proliferator-activated receptors (PPARs) are transcriptional regulators and members of a nuclear receptor family that include the closely related PPARα, PPARβ/δ and PPARγ, [Desvergne, 1999 #55]. PPARs play important roles in regulation of adipocyte differentiation, insulin sensitivity, anti-inflammatory responses, and antiproliferative effects in certain tumors [Lehrke, 2005 #64]. In recent years, the lipid lowering fibrates, which activate PPARα and the insulin sensitizing thiazolidinediones (TZDs), which activate PPARγ have proven useful as potent anti-inflammatory agents [Zingarelli, 2005 #45]. Ligand binding of PPAR promotes the heterodimerization with the retinoid x receptor inducing PPAR transactivation of target genes. In addition, activation of PPARs can also lead to transrepression, a process that has been shown to negatively interfere with key transcription factors (e.g., NF-κB, AP-1, STAT1, and sp1) involved in inflammatory responses. Stimulation of PPARs down-regulated the expression of pro-inflammatory genes such as IL-1β, IL-6, inducible nitric oxide synthase, cyclooxygenase-2 and various chemokines [Brown, 2007 #59]. Additionally, PPARs were found to decrease endothelial-leukocyte interactions in models of atherosclerosis [Kurebayashi, 2005 #2; Zandbergen, 2007 #58]. Although these inhibitory effects on monocyte adhesion by PPAR activation have been in part attributed to suppression of adhesion molecules in the endothelial cells, the precise mechanism remains elusive. Recently, the effects of PPAR on the Rho pathway was identified in smooth muscle cells [Wakino, 2004 #19] in which PPARγ stimulation via the phosphatase SHP-2 inactivated RhoA/Rho kinase signaling. These results could provide an intriguing possibility for the modulation of GTPases by PPAR isotypes, since GTPases are important mediators of leukocyte-endothelial interaction.
While much is known about key pathways involved in leukocyte extravasation, signaling events in the BMVEC actively participating in this process remain unclear [Wittchen, 2005 #4]. Small guanosine triphosphate (GTP)-binding proteins such as RhoA and Rac1 regulate in transendothelial leukocyte migration [reviewed in [Cernuda-Morollon, 2006 #5]]. Rac1 was shown to mediate the clustering of adhesion molecules on the surface of endothelial cells essential for leukocyte docking. Consequently, firm adhesion of leukocytes to the endothelium signals to the RhoA in endothelial cells leading to cytoskeletal rearrangement required for junctional modification and leukocyte paracellular transmigration. In inflammation, cytokine stimulation activates of the Rho family of GTPases, but it does not result in junctional disassembly unless an additional stimulus is provided like co-culture with monocytes or antibody cross-linking of ICAM-1 and VCAM-1 (simulating leukocyte docking) [Greenwood, 2003 #43; Muro, 2003 #44; Persidsky, 2006 #26]. Therefore, the Rho family of GTPases provides a key molecular switch routing intracellular signaling events needed for transendothelial migration.
One area where PPAR anti-inflammatory effects have remained largely unexplored is in the context of neuroinflammation. Since enhanced leukocyte migration across the BBB is a major feature of neuroinflammation, we tested whether PPARγ activation in cytokine stimulated human BMVEC could control monocyte adhesion and migration. We found that PPARγ activation in BMVEC resulted in significant reduction of monocyte adhesion and migration in our BBB culture model. Analysis of the Rho family GTPases in cytokine stimulated and TZD treated BMVEC resulted in dose dependent inhibition of Rac1 and RhoA that paralleled diminished monocyte adhesion and migration across BMVEC monolayer. Also, we showed that TZD stimulation of PPARγ in activated BMVEC resulted in a significant decrease in the adhesion and transmigration of HIV-1 infected monocytes across brain endothelium. Together these observations provide a novel inhibitory mechanism on monocyte adhesion/migration by PPARγ ligands acting in endothelial cells forming the BBB.
Materials and Methods
Human BMVEC, monocytes, and viral infection
Primary human BMVEC were isolated from tissue derived from surgical removal of epileptogenic cerebral cortex, as described previously [Persidsky, 1997 #27] and provided by Drs. Marlys Witte and Michael Bernas (University of Arizona, Tucson, AZ). The procedures were approved by the University of Nebraska Medical Center Institutional Review Board. BMVEC were maintained in DMEM/F-12 media containing 10% horse serum, endothelial growth factor (0.1mg/ml, R&D Systems, Minneapolis, MN), heparin (100mg/ml, Sigma, St. Louis, MO), amphotericin B (2.5μg/ml, Invitrogen, Carlsbad, CA), and gentamicin (50μg/ml, Invitrogen). For all experiments only BMVEC cultures of low passage (passage 2–5) were used. For barrier formation, the cells were plated confluent in a given culture vessel and maintained in media containing the above components but lacking the rEGF, which allows BMVEC to display typical morphological and functional barrier characteristics after three to five days in culture. BMVEC cultures were routinely evaluated for expression of endothelial cell markers [von Willebrand factor, CD31/PECAM-1, and TJ proteins (ZO-1, occludin, claudin-5)], and the lack of macrophage (CD68 and CD163), astrocyte (glial fribrillary acidic protein; GFAP) and pericyte (α-myosin) markers. Barrier formation by BMVEC was confirmed by measurement of transendothelial electrical resistance, TEER (representative TEER analysis from three distinct donors is shown as supplemental data).
Primary human peripheral blood monocytes were obtained from HIV-1 and hepatitis B seronegative donors by leukopheresis and were purified by counter-current centrifugal elutriation [Gendelman, 1988 #28]. The monocytes were maintained in DMEM media with 10% heat inactivated pooled human serum, penicillin 100 (U/ml), streptomycin (100 U/ml) and L-glutamine (2mM) and used within 24 h of isolation. Monocytes were infected with HIV-1ADA at a multiplicity of infection of 0.01–0.1 virus/target cell for 4 h [Gendelman, 1988 #28]. Prior to infection, the HIV-1 cell-free stocks were treated with DNase I for 30 min at 37°C as described [Ghorpade, 1998 #29]. All reagents were prescreened for endotoxin (<10 pg/ml, Associates of Cape Cod, Woods Hole, MA) and mycoplasma contamination (Gen-probe II, Gen-probe, San Diego, CA).
Microvessels isolation
Brain tissues (cortex) from patients without neuro-cognitive impairment or neuropathologic changes were obtained from the National NeuroAIDS Tissue Consortium and our Center for Neurovirology and Neurodegenerative Diseases brain bank. The clinical histories of brain tissue donors were described in [Persidsky, 2006 #26]. The isolation procedure of microvessels was performed as described in [Brooks, 2005 #52]. Protein was extracted from each sample for Western blot. For immunofluorescent microscopy, 50μl of microvessel suspension was spread on poly-l-lysine- or fibronectin-coated glass coverslips, air-dried, fixed for 20 min with a 4% paraformaldehyde (pH 7.4), rinsed with PBS, air-dried and used for immufluorescent staining with Abs to tight junction protein, claudin-5, von Willebrand Factor; CD163 (macrophage marker) was used to confirm the vascular identity of the isolated microvessels as described [Nag, 2003 #53].
Monocyte adhesion assay
BMVEC were plated on 96-well plates coated with rat-tail collagen type I (BD, Franklin Lakes, NJ) at a density of 2.5 ×104 cells/well. After formation of confluent monolayers, BMVEC were treated with PPARγ agonists or antagonists for 30 min prior to simultaneous incubation with recombinant human TNFα (20ng/ml, R&D systems) for 4 h before the assay. The endothelial cells were rinsed of all treatments and fluorescently labeled monocytes [2.5 ×105 cells/well loaded with calcein-AM (Invitrogen) at 5μM/1×106 cells for 45 min] were added to the endothelial monolayers and co-cultured for 15 min at 37°C. After adhesion, the monolayers were washed three times with PBS and relative fluorescence was acquired on a fluorescence plate reader, Spectramax M5 (Molecular Devices, Sunnyvale, CA). The data was calculated based on the standard curve derived from fluorescent intensity of known amounts of labeled monocytes. Results are represented as the fold difference, which is the number of monocytes that attached to endothelial cells with experimental condition over the number of monocytes that attached to endothelial cells without cytokine stimulation or drug treatment.
Transendothelial migration assay
The transendothelial migration assay was a fluorescence-based assay. Briefly, BMVEC were plated on rat-tail collagen type I coated FluoroBlok™ tinted tissue culture inserts (with 3μm pores, BD) at a density of 2.5 ×104 cells/insert. Because monolayers were not visible on these inserts, manual readings of transendothelial resistance (TEER) were taken with a voltmeter (EVOM, World Precision Instruments, Sarasota, FL) to confirm monolayer formation as described [Persidsky, 2006 #26]. Thereafter, endothelial cells were treated with PPAR acting compounds for 30 min and then co-incubated for an additional 4 h with TNFα (20ng/ml). The inserts were washed of all treatments, and relevant β-chemokine, recombinant human monocyte chemotactive protein (MCP) -1 (CCL2/MCP-1, 30ng/ml, R&D) was introduced into the lower chamber in order to create a chemokine gradient present in neuroinflammatory disorders [Persidsky, 1999 #30; Eugenin, 2006 #54]. Labeled monocytes (2.5 ×105 cells/insert loaded with calcein-AM at 5μM/1×106 cells for 45 min) were placed on the upper chamber, and migration was allowed to continue up to 2 h at 37°C. Migration was monitored by taking the relative fluorescence with a fluorescence plate reader (Molecular Devices) of the labeled monocytes found in the lower chamber. The number of migrated monocytes was then derived by plotting against standard curves of the relative fluorescence from known numbers of monocytes. The data is presented as the fold difference, which is the number of migrating monocytes from the indicated experimental condition over the monocytes that migrated across untreated (no drug and no cytokine) endothelial cells without the presence of chemoattractant (left y-axis). Additionally, the data is shown as percent of input (right y-axis), which indicates the percent of cells that migrated from the number of cells inputted.
Endothelial cell transfection
Transfection of primary BMVEC was performed by nucleofection using the Nucleofector device (Amaxa System, Gaithersburg, MD) according to protocol suggested by manufacturer. Briefly, for each transfection reaction, 5.0×105 trypsinized endothelial cells were re-suspended in 100 μl of nucleofection reagent along with 1μg of vector DNA. Each reaction was transferred to an electroporation cuvette and placed in the nucleofector device for electroporation. The electroporated cells were plated in the configuration necessary for migration assay, adhesion assay, or Western blot. BMVEC were transfected with DNA constructs containing coding sequences for the following proteins: wild type RhoA (WT), constitutively active RhoA (G14V), dominant negative RhoA (T19N), WT Rac1, constitutively active Rac1 (G12V), and dominant negative Rac1 (T17N). Constructs were obtained from the University of Missouri-Rolla cDNA Resource Center (www.cdna.org). Transfection efficiency with the Amaxa system typically reached ~80%, as determined by flow cytometry.
The endothelial cells were also transfected with small hairpin RNA (shRNA) expressing vectors directed to PPARγ (GenBank™ accession number NM_138711). A scrambled sequence control and an empty vector control were also transfected. The silencing oligonucleotides were composed of the following: bases 1–21 antisense, 22–32 loop sequence, and 33–54 sense sequence. PPARγ shRNA sequence 1 (shPPARγ-1): 5′-TATTTGAGGAGAGTTACTTGGTTG ATATCCGCCAAGTAACTCTCCTCAAATA-3′, PPAR shRNA sequence 2 (shPPARγ-2) 5′-TAATGTGGAGTAGAAATGCTGTTGATATCCGCAGCATTTCTACTC CACATTA-3′, and PPARγ shRNA sequence 3 (shPPARγ-3) 5 ′-TGTTCCGTGACAATCTGTCTGTTGATATCCGCAGACAGATTGTCACGGAACA-3′. All oligonucleotides were cloned into pRNAT-H1.1/Adeno vectors (Invitrogen) and were provided by GenScript (Piscataway, NJ).
Affinity precipitation and Western blot
Precipitations of the active form of Rho (Rho-GTP) were performed from endothelial cell lysates by affinity purification using the EZ-Detect Rho activation kit from Pierce (Rockford, IL). In brief, each lysate (400 μg of total protein) was incubated with GST-rhotekin-RBD protein (400 μg) and introduced to an immobilized glutathione disc resin and incubated for 1 hour at 4°C with gentle agitation. After incubation, the resin was washed 3 times with 1X Mg2+ lysis buffer followed by centrifugation at 14,000×g for 30 seconds. The discs were then re-suspended in Laemmli sample buffer containing 2-β-mercaptoethanol and boiled for 5 min at 95°C. Relevant controls such as guanosine 5′-O-(3-thio) triphosphate (GTPγS, for positive) and guanine diphosphate (GDP, for negative) were performed with untreated lysates and affinity precipitated as above.
Assays for active Rac1 (Rac-GTP) or Cdc42 (Cdc42-GTP) from cell lysates were also performed by affinity purification using the Rac1 or Cdc42 activation assays from Cytoskeleton, Inc. (Denver, CO). Briefly, 2mg of total protein from endothelial cellular lysates were incubated with 20μg of PAK-PBD (p21 binding domain of p21 activated kinase 1) conjugated beads for 1 hour at 4°C. After incubation, the PAK-PBD beads, which bind specifically to the active form of Rac1 or Cdc42, were washed twice with 1X wash buffer (25mM Tris pH 7.5, 30mM MgCl2, and 40mM NaCl) by centrifugation at 5000×g for 3 min at 4°C. The rinsed beads were then resuspended in 10μl of Laemmli buffer and analyzed by Western blot using specific Abs to distinguish between Rac1 and Cdc42.
Total protein lysates (10μg) or precipitated proteins (quantities indicated above) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 12% gels (Pierce), then transferred to nitrocellulose membranes and incubated with Abs against RhoA (1:500, Pierce), Rac1 (1:250, Cytoskeleton Inc.), Cdc42 (1:250 Cytoskeleton, Inc.), PPARγ, PPARβ/δ, PPARα (1:500, Abcam, Cambridge, MA) and hemagglutinin epitope (1:1000, Covance, Berkeley, CA). For detection of tight junction proteins, membranous and cytoplasmic extractions were performed from lysed BMVEC according to the manufacturer protocol included with the ProteoExtract® subcellular proteome extraction kit (Calbiochem, San Diego, CA). Western blots were then performed using the following antibodies: occludin (1:500, US Biological Inc., Swampscott, MA), claudin-5 (1:500, US Biological Inc.) and ZO-1 (1:500, US Biological Inc.). Bound antibodies were detected with horseradish peroxidase-conjugated secondary antibodies (1:5000, Pierce) and exposed to Supersignal West Pico chemiluminescent substrate (Pierce). Signal visualization was obtained using the gel documentation system, G:Box Chemi HR16 (Syngene, Frederick, MD).
ELISA based PPAR DNA binding assay and GTPase activation assay
BMVEC cells were treated as outlined in the figure legends, and analysis of PPARγ and PPARα DNA binding was performed as instructed by the manufacturer using the transcription factor ELISA kits for PPARα and PPARγ from Panomics Inc., (Fremont, CA). For GTPase ELISAs, BMVEC were treated as shown in the figure legend and quantitation of RhoA and Rac1 GTPase activation was assessed following the manufacturer recommendations using the G-LISA™ small G-protein activation assays from Cytoskeleton Inc.
Flow Cytometry
BMVEC were treated with TNFα (20ng/ml, 1 h) and/or rosiglitazone. Cells were lifted with 0.5mM EDTA at 4°C, washed with flow cytometry buffer (eBioscience, San Diego, CA) then incubated with APC-conjugated VCAM-1 antibody (BD) or PE-conjugated ICAM-1 antibody (BD) for 45 min at 4°C. Cells were washed, fixed with 2% paraformaldehyde, acquired on a FACS Calibur™ (BD), and analyzed using CellQuest Pro software (BD Immunocytometry System).
ICAM-1 antibody-crosslinking
ICAM-1 antibody crosslinking was conducted as previously described [Etienne-Manneville, 2000 #56]. Briefly, BMVEC were seeded at 1.3×105 in 6-well tissue culture plates and maintained under normal growth conditions for 5 days. Thereafter, the cells were incubated with TNFα (20ng/ml) for 4 h in the absence of growth factors but with serum, and then washed with 1x PBS. Treatment with PPARγ was performed for 30 min with or without TNFα for 4 h as indicated. ICAM-1 crosslinking was conducted using mouse anti-human ICAM-1/CD54 Ab (R&D Systems) which was added to the endothelial cells for 10 min. Unbound Ab was removed by rinsing with PBS and then cells were incubated for 15 min with rabbit anti-mouse antibodies (Santa Cruz Biotech, Santa Cruz, CA). Cells were rinsed with PBS and then lysed for subsequent GTPase analysis (see above).
Transendothelial electrical resistance (TEER)
TEER was measured in real-time using the ECIS system model 1600R, (Applied Biophysics, Troy, NY). Primary BMVEC were grown to confluence on collagen type I coated ECIS cultureware arrays (8W10E). Measurements were recorded with the following settings: current (1-volt), frequency (400Hz), at 5 min intervals. Treatments were performed as indicated in the figure legends and data were represented as normalized resistance, which is the resistance measured post treatment over the resistance acquired before treatment introduction (typically ~2600 Ω/cm2).
Statistical analysis
The experiments were independently performed multiple times (at least three for all presented data) allowing statistical analyses. In each individual experiment, every condition was evaluated in three replicates. The data collected were analyzed using Prism v4.0 (GraphPad, San Diego, CA). The differences between the means were evaluated by Student t test, and statistical significance was considered at p values less than 0.01. All results were expressed as mean ± SEM.
Results
PPARγ agonist decreased monocyte adhesion and transendothelial migration
Because PPAR isotype distribution varies depending on the cell or tissue type and since no prior isotype expression has been documented in human brain endothelial cells, we sought to identify the expression profile in isolated human brain microvessels as well as in primary cultures of human BMVEC. Additionally, given that it is possible for primary cells to change a particular gene expression profile due to passage and culturing conditions, it was important to rule out whether there was a difference in the PPAR isotype expression between culture cells and intact microvessels. To this end, western blot analysis of lysates from isolated microvessels and primary cultures were probed for each of the known PPAR isotypes. Figure 1A shows the expression of PPARα, PPARβ/δ, and PPARγ in both sets of lysates. As seen in the Figure 1, all three isotypes were expressed in brain microvessels and were also maintained in BMVEC cultures. Next, we examined PPARα and PPARγ activation by oligo binding ELISA using two PPARα agonists (fibrates) and three PPARγ agonists (TZDs). As shown in Figure 1B, PPARα oligo binding increased in a dose dependent manner with both fenofibrate and clofibrate, with fenofibrate inducing better binding (EC50 =5.6 μM) compared with clofibrate (EC50 =16.5 μM). Analysis of PPARγ activation by TZDs, rosiglitazone, pioglitazone and ciglitazone, resulted in rosiglitazone as a better activator (Rosi EC50 =2.0μM, Pio EC50 =2.6μM and Cig EC50 =2.3μM), Figure 1C. Therefore, we used fenofibrate for PPARα activation and rosiglitazone for PPARγ activation in subsequent analyses.
Figure 1.

PPARγ agonist, rosiglitazone, inhibited monocyte adhesion and migration across human BMVEC in a dose dependent manner. (A) PPAR protein expression profile derived from lysates of primary cultured brain microvascular endothelial cells (BMVEC) and isolated human brain microvessels. Passage 2 BMVEC monolayers and microvessels were lysed and analyzed by western blot using specific antibodies for the three known PPAR isotypes, PPARα (top panel), PPARβ/δ (middle panel), and PPARγ (lower panel). (B and C) BMVEC were treated with the indicated PPAR agonist (fibrate or TZD) in increasing concentrations, after 4 h nuclear extractions were collected and introduced to oligo binding ELISA for PPARα and PPARγ as described in the materials and methods. The values represent the mean ± SEM. (D) Adhesion assays of BMVEC monolayers pretreated for 30 min with increasing concentrations of rosiglitatozone, pioglitazone, and fenofibrate with or without subsequent co-incubation with TNFα (20ng/ml) for additional 4 h. All treatments were removed and calcein-AM labeled monocytes were introduced to the BMVEC, as described in the material methods. Monocytes were allowed to adhere for 15 min to the BMVEC, unattached cells were rinsed and the fluorescence measured. The data is represented as mean ± SEM fold difference, which is the adhesion value from treated BMVEC over the basal adhesion value from untreated cells. Where indicated, the addition of PPARγ antagonist GW9662 (50μM) was used as control. (E) Transendothelial monocyte migration across pre-treated BMVEC monolayers towards CCL2/MCP-1 (30ng/ml) was evaluated after 2 hours as described in the methods section. Data is represented as mean ± SEM fold difference of migration, which is the value from migration of treated cells over the “spontaneous” migration of untreated cells without chemoattractant. All data collected are from at least three independent experiments performed in triplicate. Asterisk denotes statistically significant differences (p<0.01) between untreated and rosiglitazone treated and cytokine stimulated BMVEC.
Since monocyte adhesion to brain endothelial cells is the initial step in migration across the BBB during neuroinflamation, and both PPARα and PPARγ can inhibit inflammatory responses in the vasculature, we treated BMVEC with PPARα and PPARγ ligands and tested monocyte adhesion. First BMVEC were treated with PPAR agonist for 30 min and then co-incubated with TNFα (20ng/ml) for 4 h. Treatments were removed and adhesion assay was performed as indicated in the materials and methods. Monocyte adhesion increased 15-fold when endothelial cells were stimulated with cytokine as shown in Figure 1D. Pre-treatment of BMVEC with various concentrations of PPARγ agonist led to a dose-dependent decrease in monocyte adhesion in both unstimulated and stimulated endothelial cells (Figure 1D). At maximum, the reduction in monocyte adhesion seen in TNFα activated BMVEC after treatment with rosiglitazone resulted in an 8-fold decrease in adhesion (p<0.001) as compared to control. In addition to rosiglitazone, pioglitazone also produced a decrease in monocyte adhesion, resulting in a 4-fold decrease at the highest concentration tested. Surprisingly, no effect on monocyte adhesion was seen with the PPARα agonist, fenofibrate. This observation is in contrast to previous analysis reporting that PPARα activation could decrease attachment of monocytes to endothelial cells [Rival, 2002 #31]. However, these experiments were performed in non-brain derived endothelial cells. Thus, our data demonstrates that in brain endothelial cells, PPARγ activation can decrease monocyte adhesion but not when PPARα is stimulated.
Next, we studied monocyte migration in order to determine whether PPARγ stimulation in BMVEC also could have an effect on monocyte transendothelial migration. In these experiments, we utilized CCL2/MCP-1 as a relevant chemokine produced during neuroinflammatory conditions such as HIV-1 encephalitis [Persidsky, 1999 #30]. Figure 1E shows the results of monocyte migrations across BMVEC monolayers, the results are represented as normalized fold values to monocytes migrating across BMVEC without the presence of CCL2/MCP-1. Basal migration across resting endothelial cells without chemoattractact is indicated as 1-fold, stimulation of endothelial cells with TNFα and without CCL2/MCP-1 results in 1.3-fold increase, endothelial cells with CCL-2/MCP-1 in lower chamber gives a 2-fold increase in migration, and BMVEC TNFα stimulation with addition of CCL2/MCP-1 results in 4.3-fold increase monocyte migration. Pretreatment of BMVEC with increasing concentrations of rosiglitazone (two shown) in the above treatment conditions produced significant decreases in transendothelial migration. Particularly when CCL2 and TNFα were present, rosiglitazone produced a 21% decrease for 5μM concentration and 35% at 50μM concentration (p<0.01). Similar inhibitory results on migration were also obtained with other PPARγ agonist, albeit at higher concentration (data not shown). Also shown in Figure 1E, no significant change in migration of monocytes was observed with the PPARα agonist, fenofibrate.
Knockdown expression by PPARγ specific shRNA abolished the effect of rosiglitazone on monocyte adhesion
To eliminate the possibility that the effects of rosiglitazone were not mediated through other cellular targets [Chawla, 2001 #46] or other PPAR family members, BMVEC were transfected with several PPARγ-specific shRNA. Three different shRNA sequences were evaluated for knocking down endogenous PPARγ. As shown in Figure 2A, transfected shPPARγ-2 lowered endogenous PPARγ by 85%, as compared with ~60% for both shPPARγ-1 and shPPARγ-3. Since transfection with shPPARγ-2 showed the most prominent effect on PPARγ protein reduction, a control scramble sequence (shPPARγ-2-SC) of shPPARγ-2 was generated and transfected, which resulted in no observable PPARγ knock down (Figure 2A). Next, we performed adhesion assays using transfected shPPARγ-2 and shPPARγ-2-SC in BMVEC, which had been treated with rosiglitazone and stimulated with TNFα (Figure 2B). The effect of rosiglitazone on monocyte adhesion was abolished in the shPPARγ-2 transfected BMVEC, but not in PPARγ-2-SC endothelial cells. These results indicated that endogenous PPARγ was indeed the primary target for the effect of rosiglitazone on monocyte adhesion since shRNA-mediated knockdown of PPARγ brought monocyte adhesion to control levels in the presence of drug. The PPARγ dependent mechanism was also observed in migration assays, where PPARγ knockdown resulted in the elimination of most of the migratory inhibition rendered by rosiglitazone (Figure 2C).
Figure 2.
shRNA mediated PPARγ knockdown in BMVEC eliminates the effects of rosiglitazone on monocyte adhesion and migration. (A) Western blot analysis showing knockdown of endogenous PPARγ expression in BMVEC (72 hours post transfection) with vectors coding shRNA sequences directed to PPARγ. Three candidate sequences were evaluated denoted as shPPARγ-1, shPPARγ-2 and shPPARγ-3. The vector with shPPARγ-2-SC, lane 5, represents the scrambled control for shPPARγ-2, lane 3. (B) Adhesion assays of BMVEC expressing shPPARγ-2, control scramble shPPARγ-2-SC, and mock transfected (NT). After 5 days post-transfection, the cells were treated with increasing concentration of rosiglitazone, and adhesion assays were performed. (C) Migration assays of transfected BMVEC with the knockdown sequence and control. After 5 days, cells were pretreated with rosiglitazone along with or without cytokine stimulation for 4 h; migration was then performed for 2 h and values collected. The data for the adhesion and migration is represented as mean ± SEM fold difference.
PPARγ activation did not affect the expression of adhesion molecules in cytokine stimulated BMVEC
PPARγ ligands have been shown to suppress the transcriptional activity of factors mediating the up-regulation of adhesion molecules (i.e. NF-κB and STATs). Thus we investigated whether this mechanism could be responsible for the observed inhibition on monocyte adhesion. Both, ICAM-1/CD54 and VCAM-1/CD106 are critical for firm monocyte attachment to the endothelium. Representative histograms of BMVEC surface expression of ICAM-1 and VCAM-1 by flow cytometry are shown in Figure 3A. Unexpectedly, the up-regulation in surface expression of both ICAM-1 and VCAM-1 seen after 4 h stimulation with TNFα was not decreased with prior pre-treatment with 50 μM rosiglitazone. In addition, surface expression of ICAM-1 and VCAM-1 was also unchanged in resting cells. Of note, using natural PPAR ligands in human aortic endothelial cells, others have also shown the lack of regulation in adhesion molecule expression by PPARγ [Schleser, 2006 #60].
Figure 3.
PPARγ activation did not alter ICAM-1 and VCAM-1 surface expression of stimulated BMVEC or induce changes to barrier properties. (A) Flow cytometry of BMVEC ICAM-1/CD54 and VCAM-1/106 surface staining following addition of rosiglitazone (RS, 50μM) for 30 min and simultaneous incubation with TNFα (20ng/ml) for 4 h. (B), BMVEC grown to confluence on ECIS electrode chamber slides were used to analyze for differences in TEER. The measurements shown represent recordings acquired at 5 min intervals at the parameters described in the materials and methods. The single arrow designates the pre-treatment with 50μM of rosiglitazone following co-incubation with TNFα as shown by the double arrow. Addition of lindsidomide at 100μM was used as a control for lowering TEER. The data is presented as normalized resistance, which is the resistance measured post treatment over the resistance acquired before treatment introduction (typically ~2600 Ω/cm2). (C) Western blot analysis of TJ proteins ZO-1, occludin-1, and claudin-5 from the cytosolic (Cyto) and membranous (Mem) fractions of lysed BMVEC are shown; treatments are indicated in the figure.
PPARγ activation did not affect endothelial barrier properties
The paracellular (between cells) migration of monocytes across the BBB has been shown to be dependent in the orchestrated events occurring in endothelial cells that lead to tight junction (TJ) disassembly and permit migration. Thus, it is possible that PPARγ could be increasing barrier “tightness” via the TJ and therefore, limiting monocyte migration. To test this possibility, we analyzed changes in the TEER, and also on the expression and localization of TJ proteins after PPARγ treatment. Endothelial monolayers were cultured on coated electrode chamber arrays and incubated with the indicated compounds and in some cases co-incubated with TNFα. As Figure 3B shows, the TEER measurements of treated BMVEC taken in real-time for 6 h did not produce fluctuations in TEER of either rosiglitazone alone or when present with TNFα. Also shown in Figure 3B, the immediate and sustained drop in TEER measurement was produced by the nitric oxide donor, linsidomine, used as control for inducing barrier leakiness. Of note, application of TNFα at 20ng/ml concentrations, which was the concentration used in the functional assays reported here, resulted in only a slight and a transient decrease in TEER.
To further confirm the results of the TEER analysis, the expression and localization of TJ proteins, ZO-1, occludin, and claudin-5 were investigated. Cytosolic and membranous fractions from treated BMVEC, as indicated in Figure 3C, were collected and probed for the TJ proteins shown. As presented in Figure 3C, we found no differences in the expression or intracellular distribution of any of the three TJ proteins. Thus, effects on monocyte migration cannot be explained by changes in TJ protein content or distribution in the BMVEC after PPARγ activation.
PPARγ stimulation inhibited RhoA and Rac1 GTPases
Central to monocyte-endothelial interactions is the modulation of cytoskeletal components and surface adhesion molecules by the Rho family of small GTPases [Wojciak-Stothard, 1999 #20; Cernuda-Morollon, 2006 #5]. During neuroinflammation, brain endothelial cells up-regulate adhesion molecules such as ICAM-1 and VCAM-1 leading to increased leukocyte-endothelial recruitment and trafficking [Adamson, 2002 #33]. Pro-inflammatory cytokines including TNFα, IL-1β, and IL-6 induce the activation of the GTPases in endothelial cells [Nwariaku, 2003 #34] leading to receptor clustering (Rac1), important for leukocyte adhesion and cytoskeletal and TJ rearrangement (RhoA), important in transendothelial migration. [Wittchen, 2005 #4; Persidsky, 2006 #26]
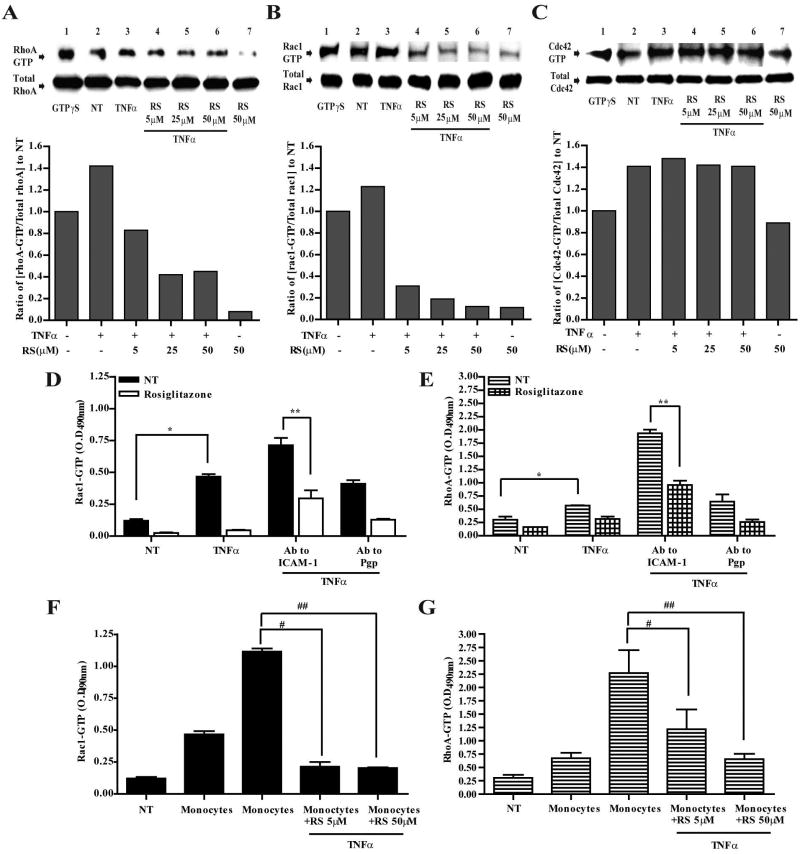
To evaluate whether PPARγ stimulation modulated the activation state of members of the Rho family of GTPases, we performed affinity purification of the active forms of RhoA, Rac1, and Cdc42 in TNFα treated BMVEC. After treatment, cells were lysed and subjected to affinity purification-based pull-down of the GTP bound active forms of RhoA, Rac1, and Cdc42. The pull-down assays used the binding domain of the effector protein that targets the particular GTPase. In agreement with previous reports, TNFα stimulation increased the level of both GTP bound RhoA and Rac1 in BMVEC (Figure 4A,B). PPARγ agonist, rosiglitazone, significantly decreased binding of active RhoA and Rac1 in a dose-dependent manner (Figure 4A,B). It is interesting to note that PPARγ stimulation appeared to inhibit more effectively Rac1 (~60% at 5μM) than RhoA (~20% at 5μM). While TNFα induced Cdc42 activation/GTP binding, surprisingly rosiglitazone did not reduce Cdc42-GTP binding suggesting that PPARγ could modulate the activation status of only certain GTPases. PPARγ stimulation of the GTPases did not change protein expression since total protein expression of GTPases was not affected. Thus, these results suggest that PPARγ via GTPase regulation could be the underlying mechanism preventing monocyte adhesion and migration across activated endothelium after PPARγ stimulation.
Figure 4.
PPARγ regulates activation of RhoA and Rac1 GTPases. GTPases from whole cell extracts were affinity immunoprecipitated from BMVEC after the indicated treatments. The top panels show western blots of the active GTP bound RhoA (A), Rac1 (B), and Cdc42 (C) following affinity precipitation (top panel). Together with the active form of the GTPase are immunoblots showing the total GTPase (lower panels) content of inactive plus the active form from whole cell lysates. The included densitometry analysis depicts the ratios from the normalized values of the active GTPases from all treatments and compared to the normalized value from untreated cells. (D–G), ELISA based GTPase activation assays of Rac1 (D) and RhoA (E) were performed after BMVEC crosslinking of ICAM-1 surface molecules with specific antibodies. Monocyte mediated activation of Rac1 (F) and RhoA (G) was performed by using BMVEC lysates collected after monocyte adhesion, monocytes were removed as previously described [Persidsky, 2006 #26]. The data is presented as the average ± SEM of three independent experiments each performed in triplicate. Symbols in the graph represents statistical significance of (p<0.01). (*) compares values from untreated cells and TNFα treated; (**) indicates values from untreated cells compared with those from TNFα, rosiglitazone and crosslinked with ICAM-1; (#) compares values from TNFα and monocytes with TNFα, monocytes and rosiglitazone (5μM); (##) compares values from TNFα and monocytes with TNFα, monocytes and rosiglitazone (50μM).
PPARγ prevents RhoA and Rac-1 activation after ICAM-1 crosslinking or monocyte engagement
Although GTPases are activated by pro-inflammatory cytokines as seen in Figure 4A and B, additional GTPase activation occurs after leukocyte-endothelial engagement. Antibody crosslinking of adhesion molecules was used to emulate this interaction [Etienne-Manneville, 2000 #56] leading to cytoskeletal rearrangement needed for leukocyte docking and extravasation. With Ab crosslinking to ICAM-1, we proceeded to investigate whether PPARγ mediated inhibition of RhoA and Rac1 could also be seen if further GTPase activation was induced. Using quantitative GTPase ELISA for Rac-1 and RhoA, we not only observed the induction in GTPase activation after addition of TNFα (20ng/ml for 4 h), but also found the further activation of the two GTPases triggered by ICAM-1 Ab crosslinking (Figure 4D and E). BMVEC exposed to rosiglitazone along with TNFα stimulation and ICAM-1 crosslinking showed inhibition of Rac1 and RhoA. Lastly, application of monocytes to BMVEC monolayers resulted in Rac1 and RhoA activation (as seen with Ab crosslinking) (Figure 4G and F); rosiglitazone blocked these changes.
It is important to note that rosiglitazone inhibition of Rac1 and RhoA in these analyses, like the pull-down assays (Figure 4A and B), also appeared to be more efficient for Rac1. These effects on Rac1 may explain rosiglitazone effects in BMVEC inhibition of monocyte adhesion, since maximal inhibition is seen with 5μM rosiglitazone similarly to that observed with Rac1 inhibition (Figure 4D). On the other hand, RhoA inhibition by rosiglitazone in the endothelial cells appeared less sensitive (Figure 4E) at low concentrations, possibly explaining the effect on monocyte migration where the maximal inhibitory effect is seen with 50μM (Figure 1E). Lastly, analysis of GTPase activity was observed to be similar to those with Ab crosslinking when actual monocytes were employed (Figure 4F and G). Taken together the analysis on the GTPases provides support for the notion that in BMVEC PPARγ negatively modulates Rac1 and RhoA thus affecting monocyte adhesion and migration.
PPARγ inhibits Rac1 mediated monocyte adhesion to activated brain endothelium
To further elucidate whether PPARγ inhibitory action on Rac1 and RhoA could influence monocyte adhesion, we examined monocyte adhesion to BMVEC expressing RhoA and Rac1 mutants. BMVEC were transfected with low concentrations (as outlined in the materials and methods) of DNA constructs expressing wild type (WT), constitutively active (CA) and dominant negative (DN) forms of Rac1 and RhoA. Inserts panels in Figure 5A and B show the expression of the various mutants: the first row denotes the detection of the 3xHA peptide tag fused to the mutants; the second row indicates the GTPase expression (endogenous plus mutant); and the third row shows the internal control (α-actin). After transfection, the cells were allowed to form monolayers and then treated as indicated (Figure 5A and B). Higher concentrations of rosiglitazone were omitted since maximal inhibition on adhesion was reached at 5μM concentrations (as shown on Figure 1). As shown in Figure 5A, the expression of Rac-CA resulted in a 4-fold increase in monocyte adhesion even in unstimulated BMVEC as compared to controls (transfected with Rac-WT). TNFα stimulation alone in endothelial cells produced increased monocyte adhesion in Rac-WT as previously observed, and yet 20% higher in Rac-CA expressing cells. Rosiglitazone application led to a 23% and 35% decrease in monocyte adhesion for 2μM and 5μM respectively in Rac-WT; the Rac-CA transfectants completely counteracted the actions of rosiglitazone in activated BMVEC pointing to Rac1 as a critical mediator of monocyte adhesion in BMVEC. Interestingly, expression of Rac-DN led to a decrease in baseline adhesion to the cytokine stimulated monolayers (~36% as compared to Rac-WT), similar to the inhibition seen in rosiglitazone treated cells at the maximal effective dose (5μM, Figure 5A).
Figure 5.

Stimulation of PPARγ in activated BMVEC prevents Rac-1 mediated monocyte adhesion. BMVEC were transfected with expression vectors for wild type (WT), constitutively active (CA) and dominant negative (DN) Rac1 and RhoA. Five days post-transfection the cells were treated with two increasing concentrations of rosiglitazone (2μM and 5μM). Figure inserts in A and B show the BMVEC expression of the mutant GTPase by antibodies that detected the hemagglutin epitope (HA) fused to the expressing GTPase (top row), along with the proteins detected by RhoA or Rac1 antibodies (middle row), and actin (bottom row). After transfection and treatments, the BMVEC were used in adhesion assays. The data is presented as the mean ± SEM adhesion fold difference of transfectants with the Rac1 (A) and RhoA (B) mutants. Asterisk indicates statistical significance (p<0.01) when compared with the value from stimulated adherent cells without PPARγ agonist.
In stark contrast to the functional analysis performed with the Rac1 mutants, the RhoA mutants had no effect on the response of rosiglitazone treated endothelial cells, Figure 5B. TNFα stimulation of BMVEC led to ~10-fold increase in monocyte adhesion regardless of the RhoA mutant the endothelial cells were transfected with. The expression of Rho-CA and Rho-DN did not change the inhibitory effect of PPARγ agonist on monocyte adhesion as compared to control Rho-WT suggesting that although TNFα induced RhoA activation and PPARγ mediated its partial inhibition, PPARγ effects on RhoA did not contribute to monocyte adhesion to activated BMVEC. It is important to point out that all inhibitory effects on monocyte adhesion produced by rosiglitazone were reversed by co-incubation with high affinity PPARγ antagonist ligand (GW9662) ensuring that over-expression of the mutants did not alter the specific action of the agonist (fig. 5A).
RhoA mediated monocyte migration across brain endothelium was attenuated in response to endothelial PPARγ activation
Previous studies identified the importance of RhoA in transendothelial migration [Wittchen, 2005 #4]. It was demonstrated that RhoA is a key mediator of TJ “gating”, which promotes leukocyte trafficking across brain endothelial cells [Stamatovic, 2005 #48; Persidsky, 2006 #26]. We performed experiments to determine whether mutant GTPase expression in endothelial cells could affect PPARγ inhibitory function of monocyte migration. Monocyte migration towards CCL2/MCP-1 was enhanced across TNFα activated BMVEC expressing Rho-CA (5.1-fold) and was significantly diminished by Rho-DN (1.8-fold) as compared to cells transfected with the Rho-WT (4.1-fold, p<0.001, Figure 6A). While treatment with rosiglitazone diminished monocyte migration in BMVEC transfected with control Rho-WT in a dose-dependent fashion (21% with 5μM or 35% with 50μM), minimal inhibition (11%) was achieved in rosiglitazone-treated BMVEC expressing Rho-CA regardless of drug concentration. Similarly, no change in monocyte migration was found in BMVEC transfected with Rho-DN after PPARγ agonist treatment as compared to cells transfected with Rho-DN but without rosiglitazone application. Therefore, under these experimental conditions, transfection of cells with Rho-CA mostly counteracted the effects of PPARγ on monocyte migration, suggesting the role of PAPRγ in regulating the role of RhoA in transendothelial migration.
Figure 6.

Activation of PPARγ in BMVEC inhibits RhoA mediated transendothelial migration of monocytes. BMVEC were transfected with GTPase mutants and allowed to express the transgene for 5 days followed by treatment with or without PPARγ agonist, rosiglitazone, at 5μM and 50μM with or without TNFα co-incubation. The figure shows CCL2/MCP-1 driven monocyte migration across BMVEC expressing RhoA (A) and Rac1 (B) mutants. The migration assay was conducted for 2 h and the relative fluorescence of labeled monocytes was measured. Asterisk indicates statistical significance (p<0.01) when compared with the value from stimulated cells in the presence of CCL2/MCP-1 but without rosiglitazone. Data represented as the migration fold difference mean ± SEM from at least three independent experiments performed in triplicate.
We next analyzed the impact of rosiglitazone on monocyte migration in BMVEC transfected with Rac1 mutants. As expected, CCL2/MCP-1 driven monocyte migration was enhanced across TNFα stimulated BMVEC expressing Rac-CA (15%) as compared to cells transfected with the Rac-WT (p<0.01, Figure 6B), while Rac-DN expression led to diminution of cell passage (23%). The range of these changes in migration was much less prominent as compared to results obtained with respective RhoA mutants. Monocyte migration across BMVEC expressing Rac-CA was reduced by rosiglitazone only at the higher concentration (50μM), and the same trend existed for dominant negative Rac1. These data suggested that PPARγ effects on RhoA rather than Rac1 mediated changes of monocyte migration. Moreover, these results indicated that Rac1 inactivation occurred in a seemingly dose sensitive manner, such that the effect of agonist at low concentration is more profound on Rac1 than for RhoA. These results underscored the dual modulation of PPARγ activation on RhoA and Rac1.
PPARγ stimulation in BMVEC monolayers inhibited adhesion and transendothelial migration of HIV-1 infected monocytes
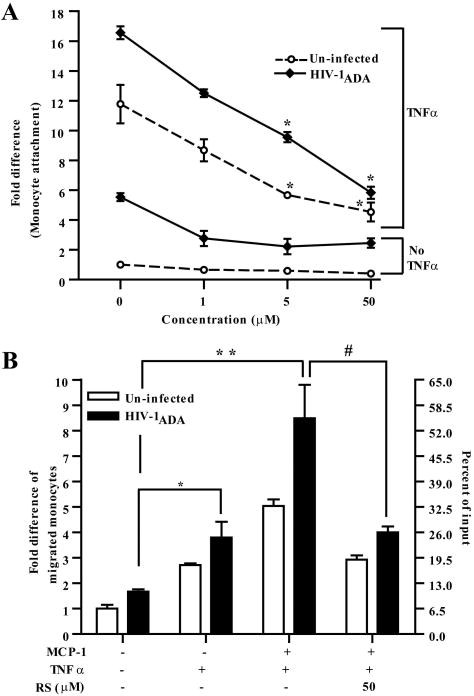
To evaluate whether activators of PPARγ could protect the brain against infiltration of HIV-1 infected monocytes, we infected monocytes with HIV-1ADA, an CCR5-tropic HIV-1 strain, for 4 h and then applied virus-infected monocytes to cytokine stimulated BMVEC pretreated with rosiglitazone. The infected monocytes were analyzed in adhesion and migration assays. As shown in Figure 7A, basal adhesion of infected monocytes to un-treated BMVEC was increased by 5.5-fold when compared to the un-infected monocyte control. Consequently, adhesion was even higher when BMVEC monolayers were TNFα stimulated, 16.5-fold vs. 11.8-fold for un-infected monocyte control. Rosiglitazone treatment (PPARγ activation) of BMVEC readily attenuated adhesion of HIV-1 infected monocytes ranging from 16.5-fold in untreated to 12.5, 9.5, and 5.8 for rosiglitazone concentrations 1, 5, and 50μM respectively (p<0.01). Therefore, these data suggest that PPARγ stimulation in BMVEC prevented adhesion to HIV-1 infected monocytes to unstimulated as well as stimulated brain endothelium. Next, we examined rosiglitazone effects on transendothelial migration of infected monocytes. As shown in Figure 7B, migration of HIV-1 infected monocyte was significantly enhanced in response to CCL2/MCP-1 as compared to uninfected cells (p<0.001). Furthermore, HIV-1 infected monocytes showed 1.7–fold enhanced migration across TNFα activated endothelium as compared to uninfected cells. Addition of rosiglitazone to the cytokine stimulated monolayers resulted in ~50% inhibition in the migration of infected or uninfected cells. These observations point to novel therapeutic approaches in using PPARγ agonists to prevent infiltration of HIV-1 infected monocytes across the BBB.
Figure 7.
PPARγ activation in BMVEC inhibited adhesion and transendothelial migration of HIV-1 infected monocytes. (A) Adhesion and (B) migration assays using uninfected or HIV-1ADA (MOI 0.01) infected monocytes were performed. For adhesion assays the endothelial cells were exposed to increasing concentrations of rosiglitazone, 1μM, 5μM and 50μM and where indicated cells were also simultaneously incubated with TNFα (20ng/ml) for 4 h. Data is represented as adhesion fold differences as described earlier. For migration assays BMVEC monolayers were pre-incubated with rosiglitazone (50μM) with or without TNFα (20ng/ml) following the same treatments times as before. Data is represented as the migration fold difference mean ± SEM. (*) indicates statistical significance (p<0.01) when compared with the value from migrated cells towards CCL2/MCP-1 but without cytokine stimulation. (**) represents statistical significance (p<0.01) when compared with values from uninfected cells migrating towards CCL2/MCP-1 and monolayers treated with cytokine. (#) indicates statistical significance (p<0.01) of values from infected cells migrated towards CCL2/MCP-1 on monolayers treated with TNFα and rosiglitazone compared with infected cells migrating towards CCL2/MCP-1 but without rosiglitazone treatment.
Discussion
The responses of the endothelium to neuroinflammatory conditions play a significant role in the regulation of immune cell infiltration into the brain parenchyma [Grigoriadis, 2006 #21; Minghetti, 2005 #22]. Whether CNS inflammation arise from a autoimmune (multiple sclerosis) or from a pathogen driven disorder as in the case of HIV-1 encephalitis, immune cell migration has damaging effects to the BBB and to the neuronal environment [Abbott, 2006 #63; Persidsky, 2006 #26]. Once immune cells cross the BBB, a cascade of events, in part orchestrated by astrocytes and other glia cells, contributes to potentiation of CNS inflammation. In this report we explored the anti-inflammatory effects of PPAR ligands in preventing interactions between brain endothelium and monocytes. Using primary human BMVEC, we modeled the human BBB in vitro and simulated a condition of chronic inflammation (cytokine stimulated BMVEC) and then performed quantitative analysis of monocyte adhesion and transendothelial migration. Our analyses indicated that PPARγ activation by potent TZD, such as rosiglitazone led to a significant reduction in monocyte adhesion and migration in resting and stimulated BMVEC. This process was highly specific for PPARγ, as shown by knockdown of PPARγ in BMVEC and failure of selective PPARα agonist to exhibit similar effects. In this study, we demonstrated the inhibition of Rac1 and RhoA GTPases by PPARγ stimulation in brain endothelium and provided evidence that this is the mechanism preventing monocyte engagement and passage across brain endothelium. Lastly as prove of concept, we showed that synthetic PPARγ agonist hindered adhesion and migration of HIV-1 infected monocytes. Taken together, our data suggests that PPARγ activation in brain endothelium may be a strategy for counteracting CNS inflammation contributed by immune cell infiltration.
It is recognized that PPARs agonists could be useful in attenuating inflammatory responses [Zandbergen, 2007 #58; Li, 2007 #57]. PPARs are nuclear hormone receptors, which are ligand activated and act as transcription factors. The three isotypes of PPARs (PPARα, PPARγ, and PPARβ/δ) are expressed in endothelium and have been studied as agents to curb the inflammation in atherosclerosis [Zandbergen, 2007 #58]. While up to date, no studies have been performed to evaluate the anti-inflammatory effect of PPARs activation in human brain endothelial cells, recent study demonstrated inhibitory effect of PPARγ stimulation on CD4+ lymphocyte adhesion and migration across monolayers of mouse brain endothelial cells [Klotz, 2007 #67]. As CNS inflammation is attributed in part to the infiltration of immune cells across brain endothelium, we first evaluated whether activation of PPARα and PPARγ could prevent monocyte adhesion to endothelial cells and transendothelial migration. Using PPARγ and PPARα stimulators, we demonstrated that PPARγ stimulation and not PPARα exerted a striking dose-dependent inhibition of monocyte adhesion and migration across activated BMVEC. This observation differs from those reported for aortic vascular endothelium where either PPARα and PPARγ activation prevents monocyte adhesion [Kurebayashi, 2005 #2; Jackson, 1999 #3]. Although the rosiglitazone is a highly specific PPARγ agonist, it is possible that the observed effects occur via other intracellular factors independent of PPARγ as suggested by Chawla and colleagues [Chawla, 2001 #46]. Using targeted shRNA down-regulation of endogenous PPARγ, we observed near complete reversal of monocyte adhesion/migration when the BMVEC were treated with rosiglitazone indicating specificity of PPARγ effects in BMVEC.
To address the underlying mechanism of diminished monocyte adhesion, we analyzed the expression of adhesion molecules ICAM-1 and VCAM-1 in TNFα stimulated BMVEC. Although PPARγ stimulation was shown to down-regulate ICAM-1 and VCAM-1 in some experimental systems [Arnold, 2007 #62][Klotz, 2007 #67], we did not find an appreciable effect on surface expression of adhesion molecules. Consistent with our data, others previously reported a lack of effect on ICAM-1 and VCAM-1 regulation after activation of PPARγ in TNFα stimulated human aortic endothelial cells using the natural ligands belonging to the conjugated linoleic acid class of fatty acids [Schleser, 2006 #60]. It is possible that the effect on the expression of adhesion molecules by PPARγ could occur if treatment with PPARγ ligands was extended beyond the few hours performed in this study [Arnold, 2007 #62][Klotz, 2007 #67]. Similarly, the PPARγ agonist, rosiglitazone did not change barrier function of BMVEC monolayers (measured by TEER) or distribution/level of TJ proteins in brain endothelium.
We next considered the possibility that PPARγ activation could inhibit the Rho family of GTPases, given that leukocyte adhesion and migration are tightly regulated by GTPase function in endothelial cells [Wittchen, 2005 #4; Cernuda-Morollon, 2006 #5]. All Rho family members act as molecular switches by binding GDP, rendering inactivation, or GTP to shift into the activation state. GTP bound GTPases act on effectors that then induce changes to cytoskeletal dynamics and gene regulation [Wennerberg, 2004 #9]. In the endothelium, Rho GTPases are activated in response to inflammatory stimuli (such as TNFα, IL-1β, and bacterial toxins), hypoxia, shear stress [Li, 1999 #16; Munro, 2005 #17; Wojciak-Stothard, 1998 #15; Turner, 2005 #18; Stamatovic, 2003 #47; Stamatovic, 2005 #48], or endothelial-leukocyte interactions [Adamson, 2002 #33; Persidsky, 2006 #26]. RhoA and Rac1 were activated in BMVEC after TNFα treatment and further enhanced by monocyte-BMVEC engagement or simulation of this process by ICAM-1 antibody crosslinking. We found that PAPRγ activation markedly decreased the level of active GTP bound Rac1 and RhoA. The maximal inhibition of Rac1 occurred at low concentrations of PPARγ agonist whereas most prominent RhoA inhibition was achieved at higher doses. Using BMVEC expressing CA and DN mutants of Rac1 and RhoA, we showed that PPARγ inhibition of Rac1 impacted monocyte adhesion; whereas, RhoA suppression affected monocytes transendothelial migration. Expression of the Rac-CA mutant had an overriding effect on monocyte adhesion even when PPARγ was activated. Conversely, Rho-CA did not counteract the inhibitory effect of the PPARγ agonist on monocyte adhesion. In the case of migration, the Rho-CA mutant showed significant increase in monocyte migration with a minimal inhibitory effect achieved by PPARγ activation. The Rac-CA mutant was able to supersede PPARγ induced inhibition on monocyte migration only at the lower and not at the higher concentrations of rosiglitazone, while the Rho-CA showed an equipotent response to all doses. Taken together these results suggest a dose dependent inhibition on Rac1 and RhoA GTPase activity. To our knowledge this is the first time that PPARγ agonist are reported to negatively regulate both RhoA and Rac1 in brain endothelium or endothelial cells of any origin. The only available study on RhoA inhibition by PPARγ stimulation was performed in smooth muscle where PPARγ in aortic smooth muscle cells could induce the upregulation of the protein tyrosine phosphatase SHP-2, leading to inhibition of the RhoA/Rho kinase pathway [Wakino, 2004 #19]. Additional studies will be necessary to elucidate whether a similar mechanism is operational in the endothelium as wells as how PPARγ may regulate function of multiple GTPases and whether this mechanism occurs by transcription-dependent or independent mechanisms.
A number of neuroinflammatory disorders (like multiple sclerosis, encephalitis) are associated with monocyte infiltration across BBB leading to neuronal injury and neurological demise [Hawkins, 2005 #65]. HIV-1 encephalitis is characterized by monocyte and macrophage accumulation in affected brain tissue [Glass, 1995 #49]. Perivascular macrophages serve as a source of neurotoxins and viral reservoir, and they are a driving force of chronic inflammation providing chemokines and homing cues for circulating HIV-1 infected cells [Williams, 2001 #50; Williams, 2005 #51]. A pool of perivascular virus-infected macrophages is derived from monocytes migrating across the BBB and suppression of monocyte infiltration may ameliorate neuronal demise [Williams, 2005 #51]. PPARγ stimulation significantly diminished adhesion and migration of HIV-1 infected monocytes across BMVEC monolayers. The evidences provided here of PPARγ mediated inhibition on Rac1 and RhoA GTPases extend our previous findings showing that RhoA activation is required in the BMVEC during interactions with HIV-1 infected monocytes, and its suppression prevents monocyte migration and BBB dysfunction [Persidsky, 2006 #26]. Taken together, these findings suggest a novel role for PPARγ at the BBB, which offers a therapeutic strategy to diminishing infiltration of leukocytes into the brain parenchyma.
Acknowledgments
We thank Dr. Georgette Kanmonge for generously providing human microvessel lysates; Dr. Anuja Ghorpade for critical reading of this manuscript; Ms. Robin Taylor and Ms. Debra Baer for excellent editorial support.
Footnotes
Abbreviations used in this paper: BBB, blood brain barrier: BMVEC, human brain microvascular endothelial cells; PPAR, peroxisome proliferator-activated receptors; TEER, transendothelial electrical resistance; TZD, thiazolidinediones.
This work was supported by grants from the National Institutes of Health (AA015913, MH65151 to Y.P.).
Disclosures
The authors have no financial conflict of interest.
References
- 1.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Kanwar JR. Anti-inflammatory immunotherapy for multiple sclerosis/experimental autoimmune encephalomyelitis (EAE) disease. Curr Med Chem. 2005;12:2947–2962. doi: 10.2174/092986705774462833. [DOI] [PubMed] [Google Scholar]
- 3.Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 4.Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich JB. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol. 2002;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 7.Quandt J, Dorovini-Zis K. The beta chemokines CCL4 and CCL5 enhance adhesion of specific CD4+ T cell subsets to human brain endothelial cells. J Neuropathol Exp Neurol. 2004;63:350–362. doi: 10.1093/jnen/63.4.350. [DOI] [PubMed] [Google Scholar]
- 8.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 9.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 11.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 12.Kurebayashi S, Xu X, Ishii S, Shiraishi M, Kouhara H, Kasayama S. A novel thiazolidinedione MCC-555 down-regulates tumor necrosis factor-alpha-induced expression of vascular cell adhesion molecule-1 in vascular endothelial cells. Atherosclerosis. 2005;182:71–77. doi: 10.1016/j.atherosclerosis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakino S, Hayashi K, Kanda T, Tatematsu S, Homma K, Yoshioka K, Takamatsu I, Saruta T. Peroxisome proliferator-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res. 2004;95:e45–55. doi: 10.1161/01.RES.0000142313.68389.92. [DOI] [PubMed] [Google Scholar]
- 15.Wittchen ES, van Buul JD, Burridge K, Worthylake RA. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Curr Opin Hematol. 2005;12:14–21. doi: 10.1097/01.moh.0000147892.83713.a7. [DOI] [PubMed] [Google Scholar]
- 16.Cernuda-Morollon E, Ridley AJ. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ Res. 2006;98:757–767. doi: 10.1161/01.RES.0000210579.35304.d3. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, Adamson P. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. 2003;171:2099–2108. doi: 10.4049/jimmunol.171.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 19.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 21.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman HE. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72:3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738–743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nag S. Morphology and molecular properties of cellular components of normal cerebral vessels. Methods Mol Med. 2003;89:3–36. doi: 10.1385/1-59259-419-0:3. [DOI] [PubMed] [Google Scholar]
- 25.Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 27.Rival Y, Beneteau N, Taillandier T, Pezet M, Dupont-Passelaigue E, Patoiseau JF, Junquero D, Colpaert FC, Delhon A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002;435:143–151. doi: 10.1016/s0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 28.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 29.Schleser S, Ringseis R, Eder K. Conjugated linoleic acids have no effect on TNF alpha-induced adhesion molecule expression, U937 monocyte adhesion, and chemokine release in human aortic endothelial cells. Atherosclerosis. 2006;186:337–344. doi: 10.1016/j.atherosclerosis.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson P, Wilbourn B, Etienne-Manneville S, Calder V, Beraud E, Milligan G, Couraud PO, Greenwood J. Lymphocyte trafficking through the blood-brain barrier is dependent on endothelial cell heterotrimeric G-protein signaling. Faseb J. 2002;16:1185–1194. doi: 10.1096/fj.02-0035com. [DOI] [PubMed] [Google Scholar]
- 32.Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol. 2003;95:1889–1895. doi: 10.1152/japplphysiol.00225.2003. [DOI] [PubMed] [Google Scholar]
- 33.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 34.Grigoriadis N, Grigoriadis S, Polyzoidou E, Milonas I, Karussis D. Neuroinflammation in multiple sclerosis: evidence for autoimmune dysregulation, not simple autoimmune reaction. Clin Neurol Neurosurg. 2006;108:241–244. doi: 10.1016/j.clineuro.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- 36.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Wang N. Peroxisome proliferator-activated receptor-gamma in vascular biology. Cardiovasc Hematol Disord Drug Targets. 2007;7:109–117. doi: 10.2174/187152907780830932. [DOI] [PubMed] [Google Scholar]
- 38.Klotz L, Diehl L, Dani I, Neumann H, von Oppen N, Dolf A, Endl E, Klockgether T, Engelhardt B, Knolle P. Brain endothelial PPARgamma controls inflammation-induced CD4(+) T cell adhesion and transmigration in vitro. J Neuroimmunol. 2007 doi: 10.1016/j.jneuroim.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 40.Arnold R, Neumann M, Konig W. Peroxisome proliferator-activated receptor-gamma agonists inhibit respiratory syncytial virus-induced expression of intercellular adhesion molecule-1 in human lung epithelial cells. Immunology. 2007;121:71–81. doi: 10.1111/j.1365-2567.2006.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest. 1999;103:1141–1150. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munro P, Lemichez E. Bacterial toxins activating Rho GTPases. Curr Top Microbiol Immunol. 2005;291:177–190. doi: 10.1007/3-540-27511-8_10. [DOI] [PubMed] [Google Scholar]
- 44.Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 45.Turner NA, O’Regan DJ, Ball SG, Porter KE. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. Faseb J. 2005;19:804–806. doi: 10.1096/fj.04-2852fje. [DOI] [PubMed] [Google Scholar]
- 46.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 47.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 48.Glass J, Fedor H, Wesselingh S, McArthur J. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 49.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]