Abstract
Ethnopharmacological relevance
Berberine is an isoquinoline alkaloid isolated from the root and bark of plants such as goldenseal, Berberis, and Chinese goldthread. Berberine-containing crude drugs have been used as an antimicrobial remedy against gastrointestinal infections for thousands of years. It is also widely used in Asian countries for diabetes, hypertension, and hypercholesterolemia therapy.
Aim of the Study
Potential drug-drug interactions are of concern because of the wide usage of berberine. A few studies have reported interactions between berberine and cytochromes P450 (CYPs) in vitro, but little is known about whether berberine influences CYPs in vivo, especially after repeated administration. In this study, eight-week-old male C57BL/6 mice were given berberine orally (0,10, 30, 100, 300 mg/kg, i.g., daily for 14 days), and the effect of berberine on over 20 major Cyps and related nuclear receptors in mice livers were examined at both the mRNA and enzyme activity levels.
Results
In general, liver function of mice treated with various doses of berberine had no significant change, and repeated oral administration of the 3 lower doses of berberine for 14 days did not affect the expression of genes examined. However, after the highest dose of berberine (300 mg/kg), Cyp3a11 and Cyp3a25 mRNA decreased 67.6 and 87.4%, respectively, whereas Cyp1a2 mRNA increased 43.2%, and enzyme activities of Cyp3a11 and Cyp2d22 decreased 67.9 and 32.4%, respectively. Cyp2a4, 2b10 and Cyp2c29 were not altered at both mRNA and enzyme activity levels.
Conclusions
If studies in mice extrapolate to humans, lower doses of berberine appear to present a low risk of producing drug-drug interactions as a result of changed Cyp enzyme activity. However, high doses of berberine may suppress Cyp activities and result in drug-drug interactions.
Keywords: Berberine, liver, drug-drug interactions
1. Introduction
Berberine is a widely used plant extract and dietary supplement against viral, bacterial, and fungal infections as well as parasites (Seddon, 2000). This chemical is a quaternary ammonium salt that belongs to the protoberberine group of isoquinoline alkaloids, and can be found in the root and bark of herbs, such as goldenseal, berberis, and chinese goldthread (Zhang et al., 2010). In recent years, berberine has been used for cardiovascular, nervous, and endocrine system diseases as well as cancer therapy (Kulkarni and Dhir, 2010; Vuddanda et al., 2010; Zhang et al., 2011; Zhang et al., 2008). Goldenseal, a main source of berberine, was the sixth most commonly used herbal supplement for children in America in 2007 (Barnes et al., 2008).
The effects of berberine on drug processing genes, such as the Cytochrome P450 (CYP) superfamily and transporters, which are important in controlling the elimination of xenobiotics, have been occasionally reported. The CYP superfamily has an important role in the metabolism of xenobiotics. Co-administration of drugs that are metabolized by, as well as those that induce or inhibit CYPs, can cause drug-drug interactions that may result in severe adverse effects. It has been shown that berberine inhibits CYP2D6 (IC50 = 45 μM) and CYP3A4 (IC50 ~ 400 μM) activities in human liver microsomes (HLM) (Etheridge et al., 2007). Goldenseal, which contains berberine, hydrastine, and other alkaloids, also inhibits enzyme activities of CYPs. More specifically, in human subjects administered goldenseal extract for 28 days, CYP2D6 and CYP3A4/5 were strongly inhibited (Gurley et al., 2005; 2008); goldenseal extract (1,070 mg) administered for 14 days interfered with the metabolism of a probe drug for CYP2D6 (Streetman et al., 2000). However, the role that berberine played in these in vivo effects produced by goldenseal is not clear.
Specific members of the CYP1A, 2B, 2D, 2E, 3A, and 4A families are regulated by nuclear receptors. For example, the aryl hydrocarbon receptor (AhR) regulates the expression of Cyp1a1/2, whereas Cyp3a11 and Mdr1a are target genes of the pregnane X receptor (PXR); hepatocyte nuclear factor 4α (HNF4α) is thought to be important for Cyp3a25 and Cyp2d22 regulation; constitutive androstane receptor (CAR) plays a critical role in modulating Cyp2b10; HNF1α may affect Cyp2e1 expression; and Cyp4a14 induction is mediated by peroxisome proliferator-activated receptor alpha (PPARα) (Down et al., 2007; Waxman, 1999; Zhou et al., 2009).
Nuclear receptors are also potential targets of berberine. In human hepatic cell lines, berberine reduces insulin resistance through the glucocorticoid receptor, and induces expression of AhR-dependent genes, such as CYP1A1, via the AhR receptor (Dvorak and Vrzal, 2011). In diabetic rat retina, berberine increased PPARα expression (Zhou and Zhou, 2007). Improved glucose and lipid metabolism are also observed in both blood and liver of diabetic rats, possibly through induction of PPARα/δ as well as decreased PPARγ protein expression in liver (Zhou et al., 2008). In cancer cells, berberine up-regulated multidrug-resistance transporter expression (Lin et al., 1999), and the induction of P-glycoprotein is partially regulated by PXR (Harmsen et al., 2010). Berberine also prevented fructose-induced insulin resistance in rat islet cells by promoting the expression of HNF4α (Gao et al., 2008). However, information about effects of berberine on these nuclear receptors as well as whether drug processing genes are altered after in vivo exposure is limited.
In the current study, a wide range of berberine doses (10, 30, 100, 300 mg/kg) were given to 8-week old C57BL/6 male mice orally. The effects of berberine on the expression of over 20 drug processing genes, including major Cyps and related nuclear receptors were systematically profiled. In addition, major Cyp enzyme activities were also characterized.
2. Materials and Methods
2.1. Chemicals and Reagents
Dextrorphan and resorufin were supplied by Cerilliant (Round Rock, TX), and 15α-hydroxytestosterone and 16β-hydroxytestosterone were obtained from Steraloids (Newport, RI). Cypex (Dundee, Scotland) provided 7-ethoxyresorufin for this study, and 6β-hydroxytestosterone-d3 was supplied by Toronto Research Chemicals (North York, Ontario, Canada). Dextrorphan-d3 was supplied by High Standard Products (North Wales, PA). The recombinant human CYPs and HLM were obtained from XenoTech (Lenexa, KS, USA). berberine chloride and all other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Animals Treatments
Male C57BL/6 mice (22 ± 2g, 8-weeks old) were obtained from Charles River Laboratories, Inc. (Wilmington, MA). All mice were maintained under a standard 12-h dark and 12-h light cycle with water and chow provided ad libitum. Animal studies were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. berberine (0, 10, 30, 100, 300 mg/kg, once daily, i.g. dissolved in saline) was administered to mice (n = 6) for 2 weeks. Livers were collected and frozen in liquid nitrogen, and stored at −80°C before use.
2.3. Histopathology
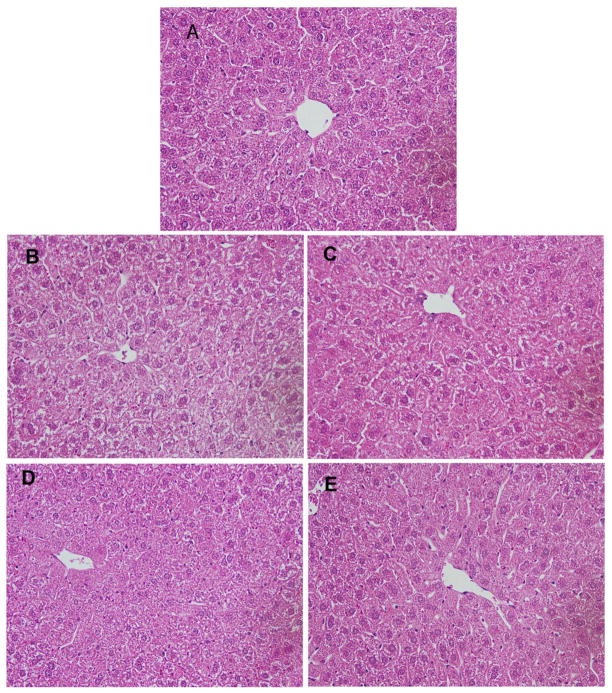
At the end of the 14 days treatment, liver were removed, weighed, and fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 6 μm, and stained with H&E for histological analysis. Pathological assessments were done in a blind fashion. Incidence of hepatic lesions was determined by histologic analysis of the same portion of the liver from each animal. Representative microphotos were taken for comparison.
2.4. Blood biochemistry
The serum activities of alanine aminotransferase (ALT) were quantified with a BioTech Synergy 2 multi-detection microplate reader (BioTek Instruments, Inc., Winooski, Germany). The potential hepatotoxicity of animals treated with the different doses of berberine was examined.
2.5. Total RNA extraction
Total liver RNA was isolated using RNAzol Bee reagent (Tel-Test Inc., Friendswood, TX) per the manufacturer’s protocol, and concentrations were quantified with a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE) at 260 nm. Formaldehyde-agarose gel eletrophoresis was used to evaluate the integrity of these total RNA samples, which were confirmed by visualization of 18s and 28s rRNA bands. Diethyl pyrocarbanate (DEPC)-treated double-distilled water was used to dilute each RNA sample to 50 ng/μl for real-time PCR quantification.
2.6. Synthesis of cDNA and Real-time PCR quantification
Reverse transcription of RNA to cDNA was performed using the Applied Biosystems High Capacity Reverse Transcriptase kit (Applied Biosystems, Foster City, CA).. Primers for RT- PCR (Table 1) were synthesized by Integrated DNA Technologies (Coralville, IA). Reactions were seeded in 384-well optical reaction plates (Applied Biosystems) and fluorescence was quantified using Applied Biosystems 7900 Real Time PCR System.
Table 1.
RT-PCR primers for related genes
| Gene Name | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| Cyp1a1 | NM_009992 | tgcccttcattggtcacatg | cacgtccccatactgctgact |
| Cyp1a2 | NM_009993 | gacatggcctaacgtgcag | ggtcagaaagccgtggttg |
| Cyp1b1 | NM_009994 | cctttccttggccactgatc | ctggaaaacgtcgccatagc |
| Cyp2a4 | J03549 | ggaagacgaacggtgctttc | cccgaagacgattgagctaatg |
| Cyp2b9 | M21855 | tctctgtggcaagccctgtt | ggtgtgctggaggtatttttcc |
| Cyp2b10 | AF128849 | aaggagaagtccaaccagca | ctctgcaacatgggggtact |
| Cyp2c37 | NM_010001 | tcctgggctgtgctccttgc | tgcaaatctgcaaccaagggctg |
| Cyp2d9 | NM_010006 | aaggctggctgacaaggccc | tcggggtgcttggacagggt |
| Cyp2d22 | NM_019823 | cagtggttgtactaaatgggct | gctaggactataccttgagagcg |
| Cyp2e1 | NM_021282 | tccctaagtatcctccgtga | gtaatcgaagcgtttgttga |
| Cyp2f2 | NM_00781 | gtcactcgggacacaccttt | ttgaactcctgaggcgtctt |
| Cyp2j5 | NM_010007 | cagacatggaaggagcaaagg | gaatgcgctcctccaagct |
| Cyp3a11 | NM_007818 | acaaacaagcagggatggac | ggtagaggagcaccaagctg |
| Cyp3a25 | Y11995 | tggaggcctgaactgctaaag | taaccagcagcacccaggtt |
| Cyp4a14 | Y11638 | ggtgaggctgattgagtcttgag | ctccagattgatccaggatgga |
| AhR | NM_013464 | accagaactgtgagggttgg | ctcccatcgtatagggagca |
| CAR | NM_009803 | ctcaaggaaagcagggtcag | agttcctcggcccatattct |
| HNF4a | NM_008261 | cggagcccctgcaaagt | ccagtctcacagcccattcc |
| HNF1 | M57966 | cccaatatctgcgtggtaagtg | cagttacaccaacgaccgtcagt |
| PXR | AF031814 | cccatcaacgtagaggagga | tctgaaaaaccccttgcatc |
| PPARa | X89577 | ccatacaggagagcagggattt | ttacctacgctcagccctcttc |
| Mdr1a | M14757 | ccaggctcgccagtgatg | cccgaggtttgctacattctg |
| Gapdh | M32599 | agtatgactccactcacggcaaat | gtctcgctcctggaagatggt |
2.7. Microsomal CYPs Enzyme Activities
The metabolic rate of testosterone 6β-hydroxylation was used as the marker for Cyp3a11/13 activity (Attar et al., 2005), testosterone 15α-hydroxylation metabolic rate for Cyp2a4 (Lavery et al., 1999), testosterone 16β-hydroxylation metabolic rate for Cyp2b10 and Cyp2c29 (Claus et al., 2011; Usmani et al., 2003), 7-ethoxyresorufin O-dealkylation metabolic rate for Cyp1a1/2 (Bullock et al., 1995), and dextromethorphan O-demethylation metabolic rate for Cyp2d22 (Bisaga et al., 2008). Each enzymatic assay was duplicated, and carried out at 37°C for 10 min in a 200 μl reaction mixture containing 50 mM PBS, 1 mM EDTA, and 3 mM MgCl2 (pH 7.4), and a NADPH generating system (1 mM NADP, 5 mM glucose 6-phosphate, 1 u/ml of glucose 6-phosphate dehydrogenase). Protein concentrations were 0.04, 0.015, and 0.01 mg/incubation, and substrate concentrations were 250, 10, and 80 μM for the testosterone hydroxylation, 7-ethoxyresorufin O-dealkylation, and dextromethorphan O-demethlyation assays, respectively. Substrate reactions were stopped by adding 150 μl acetonitrile and 25 μl internal standards (6β-hydroxytesosterone-d3 or dextrorphan-d3) for the testosterone hydroxylation and dextromethorphan O-demethlyation assays. Substrate reactions were stopped by adding 175 μM acetonitrile for the 7-ethoxyresorufin O-dealkylation assay.
All analyses of P450-enzyme activities were performed with validated high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) methods (Paris et al., 2009; Parkinson et al., 2006). The mass spectrometry equipment was an ABI Sciex (Applied Biosystems/MDS Sciex, Foster City, CA), API 2000 for the dextromethorphan O-demethlyation assay, and API 3000 for the testosterone hydroxylation assay. Shimadzu HPLC pumps and autosampler systems (Shimadzu, Kyoto, Japan) were used. The HPLC columns used were as follows: Waters Atlantis dC18 (5 μM 100 × 2.1 mm) (Waters, Milford, MA) preceded by a Phenomenex Security Guard Luna C8 (4 × 2.0 mm) (Phenomenex, Torrance, CA) for the dextromethorphan O-demethlyation assay, and Phenomenex hyperclone 150 (5 μM 150 × 2.0 mm) (Phenomenex) preceded by a Phenomenex Security Guard Luna C8 (4 × 2.0 mm) (Phenomenex) for the testosterone hydroxylation assay. Formic acid-based mobile phase for the dextromethorphan O-demethlyation assay and ammonium acetate-based mobile phase for the testosterone hydroxylation assay was used for all sample analyses, with flow rates of 600 μl per min for the dextromethorphan O-demethlyation assay and 450 μl or 500 μl for testosterone hydroxylation assay. The column was maintained at ambient temperature for the dextromethorphan O-demethlyation assay and at 50°C for the testosterone hydroxylation assay. Metabolites were quantified by back calculation of a weighted (1/x), linear, least-squares regression.
The formation of 7-ethoxyresorufin O-dealkylation metabolite was determined fluorimetrically (λex ~590 nm, λem ~645 nm) with a BioTech Synergy HT multi-detection microplate reader (BioTek Instruments, Inc., Winooski, Germany) with KC4 Signature software (version 3.4 Rev 21, BioTek Instruments, Inc. Winooski, Germany).
2.8. Data Analysis
Serum ALT activities, relative mRNA expression, and liver microsomal enzyme activities were analyzed by SPSS 11.0 using one way ANOVA followed by a Duncan’s post hoc test (log transformed when the data did not pass the homogeneity of variance test).
3. Results
3.1. Liver function of mice treated with various doses of berberine
To evaluate liver function of mice treated with different doses of berberine, serum activities of ALT and histopathology of liver sections after berberine treatment were examined. Figure There was no significant alteration in serum ALT activities of mice treated with various doses of berberine. Representative histopathology microphotos are shown in Figure Figure 1. Liver sections from mice treated with various doses of berberine (Figure Figure 1B-1E) did not show morphological alterations as compared to control (Figure Figure 1A).
Figure 1. Histopathological examination of liver sections from mice treated with berberine.
Liver sections of C57BL/6 male mice treated with various doses of berberine showed largely normal appearance. For each dose group, 6 sections were made and the microphotos show representative foci of control (A), 10 mg/kg (B), 30 mg/kg (C), 100 mg/kg (D), and 300 mg/kg (E) groups. Tissues were fixed in formalin and stained with H&E. Magnitude (400×).
3.2. Regulation of AhR, Cyp1a1, 1a2, and 1b1 mRNA by various doses of berberine in livers of mice
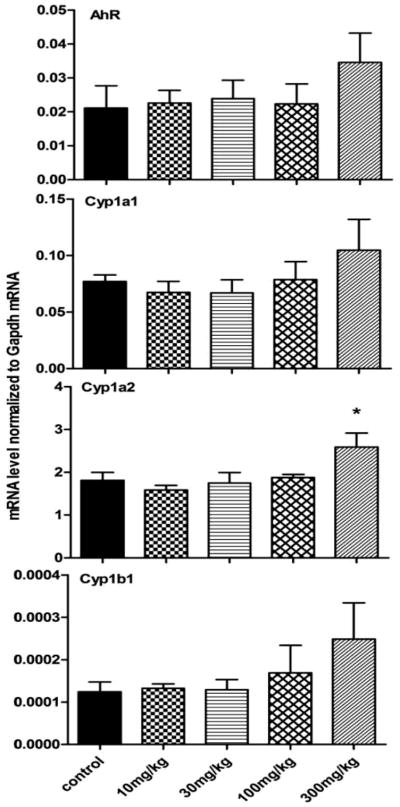
Effects of berberine on the expression of AhR, Cyp1a1, 1a2, and 1b1 mRNAs are shown in Figure 2. There is no significant effect of the 3 lower doses of berberine on the expression of these genes. The gene expression of the Cyp1 family tended to increase 30–100% after 300 mg/kg berberine treatment for 14 days, but only the up-regulation of Cyp1a2 was statistically significant, which was 43.2% higher in 300 mg/kg berberine-treated group compared with controls. The mRNA expression of AhR also had a tendency to increase in the highest dose group. However, the change was not statistically significant.
Figure 2. Regulation of AhR, Cyp1a1, 1a2, and 1b1 by various doses of berberine in livers of mice.
The gene expression of the Cyp1 family tend to increase 30–100% after 300 mg/kg berberine treatment for 14 days, but only the up-regulation of Cyp1a2 was statistically significant, which was 43.2% higher in 300 mg/kg berberine-treated group compared with control. There was no significant change in AhR mRNA expression.
3.3. Regulation of HNF4α, Cyp2d22, and Cyp3a25 by various doses of berberine in livers of mice
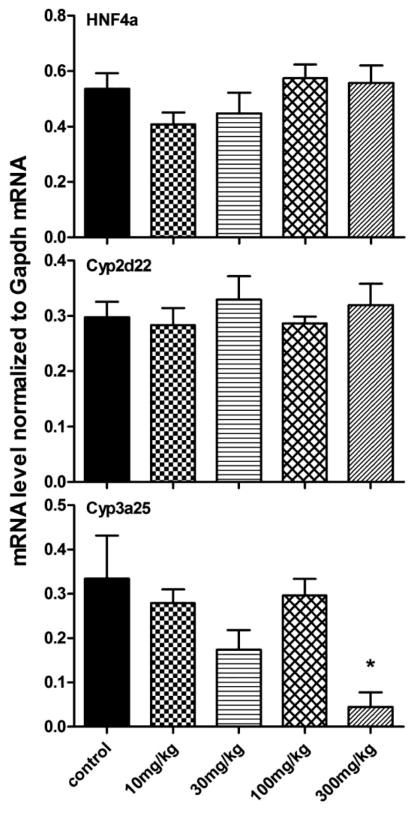
HNF4α, Cyp2d22, and Cyp3a25 mRNA expression in livers of mice treated with berberine are shown in Figure 3. Similar to the other group of genes, lower doses of berberine did not have a significant effect on these genes, but Cyp3a25 mRNA decreased 87.4% with the 300 mg/kg dose of berberine, and the mRNA of HNF4α and Cyp2d22 were not significantly changed.
Figure 3. Regulation of HNF4α, Cyp2d22, and 3a25 by various doses of berberine in livers of mice.
Lower doses of berberine did not have a significant effect on these genes, but Cyp3a25 mRNA decreased 87.4% with the 300 mg/kg dose of berberine, and the mRNA of HNF4α and Cyp2d22 were not significantly changed.
3.4. Regulation of PXR, Cyp3a11, and Mdr1a by various doses of berberine in livers of mice
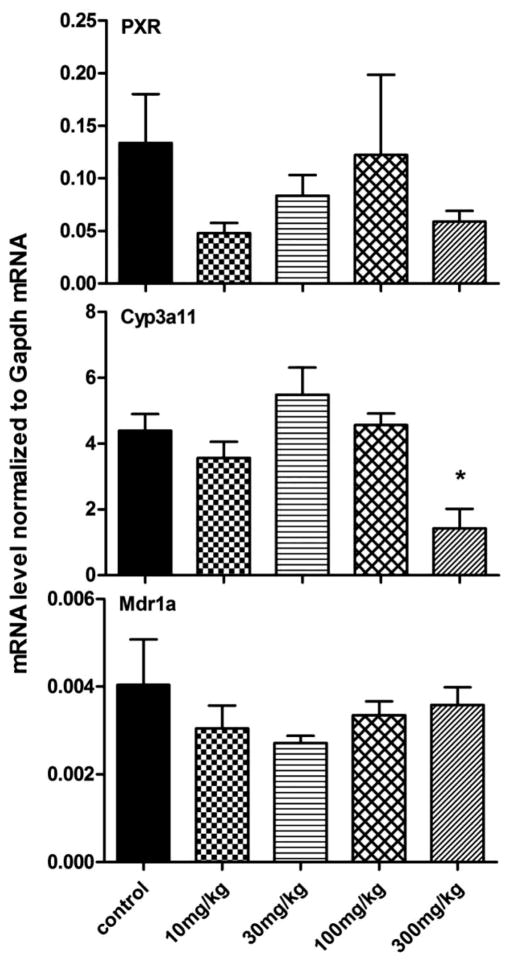
The mRNA expressions of PXR, Cyp3a11, and Mdr1a in liver of mice treated with berberine are shown in Figure 4. The highest dose of berberine (300 mg/kg) decreased Cyp3a11 mRNA by 67.6% but PXR and Mdr1a were not affected.
Figure 4. Regulation of PXR, Cyp3a11, and Mdr1a by various doses of berberine in livers of mice.
The highest dose of berberine (300 mg/kg) decreased Cyp3a11 mRNA by 67.6% but PXR and Mdr1a were not affected.
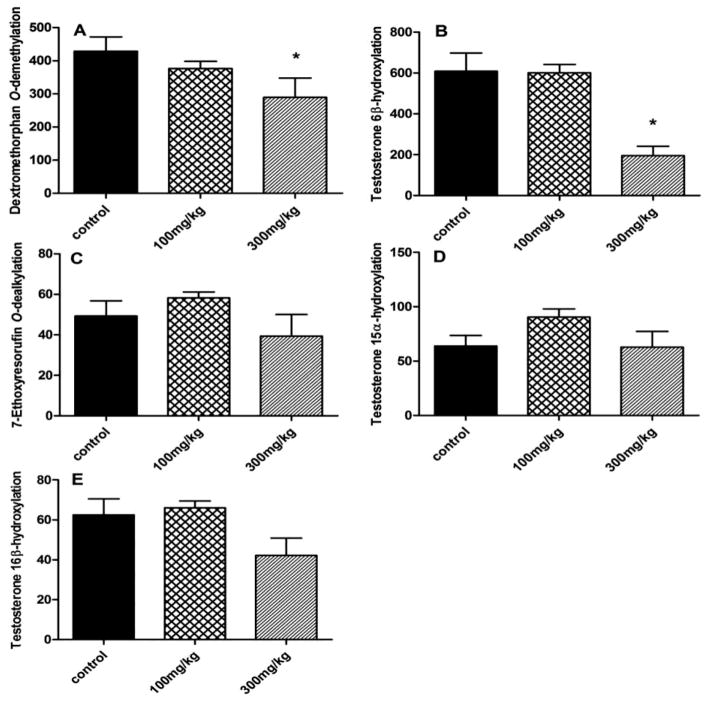
3.5. Influence of berberine on Cyp activities in livers of mice
After oral treatment with berberine (100 and 300 mg/kg) for 2 weeks, CYP enzyme activities in livers of mice were quantified by the metabolic rate of a probe drug for each major Cyp. The highest dose of berberine (300 mg/kg, daily, i.g.) inhibited dextromethorphan O-demethylation (Cyp2d22) by 32.4% (Figure 5A) and testosterone 6β-hydroxylation (Cyp3a11) by 67.9% (Figure 5B). There were no significant change in 7-ethoxyresorufin O-dealkylation (Cyp1a1/2) (Figure 5C), testosterone 15α-hydroxylation (Cyp2a4) (Figure 5D), and testosterone 16β-hydroxylation (Cyp2b10 and Cyp2c29) (Figure 5E) in livers of berberine-treated mice.
Figure 5. Influence of berberine on major Cyp enzyme activities in livers of mice.
The major Cyp enzyme activities in mice were quantified after 2-weeks of daily berberine treatment. (A) Dextromethorphan O-demethylation was used as a marker for Cyp2d. The highest dose of berberine inhibited dextromethorphan O-demethylation 32.4%. (B) Testosterone 6β-hydroxylation was used as a marker for Cyp3a11/13 activity. The highest dose of berberine decreased testosterone 6β-hydroxylation by 67.9%. (C) The metabolism of 7-ethoxyresorufin O-dealkylation was the marker for Cyp1a1/2 activity. (D) Testosterone 15α-hydroxylation was used as the marker for Cyp2a4. (E) Testosterone 16β-hydroxylation was used as the marker for Cyp2b10 and Cyp2c29 activities.
The expressions of CAR, HNF1α, PPARα, Cyp2a4, 2b10, 2c37, 2e1, 2f2, 2j5, and Cyp4a14 in liver of mice are illustrated in Table 2, and there was no significant change in the expression of these genes after repeated administration of berberine for 14 days.
Table 2.
Effect of berberine on the mRNA expression of nuclear receptors and related genes with no significant changes
| Genes | mRNA expression normalized to Gapdh (treatment/control)
|
|||
|---|---|---|---|---|
| berberine (10mg/kg) | berberine (30mg/kg) | berberine (100mg/kg) | berberine (300mg/kg) | |
| Cyp2a4 | 81% | 64% | 106% | 60% |
| CAR | 120% | 175% | 157% | 152% |
| Cyp2b10 | 58% | 59% | 71% | 55% |
| Cyp2c37 | 107% | 99% | 141% | 128% |
| HNF1α | 88% | 89% | 79% | 104% |
| Cyp2e1 | 94% | 96% | 96% | 124% |
| Cyp2f2 | 97% | 95% | 88% | 123% |
| Cyp2j5 | 68% | 92% | 66% | 156% |
| PPARα | 46% | 63% | 65% | 133% |
| Cyp4a14 | 97% | 53% | 92% | 74% |
4. Discussion
Exposure of berberine in laboratory animals and humans is highly variable. In previous reports, oral doses of berberine to mice usually range from 5 to 400 mg/kg/day, and developmental toxicity evaluation of berberine in mice showed that the no observed adverse effect level is over 450 mg/kg/day (Jahnke et al., 2006). In the current study, no obvious liver damage was triggered with doses up to 300 mg/kg, which implies berberine is well-tolerated in mice. Dosages of berberine used in humans are also vary in different situations, for instance, berberine dosages of 300 to 1200 mg are often used to treat diarrhea; berberine 200 mg/day (t.i.d, orally for 6 days) has been recommended to treat giardiasis in children under 10 years of age; in heart disease patients, berberine is used intravenously at the rate of 0.02–0.2 mg/min/kg to improve cardiac function; and in hypercholesterolemic patients, 500 mg berberine is given orally twice daily for 3 months (Jahnke et al., 2006). Therefore, a wide range of doses in the current study might be necessary.
Very little is known about what doses of berberine might alter the expression of drug processing genes in vivo. The present study systematically examined the effects of various doses of berberine on major Cyps and related nuclear receptors at the mRNA and enzyme activity level. The data reveal that lower doses of repeated berberine administration have little effect on these drug processing genes. However, after the high dose of berberine (300 mg/kg; i.g.; once daily) for 2 weeks, Cyp3a11 and 3a25 mRNA expression in liver of C57BL/6 mice decreased 67.6 and 87.4%, respectively, whereas Cyp1a2 mRNA expression had a 43.2% increase. The enzyme activities of Cyp3a11/13 and Cyp2d22 also decreased 67.9 and 32.4%, respectively, after the high dose of berberine.
After treatment of mice for two weeks with a high dose of berberine, there was a slight but statistically significant increase in Cyp1a2 mRNA expression (Figure 2), however, the enzyme activity of Cyp1a2 was not altered (Figure 5C). Studies performed in hepatic cell lines indicate that berberine has an effect on the AhR-Cyp1 signaling pathway. It was reported that berberine was an inhibitor (IC50=2.5 mM) of CYP1A1 catalytic activity in HepG2 cells as well as with recombinant CYP1A1 protein (Vrzal et al., 2005). In contrast, others demonstrated that berberine reduced insulin resistance through the glucocorticoid and Ah receptor, and induced the expression of AhR-dependent genes, such as CYP1A1 (Dvorak and Vrzal, 2011). The effective concentrations in these in vitro studies varied from 1 to 50 μM, and it is not clear whether the concentrations of berberine used in these studies might be too high to predict what would happen in vivo. AhR activation and the significant induction of Cyp1a enzyme activity can increase the biotransformation of various procarcinogens and promutagens to carcinogens and mutagens that covalently bind to important functional macromolecules such as DNA, which can result in carcinogenic transformation of cells (Zhou et al., 2010). However, data in the present study showed that only mice treated with a high dose of berberine had a marginal induction of Cyp1a2 mRNA, moreover, this is not accompanied by an increase in Cyp1a2 enzyme activity, which indicates that berberine has a low potential to produce AhR-Cyp1a-mediated drug-drug interactions.
The effect of berberine on the activation of PXR and its target genes are controversial. Cyclosporin A bioavailability increased in renal transplant patients after berberine administration, which might be due to a decrease in or inhibition of CYP3A4 in liver and/or intestine (Xin et al., 2006). However, in cancer cell lines berberine up-regulated PXR-target genes (Lin et al., 1999). The present study indicates that the high dose of berberine markedly decreases Cyp3a11 mRNA expression by 67.6% (Figure 4). The enzyme activity of Cyp3a11 was also inhibited 67.9% by 300 mg/kg of berberine (Figure 5B). Moreover, data from our previous study imply that Cyp3a11 plays an important role in berberine biotransformation in mice (Guo et al., 2011), and therefore the decrease in Cyp3a11 enzyme activity might also result in pharmacokinetic changes of other CYP3a substrates.
Dextromethorphan O-demethylation activity, which is catalyzed by Cyp2d22, decreased about 30% in mice treated with 300 mg/kg berberine (Figure 5A); however, the mRNA expression of Cyp2d22 did not change (Figure 3). Our previous data (Guo et al., 2011) indicate that Cyp2d22 is a major enzyme that catalyzes the metabolism of berberine in livers of mice and human, and thus berberine might decrease the metabolism of other Cyp2d22 substrates.
Cyp3a11 and 2d22 in mice are homologs of human CYP3A4 and 2D6 (Inoue et al., 2011; Singh et al., 2009), respectively. These two major drug metabolizing enzymes catalyze the biotransformation of nearly 70% of drugs. Moreover, a hypo-active CYP2D6 exists in up to 10% of Caucasians and 1% of Asians (Ozdemir et al., 2007). In the present study, enzyme activities of both Cyp3a11 and 2d22 were decreased after a high dose of berberine (300 mg/kg) was administrated. Therefore, drug-drug interactions are of concern when high-dose berberine as well as substrate, inducer, or inhibitor of CYP2D6 and CYP3A4 are administered.
berberine is reported to have potential benefits to hyperlipidemic patients and moderate lipid metabolism though activation of PPARs (Zhang et al., 2011), which are important transcription factors for lipid metabolism in liver. However, the reports are not consistent. In 3T3-L1 adipocytes, berberine was a PPARα and γ dual inhibitor, and suppressed differentiation of adipocytes (Huang et al., 2006). In white adipose tissue of diabetic rats, as well as RNA-interference targeted cyclin dependent kinase 9 treated 3T3-L1 cells, berberine increased the expression of PPARs, which is associated with its hypoglycemic and hypolipidemic effects (Zhou and Zhou, 2010). In type 2 diabetic hamsters, berberine increased the mRNA levels of liver X receptor α and PPARα, and decreased mRNA levels of Sterol Regulatory Element Binding Proteins (Liu et al., 2010). However, data from the current study indicated that berberine did not alter PPARα mRNA or its target gene Cyp4a14 in C57BL/6 mice. The difference in models and dosages used in these studies may explain the discrepancy of the results.
In summary, the present study indicates that repeated administration of lower doses of berberine (10, 30, and 100 mg/kg/day) for 14 days does not affect the mRNA expression of major Cyps and related nuclear receptors. Moreover, enzyme activities correlated well with mRNA expression. Thus, berberine at these doses would not likely result in drug-drug interactions mediated by major Cyps if the data are extrapolatable from mice to humans. However, a high dose of berberine (300 mg/kg) inhibited the mRNA expression of Cyp3a11 and Cyp3a25 mRNA expression as well as Cyp3a11 and Cyp2d22 enzyme activity. In contrast, mRNA expressions of CAR, HNF1α, PPARα, Cyp2a4, 2b10, 2c37, 2e1, 2f2, 2j5, and Cyp4a14, as well as enzyme activities of Cyp2a4, 1a1/2, 2b10 and Cyp2c29 were not changed even after a high dose of berberine. Therefore, the present dose-response study in mice suggests that potential drug-drug interactions are unlikely at low doses of berberine, but need to be considered when high doses of berberine or berberine-containing products are used.
Acknowledgments
The authors thank all members of Dr. Klaassen’s laboratory for technical support, tissue collection, and reviewing the manuscript. This work was supported by the following grants: National Institute of Health (ES-009649, ES-019487, DK-081461, and RR021940); National Scientific Foundation of China (No.30801421); Hunan Provincial Innovation Foundation for Postgraduate (No. 2009bsxt020); Huge Project to Boost Chinese Drug Development (No.2009ZX09501-032); 863 Projects (No.2009AA022710, 2009AA022703, 2009AA022704)
Abbreviation List
- AhR
Aryl hydrocarbon receptor
- CAR
constitutive androstane receptor
- CYP
Cytochrome P450
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- HLM
Human liver microsomes
- HNF
Hepatocyte nuclear factor
- HPLC-MS
high-performance liquid chromatography-tandem mass spectrometry
- MDR1a
Multi-drug-resistance 1a
- MLM
mouse liver microsomes
- PPARα
Peroxisome proliferator-activated receptor alpha
- PXR
pregnane X receptor
- RT-PCR
reverse transcription-quantitative polymerase chain reaction
Footnotes
Conflict of interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attar M, Ling KH, Tang-Liu DD, Neamati N, Lee VH. Cytochrome P450 3A expression and activity in the rabbit lacrimal gland: glucocorticoid modulation and the impact on androgen metabolism. Investigative Ophthalmology and Visual Science. 2005;46:4697–4706. doi: 10.1167/iovs.05-0139. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- Bisaga A, Kos T, Wojcikowski J, Daniel WA, Popik P. Brain levels of dextromethorphan and the intensity of opioid withdrawal in mice. Drug and Alcohol Dependence. 2008;95:147–151. doi: 10.1016/j.drugalcdep.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Bullock P, Pearce R, Draper A, Podval J, Bracken W, Veltman J, Thomas P, Parkinson A. Induction of liver microsomal cytochrome P450 in cynomolgus monkeys. Drug Metabolism and Disposition. 1995;23:736–748. [PubMed] [Google Scholar]
- Claus SP, Ellero SL, Berger B, Krause L, Bruttin A, Molina J, Paris A, Want EJ, de Waziers I, Cloarec O, Richards SE, Wang Y, Dumas ME, Ross A, Rezzi S, Kochhar S, Van Bladeren P, Lindon JC, Holmes E, Nicholson JK. Colonization-induced host-gut microbial metabolic interaction. MBio. 2011;2 doi: 10.1128/mBio.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down MJ, Arkle S, Mills JJ. Regulation and induction of CYP3A11, CYP3A13 and CYP3A25 in C57BL/6J mouse liver. Archives of Biochemistry and Biophysics. 2007;457:105–110. doi: 10.1016/j.abb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Dvorak Z, Vrzal R. Berberine reduces insulin resistance: the roles for glucocorticoid receptor and aryl hydrocarbon receptor. Fertility and Sterility. 2011;95:e7. doi: 10.1016/j.fertnstert.2010.11.014. author reply e8–9. [DOI] [PubMed] [Google Scholar]
- Etheridge AS, Black SR, Patel PR, So J, Mathews JM. An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents. Planta Medica. 2007;73:731–741. doi: 10.1055/s-2007-981550. [DOI] [PubMed] [Google Scholar]
- Gao Z, Leng S, Lu F, Xie M, Xu L, Wang K. Effect of berberine on expression of hepatocyte nuclear factor-4alpha in rats with fructose-induced insulin resistance. Journal of Huazhong University of Science and Technology Medical Sciences. 2008;28:261–265. doi: 10.1007/s11596-008-0307-2. [DOI] [PubMed] [Google Scholar]
- Guo Y, Li F, Ma XC, Cheng XG, Zhou HH, Klaassen CD. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica. 2011 doi: 10.3109/00498254.2011.597456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clinical Pharmacology and Therapeutics. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Swain A, Hubbard MA, Hartsfield F, Thaden J, Williams DK, Gentry WB, Tong Y. Supplementation with goldenseal (Hydrastis canadensis), but not kava kava (Piper methysticum), inhibits human CYP3A activity in vivo. Clinical Pharmacology and Therapeutics. 2008;83:61–69. doi: 10.1038/sj.clpt.6100222. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemotherapy and Pharmacology. 2010;66:765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zhang Y, Gong Z, Sheng X, Li Z, Zhang W, Qin Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochemical and Biophysical Research Communications. 2006;348:571–578. doi: 10.1016/j.bbrc.2006.07.095. [DOI] [PubMed] [Google Scholar]
- Inoue S, Yoshinari K, Sugawara M, Yamazoe Y. Activated sterol regulatory element-binding protein-2 suppresses hepatocyte nuclear factor-4-mediated Cyp3a11 expression in mouse liver. Molecular Pharmacology. 2011;79:148–156. doi: 10.1124/mol.110.068577. [DOI] [PubMed] [Google Scholar]
- Jahnke GD, Price CJ, Marr MC, Myers CB, George JD. Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Research Part B, Developmental and Reproductive Toxicology. 2006;77:195–206. doi: 10.1002/bdrb.20075. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytotherapy Research. 2010;24:317–324. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]
- Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Molecular and Cellular Biology. 1999;19:6488–6499. doi: 10.1128/mcb.19.10.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Liu TY, Wu CW, Chi CW. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. British Journal of Cancer. 1999;81:416–422. doi: 10.1038/sj.bjc.6690710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li G, Zhu H, Huang L, Liu Y, Ma C, Qin C. Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRalpha and PPARalpha transcriptional programs. Endocrine Journal. 2010;57:881–893. doi: 10.1507/endocrj.k10e-043. [DOI] [PubMed] [Google Scholar]
- Ozdemir V, Bertilsson L, Miura J, Carpenter E, Reist C, Harper P, Widen J, Svensson JO, Albers LJ, Kennedy JL, Endrenyi L, Kalow W. CYP2D6 genotype in relation to perphenazine concentration and pituitary pharmacodynamic tissue sensitivity in Asians: CYP2D6-serotonin-dopamine crosstalk revisited. Pharmacogenetics and Genomics. 2007;17:339–347. doi: 10.1097/FPC.0b013e32801a3c10. [DOI] [PubMed] [Google Scholar]
- Paris BL, Ogilvie BW, Scheinkoenig JA, Ndikum-Moffor F, Gibson R, Parkinson A. In vitro inhibition and induction of human liver cytochrome p450 enzymes by milnacipran. Drug Metabolism and Disposition. 2009;37:2045–2054. doi: 10.1124/dmd.109.028274. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Leonard N, Draper A, Ogilvie BW. On the mechanism of hepatocarcinogenesis of benzodiazepines: evidence that diazepam and oxazepam are CYP2B inducers in rats, and both CYP2B and CYP4A inducers in mice. Drug Metabolism Reviews. 2006;38:235–259. doi: 10.1080/03602530600570081. [DOI] [PubMed] [Google Scholar]
- Seddon PJGaKR. Berberine. Alternative Medicine Review. 2000;5:175–177. [PubMed] [Google Scholar]
- Singh S, Singh K, Patel DK, Singh C, Nath C, Singh VK, Singh RK, Singh MP. The expression of CYP2D22, an ortholog of human CYP2D6, in mouse striatum and its modulation in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease phenotype and nicotine-mediated neuroprotection. Rejuvenation Research. 2009;12:185–197. doi: 10.1089/rej.2009.0850. [DOI] [PubMed] [Google Scholar]
- Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Usmani KA, Rose RL, Hodgson E. Inhibition and activation of the human liver microsomal and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metabolism and Disposition. 2003;31:384–391. doi: 10.1124/dmd.31.4.384. [DOI] [PubMed] [Google Scholar]
- Vrzal R, Zdarilova A, Ulrichova J, Blaha L, Giesy JP, Dvorak Z. Activation of the aryl hydrocarbon receptor by berberine in HepG2 and H4IIE cells: Biphasic effect on CYP1A1. Biochemical Pharmacology. 2005;70:925–936. doi: 10.1016/j.bcp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opinion on Investigational Drugs. 2010;19:1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Archives of Biochemistry and Biophysics. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- Xin HW, Wu XC, Li Q, Yu AR, Zhong MY, Liu YY. The effects of berberine on the pharmacokinetics of cyclosporin A in healthy volunteers. Methods and Findings in Experimental and Clinical Pharmacology. 2006;28:25–29. doi: 10.1358/mf.2006.28.1.962774. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Cai L, Zhong G, Luo W. Simultaneous determination of jatrorrhizine, palmatine, berberine, and obacunone in Phellodendri Amurensis Cortex by RP-HPLC. Zhongguo Zhong Yao Za Zhi (China Journal of Chinese Materia Medica) 2010;35:2061–2064. [PubMed] [Google Scholar]
- Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, Sun X, Sun Q, Xiang H, Wang H. Berberine Moderates Glucose and Lipid Metabolism through Multipathway Mechanism. Evidence-Based Complementary and Alternative Medicine. 2011 doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, Ren G, Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. Journal of Clinical Endocrinology and Metabolism. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhou S. Berberine regulates peroxisome proliferator-activated receptors and positive transcription elongation factor b expression in diabetic adipocytes. European Journal of Pharmacology. 2010;649:390–397. doi: 10.1016/j.ejphar.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Zhou JY, Zhou SW. Effect of berberine on PPARalpha/delta/gamma expression in type 2 diabetic rat retinae. Yao Xue Xue Bao (Acta Pharmaceutica Sinica) 2007;42:1243–1249. [PubMed] [Google Scholar]
- Zhou JY, Zhou SW, Zhang KB, Tang JL, Guang LX, Ying Y, Xu Y, Zhang L, Li DD. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biological and Pharmaceutical Bulletin. 2008;31:1169–1176. doi: 10.1248/bpb.31.1169. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Liu JP, Lai XS. Substrate specificity, inhibitors and regulation of human cytochrome P450 2D6 and implications in drug development. Current Medicinal Chemistry. 2009;16:2661–2805. doi: 10.2174/092986709788681985. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Wang B, Yang LP, Liu JP. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metabolism Reviews. 2010;42:268–354. doi: 10.3109/03602530903286476. [DOI] [PubMed] [Google Scholar]