Abstract
Background and Purpose
This study aimed to examine the temporal relationship between tissue perfusion and apparent diffusion coefficient (ADC) changes within 6 hours of ischemic stroke onset and how different reperfusion patterns may affect tissue outcome in ADC lesion.
Methods
Thirty-one participants were sequentially imaged at 3 hours, 6 hours and 1 month post stroke. Three regions of interest (ROIs) were defined within initial ADC lesions: ROI(1)reperf3hr hyper-acute reperfusion (within 3 hours), ROI(2)reperf6hr acute reperfusion (3-6 hours), and ROI(3)nonreperf no reperfusion (by 6 hours). For each ROI, changes of ADC (ΔADC) from 3 to 6 hours and risks of infarction were examined.
Results
The magnitude of initial ADC reduction was similar in all three ROIs (P=0.51). ΔADC were strongly associated with reperfusion (P<0.0001) but not with initial ADC reduction (P=0.83). ΔADC in ROI(1)reperf3hr, ROI(2)reperf6hr were significantly larger than that of ROI(3)nonreperf (P<0.05). Positive ΔADC were obtained from 3 to 6 hours in ROI(1)reperf3hr that had restored perfusion prior to 3 hours, demonstrating a temporal delay between reperfusion and ADC changes. Risk of infarction were significantly higher in ROI(3)nonreperf than those in ROI(1)reperf3hr and ROI(2)reperf6hr.
Conclusions
Improvement of ADC did not occur coincidently with reperfusion but showed a temporal delay. Regions with similar initial ADC reductions at 3 hours had different evolution of ADC and infarction risks depending on when or if tissue reperfused. These findings provide a physiological basis for the observation that a single ADC measurement at a fixed time after stroke onset may not accurately predict tissue outcome.
Keywords: ischemic stroke, ADC recovery, reperfusion, risk of infarction
Introduction
Magnetic resonance diffusion weighted imaging (DWI) is widely utilized in clinical practice to depict acute ischemic stroke lesions. It has been demonstrated that compromised blood flow leads to a reduction of the apparent diffusion coefficient (ADC) during ischemia 1, 2. ADC reduction may be observed as early as minutes after stroke onset 3. Conversely, DWI lesions have been found to reverse in various settings including shortly after thrombolysis or a few days after stroke onset 4–6. However, the temporal behavior of ADC lesion improvement after reperfusion during the first hours after stroke onset has not been documented in humans. Moreover, it has not been thoroughly investigated whether the presence of reperfusion and its timing directly affect the final fate of an ADC lesion. In a rapid sequential DWI study in cats, Davis et al found that ADC reduction and recovery did not occur concurrently with stroke onset or reperfusion but rather evolved progressively over 5–10 minutes after these events 7. Based on this animal study, we hypothesized that a temporal delay may also exist between tissue reperfusion and ADC improvement in acute human stroke. This temporal delay may explain, in part, why ADC reduction is not a reliable predictor of ischemic tissue outcome 8, particularly since DWI images are usually acquired at a single time point after stroke onset in current clinical practice. An improved understanding of the relationship between perfusion and diffusion changes may aid in clinical decision-making using MRI.
In this study of sequential MR imaging in patients with acute ischemic stroke, we examined the temporal evolution of abnormal ADC in brain regions exhibiting three different reperfusion patterns during the hyper-acute phase of ischemia. The infarction risk for each of these patterns was measured and compared.
Participants and Methods
Participants and Inclusion Criteria
This is a retrospective analysis of data from a prospectively collected observational study of serial MRIs performed in acute ischemic stroke patients at a large tertiary care referral center, admitting over 800 ischemic stroke patients per year. After Institutional Review Board approval, the study enrolled consecutive patients within 3.5 hours of stroke onset based on the following pre-specified inclusion criteria: clinically-suspected acute cortical ischemic stroke; age ≥ 18 years; NIHSS ≥ 5; and patient or patient’s next of kin capable of informed consent. Exclusion criteria included bilateral strokes or any acute endovascular or surgical intervention. Both tPA-treated and untreated patients were included. Patients were given IV tPA according to NINDS tPA trial protocol 4. In tPA-treated patients, tPA administration was begun prior to all MR imaging studies without causing any delay in time-to tPA-treatment or any deviation from standard monitoring practices.
Magnetic Resonance Imaging
Thirty-one participants were serially scanned with MRI at 3 time points (tp): within 3.5 hours (tp1), at 6 hours (tp2), and at 1 month (tp3) after stroke onset. Tp1 scan was acquired as early as possible. Participants treated with tPA were imaged immediately after initiation of tPA infusion. One month follow-up scans were obtained in twenty-six participants, the remaining five participants were not available due to premature death (n=1) or early withdrawal (n=4) from the study.
MR images were acquired on a 3T Siemens whole body Trio system (Siemens Medical Systems, Erlangen, Germany). Imaging protocols, including DWI, FLAIR, T1, and PWI using dynamic susceptibility contrast (DSC), were identical for both tp1 and tp2. DWI images were acquired with a single-shot, spin echo, echo planar imaging (EPI) sequence (TR/TE=2900/90 ms, b= 0, 500, 1000 s/mm2; 3-axis diffusion encoding; 20 slices with a slice thickness of 5 mm). The imaging parameters for the FLAIR sequence were: TR/TE = 10000/115 ms; inversion time (TI) = 2500 ms; a matrix of 512 × 416 pixels, and 20 slices. T1 weighted images were obtained using a 3D MPRAGE sequence (TR/TE=1520/3.69 ms, TI=800ms, Flip Angle=8, matrix = 256 × 256 × 144, voxel size= 1 × 1 × 1 mm3, parallel imaging with an acceleration factor of 2). PWI images were acquired with a T2*-weighted gradient echo EPI sequence (TR/TE= 1500/43 ms, 14 slices with a slice thickness of 5 mm, matrix= 128×128). This sequence was repeated 50 times and Gadolinium diethylenetriamine penta-acetic acid (Gd-DTPA, 0.1 mmol/kg) was injected at the completion of the 5th measure.
Data Analysis
In PWI images, the change of MR signal induced by the bolus passage of contrast agent was first converted to ΔR2*/concentration curve. Subsequently, voxels within the MCA of the contralateral hemisphere were manually chosen to obtain an arterial input function. A singular value decomposition (SVD) method was utilized to calculate cerebral blood flow (CBF), cerebral blood volume (CBV) and derive the mean transit time (MTT=CBV/CBF) 9
Six parameter rigid image registration was performed to align MTT, ADC, and FLAIR images from the same participant across all time points using FSL 3.2 (FMRIB, Oxford, UK). To minimize the inclusion of cerebrospinal fluid (CSF), voxels with an ADC value greater than 100×10−5 mm2/s were removed from data analysis 6. Blood from early hemorrhagic transformation (HT) may affect ADC at tp2. To minimize its effect on ADC, we examined the T2* weighted DSC images at tp2 for each patients. Hypointense regions were then manually outlined as HT and excluded from data analysis. Mean MTT and ADC values were obtained from a ROI that encompasses the whole contralateral hemisphere for each participant.
Voxels with ADC values < mean-2*SD of the contralateral hemisphere were defined as abnormal. Three regions of interest (ROIs) were defined within tp1 abnormal ADC regions corresponding to three different reperfusion patterns to (1) evaluate the temporal evolution of each of these three ADC lesions and (2) their respective tissue outcomes. MTT was chosen to define perfusion status because MTT is uniform across gray and white matter (unlike CBF or CBV), allowing for use of a single threshold across both gray and white matter 9. Hypoperfusion was defined using MTT > 4 seconds longer than the mean contralateral hemispheric. Fig 1 shows a schematic representation of the definition of three ROIs. ROI(1)reperf3hr was defined as voxels with abnormal ADC at tp1 but normal MTT at tp1 and tp2 (labeled as “1”, Fig. 1), representing an ADC lesion that reperfused prior to tp1 imaging (<3hr). ROI(2)reperf6hr was defined as voxels with abnormal ADC and MTT at tp1, but normal MTT at tp2 (labeled as “2”, Fig. 1), representing an ADC lesion that reperfused between tp1 (3 hours) and tp2 (6 hours). ROI(3)nonreperf was defined as voxels with abnormal ADC at tp1 and abnormal MTT at tp1 and tp2 (labeled as “3”, Fig. 1), representing an ADC lesion that did not reperfuse. Note our definition of ADC lesion was based on tp1 ADC maps only and three ROIs were defined using tp1 ADC and tp1 and tp2 MTT. Therefore, no ADC lesion is delineated at tp2 in Fig. 1 to avoid confusion. In all ROIs, isolated regions smaller than 1 ml were removed to minimize artifacts due to potential misalignment and spurious findings caused by random noise.
Fig 1.
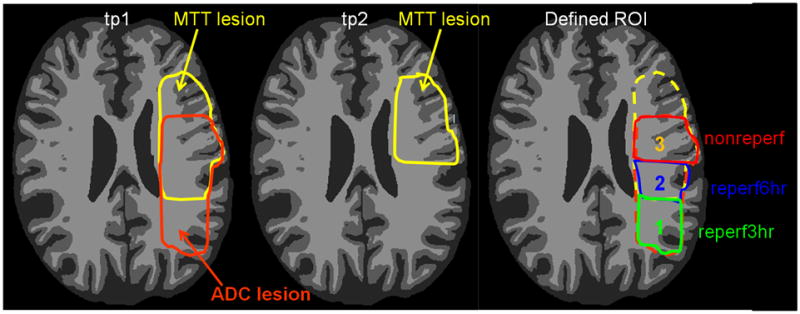
A schematic representation of the definition of three ROIs within tp1 abnormal ADC regions. Yellow color outlines MTT lesion at tp1 and tp2, and orange color outlines ADC lesion at tp1. Region 1 represents ROI(1)reperf3hr that exhibits abnormal ADC at tp1but normal MTT at tp1 and tp2; Region 2 represents ROI(2)reperf6hr that has abnormal ADC and MTT at tp1 and normal MTT at tp2; and region 3 represents ROI(3)nonreperf that shows abnormal ADC at tp1 and abnormal MTT at tp1 and tp2.
Mean ADC values from these ROIs at tp1 and tp2 were obtained to examine the temporal evolution of ADC between the two tps on an individual participant basis. ADC changes from tp1 to tp2 were computed as ΔADC=ADC_tp2-ADC_tp1 in all three ROIs. A positive ΔADC was considered as an improvement of ADC. A generalized linear model (SAS 9.2, SAS Institute Inc., Cary, NC, USA) was utilized to perform an analysis of covariance (ANCOVA) to evaluate whether reperfusion status (reperfusion <3hr, reperfusion 3-6 hr, and without reperfusion) and/or tp1 ADC values might affect ΔADC. ΔADC was the dependent variable, while tp1 ADC values and reperfusion status were the independent variables in the model.
Final lesions were manually outlined as hyperintense regions on tp3 FLAIR. The defined final lesions were mapped onto the tp1 and tp2 images. Based on the overlap between final lesion and each ROI, risk of infarction was calculated as the ratio of the number of infarcted voxels to the total number of voxels in a specific ROI on an individual patient basis. To evaluate whether the risk of infarction differed among all three ROIs, one-way analysis of variance (ANOVA) with Newman-Keuls multiple comparison post test was performed. P<0.05 was considered statistically significant.
Results
Thirty-one acute ischemic stroke patients were enrolled after obtaining written informed consent. Their characteristics are summarized in Table 1. At tp1, the mean ADC of contralateral hemispheres was 81.1± 2.0×10−5 mm2/s, which is similar to published reports 6, 10. The threshold for abnormal ADC, defined as two standard deviations below the normal value at tp1, was 64.3± 3.9×10−5 mm2/s (approximately 21% below normal values).
Table 1.
Participant Characteristics
| Participants | n=31 |
| Age, year (mean±SD) | 61±14 |
| Women, n (%) | 13 (42%) |
| Stroke Syndrome | R MCA: 17, L MCA:13, L ICA:1 |
| tPA treated, n (%) | 23 (72%) |
| NIHSS | |
| Admission | 15±6 |
| tp1 | 14±6 |
| tp2 | 12±7 |
| tp3 | 8±7 |
| Time from Symptom Onset | |
| Admission | 0.9±0.6 |
| Treatment (OTT) | 1.9±0.4 |
| tp1 (hr) | 2.9±0.8 |
| tp2 (hr) | 6.3±0.3 |
| tp3 (day) | 29±10 |
| Time from tPA-Treatment to scan | |
| tp1 (hr) | 0.7±0.4 |
| tp2 (hr) | 4.0±1.2 |
| ROI Volumes (ml) | |
| ROIreperf3hr | 11.3±14.0 |
| ROIreperf6hr | 6.8±4.3 |
| ROInonreperf | 35.6±37.0 |
MCA=middle cerebral artery, ICA=internal cerebral artery, L=left, R=right.
Representative tp1 and tp2 MTT, tp1 and tp2 ADC, one month FLAIR and overlaid ROIs from three participants are shown in Figure 2.
Fig 2.
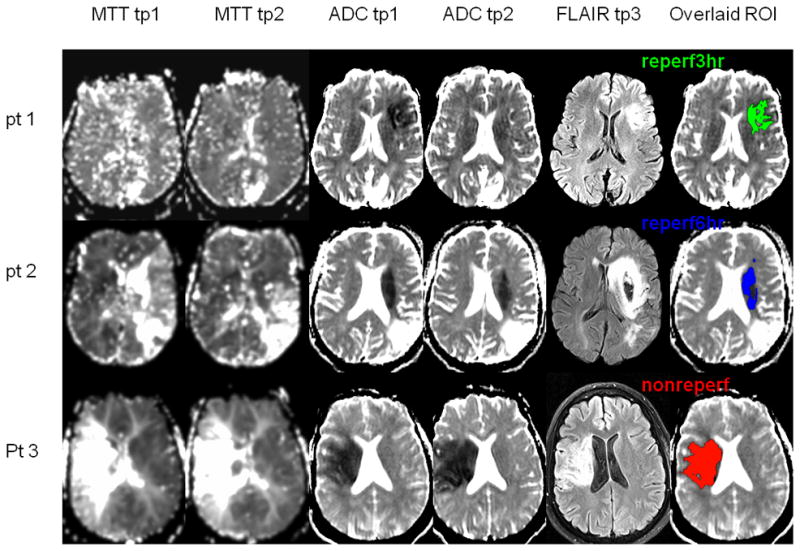
Representative MRI images of the participants exhibiting the three ROIs defined in Figure 1. Shown are MTT tp1 and tp2 (1st and 2nd columns), ADC tp1 and tp2 (3rd and 4th columns), and FLAIR at tp3 (5th column). Each representative ROI (defined in Figure 1) was overlaid onto the tp1 ADC (6th columns): pt 1, ROIreperf3hr (green); pt 2, ROIreperf6hr (blue); and pt 3, ROInonreperf (red).
Temporal change of ADC
In this study, two sequential MR scans were used to determine reperfusion status within the first 6 hrs after stroke onset in both tPA treated and untreated patients. Given that only 8 patients did not receive tPA treatment in our study cohort, we did not perform separate analyses for tPA treated and untreated patients.
Tp1 ADC values were similarly reduced in all three ROIs (P=0.51) regardless of the initial or different subsequent reperfusion status (Fig 3a-c). The median and interquartile range (IQR) of tp1 ADC were 54.2 (IQR [52.5, 56.9]) ×10−5 mm2/s, 52.3 (IQR [49.8, 55]) ×10−5 mm2/s, and 52 (IQR [48.9, 55.8]) ×10−5 mm2/s for ROI(1)reperf3hr, ROI(2)reperf6hr and ROI(3)nonreperf, respectively.
Fig 3.
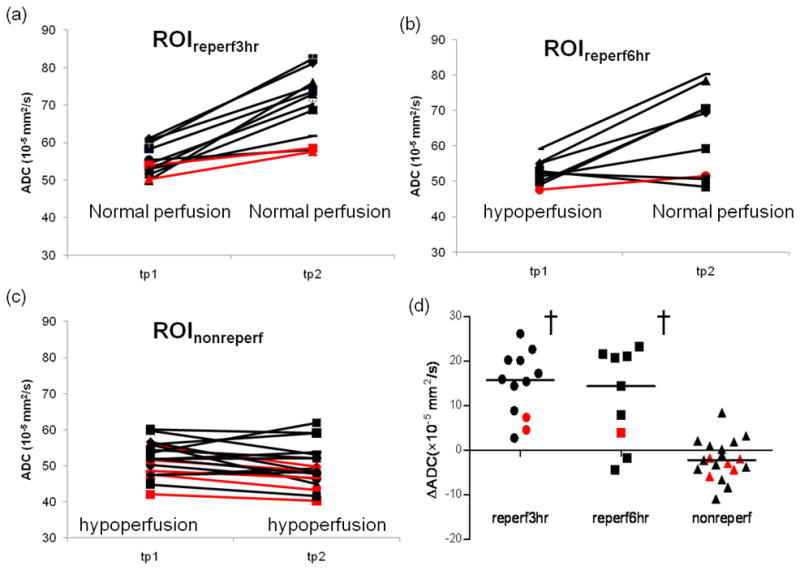
Mean ADC from ROIreperf3hr (a), ROIreperf6hr (b), and ROInonreperf (c) for each individual participant at tp1 and tp2 after stroke onset. Perfusion status was labeled at both tp1 and tp2 in (a) to (c). A scatter plot of ΔADC in the three ROIs (d). † denotes statistical different ΔADC between a ROI and ROInonreperf. tPA treated patients were marked in black, while untreated patients were highlighted using red.
ROI(1)reperf3hr, representing hyper-acute reperfusion (< 3 hours), was identified in 12 participants. Of which, 2 (17%) were not treated with tPA (marked in red, Fig 3a). ROI(2)reperf6hr, representing reperfusion between tp1 (3 hours) and tp2 (6 hours), was detected in 9 participants. Of which, 1 (11%) was not treated with tPA (marked in red, Fig 3b). ROI(3)nonreperf, representing initially abnormal ADC and abnormal perfusion at tp1 and without evidence of reperfusion at tp2, was detected in 19 participants. Of which, 5 (26%) were untreated patients.
Scatter plots of ΔADC in the three predefined ROIs were given in Figure 3d. ΔADC were strongly associated with reperfusion status (P<0.0001, ANCOVA analysis) but was not associated with tp1 ADC (P=0.83). The median (IQR) ΔADC were 15.7 (IQR [8.4, 20.1]) ×10−5 mm2/s, 14.3 (IQR [3.9, 21.1]) ×10−5 mm2/s, and −2.3 (IQR [−4.4, 0.6]) ×10−5 mm2/s in ROI(1)reperf3hr, ROI(2)reperf6hr and ROI(3)nonreperf, respectively. ΔADC were not significantly different for ROI(1)reperf3hr, ROI(2)reperf6hr, but ΔADC in both reperfused ROIs were significantly larger than that of ROI(3)nonreperf (P<0.05). That ROI(1)reperf3hr showed a similar increase in ADC to ROI(2)reperf6hr even though already reperfused at 3 hours, demonstrates that a temporal dissociation between reperfusion and ADC change occurred in this tissue. The small number of non-tPA-treated patients prevents us from discerning whether tPA induced reperfusion or spontaneous reperfusion might have a different impact on subsequent ADC evolution.
Risk of Infarction
Risks of infarction were plotted for all ROIs in Figure 4. The median and IQR risks of infarction were 64.2% (IQR [56.2%, 82.7%]) for ROI(1) reperf3hr, 84.3% (IQR [30.2%, 88.5%]) for ROI(2) reperf6hr, and 94.4% (IQR [83.5%, 97.9%]) for ROI(3)nonreperf (Fig. 4). One-way ANOVA analysis showed significantly different risks of infarction among the three ROIs (P<0.01). Newman-Keuls post tests further revealed that risks of infarction in ROI(3)nonreperf were significantly higher than those in ROI(1)reperf3hr and ROI(2) reperf6hr (P<0.05). The fact that all three groups had similar initial ADC reductions but different risks of infarction highlights the failure of a single ADC measurement at 3 hours after stroke onset to accurately reflect tissue outcome and the importance of concurrent and subsequent perfusion status in this determination.
Fig 4.
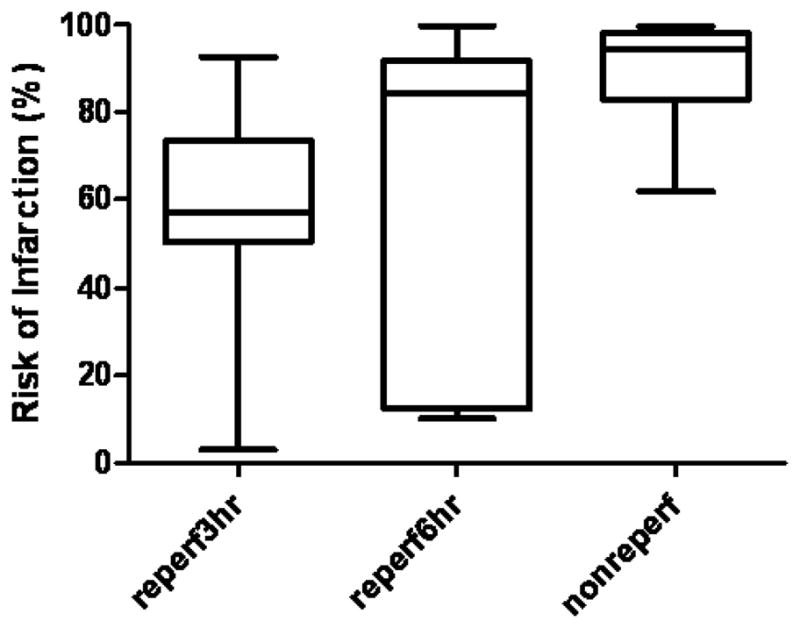
Risk of infarction in the three ROIs (box=25th-75th percentile; bars = 10th-90th percentile). † denotes statistical difference between a specific ROI and ROInonreperf using Newman-Keuls multiple comparison.
Discussion
DWI is widely utilized in the clinical setting of acute stroke to delineate ischemic injury. Given its common use, it is of critical importance to improve our understanding of ADC evolution during and after ischemia. Consistent with previous reports, we found that acute changes in ADC are dependent on tissue reperfusion, but not on initial severity of ADC reduction 6. Moreover, a temporal delay between reperfusion and ADC improvement was observed in some tissue. Regions with similar ADC reductions at 3 hours had different tissue outcomes depending on whether or when reperfusion occurred.
Temporal delay between reperfusion and ADC improvement
Despite the clinical utility of ADC to identify ischemic injury 11, challenges remain to explain why acute ADC lesions might or might not undergo infarction12, 13 and why an absolute ADC threshold for infarction has not been identified 14. We have shown that similar ADC abnormalities at tp1 correspond to different tissue fates, depending on concurrent and subsequent perfusion status (Fig 4). Positive ΔADC were obtained from tp1 to tp2 in ROI(1)reperf3hr that had restored perfusion prior to tp1 (Fig. 3a). It demonstrates that depending on the elapsed time after reperfusion, different ADC values might be obtained (e.g. tp1 and tp2 ADC in ROI(1)reperf3hr ) in tissue with already improved perfusion (Fig. 3a). Our findings are consistent with a previous animal study showing ADC reversal subsequent to tissue reperfusion 7 and previous MR spectroscopy and PET studies demonstrating heterogeneous cellular metabolic injury in regions with similar ADC 2, 15. Taken together, this evidence may help to explain why a single time-point ADC threshold to predict tissue outcome has not been identified.
The biophysical mechanisms of ADC lesion reversal have not been fully determined. Previous animal studies 16, 17 demonstrated that extracellular [K+] began to revert to normal about 40 minutes after reperfusion. Meanwhile, electrical excitability was shown to recover after 8 to 15 minutes; synaptic excitability and low-frequency spontaneous electrocortical activity were restored after 30 to 60 minutes and one to two hours, respectively, after reperfusion. This progressive recovery of energy metabolism and ion homeostasis after reperfusion may be related to delayed ADC improvement after reperfusion.
ADC improvement vs. Tissue recovery
The median risks of infarction for the two ROIs that exhibited reperfusion, ROI(1)reperf3hr and ROI(2) reperf6hr, were 64.2% and 84.3%, respectively, indicating that regions with acute ADC improvement (positive ΔADC) within 6 hrs after symptom onset still showed a high probability of infarction. This finding is consistent with previous animal and human studies 12, 13, 18.
Several mechanisms may be responsible for the discrepancy between acute ADC increase and 1 month tissue infarction. Ringer et al examined the histological condition of ischemic tissue exhibiting ADC reversal in rats after 30 minutes of middle cerebral artery occlusion (MCAO)19, followed by successful reperfusion. MRI scans were compared with histology by using neuronal, astrocytic markers, and heat shock protein 72 (HSP72) 19. Their results suggested that neurons already exhibited structural damage and stress despite ADC lesion reversal, while astrocytes were morphologically intact. Other studies reported that reversed ADC regions had varying degrees of neuronal injury 13, 18, 20. Moreover, several factors such as calcium overload, free radical formation, and lactic acidosis 21, 22 might trigger a delayed mitochondrial dysfunction leading to the death of these regions with normalized ADC 23–25.
Study limitations and other issues
In this study, only two time points were acquired during the hyper-acute phase of ischemia to characterize lesion evolution. While additional time-points will be needed to fully document the temporal relationship between perfusion and diffusion changes during hyper-acute and acute stages of ischemia, this is impractical for human studies. In addition, the number of non-tPA-treated patients was too few to determine whether tPA treatment might uniquely impact our findings. Given these limitations, to the best of our knowledge, this study is the first to reveal that a temporal delay exist between reperfusion and ADC improvement in human acute stroke.
Of note, there is an important difference between the previously reported pseudo-normalization and the ADC evolution observed in our study. Pseudonormalization (normal ADC, but subsequent infarct evolution on T2 weighted images) occurs between 1 and 7 days after stroke onset in humans. This phenomenon might be due to increased cerebral water content associated with vasogenic edema 6, 26–28. In contrast, ADC improvement in our study which occurred during the first 6 hours after ischemia onset is unlikely caused by vasogenic edema but rather in response to tissue reperfusion. Therefore, the underlying pathophysiological mechanism of the acute ADC improvement in this study likely reflects a different process from ADC pseudo-normalization observed days after stroke.
Clinical implications
Regions with a similar ADC reduction at 3hrs had different final outcomes depending on whether and when reperfusion occurred. In regions with reperfusion within 3 hrs after stroke onset, less than 40% of tissue survived. In regions with reperfusion between 3–6 hrs after stroke onset, risk of infarction varied in a large range with a median risk of infarction greater than 80%. Because ADC may still increase over time in tissue that already reperfused, different ADC values could be obtained depending on the elapsed time after reperfusion. Therefore, a single time-point ADC measurement does not fully reflect the disease process and outcome. Beyond diagnosing acute ischemic, interpretation of ADC for tissue outcome, particularly in the presence of reperfusion, must be performed with caution.
Acknowledgments
Sources of Funding
This study was supported by grants from National Institute of Health (NIH 5P50NS055977, NIH 5R01NS054079) and American Heart Association (AHA 0730321N).
Footnotes
The authors have nothing to disclose.
References
- 1.Lin W, Lee JM, Lee YZ, Vo KD, Pilgram T, Hsu CY. Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke. 2003;34:64–70. doi: 10.1161/01.str.0000048151.28173.0d. [DOI] [PubMed] [Google Scholar]
- 2.Guadagno JV, Jones PS, Fryer TD, Barret O, Aigbirhio FI, Carpenter TA, Price CJ, Gillard JH, Warburton EA, Baron JC. Local relationships between restricted water diffusion and oxygen consumption in the ischemic human brain. Stroke. 2006;37:1741–1748. doi: 10.1161/01.STR.0000232437.00621.86. [DOI] [PubMed] [Google Scholar]
- 3.Moseley ME, Mintorovitch J, Cohen Y, Asgari HS, Derugin N, Norman D, Kucharczyk J. Early detection of ischemic injury: Comparison of spectroscopy, diffusion-, t2-, and magnetic susceptibility-weighted mri in cats. Acta Neurochir Suppl (Wien) 1990;51:207–209. doi: 10.1007/978-3-7091-9115-6_70. [DOI] [PubMed] [Google Scholar]
- 4.Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- 5.Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW, Dunn B, Warach S. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: Insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55:105–112. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- 6.Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, Zeumer H, Rother J. Severe adc decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- 7.Davis D, Ulatowski J, Eleff S, Izuta M, Mori S, Shungu D, van Zijl PC. Rapid monitoring of changes in water diffusion coefficients during reversible ischemia in cat and rat brain. Magn Reson Med. 1994;31:454–460. doi: 10.1002/mrm.1910310416. [DOI] [PubMed] [Google Scholar]
- 8.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: Evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 10.Nagesh V, Welch KM, Windham JP, Patel S, Levine SR, Hearshen D, Peck D, Robbins K, D’Olhaberriague L, Soltanian-Zadeh H, Boska MD. Time course of adcw changes in ischemic stroke: Beyond the human eye! Stroke. 1998;29:1778–1782. doi: 10.1161/01.str.29.9.1778. [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 12.Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, Villablanca JP, Liebeskind DS, Gobin YP, Vinuela F, Alger JR. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Han SS, Tatlisumak T, Liu KF, Garcia JH, Sotak CH, Fisher M. Reversal of acute apparent diffusion coefficient abnormalities and delayed neuronal death following transient focal cerebral ischemia in rats. Ann Neurol. 1999;46:333–342. doi: 10.1002/1531-8249(199909)46:3<333::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, Eckert B, Wittkugel O, Weiller C, Zeumer H, Rother J. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–519. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- 15.Nicoli F, Lefur Y, Denis B, Ranjeva JP, Confort-Gouny S, Cozzone PJ. Metabolic counterpart of decreased apparent diffusion coefficient during hyperacute ischemic stroke: A brain proton magnetic resonance spectroscopic imaging study. Stroke. 2003;34:e82–87. doi: 10.1161/01.STR.0000078659.43423.0A. [DOI] [PubMed] [Google Scholar]
- 16.Heiss WD. Experimental evidence of ischemic thresholds and functional recovery. Stroke. 1992;23:1668–1672. doi: 10.1161/01.str.23.11.1668. [DOI] [PubMed] [Google Scholar]
- 17.Hossmann KA, Sakaki S, Zimmerman V. Cation activities in reversible ischemia of the cat brain. Stroke. 1977;8:77–81. doi: 10.1161/01.str.8.1.77. [DOI] [PubMed] [Google Scholar]
- 18.van Lookeren Campagne M, Thomas GR, Thibodeaux H, Palmer JT, Williams SP, Lowe DG, van Bruggen N. Secondary reduction in the apparent diffusion coefficient of water, increase in cerebral blood volume, and delayed neuronal death after middle cerebral artery occlusion and early reperfusion in the rat. J Cereb Blood Flow Metab. 1999;19:1354–1364. doi: 10.1097/00004647-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke. 2001;32:2362–2369. doi: 10.1161/hs1001.096058. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Silva MD, Sotak CH, Fisher M. Temporal evolution of ischemic injury evaluated with diffusion-, perfusion-, and t2-weighted mri. Neurology. 2000;54:689–696. doi: 10.1212/wnl.54.3.689. [DOI] [PubMed] [Google Scholar]
- 21.Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: Acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Murphy AN, Fiskum G, Beal MF. Mitochondria in neurodegeneration: Bioenergetic function in cell life and death. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda S, Katsura KI, Tsuchidate R, Siesjo BK. Secondary bioenergetic failure after transient focal ischaemia is due to mitochondrial injury. Acta Physiol Scand. 1996;156:149–150. doi: 10.1046/j.1365-201X.1996.449170000.x. [DOI] [PubMed] [Google Scholar]
- 25.Siesjo BK, Hu B, Kristian T. Is the cell death pathway triggered by the mitochondrion or the endoplasmic reticulum? J Cereb Blood Flow Metab. 1999;19:19–26. doi: 10.1097/00004647-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37:231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 27.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (adc) abnormality in human stroke. Neurology. 1997;49:113–119. doi: 10.1212/wnl.49.1.113. [DOI] [PubMed] [Google Scholar]
- 28.Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME. Evaluation of early reperfusion and i.V. Tpa therapy using diffusion- and perfusion-weighted mri. Neurology. 1999;52:1792–1798. doi: 10.1212/wnl.52.9.1792. [DOI] [PubMed] [Google Scholar]


