Abstract
Synchronized oscillatory neuronal activity in the beta frequency range has been observed in the basal ganglia of Parkinson’s disease patients and hypothesized to be antikinetic. The unilaterally lesioned rat model of Parkinson’s disease allows examination of this hypothesis by direct comparison of beta activity in basal ganglia output in non-lesioned and dopamine cell lesioned hemispheres during motor activity. Bilateral substantia nigra pars reticulata (SNpr) recordings of units and local field potentials (LFP) were obtained with EMG activity from the scapularis muscle in control and unilaterally nigrostriatal lesioned rats trained to walk on a rotary treadmill. After left hemispheric lesion, rats had difficulty walking contraversive on the treadmill but could walk in the ipsiversive direction. During inattentive rest, SNpr LFP power in the 12–25 Hz range (low beta) was significantly greater in the dopamine-depleted hemisphere than in non-lesioned and control hemispheres. During walking, low beta power was reduced in all hemispheres, while 25–40 Hz (high beta) activity was selectively increased in the lesioned hemisphere. High beta power increases were reduced by L-DOPA administration. SNpr spiking was significantly more synchronized with SNpr low beta LFP oscillations during rest and high beta LFP oscillations during walking in the dopamine-depleted hemispheres compared with non-lesioned hemispheres. Data show that dopamine loss is associated with opposing changes in low and high beta range SNpr activity during rest and walk and suggest that increased synchronization of high beta activity in SNpr output from the lesioned hemisphere during walking may contribute to gait impairment in the hemiparkinsonian rat.
Keywords: Parkinson’s disease, basal ganglia, substantia nigra pars reticulata, beta frequency, local field potentials, gait, synchronization, dopamine, movement, 6-hydroxydopamine
INTRODUCTION
Oscillatory neuronal activity in the 12–30 Hz beta frequency range is prominent in recordings from the subthalamic nucleus (STN) and internal segment of the globus pallidus (GPi) in Parkinson’s disease (PD) patients undergoing implantation of deep brain stimulation (DBS) electrodes. The presence of these patterns in STN and GPi recordings has lead to the hypotheses that excessive beta range activity contributes to the symptomatology of PD and is antikinetic in nature. This view is supported by evidence that beta activity in local field potential (LFP) recordings is reduced in the STN and GPi of PD patients during movement or following dopaminergic therapies (Alegre et al., 2005; Alonso-Frech et al., 2006; Brown, 2003, 2007; Brown et al., 2001; Brown and Williams, 2005; Cassidy et al., 2002; Doyle et al., 2005; Foffani et al., 2005; Kempf et al., 2007; Kuhn et al., 2004; Levy et al., 2000, 2002; Marsden et al., 2001; Priori et al., 2002, 2004; Weinberger et al., 2006; Williams et al., 2003). Additional studies in PD patients have explored relationships between STN beta range activity and PD symptoms, showing that L-DOPA’s therapeutic effects on bradykinesia and rigidity, but not tremor, correlate with reduction of STN peak LFP power within the 8–35 Hz range (Bronte-Stewart et al., 2009; Foffani et al., 2005; Kuhn et al., 2009; Priori et al., 2004; Ray et al., 2008; for review, see Brown and Eusebio, 2008).
Collectively, these observations have provided incentive to determine the extent to which animal models of PD show similar changes in beta activity in the basal ganglia in conjunction with loss of dopamine and motor dysfunction. Such models allow direct comparisons of synchronized activity in basal ganglia circuitry in control and dopamine-depleted subjects over a range of behaviors, and permit insight into the origins and consequences of increased synchronization in basal ganglia output in the beta range.
To date, studies in the rodent model of PD have supported the hypothesis that loss of dopamine has a significant effect on synchronization and oscillatory activity in basal ganglia circuits. Marked increases are observed in the incidence of oscillatory activity in the ~1 Hz range in basal ganglia spike trains recorded throughout the lesioned hemisphere of systemically anesthetized rats with unilateral dopamine cell lesions. This activity is coherent with the ~1 Hz oscillatory firing patterns dominant in the cortex of the anesthetized rat, and phase relationship data show that loss of dopamine dramatically affects the extent to which the 1 Hz cortical oscillations entrain activity throughout the basal ganglia and modify basal ganglia output (Belluscio et al., 2003; Kasanetz et al., 2002; Magill et al., 2001; Mallet et al., 2006; Murer et al., 2002; Parr-Brownlie et al., 2007, 2009; Tseng et al., 2001b; Walters and Bergstrom, 2009; Walters et al., 2007; Zold et al., 2007). Increased beta frequency activity has also been observed in the STN and the external segment of the globus pallidus (GPe) of the anesthetized hemiparkinsonian rats during periods of desynchronized cortical activity (Mallet et al., 2008a, 2008b). In awake immobilized, locally anesthetized rats increases in oscillatory activity in the 4–30 Hz range in the entopedunclular nucleus (EPN, the rat homolog of the GPi), GPe and STN in the dopamine cell lesioned hemisphere have been reported (Ruskin et al., 2002; Tierney et al., 2003; Walters et al., 2009). Enhanced synchronization between GPe and STN (Mallet et al., 2008a; Tierney et al., 2003; Walters et al., 2009) and between STN and cortex (Sharott et al., 2005) in beta frequencies has also been observed in the dopamine-depleted hemisphere.
The present study was undertaken to determine whether expression of beta range activity is increased in basal ganglia output from the substantia nigra pars reticulata (SNpr) in the rodent model of PD, as observed in STN and GPi of PD patients, and whether beta activity is differentially expressed during rest, during episodes of walking on a circular treadmill and after L-DOPA treatment. The SNpr nucleus, through its main projection sites (the motor and parafascicular nuclei of the thalamus, the superior colliculus and the pedunculopontine nucleus) is thought to play a role in behaviors affected in the unilateral rodent model of PD, such as motor activity, orientation, axial posture and gait (Anderson and Yoshida, 1977; Arnt and Scheel-Kruger, 1979; Aziz et al., 1998; Bentivoglio et al., 1979; Burbaud et al., 1998; Deniau and Chevalier, 1992; DiChiara et al., 1977; Henderson et al., 2005; Herkenham, 1979; Hikosaka and Wurtz, 1983; Lestienne and Caillier, 1986; Takakusaki et al., 2003; Wichmann et al., 2001). For these studies, LFP and unit activity were recorded bilaterally from chronically implanted electrodes in the SNpr nuclei of hemiparkinsonian rats. Unilateral or bilateral SNpr recordings were also conducted in neurologically intact (control) rats. SNpr activity was recorded while rats were at rest and while walking on a circular treadmill. Electromyographic (EMG) activity was recorded bilaterally from the scapularis muscles to confirm motor activity.
MATERIALS AND METHODS
All experimental procedures were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the NINDS Animal Care and Use Committee. Every effort was made to minimize the number of animals used and their discomfort.
Subjects
Male Long Evans rats (Taconic Farm, Rockville, MD, USA), weighing 250–300 g), were housed with ad libitum access to chow and water in environmentally controlled conditions with a reversed 12:12 h light:dark cycle (lights on at 1800 h).
Behavioral Paradigm
A circular rotating treadmill was custom built by the NIMH Engineering Department, NIH (Fig. 1). A round plexiglass bowl, with an outer 20 cm diameter and an inner cylinder wall of 4.7 cm diameter, was secured to a moving platform (Fig. 1). The speed of rotation was set at 9 rpm, a speed compatible with a steady walking pace for the rat, and a stationary paddle placed behind the rat gently encouraged walking.
Figure 1.
Photograph of the circular treadmill. Rats were trained to walk in the treadmill at a rate of 9 rpm in ipsiversive and contraversive directions. During recordings, rats would walk for 5 min in the rotating treadmill and then rest in the stationary treadmill for 10–20 min between walking epochs.
Rats were trained during a 2 week period prior to surgery for a total of 5 sessions and during recovery for 1 session. In each training session, rats were acclimated to the circular treadmill and then encouraged to walk for 5 min in either ipsiversive or contraversive directions with 10 min rest periods between walking epochs for a total of 2 cycles in each direction.
Surgical Procedures
Rats (n=24) were anesthetized with 75 mg/kg ketamine (i.p. with a 38 mg/kg supplemental dose) and 0.5 mg/kg medetomidine (i.p.). The incision area was shaved and a long acting local anesthetic (1% mepivacaine HCl solution) injected along the intended incision lines. Ophthalmic ointment was applied to prevent corneal dehydration and lidocaine gel placed in the ear canals. Rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) fitted with atraumatic earbars with their skull leveled in the dorsal-ventral plane. A heating pad was used to maintain body temperature at ~37°C. Unilateral lesion of the nigrostriatal pathway and implantation of recording and EMG electrodes were performed during the same surgery.
After completion of surgical procedures, atipamezole (0.3–0.5 mg/kg, i.p.) was administered to reverse the anesthetic effect of medetomidine. During the first week of postoperative recovery, the rat’s diet was supplemented with fruit and gelatin. Rats were trained for the step test (see below) from the first post-operative day, and retrained at the rotating treadmill walking task from the fifth post-operative day.
Unilateral lesion of the nigrostriatal pathway
During the surgical procedure to implant recording electrodes, 16 animals were given an unilateral intracerebral injection of 6-hydroxydopamine (6-OHDA) to destroy the dopaminergic nigrostriatal pathway. The 6-OHDA injection was preceded by administration of 15 mg/kg desmethylimipramine (i.p.) to protect noradrenergic neurons. Standard stereotaxic procedures were used to target the left medial forebrain bundle: anterior 4.4 mm from the lambdoid suture, lateral +1.2 mm from the sagittal suture and ventral 8.3 mm from the skull surface. Six µg of 6-OHDA HBr in 3 µl of 0.9% saline with 0.01% ascorbic acid was infused via a 30 gauge stainless steel cannula into the medial forebrain bundle at a rate of 1 µl/min over 3 min via a syringe pump (Harvard Apparatus, Holliston, MA, USA). The cannula remained at the target site for 3 min after the infusion was completed to prevent diffusion of the neurotoxin.
The efficacy of the 6-OHDA injection was assessed behaviorally after 5–7 days using the step test (Olsson et al., 1995; Schallert and Tillerson, 1999). In the step test, rats were held with their torso, hindlimbs, and one forelimb supported by the investigator above the surface of the tabletop, so that the remaining forelimb was in contact with the table. Rats were then moved laterally across a total distance of ~90 cm in 6s with the torso moving towards the free forelimb, and then with the torso moving away from the free forelimb on the table surface. This was repeated with the opposite forelimb in contact with the table. This procedure was performed 3 times on the test date and the total number of steps taken by the limb contralateral to the lesion was compared to the total number taken by the ipsilateral limb. Only rats that demonstrated a strong unilateral motor deficit as revealed by the step test (total number of steps by contralateral limb < 5% of the total number of steps taken by ipsilateral limb) were used for electrophysiological recordings. The extent of dopamine cell degeneration was assessed post mortem using immunohistochemistry for tyrosine hydroxylase (TH) (see below).
Recording electrode placement
Holes were drilled over the target coordinates for the SNpr: anterior 3.0 mm from the lambdoid suture, lateral ± 2.2 mm from the sagittal suture and ventral 8.0 mm from the skull surface. The first group of 18 rats was implanted in the left and right SNpr with electrode bundles consisting of 8 stainless steel insulated microwires, with an additional scraped 9th wire for reference; a bundle had an initial diameter of ~350 µm (NB Labs, Denison, TX, USA). Electrode bundles were secured to the skull with screws and dental cement. Ground wires from each of the two bundles were connected to a screw located above the cerebellum (from bregma, posterior 11.0 mm and lateral −1.5 mm). In a second group of rats (n=6) electrode arrays were implanted bilaterally in the SNpr. The arrays consisted of 8 stainless steel microwires, plus a 9th wire with insulation removed for ~1.5 mm at the tip (NB Labs), arranged in a 3 × 3 matrix with ~200 µm between wires and with the 9th reference wire at one corner of the array. Microwire arrays allowed assessment of LFPs between individual recording electrodes spaced ~200 to 600 µm apart. For both electrode configurations, recording wires had impedances of ~0.6 MΩ, measured in saline at 135 Hz, and 9th wires had impedances of ~0.4 MΩ. Data presented below were obtained from experiments with bundle electrodes unless stated otherwise.
Implantation of EMG electrodes
All rats were chronically implanted with EMG electrodes inserted bilaterally into the scapularis muscle. Bipolar electrodes were made as described by Loeb and Gans (1986) with 2 stranded stainless steel wires coated with Teflon (Cooner Wire, Chatsworth, CA, USA) and inserted into the scapular head of the deltoid by the pull-through method (Hyland and Reynolds, 1993) and secured to the muscle by sutures. Sites of EMG implantations were verified and electrode integrity was checked after recordings were completed.
Unit, Local Field Potential (LFP) and EMG Recordings
Seven days post lesion, spikes and LFPs were recorded extracellularly from rats with electrodes chronically implanted into the SNpr. In rats with 6-OHDA-induced dopamine cell lesions, SNpr neurons were recorded from both ipsilateral (lesioned) and contralateral (non-lesioned) hemispheres (15/16 rats). In neurologically intact (control rats), SNpr neurons were recorded from one hemisphere (6/8 rats) or from both hemispheres (2/8 rats). Recordings were also performed 21 days post lesion.
To determine the effects of dopamine replacement on the expression of LFP power during walk and rest episodes, hemiparkinsonian rats were administered L-DOPA (4–5 mg/kg with 15 mg/kg of the dopa decarboxylase inhibitor benserazide, i.p.) 21 days after lesion (6/16 rats) following baseline recordings.
All recordings were conducted in freely moving rats. Action potentials were amplified (1000×), band pass filtered (154–9000 Hz) and monitored on oscilloscopes (Hewlett-Packard, Palo Alto, CA, USA) and audiomonitors (Grass, West Warwick, RI, USA). LFPs were amplified (1000×) and band pass filtered (3–170 Hz) (Plexon Inc., Dallas, TX, USA). EMGs were amplified (1000×) and band pass filtered (100 Hz-1 kHz). Spikes, LFPs and EMG were recorded at 40 kHz, 1 kHz, and 1 kHz, respectively (Cambridge Electronic Design (CED), Cambridge, UK). Spikes and LFPs were referenced to the 9th wire in the SNpr from the same bundle or array. Discriminated spike signals and LFPs were collected using Plexon and Power1401 (CED) data acquisition systems. Signals were digitized, stored and analyzed using Spike2. Spikes were sorted off-line using the Spike 2 template algorithm and principal component analysis.
Histology
Histological verification of recording sites and dopamine cell lesion
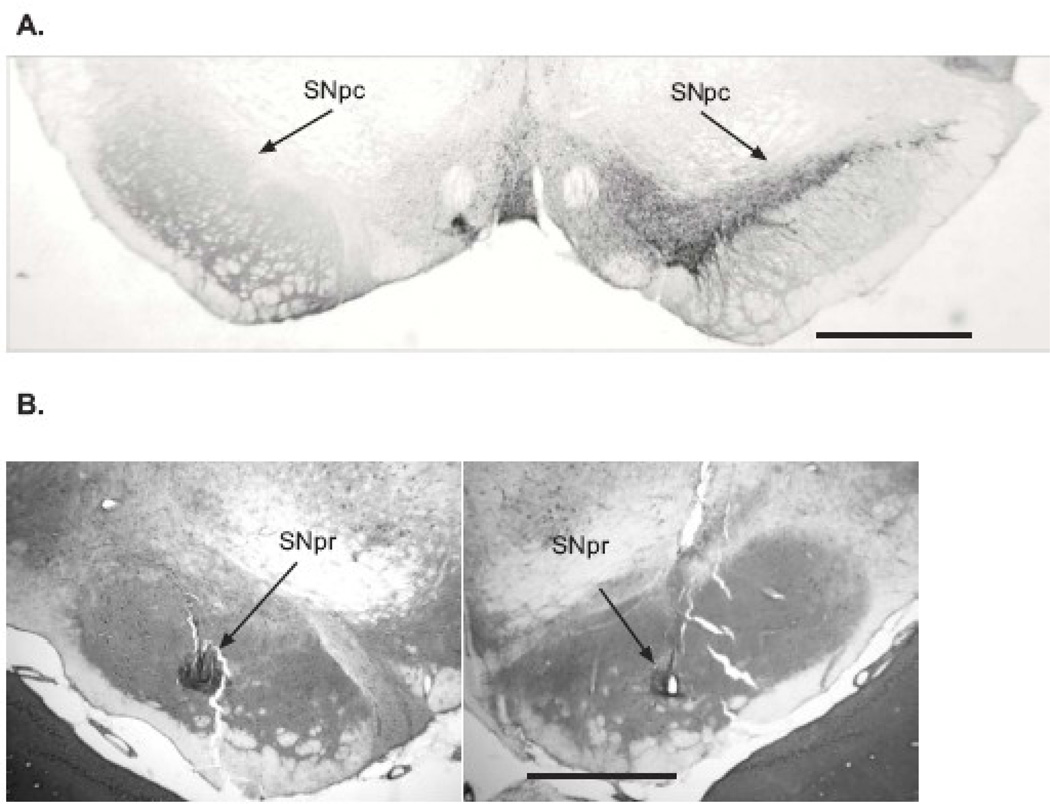
After completion of recordings, rats were anesthetized with urethane (1.6 g/kg, i.p.) and recording sites marked. A positive current of 10 µA was passed for 10 s through 4 of the microwires to mark the placement of the recordings. Rats were perfused intracardially with 200 mL cold saline followed by 200 mL 4% paraformaldehyde in phosphate buffer solution (PBS). Brains were post-fixed in paraformaldehyde solution overnight and then immersed in 20% sucrose in phosphate buffered saline (0.1 M, pH 7.4). Coronal sections of 40 µm were collected in PBS. Sections used for electrode placement localization were mounted on glass slides and stained with cresyl violet and 5% potassium ferricyanide/9% HCl to verify recording sites. Only recordings from electrodes confirmed to be in the SNpr were used for analyses (Fig. 2).
Figure 2.
Immunohistochemical results and electrode microwire placement. (A) Coronal section of the substantia nigra pars compacta (SNpc) illustrating immunohistochemistry of tyrosine hydroxylase (TH). Note the lack of TH staining in the SNpc of the lesioned (left) hemisphere compared with the non-lesioned (right) hemisphere. Location of section: bregma = −5.04 mm (Paxinos and Watson, 1986). Mean loss of TH+ stained = 86%, range = 76–97%. (B) Coronal section of the substantia nigra pars reticulata (SNpr) illustrating microwire placement. Sections were stained with cresyl violet and potassium ferricyanide to reveal the iron deposits; recording sites are indicated by arrows. Note the small electrolytic lesion clearly evident in the right section. Location of section: left figure, bregma = −5.02 mm and right figure, bregma = −5.28 mm (Paxinos and Watson, 1986). Scale bars: 1 mm
Tyrosine hydroxylase immunohistochemistry
The extent of the 6-OHDA lesion was examined quantitatively in the substantia nigra by staining for TH in the group of lesioned rats (n=10) (Fig. 2). Brain sections were washed 3 times in PBS (0.01 M, pH 7.4) prior to incubation with rabbit polyclonal anti-TH antibody (1:200 dilution) for 12–18 h at room temperature, with moderate agitation. Sections were rinsed 3 times in PBS and incubated with secondary antibody biotinylated anti-rabbit IgG (1:200 dilution). After 1 h of incubation, sections were rinsed three times in PBS and incubated with avidin-biotin-perioxidase complex for 60 min. Sections were rinsed in 50 mM Tris (pH 7.4) and reacted with 0.05% 3,3’-diaminobenzidine tetrahydrochloride and 0.01% H2O2 for 2–10 min, until intense staining. Sections were washed in 50 mM Tris then mounted on slides, dehydrated and prepared for light microscopy. TH+ soma were counted using the MicroBrightfield StereoInvestigator System (MBF, Inc., Williston, VT, USA). The optical fractionator method (Manfredsson et al., 2007) was used to quantitate the total number of cell bodies containing TH in four sections spaced through the substantia nigra, a distance of 120 µm between quantified sections, starting at −5.3 mm from bregma (Paxinos and Watson, 1986), under 40× magnification. Data are expressed as % of lesioned side compared to the contralateral non-lesioned side.
Data Analysis
Baseline (walk/rest cycles) spike train and LFP data were recorded for ~80 min. Rest periods, categorized as inattentive rest states, were defined by lack of EMG activity and videotaped records showing no activity. During these periods, rats were awake but not attentive to their environment; care was taken to avoid environmental stimuli that would alert them. Epochs of 180 s representative of the overall recording and free of major artifacts were used to assess mean firing rates during rest and walk periods. For each rat, data from at least three spike trains per hemisphere were used. Epochs of 100 s, taken from within the 180 s epoch, were used for power analysis and spike-triggered waveform averages (STWA). For analysis of walk epochs, data were taken only from periods when rats were walking in the ipsiversive direction because rats with left hemispheric lesions experienced difficulty walking in the contraversive direction. To assess the effects of dopamine replacement, LFP power at time points 30 min after i.p. administration of L-DOPA was analyzed.
For analysis of power in the beta frequency range, LFP recordings were band pass filtered at 8–100 Hz using an IIR filter (Spike2) and smoothed to 250 Hz. LFP power was analyzed by fast Fourier transform (FFT) with a frequency resolution of ~1 Hz. Total power values in 12–25 Hz (low beta) and 25–40 Hz (high beta) frequency ranges were calculated. Data from four resting periods and two walking periods were averaged. LFP power was also analyzed in the 6–12 Hz range from unfiltered LFPs smoothed to 250 Hz.
To assess relationships between spikes and LFPs, STWAs in the 6–12 and 12–25 Hz range were generated from LFPs smoothed to 250 Hz and then band pass filtered at 6–12 or 12–25 Hz using an FIR filter (Spike2). STWAs in the 25–40 Hz range were generated from LFPs band pass filtered at 25–40 Hz using a FIR filter (Spike2). In addition, the order of the interspike intervals from these epochs was randomized (using a Spike2 script) to provide a second set of scrambled spike trains and STWAs were also generated from the scrambled data. Amplitudes of the peak-to-trough values at or around zero time were compared for STWAs generated from scrambled and unscrambled spike trains. STWAs were calculated 0.15 s before and after the spike trigger over a 100 s epoch. SNpr spike trains from faster firing neurons were selected from the non-lesioned and lesioned hemispheres of 5 rats for these analyses. Each selected spike train was compared during both rest and walk epochs for both beta frequency ranges and the interspike intervals from the same epochs were randomized for comparative analyses. Two to three spike trains per hemisphere were analyzed, representing ~65% of all cells recorded.
To visualize spectral power changes over time, time frequency scalograms were constructed from continuous wavelet transform of the LFP data using 128 Morlet wavelets with 512 points (Time-Frequency Toolbox; http://tftb.nongnu.org) with Matlab 7.1.0 (The MathWorks, Natick, MA, USA). Data sets of 240 s epochs were downsampled to 250 Hz. Spectral power was plotted on a log10 scale with greater power represented by redder colors and with the threshold set at zero. Color axes were scaled so that the power levels with a given scalogram spanned the full color range and kept consistent between comparisons.
Data are reported as mean ± standard error of the mean (SEM). Total power and spike-triggered waveform data were evaluated statistically with repeated 2-way ANOVA with Student Newman-Keuls post hoc tests. Firing rate data were analyzed using a combination of two-tailed grouped and paired t-tests (corrected for multiple comparisons). Statistical analysis was performed using SyStat (SyStat Software, San Jose, CA, USA). The criterion of significance was p< 0.05.
Materials
Atipamezole HCl (Antisedan) was purchased from Pfizer Orion Pharma, New York, NY, USA; benserazide, desmethylimipramine HCl, L-DOPA, potassium hexacyanoferrate, urethane and 6-OHDA HBr from Sigma-Aldrich Co., St. Louis, MO, USA; ketamine (Ketaved) from Vedco, St. Joseph, MO, USA; medetomidine HCl (Domitor) from Pfizer Animal, Exton, PA, USA; mepivacaine (Polocaine) from AstraZeneca LP, Wilmington, DE, USA. The ocular lubricant Lacrilube was purchased from Allergan Pharmaceuticals, Irvine, CA, USA, lidocaine gel from Teva Pharmaceuticals USA, Sellersville, PA, USA and dental acrylic cement (Ortho-Jet Liquid) from Lang Dental Mfg. Co., Inc, Wheeling, IL, USA.
For histology and immunohistochemistry, the following reagents were used: cresyl violet solution and phosphate buffered 4% paraformaldehyde solution from FD Neurotechnologies, Baltimore, MD, USA; rabbit polyclonal anti-tyrosine hydroxylase antibody from Pel-Freeze Biologicals, Rogers, AR, USA; biotinylated anti-rabbit IgG, avidin-biotin-peroxidase complex (ABC) and 3,3’-diaminobenzidine tetrahydrochloride (DAB kit) from Vector Laboratories, Burlingame, CA, USA; Tris (Trizma) and potassium hexacyanoferrate (III) from Sigma; Histoclear from National Diagnostics, Atlanta, GA, USA; Permount from Fisher Scientific, Pittsburgh, PA, USA.
RESULTS
Effect of 6-OHDA-Induced Dopamine Cell Lesion on Step Test and TH Loss
One week post 6-OHDA infusion, dopamine cell lesion was associated with a marked unilateral deficit in motor function as assessed by the step test (Olsson et al., 1995). For the rats used in the present study, the total number of adjusting steps made by the contralateral limb ranged from 0 to 1.5% and averaged 0.4% of those made by the ipsilateral limb (33.3 ± 3.0 mean total steps taken by the ipsilateral limb per test), reflecting marked depletion of dopamine in the ipsilateral striatum (Parr-Brownlie et al., 2007; Schallert and Tillerson, 1999; Tseng et al., 2005).
Dopamine cell loss was further confirmed by TH immunohistochemical staining; a significant mean loss of TH+ neurons (mean 86% loss, range 76–97% as compared with non-lesioned) was observed in the substantia nigra pars compacta ipsilateral to the 6-OHDA infusion (Fig. 2).
Effect of 6-OHDA-Induced Dopamine Cell Lesion on Walking in a Circular Treadmill
Neurologically intact rats readily learned to walk on a circular treadmill rotating at a rate of 9 rpm in ipsiversive and contraversive directions (Fig. 1). After left hemispheric dopamine cell lesions, rats consistently showed difficulty in walking on the treadmill at the target speed of 9 rpm if their impaired side, contralateral to the lesion, was on the inside of the circular path, i.e. during contraversive epochs. Lesioned rats tended to frequently stop, rear or turn around during walking episodes in the contraversive direction, exhibiting problems with the adjustment of posture, orientation and gait required for contraversive turns.
However, lesioned rats maintained pace with the treadmill at 9 rpm walking in the ipsiversive direction, when their intact side was on the inside of the circular path. This allowed comparison of firing rate and LFP beta frequency activity in lesioned and non-lesioned hemispheres of the lesioned rats and in control rats during periods of comparable behavior, i.e. rest and walking at 9 rpm on the treadmill. Videotaped behavior and EMG recordings were used to identify epochs of rest and walk behavior.
Changes in SNpr LFP Power in Two Beta Frequency Ranges during Rest and Walk
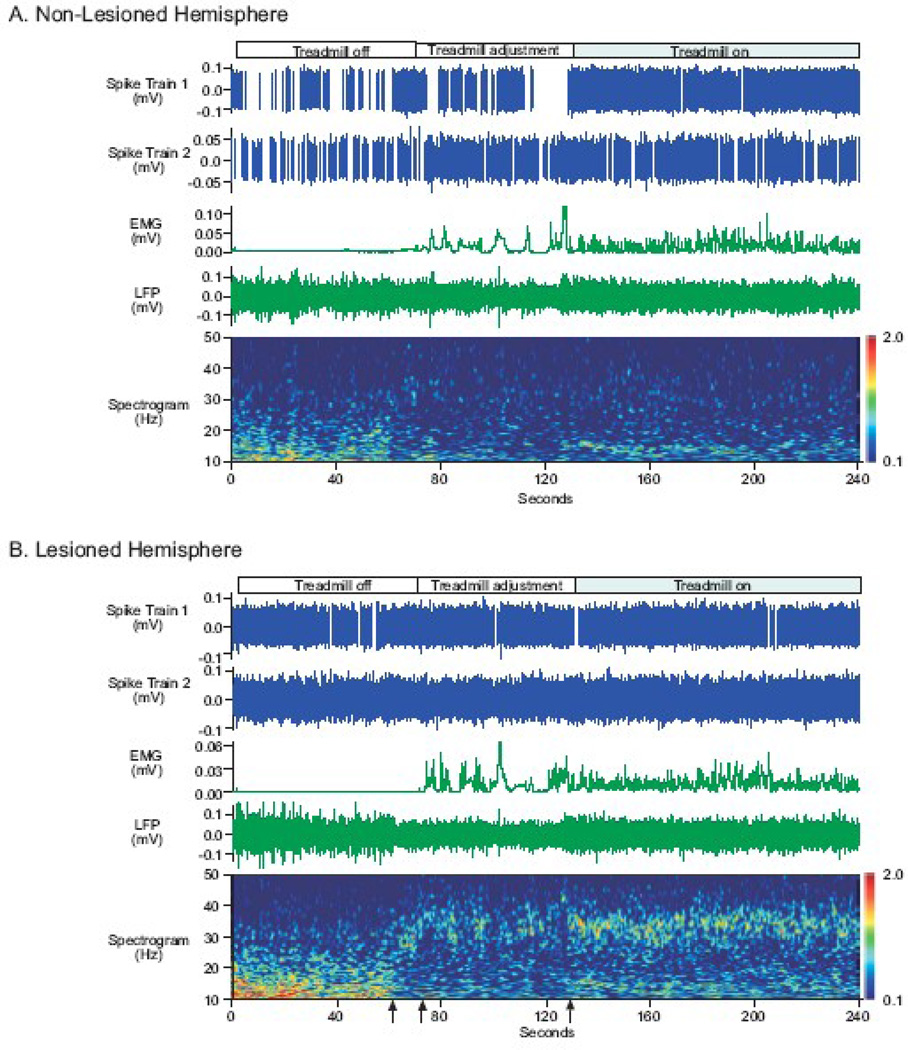
Initial inspection of wavelet-based scalograms of SNpr LFP power from the dopamine cell lesioned hemisphere showed opposite changes in LFP power in two beta frequency ranges during rest and walk epochs (Fig. 3). Beta frequency activity in the 12–25 Hz range (low beta) was affected differently from beta frequency activity in the 25–40 Hz (high beta) range in the lesioned hemisphere during rest and walk epochs. Activity in the low beta range was prominent in SNpr LFPs recorded from the lesioned hemisphere during inattentive rest and was significantly reduced during walk epochs as illustrated in the wavelet scalogram of LFP power in Figure 3. Strikingly, a distinct band of higher frequency beta activity in the range of 27–34 Hz emerged in the SNpr LFP from the lesioned hemisphere when the rat was walking. This was noted only in the lesioned hemisphere, as depicted in the plots of SNpr LFP activity in Figure 3B and not in equivalent plots obtained from the non-lesioned hemisphere (Fig. 3A) or control rats (data not shown). During the transition from inattentive rest to treadmill walking, a drop in low beta power and an increase in high beta power in the lesioned hemisphere were typically observed when the rat became alert as the experimenter approached the treadmill (Fig. 3B). Brief bursts of high beta power were evident while adjustments were made to position the rat in the desired walking direction and the treadmill was turned on. More consistent high beta power was evident during steady walking on the treadmill.
Figure 3.
SNpr spike train and LFP recordings during rest and walk on a circular treadmill from a unilaterally dopamine cell lesioned rat. Representative spike trains (blue) and LFPs (green) were recorded simultaneously from the SNpr of the non-lesioned (A) and lesioned (B) hemispheres during rest (treadmill stationary), preparation to walk (treadmill adjustment) and walking (treadmill on). EMGs (green, middle panels) were also recorded simultaneously from the scapularis muscles; the EMGs contralateral to the SNpr recording site are presented accordingly. EMG activity and video taped behavioral analyses directed the selection of epochs to analyze for rest and walk epochs. Wavelet scalograms (bottom panels) represent the time-frequency plots of spectral power. Spectral power was plotted on a log10 scale with greater power represented by redder colors.
SNpr LFP low beta power during rest was greater than low beta power during walking, as illustrated in the scalograms from the non-lesioned and lesioned hemispheres. Notably, SNpr LFP low beta power in the lesioned hemisphere was greater than low beta power in the non-lesioned hemisphere (before the first arrow on the time axis in bottom panel). When the experimenter approached the treadmill, thus alerting the rat, a change from low beta frequency power to high beta frequency (25–40 Hz) power in the lesioned hemisphere (first arrow) was observed. When the treadmill motor was turned on (second arrow) and adjustments to the rat’s posture were made to position him in the correct walking direction, high beta power appeared more prominently in the lesioned hemisphere relative to the non-lesioned hemisphere. With the onset of walking (third arrow), a prominent band of high beta SNpr LFP activity emerged in the lesioned hemisphere.
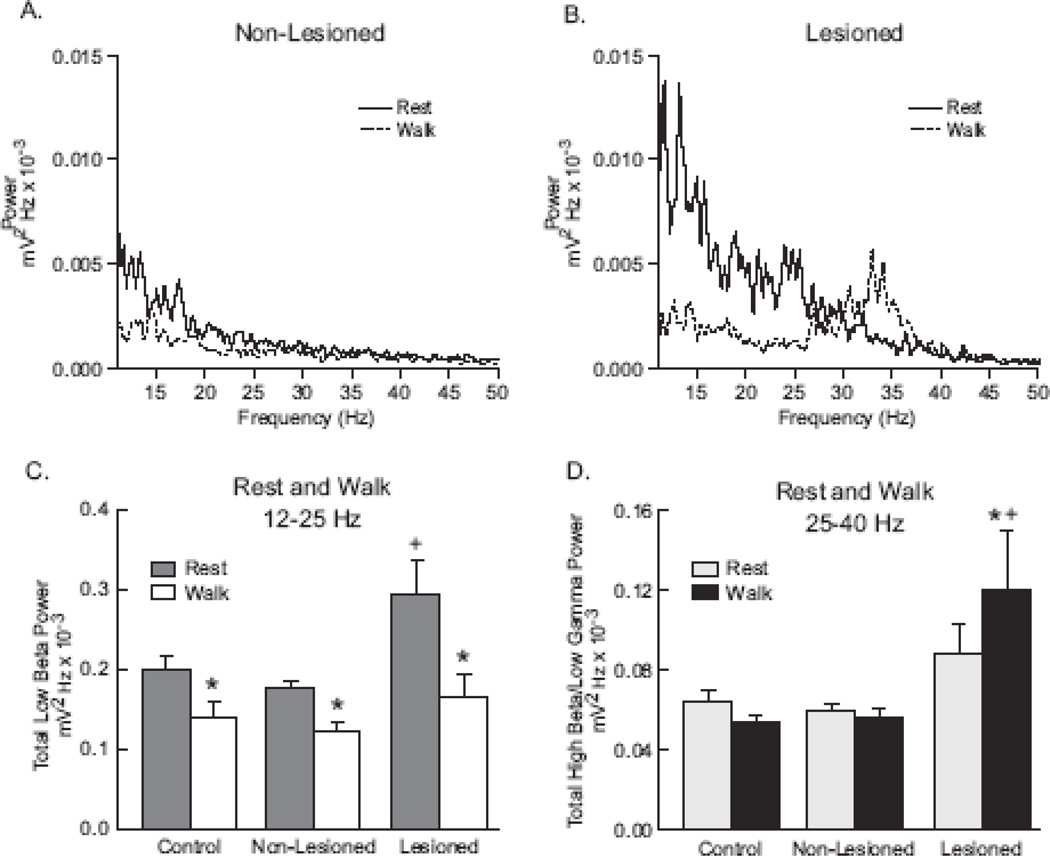
To further evaluate and quantify SNpr LFP oscillatory activity in these two frequency ranges, FFT power spectra were used to obtain total LFP power in the 12–25 Hz and 25–40 Hz ranges in the control, non-lesioned and lesioned hemispheres during rest and ipsiversive walk epochs (Fig. 4).
Figure 4.
SNpr LFP power in the beta frequency range recorded in the non-lesioned and lesioned hemispheres of the unilateral dopamine cell lesioned rat 7 days following 6-OHDA infusion and from neurologically intact (control) rats. Power spectra (A, B) are representative examples from SNpr LFP activity in the non-lesioned (A) and lesioned (B) hemispheres of an individual rat over the frequency range of 11–50 Hz. SNpr LFP activity was greater in the low beta frequency range (12–25 Hz) in both non-lesioned and lesioned hemispheres at rest (solid line) than during walking (dotted line). During walking, a prominent peak of high beta activity emerged in the lesioned hemisphere (B); this was not observed in the non-lesioned (A) or control (data not shown) hemispheres. Total power in the frequency ranges of low beta (12–25 Hz) and high beta (25–40 Hz) are presented (C, D) as the mean ± SEM for LFP recordings in the SNpr of 8 neurologically intact (control) hemispheres and 9–10 non-lesioned and lesioned hemispheres during rest and walk epochs. In the low beta frequency range (C), SNpr LFP power was significantly greater in the lesioned hemispheres compared with SNpr LFP power in the non-lesioned or control hemispheres when the animals were at rest. SNpr LFP power was significantly reduced when the animals were walking compared with epochs of rest in all 3 hemispheric states. There were no significant differences between the SNpr LFP power in the 3 hemispheres during walking. In the high beta frequency range (D), there was no significant differences in SNpr LFP power during rest among the 3 hemispheres. However, during walking high beta SNpr LFP power was significantly increased in the lesioned hemispheres.
* p<0.05 significantly different than rest within hemispheric condition
+ p<0.05 significantly different than control and non-lesioned hemispheres for rest in low beta frequency and walk in high beta frequency
Low beta frequency activity in SNpr LFPs
Spectral analysis was used to quantify SNpr oscillatory activity in the low beta range (12–25 Hz) during rest epochs in control rats and unilaterally lesioned rats in the lesioned and non-lesioned hemispheres. Results showed that low beta range SNpr LFP power was significantly increased in the lesioned hemisphere during inattentive rest as compared with SNpr LFP power in the non-lesioned and control hemispheres (Fig. 4; p=0.005, p=0.007, respectively). A significant increase of 57 ± 25 % in total SNpr power was observed in the lesioned hemisphere, compared with power in the control hemisphere. Total SNpr LFP power in non-lesioned hemispheres was not significantly different than control hemispheres. During inattentive rest, the increase in beta LFP power observed in the lesioned hemisphere did not form a well-defined peak, but was evident in a broad band below ~20 Hz. Although beta frequency activity was the focus of the present study, it was further noted that during inattentive rest, SNpr LFP power in the 6–12 Hz range showed a strong trend towards greater power in the lesioned hemisphere (p=0.059; data not shown).
Consistent with observations from recordings in the STN of PD patients, LFP power in the 12–25 Hz range in recordings from the SNpr in the hemiparkinsonian rats was significantly reduced during movement, relative to rest. During ipsiversive walk epochs, low beta total power in the lesioned hemisphere was reduced by 40 ± 7 % relative to rest in the 12–25 Hz range in SNpr LFP (Figs. 3 and 4C; p<0.001). Interestingly, significant decreases in low beta power were also observed in the non-lesioned and control hemispheres during ipsiversive walk, relative to rest; low beta power was reduced 30 ± 5 % in the non-lesioned hemispheres and 25 ± 12% in the control hemispheres (Fig. 4; p=0.043, p=0.033, respectively). Collectively, the decreases in low beta power in the lesioned, non-lesioned and control hemispheres resulted in no significant difference between hemispheres in SNpr low beta LFP power during ipsiversive walk. LFP power in the 6–12 Hz range was not significantly different between non-lesioned and lesioned hemispheres during ipsiversive walk epochs. Decreases in SNpr LFP power were also noted relative to inattentive rest epochs in all 3 hemispheric conditions during contraversive walk epochs, when the lesioned rats exhibited difficulty keeping up with the treadmill in a forward direction and tended to stop and rear (data not shown).
High beta frequency activity in SNpr LFPs
As viewed in scalograms (Fig. 3), decreases in low beta frequency activity during walk epochs were associated with the selective emergence in the lesioned hemisphere of a prominent band of SNpr LFP activity in the high beta range of 25–40 Hz, with peaks in the 27–34 Hz range (Figs. 3 and 4). FFT analysis confirmed that there was a significant increase (31 ± 11%; p<0.025) in high beta SNpr LFP power in the lesioned hemisphere during walk epochs as compared with inattentive rest epochs (Fig. 4D). No significant changes in SNpr LFP high beta power were observed in the non-lesioned and control hemispheres during walk epochs, compared with rest epochs. High beta power did not differ between hemispheres during inattentive rest epochs.
Effect of dopamine replacement on high beta frequency activity in SNpr LFPs
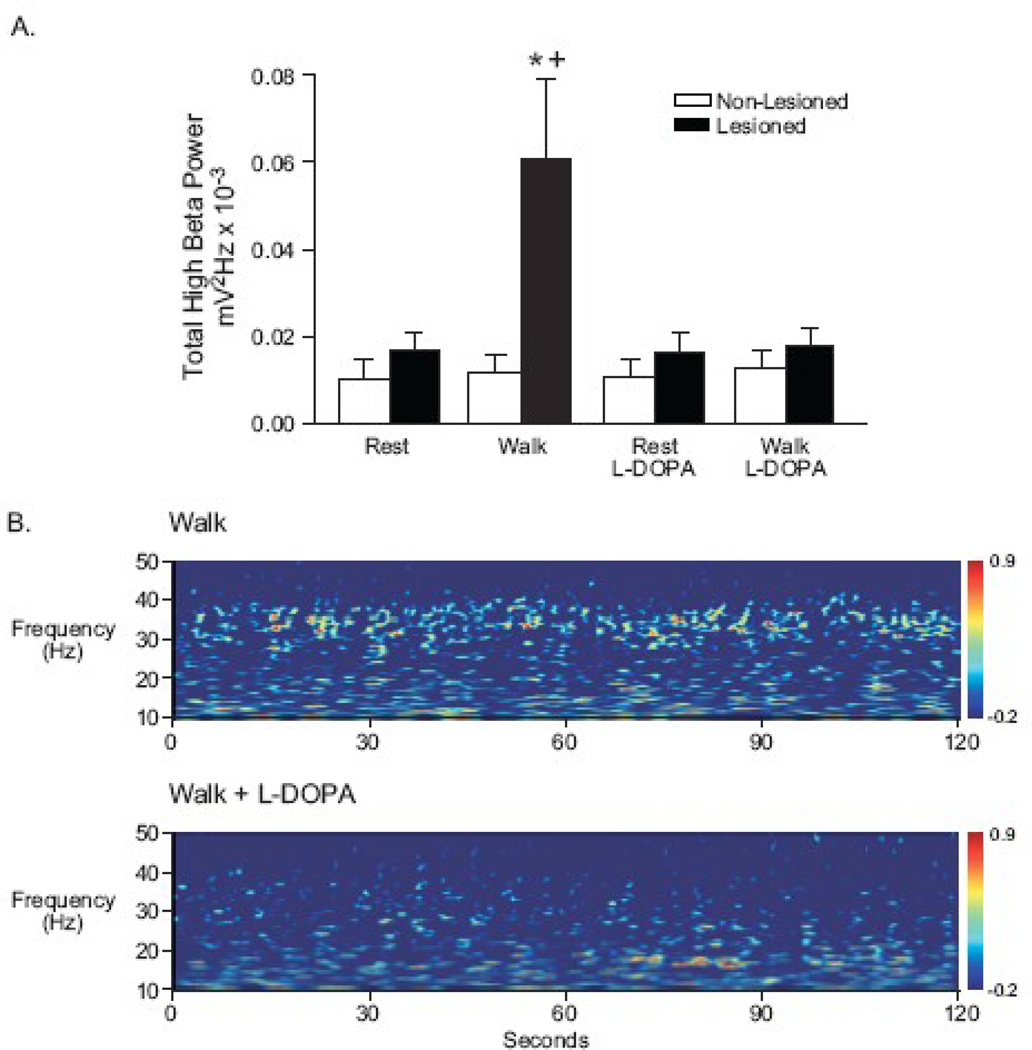
To confirm the results presented above and examine the effect of L-DOPA treatment on high beta frequency activity during rest/walk epochs, an additional set of 6 unilaterally lesioned rats was implanted with 3 × 3 electrode arrays bilaterally in the SNpr. The array configuration allowed assessment of LFPs between pairs of wires spaced ~200 µm to ~ 600 µm, as well as between individual wires and the 9th wire located within the SNpr, providing additional support for LFP activity being generated locally in the SNpr in the beta frequency range. SNpr LFP data obtained with electrode arrays were consistent with data obtained with electrodes bundles, as described above. Recordings with electrode arrays at 7 days (data not shown) and 21 days post lesion (Fig. 5) showed that high beta SNpr LFP power in the lesioned hemisphere was significantly increased during ipsiversive walking epochs, relative to SNpr LFP activity in the non-lesioned hemisphere (p<0.001). This increased power in the 25–40 Hz range observed in the lesioned hemisphere during walk epochs was also significantly greater than that observed in the lesioned hemisphere during rest epochs (p<0.001).
Figure 5.
LFP power in the high beta frequency range (25–40 Hz) recorded with electrode arrays placed bilaterally in the SNpr of the lesioned and non-lesioned hemispheres of unilateral dopamine cell lesioned rats. To confirm the results obtained with recordings with electrode bundles and observe the effects of dopamine replacement therapy, experiments were conducted with this electrode configuration in rats 21 days post lesion (n=6 rats). During walk epochs, SNpr LFP power in the high beta range was significantly increased in the lesioned hemispheres, but not in the non-lesioned hemispheres (A). L-DOPA administration (4–5 mg/kg plus 15 mg/kg benserazide, i.p., a dose that improves gait but does not induce rotation) significantly reversed the elevated SNpr LFP power. No significant differences were observed between rest and walk with and without L-DOPA in the non-lesioned hemispheres. Wavelet scalograms (B) illustrate the effect of dopamine replacement with L-DOPA on SNpr LFP high beta frequency activity in the lesioned hemisphere of an individual rat. Before L-DOPA administration, notable LFP power in the 25–40 Hz range is evident during ipsiversive walk epochs (B, top). Following L-DOPA administration (~ 30 min post i.p. injection), LFP power in significantly reduced during ipsiversive walk epochs (B, bottom). Wavelet scalograms represent the time-frequency plots of spectral power with spectral power plotted on a log10 scale with greater power represented by redder colors.
* p<0.05 significantly different than walk in non-lesioned hemispheres
+ p<0.05 significantly different than rest, rest plus L-DOPA, and walk plus L-DOPA in lesioned hemispheres
To determine the effects of dopamine replacement therapy on the expression of high beta SNpr LFP power during ipsiversive walk episodes, a low dose of L-DOPA (4–5 mg/kg, i.p.) that did not induce rotational behavior was administered to this subset of hemiparkinsonian rats 21 days post lesion. L-DOPA effectively reduced high beta activity during walk epochs to rest levels in the lesioned hemisphere (Fig. 5A; n=6 rats; p<0.001), but had no significant effect on LFP power in the non-lesioned hemisphere in this frequency range during walk or rest epochs. Wavelet scalograms illustrate the marked L-DOPA-induced reduction in high beta frequency activity in the lesioned hemisphere of an individual rat during an ipsiversive walk epoch (Fig. 5B).
SNpr Spiking Activity and its Relationship to LFP Activity
To assess whether increases in LFP power in beta frequency activity during rest and walk epochs reflected changes in local SNpr firing patterns, mean firing rates and relationships between LFP power and SNpr firing pattern were compared in recordings from both hemispheres of unilaterally lesioned rats.
SNpr firing rates
Seven days post lesion, mean SNpr firing rates in the lesioned hemisphere obtained from both bundle and array electrodes recordings were not significantly different from firing rates in the non-lesioned hemisphere (17.7 ± 2.4 Hz, n=61 spike trains, 16 rats for lesioned hemisphere and 13.6 ± 1.6 Hz, n=50 spikes trains, 15 rats for non-lesioned hemisphere) when rats were at rest or walking (data not shown). However, at 21 days post lesion, firing rates in the lesioned hemisphere were significantly greater than firing rates in the non-lesioned hemisphere when rats were at rest (21.7 ± 2.6 Hz, n=61 spike trains, 16 rats for lesioned hemisphere and 11.5 ± 1.4 Hz, n=54 spikes trains, 15 rats for non-lesioned hemisphere; p= 0.0004) or walking (data not shown; p = 0.020).
The relationship between SNpr firing rates and walking was also assessed. SNpr firing rates during walk epochs were significantly greater than rates during rest epochs in both nonlesioned (p = 0.006) and lesioned (p = 0.001) hemispheres. Mean increases of 21 ± 6 % in the non-lesioned hemisphere and 14 ± 4% in the lesioned hemisphere were observed during walk epochs.
SNpr spike – LFP relationships in low beta and high beta frequency ranges
To determine whether changes observed in SNpr LFP beta activity following loss of dopamine were correlated with changes in firing patterns in the SNpr, SNpr spike/LFP relationships were examined. Spike-triggered waveform averages (STWAs) were generated from epochs of rest and walk using LFPs filtered for activity in either the 6–12, 12–25 Hz or 25–40 Hz range. Interspike intervals from time periods used to generate the STWAs were scrambled to provide a measure of LFP relationships with randomized spiking and then STWAs derived from unscrambled spike trains were normalized by data obtained with scrambled spike trains.
Results from these STWA analyses showed that during rest SNpr spiking activity was more correlated with low 12–25 Hz beta activity in the lesioned hemisphere than in the non-lesioned hemisphere (Figs. 6A and 6C). The mean amplitude of scramble-normalized STWAs in the low beta range from the lesioned hemisphere was significantly greater than that from the non-lesioned hemisphere during rest (p < 0.001). No significant difference was observed in mean amplitudes of scramble-normalized STWAs in the low 12–25 Hz beta range between hemispheres during walk epochs (Figs. 6B and 6C). Scramble-normalized STWAs in the 6–12 Hz range were also significantly greater in the lesioned hemisphere relative to the non-lesioned hemisphere during rest and not significantly different during walk epochs (p = 0.0001; data not shown).
Figure 6.
Phase locking in the SNpr of lesioned and non-lesioned hemispheres of unilateral dopamine cell lesioned rats. Representative examples of individual rats are shown in A, B, D and E. Group results are shown in C and F. (A) SNpr spike trains recorded in the lesioned hemispheres showed increased phase locking of spikes when paired with SNpr LFP in the 12–25 Hz low beta range during rest (solid line) compared with scrambled spikes (dashed line). No phase locking of SNpr spikes with SNpr LFP was observed during walking in the 12–25 Hz range (B). (C) Normalized SNpr spike-triggered SNpr LFP waveform averages (STWA) were obtained by dividing unscrambled STWA peak to trough amplitude values by scrambled STWA amplitude values. Dashed line indicates a ratio = 1, where STWA amplitudes from scrambled and unscrambled are equal. In the low beta frequency range normalized STWAs from the lesioned hemispheres were significantly greater than STWAs from non-lesioned hemispheres during rest. In contrast, during walking, there was no significant difference between the STWAs from lesioned and non-lesioned hemispheres. (D) In the 25–40 Hz high beta range, SNpr spike trains recorded in the lesioned hemispheres demonstrated no significant phase locking of spikes with SNpr LFPs during rest. (E) However, during walking, a marked increase in phase locking compared with scrambled spikes paired with LFPs was demonstrated. (F) In the high beta frequency range normalized STWAs from the lesioned hemispheres were significantly greater than STWAs from the non-lesioned hemispheres during walking. In contrast, during rest, there was no significant difference between the STWAs from lesioned and non-lesioned hemispheres. Data in bar graphs represent 2–3 spike trains per hemisphere from 5 rats.
* p<0.05 significantly different than non-lesioned
+ p<0.05 significantly different than walk for low beta frequencies and rest in high beta frequencies in lesioned hemispheres
For the high beta frequency range (25–40 Hz), the reverse relationship was observed in STWA ratios during walk and rest epochs. During walking the mean amplitude of scramble-normalized STWAs in the high beta range was significantly greater in the lesioned hemisphere than in the non-lesioned hemisphere (Figs. 6E and 6F; p < 0.001). During rest, no significant difference between hemispheres was observed (Figs. 6D and 6F).
DISCUSSION
In the present study, recordings from electrodes implanted bilaterally in the SNpr of unilaterally lesioned rats show that loss of dopamine is associated with increased LFP power and spiking activity in the beta frequency range. This increase is specifically focused in a high beta frequency range during epochs when the rat is walking on a circular treadmill and most evident in a low beta frequency range during periods of inattentive rest. The increase in high beta power in the SNpr in the lesioned hemisphere during treadmill walking is significantly attenuated after L-DOPA administration, consistent with observations from PD patients showing that beta activity in STN and GPi LFPs in the 8–35 Hz range is reduced following L-DOPA treatment. These findings add to the accumulating evidence that loss of dopamine selectively enhances expression of synchronized and oscillatory activity in the basal ganglia in the dopamine-depleted hemisphere (Alegre et al., 2005; Alonso-Frech et al., 2006; Bergman et al., 1994, 1998; Brown, 2003, 2007; Brown et al., 2001; Cassidy et al., 2002; Chen et al., 2007; Costa et al., 2006; Doyle et al., 2005; Filion, 1979; Foffani et al., 2005; Gatev and Wichmann, 2009; Goldberg et al., 2004; Heimer et al., 2002; Kuhn et al., 2004; Leblois et al., 2006, 2007; Levy et al., 2000, 2001, 2002; Magill et al., 2001; Mallet et al., 2008a, 2008b; Murer et al., 2002; Nini et al., 1995; Parr- Brownlie et al., 2007, 2009; Priori et al., 2002, 2004; Raz et al., 2000, 2001; Sharott et al., 2005; Tseng et al., 2001b; Walters et al., 2007, 2009; Weinberger et al., 2006; Wichmann et al., 1994; Williams et al., 2002, 2003). Moreover, the novel observation that neuronal activity in the high beta range is prominent in the SNpr of the dopamine-depleted hemisphere during episodes of treadmill walking adds support to the view that increased beta synchronization in basal ganglia output may play a role in the motor impairment associated with loss of dopamine.
Beta Frequency Modulation by Loss of Dopamine and Movement
Low beta frequency power
Low beta activity in SNpr LFPs was most prominent during inattentive rest epochs and occupied a broad band below 20 Hz. Although low beta activity was evident in the SNpr LFPs in control, non-lesioned and lesioned hemispheres during rest, low beta total power was significantly greater in the lesioned hemisphere, relative to the non-lesioned hemisphere or the intact hemisphere from control rats and there was a trend for increased power in the 6–12 Hz range in the lesioned hemisphere. Low beta power was reduced in both lesioned and non-lesioned, and control hemispheres when the rats’ behavior switched from rest to walk, although the reduction in the lesioned hemisphere was ~15% greater.
The present study also demonstrates that the increase in low beta power in the SNpr LFP of the dopamine-depleted hemisphere is associated with an increase in phase-locked SNpr spiking activity in the low beta range. Spike-triggered waveforms show evidence of significantly greater phase-locking between spikes and LFPs in the low 12–25 Hz beta range in the lesioned hemisphere during rest epochs, as well as in the 6–12 Hz range, relative to the non-lesioned hemisphere and relative to walk epochs. These results provide evidence for local generation of increases in LFP power observed in the SNpr recordings in the lesioned hemisphere during inattentive rest and confirm that SNpr output is more synchronized under these conditions following dopamine cell loss. Increased spike-LFP synchrony as assessed with STWAs has also provided evidence for local generation of beta frequency activity in STN LFPs recorded in PD patients (Kuhn et al., 2005; Weinberger et al., 2006).
High beta frequency power
The transition from inattentive rest to alert rest and treadmill walking was associated with a decline in low beta power and the selective emergence of a high beta band in the lesioned hemisphere. Brief bursts of high beta power were evident as the rat was alerted by the experimenter approaching the treadmill and positioning the rat in the desired walking direction. More consistent high beta power, focused in the 27–34 Hz range, was evident during steady walking on the treadmill. Increases in LFP power in this range were not evident in the non-lesioned or control hemispheres during walking, nor were they evident during walk epochs after L-DOPA treatment. Similarly, increases in spike-LFP phase-locking in the high beta range during walk epochs were only observed in the dopamine cell lesioned hemisphere.
The association between loss of dopamine, increased SNpr beta activity and impaired movement in the hemiparkinsonian rat is consistent with correlations between increased basal ganglia beta synchronization and L-DOPA-sensitive motor dysfunction observed in PD patients. The rodent data, therefore, lend support to the general hypothesis that beta synchronization exerts a dysfunctional effect on motor circuits modulated by basal ganglia output. Nevertheless, the limitations to such a comparison should be pointed out. First, there does not seem to be a precedent for the emergence of a beta band in a higher frequency range with the transition from rest to movement in STN recordings from PD patients. However, it could be argued that the inattentive rest state of the rat is not a good parallel for the more alert state of the PD patient waiting for a movement cue. Second, patient studies lend themselves to more fine-tuned assessment of the immediate effect of movement cues and motor planning and execution on beta range STN LFP activity during the ‘off’ state than is readily feasible in rodents. In recordings from PD patients, movements are typically associated with decreases in beta power, although increases in beta activity are noted before movement, in conjunction with the movement cues and preparation, and during rebound after movement (Alegre et al., 2005; Doyle et al., 2005; Foffani et al., 2005; Kempf et al., 2007; Kuhn et al., 2004; Levy et al., 2002; Priori et al., 2002; Williams et al., 2003, 2005). The experimental design of this rodent study, on the other hand, does not permit as precise assessment of the timing of muscle activity once the animal is aroused from the inattentive rest state. However, rodent studies benefit from the possibility of direct comparisons between non-lesioned and lesioned hemispheres during complex and continuous activity such as treadmill walking. The rodent data presented here highlight the dramatic impact of the loss of dopamine neurons on synchronization of basal ganglia output in the beta range during walking.
The increase in high beta frequency observed in the SNpr in the dopamine cell lesioned hemisphere does not appear to be a rodent correlate of the increased activity in the 60–80 Hz gamma frequency band observed in the STN of PD patients (Alegre et al., 2005; Alonso-Frech et al., 2006; Androulidakis et al., 2007; Brown et al., 2001; Cassidy et al., 2002; Foffani et al., 2005; Fogelson et al., 2005; Kempf et al., 2007; Pogosyan et al., 2006; Priori et al., 2004; Trottenberg et al., 2006; Williams et al., 2002). The gamma range activity noted in the STN of PD patients is reported to be most evident after L-DOPA treatment and during movement and hypothesized to be prokinetic (for review, see Brown, 2003). In contrast, the high beta band observed in the present study was concentrated in a lower frequency range and was not prominent in the SNpr of control or non-lesioned hemispheres during movement. Moreover, its intensity in the lesioned hemisphere was significantly reduced by L-DOPA. Gamma range activity in the 45–80 Hz range has been observed in the basal ganglia, in particular in the STN and striatum of active normal rats (Berke, 2009; Brown et al., 2002; Sharott et al., 2009; Tort et al., 2008; van der Meer and Redish, 2009). In this study a modest increase in SNpr LFP power in the 45–60 Hz range was variably observed during walking, relative to rest, but notable peaks were not evident in the 60–80 Hz range in SNpr LFPs (unpublished observations).
Firing Pattern and Rate Changes with Movement and Dopamine Loss
The changes in SNpr firing patterns observed after dopamine cell lesion in the present study are consistent with the noisy signal hypothesis originally proposed by Marsden and Obeso (Marsden and Obeso, 1994; for review, see Brown and Eusebio, 2008), suggesting that increased synchronized and oscillatory activity disrupts the normal patterns of basal ganglia activity and contributes to the movement disorders of PD. Several mechanisms have been proposed for how loss of dopamine may induce changes in basal ganglia network function that promote increased beta frequency activity and enhanced beta frequency synchrony. The observations of increases in LFP power in the SNpr over a broad range of frequencies in the dopamine cell lesioned hemisphere,-the 1 Hz range during anesthesia, 6–12 Hz and 12–25 Hz ranges during inattentive rest and the 25–40 Hz range during walking, indicate that mechanisms mediating increased synchronization are not limited to a single frequency range and support the broad view that loss of filtering in basal ganglia processing of cortical input plays an important role in the emergence of dysfunctional synchronization of activity in basal ganglia output. Studies in anesthetized rats show that cortical rhythms entrain activity more effectively in the striatum following loss of dopaminergic modulation of glutamatergic input (Mallet et al., 2006; Murer et al., 2002; Tseng et al., 2001b) and/or the loss of glutamatergic synapses (Day et al., 2006), providing increased transmission of cortical oscillations to downstream basal ganglia nuclei. Direct cortical input to the STN in conjunction with dopamine-induced changes in STN activity and oscillatory interaction between the STN and GPe (Bevan et al., 2002) may also contribute to entrainment of activity in basal ganglia output in beta frequencies. Evidence for a direct subthalamo-cortical loop has led to suggestions that this circuit may contribute to increased propagation of dysfunctional oscillatory activity (Degos et al., 2008).
Studies in both anesthetized and awake rats with dopamine cell lesions support the view that cortical activity contributes to the entrainment of beta activity in the STN and these investigations also provide evidence for STN-GPe interaction in this frequency range (Degos et al., 2009; Mallet et al., 2008a, 2008b; Sharott et al., 2005; Tierney et al., 2003; Walters et al., 2009). Sharott and coworkers (2005) observed beta range activity in LFP recordings from the STN in dopamine cell lesioned rats when rats are still but alert; this STN LFP activity was coherent with beta activity in cortical electrocorticograms. In addition, an increase in beta range frequency during periods of spontaneous activity, and significant decreases in beta power following apomorphine administration were reported. Paired GPe-STN single unit and LFP recordings in locally anesthetized, immobilized rats demonstrated that GPe spiking becomes significantly more synchronized with STN LFP oscillations in the beta frequency range and coherences between GPe spiking and STN LFP and between STN spiking and STN LFP are increased after dopamine cell lesion (Tierney et al., 2003; Walters et al., 2009). In addition, this study showed that increased dopamine receptor stimulation with apomorphine induces a desynchronizing effect on GPe-STN relationships in both intact and lesioned rats, supporting a role for increased synchronization between STN and GPe in the emergence of beta frequency activity in the STN. The present results are consistent with observations of increased beta activity in the STN and GPe, both areas projecting to SNpr.
In addition to changes in firing pattern, alterations in SNpr firing rates were also observed in the lesioned hemisphere in the present study. These rate changes are consistent with the classic rate-based model of basal ganglia function that hypothesizes that loss of dopamine induces firing rate increases in basal ganglia output (Albin et al., 1989; Alexander and Crutcher, 1990; DeLong, 1990), which inhibit thalamocortical activity and promote hypoactivity and akinesia via a reduction in cortical activity. Significant increases in mean rate were observed in the SNpr 21 days post lesion, consistent with predictions of the rate model. However, the literature regarding SNpr rate changes in the anesthetized rodent PD model is inconsistent, reporting observations of no changes, increases and decreases in firing rates between intact or the non-lesioned hemispheres and lesioned hemispheres of lesioned rats (Belluscio et al., 2003; Breit et al., 2005; Burbaud et al., 1995; Diaz et al., 2003; Lee et al., 2001; MacLeod et al., 1990; Murer et al., 1997, 2002; Rohlfs et al., 1997; Sanderson et al., 1986; Tai et al., 2003; Tseng et al., 2001a, 2005; Walters et al., 2007). MPTP treatment in monkeys also produced no significant change in SNpr firing rates (Wichmann et al., 1999).
Changes in firing rate were also observed in the present study during walking, relative to rest. Mean firing rates increased during walking, as has been observed by Chang et al. (2006). These results are not consistent with rate model predictions that increased activity, i.e. walking, would be associated with a reduction in SNpr firing rates: although a subset of individual neurons in both hemispheres showed significant rate decreases. Nevertheless, with regard to changes in firing rate in basal ganglia output associated with loss of dopamine, the present results are supportive of the traditional view that increases in firing rate in basal ganglia output correlate with hypoactivity. Overall, these results are consistent with the emerging view that changes in firing pattern, in addition to firing rate, must be considered in developing an understanding of relationships between basal ganglia output and movement.
Potential Consequences of Impaired SNpr Output
These results extend to the SNpr nucleus the observations obtained from PD patients, primates and rodents in the STN and GPi showing increases in beta frequency activity with loss of dopamine. The SNpr is implicated in control of movement, orientation and gait through its projections to thalamus, superior colliculus and pedunculopontine nucleus. Evidence for a selective increase in SNpr spiking in the lesioned hemisphere in the high beta range during walking raises the possibility that synchronized high beta activity in basal ganglia output disrupts downstream activity in the thalamus, superior colliculus and/or pedunculopontine nucleus, contributing to the reduced motor performance of the hemiparkinsonian rat in the circular treadmill, most evident when the direction of his orientation was toward the affected side, contralateral to the lesion.
Dopamine loss has been demonstrated to have robust effects on gait in rodents (Chang et al., 2006; Metz et al., 2005; Shi et al., 2004). As described by Chang and coworkers (2006), after unilateral dopamine depletion, when rats are walking on a straight treadmill, the healthy limbs make larger steps while the impaired limbs follow passively. Metz and colleagues (2005) have reported similar gait changes in unilaterally dopamine depleted rats: when walking in a straight line, lesioned rats drag their impaired toes and take shorter steps, similar to the shuffled steps observed in human PD patients (Knutsson, 1972). In the present study, after unilateral loss of dopamine, rats made progress walking in the ipsiversive direction on the circular treadmill, when the affected side was on the outside of the curved track, but the rats tended to stop, rear and turn around during walking in the contraversive direction, presumably because walking in this direction required the affected side to make more demanding adjustments of gait, orientation and posture. Interestingly, during attempts to get the rats to walk ipsiversively on the treadmill, high beta frequency activity in the SNpr LFP was notable and not obviously different from that observed during the contraversive walking epochs (data not shown).
As the SNpr is not a preferred site for implantation of DBS electrodes, there are relatively little data from PD patients from recordings in this nucleus. However, a recent study (Chastan et al., 2009) compared the effects of DBS of the STN with effects of DBS of the SNpr in cases where the lowest contacts of DBS electrodes targeted to the STN were located in the SNpr. Results showed that stimulation of the STN was more efficacious than stimulation of the SNpr with respect to PD motor symptoms except with respect to gait and balance where the effects were comparable. Interestingly, there is evidence for the potential for DBS of the pedunculopontine nucleus in cases of advanced PD to address symptoms of posture and gait that are relatively resistant to improvement with STN DBS (Jenkinson et al., 2006; Mazzone et al., 2005; Plaha and Gill, 2005). These observations support the view that changes in SNpr output in PD patients may have a functional impact on downstream sites involved in control of posture and gait.
SUMMARY
Results demonstrate that recordings in behaving rats can lend insight into how changes in basal ganglia network correlate with motor dysfunction after loss of dopamine. In sum, data from the hemiparkinsonian rat show that loss of dopamine is associated with increased expression of low beta frequency activity during rest, which is reduced with movement. Moreover, recordings from rodent SNpr demonstrate increased expression of high beta activity in basal ganglia output associated with transition from inattentive rest to alert and walking on a circular treadmill While it is not clear whether increases in high beta range activity in SNpr output coincide more with ongoing preparation for the next phase of the stepping cycle, some aspect of execution of that cycle, or beta rebound after execution of the movement, data are consistent with observations from STN/GPi LFP recordings in PD patients and support the correlation between increased beta activity and impaired movement. These findings raise interesting questions regarding the functional significance of increases in synchronized and oscillatory activity in different beta frequency bands with respect to activity in thalamocortical loops and midbrain regions, such as the pedunculopontine nucleus and provide a basis for further exploration of these issues. Such studies may allow insight into whether these changes in SNpr firing pattern are causative, compensatory or simply epiphenomenonal (Eusebio and Brown, 2009).
ACKNOWLEDGEMENTS
The Intramural Research Program of the NINDS, NIH supported this research. We would like to thank Stacey Poloskey, Kalynda Gonzales and Jaime Ahluwalia for assistance in these experiments, Dr. Tilman Rosales for wavelet programming consultation and Newlin Morgan, Tom Talbot and Daryl Bandy in the Research Services Branch for design and fabrication of the rotary treadmill.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alegre M, Alonso-Frech F, Rodriguez-Oroz M, Guridi J, Zamarbide I, Valencia M, Manrique M, Obeso J, Artieda J. Movement-related changes in oscillatory activity in the human nucleus: ipsilateral vs. contralateral movements. Eur. J. Neurosci. 2005;22:2315–2324. doi: 10.1111/j.1460-9568.2005.04409.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, Zamarbide I, Alegre M, Rodriguez-Oroz M, Guridi J, Manrique M, Valencia M, Artieda J, Obeso J. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129:1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Anderson M, Yoshida M. Electrophysiological evidence for branching nigral projections to thalamus and superior colliculus. Brain Res. 1977;137:361–364. doi: 10.1016/0006-8993(77)90347-x. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Kuhn AA, Chen CC, Blomstedt P, Kempf F, Kupsch A, Schneider G-H, Doyle L, Dowsey-Limousin P, Hariz MI, Brown P. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson's disease. Brain. 2007;130:457–468. doi: 10.1093/brain/awl358. [DOI] [PubMed] [Google Scholar]
- Arnt J, Scheel-Kruger J. Behavioral differences induced by muscimol selectively injected into pars compacta and pars reticulata of substantia nigra. Naunyn-Schmiede. Arch. Pharmacol. 1979;310:43–51. doi: 10.1007/BF00499873. [DOI] [PubMed] [Google Scholar]
- Aziz TZ, Davies L, Stein J, France S. The role of decending basal ganglia connections to the brain stem in Parkinsonian akinesia. Br. J. Neurosurg. 1998;12:245–249. doi: 10.1080/02688699845078. [DOI] [PubMed] [Google Scholar]
- Belluscio MA, Kasanetz F, Riquelme LA, Murer MG. Spreading of slow cortical rhythms to the basal ganglia output nuclei in rats with nigrostriatal lesions. Eur. J. Neurosci. 2003;17:1046–1052. doi: 10.1046/j.1460-9568.2003.02543.x. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Van der Kooy D, Kuypers HGJM. Organization of the efferent projections of the substantia nigra in the rat - retrograde fluorescent double labeling study. Brain Res. 1979;174:1–17. doi: 10.1016/0006-8993(79)90800-x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci. 2009;30:848–859. doi: 10.1111/j.1460-9568.2009.06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Breit S, Lessmann L, Benazzouz A, Schulz JB. Unilateral lesion of the pedunculopontine nucleus induces hyperactivity in the subthalamic nucleus and substantia nigra in the rat. Eur. J. Neurosci. 2005;22:2283–2294. doi: 10.1111/j.1460-9568.2005.04402.x. [DOI] [PubMed] [Google Scholar]
- Bronte-Stewart HM, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp. Neurol. 2009;215:20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov. Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Brown P, Eusebio A. Paradoxes of functional neurosurgery: clues from basal ganglia recordings. Mov. Disord. 2008;23:12–20. doi: 10.1002/mds.21796. [DOI] [PubMed] [Google Scholar]
- Brown P, Kupsch A, Magill PJ, Sharott A, Harnack D, Meissner W. Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp. Neurol. 2002;177:581–585. doi: 10.1006/exnr.2002.7984. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J. Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin. Neurophysiol. 2005;116:2510–2519. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Gross CE, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behavior by subthalamic lesion in 6-OHDA lesioned rats is associated with normalization of firing rate and discharge pattern of pars reticulata neurons. Exp. Brain Res. 1995;105:48–58. doi: 10.1007/BF00242181. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Bonnet B, Guehl D, Lagueny A, Bioulac B. Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey. Brain Res. 1998;78:102–107. doi: 10.1016/s0006-8993(97)01158-x. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ. Neural responses in multiple basal ganglia regions following unilateral dopamine depletion in behaving rats performing a treadmill locomotion task. Exp. Brain Res. 2006;172:193–207. doi: 10.1007/s00221-005-0312-7. [DOI] [PubMed] [Google Scholar]
- Chastan N, Westby GW, Yelnik J, Bardinet E, Do MC, Agid Y, Welter ML. Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson's disease. Brain. 2009;132:172–184. doi: 10.1093/brain/awn294. [DOI] [PubMed] [Google Scholar]
- Chen CC, Litvak V, Gilbertson T, Kuhn A, Lu CS, Lee ST, Tsai CH, Tisch S, Limousin P, Hariz M, Brown P. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson's disease. Exp. Neurol. 2007;205:214–221. doi: 10.1016/j.expneurol.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MAL. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Day M, Wang ZF, Ding J, An XH, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun ZX, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Degos B, Deniau J-M, Cam JL, Mailly P, Maurice N. Evidence for a direct subthalamo-cortical loop circuit in the rat. Eur. J. Neurosci. 2008;27:2599–2610. doi: 10.1111/j.1460-9568.2008.06229.x. [DOI] [PubMed] [Google Scholar]
- Degos B, Deniau J-M, Chavez M, Maurice N. Chronic but not acute dopaminergic transmission interruption promotes a progressive increase in cortical beta frequency synchronization: relationships to vigilance state and akinesia. Cere. Cortex. 2009;19:1616–1630. doi: 10.1093/cercor/bhn199. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience. 1992;46:361–377. doi: 10.1016/0306-4522(92)90058-a. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Barroso-Chinea P, Acevedo A, Gonzalez-Hernandez T. Effects of dopaminergic cell degeneration on electrophysiological characteristics and GAD65/GAD67 expression in the substantia nigra: different action on GABA cell subpopulations. Mov. Disord. 2003;18:254–266. doi: 10.1002/mds.10345. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Olianas M, Del Fiacco M, Spano PF, Tagliamonte A. Intranigral kainic acid is evidence that nigral non-dopaminergic neurones control posture. Nature. 1977;268:743–745. doi: 10.1038/268743a0. [DOI] [PubMed] [Google Scholar]
- Doyle LMF, Kuhn AA, Hariz M, Kupsch A, Schneider GH, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson's disease. Eur. J. Neurosci. 2005;21:1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Brown P. Synchronisation in the beta frequency-band - The bad boy of parkinsonism or an innocent bystander? Exp. Neurol. 2009;217:1–3. doi: 10.1016/j.expneurol.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M. Effects of interruption of the nigrostriatal pathway and of dopaminergic agents on the spontaneous activity of globus pallidus neurons in the awake monkey. Brain Res. 1979;178:425–441. doi: 10.1016/0006-8993(79)90704-2. [DOI] [PubMed] [Google Scholar]
- Foffani G, Bianchi A, Baselli G, Priori A. Movement-related frequency modulation of beta oscillatory activity in the human subthalamic nucleus. J. Physiol. 2005;568:699–711. doi: 10.1113/jphysiol.2005.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N, Pogosyan A, Kuhn AA, Kupsch A, van Bruggen G, Speelman H, Tijssen M, Quartarone A, Insola A, Mazzone P, Di Lazzaro V, Limousin P, Brown P. Reciprocal interactions between oscillatory activities of different frequencies in the subthalamic region of patients with Parkinson's disease. Eur. J. Neurosci. 2005;22:257–266. doi: 10.1111/j.1460-9568.2005.04179.x. [DOI] [PubMed] [Google Scholar]
- Gatev P, Wichmann T. Interactions between cortical rhythms and spiking activity of single basal ganglia neurons in the normal and parkinsonian state. Cereb. Cortex. 2009;19:1330–1344. doi: 10.1093/cercor/bhn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex-basal ganglia networks of parkinsonian primates reflects global dynamics of the local field potentials. J. Neurosci. 2004;24:6003–6010. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer G, Bar-Gad I, Goldberg JA, Bergman H. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of parkinsonism. J. Neurosci. 2002;22:7850–7855. doi: 10.1523/JNEUROSCI.22-18-07850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Stanic D, Tomas D, Patch J, Horne MK, Bourke D, Finkelstein DI. Postural changes after lesions of the substantia nigra pars reticulata in hemiparkinsonian monkeys. Behav. Brain Res. 2005;160:267–276. doi: 10.1016/j.bbr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J. Comp. Neurol. 1979;183:487–517. doi: 10.1002/cne.901830304. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata .4. Relation of substantia nigra to superior colliculus. J. Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN. Pattern of activity in muscles of shoulder and elbow during forelimb reaching in the rat. Human Mov. Sci. 1993;12:51–69. [Google Scholar]
- Jenkinson N, Nandi D, Oram R, Stein JF, Aziz TZ. Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. NeuroReport. 2006;17:639–641. doi: 10.1097/00001756-200604240-00016. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme LA, Murer MG. Disruption of the two-state membrane potential of striatal neurones during cortical desynchronisation in anaesthetised rats. J. Physiol. London. 2002;543:577–589. doi: 10.1113/jphysiol.2002.0024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf F, Kuhn AA, Kupsch A, Brucke C, Weise L, Schneider GH, Brown P. Premovement activities in the subthalamic area of patients with Parkinson's disease and their dependence on task. Eur. J. Neurosci. 2007;25:3137–3145. doi: 10.1111/j.1460-9568.2007.05536.x. [DOI] [PubMed] [Google Scholar]
- Knutsson E. Analysis of parkinsonian gait. Brain. 1972;95:475–486. doi: 10.1093/brain/95.3.475. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp. Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Trottenberg T, Kivi A, Kupsch A, Schneider G-H, Brown P. The relatinship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp. Neurol. 2005;194:212–220. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J. Neurosci. 2006;26:3567–3583. doi: 10.1523/JNEUROSCI.5050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive parkinsonism. Eur. J. Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- Lee JI, Shin HJ, Nam DH, Kim JS, Hong SC, Shin HJ, Park K, Eoh W, Kim JH, Lee WY. Increased burst firing in substantia nigra pars reticulata neurons and enhanced response to selective D2 agonist in hemiparkinsonian rats after repeated administration of apomorphine. J. Korean Med. Sci. 2001;16:636–642. doi: 10.3346/jkms.2001.16.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestienne F, Caillier P. Role of the monkey substantia nigra pars reticulata in orienting behaviour and visually triggered arm movements. Neurosci. Lett. 1986;64:109–115. doi: 10.1016/0304-3940(86)90672-5. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Levy R, Dostrovsky JO, Lang AE, Sime E, Hutchison WD, Lozano AM. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson's disease. J. Neurophysiol. 2001;86:249–260. doi: 10.1152/jn.2001.86.1.249. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J. Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago: The University of Chicago Press; 1986. Design and construction of electrodes; pp. 109–122. [Google Scholar]
- MacLeod NK, Ryman A, Arbuthnott GW. Electrophysiological properties of nigrothalamic neurons after 6-hydroxydopamine lesions in the rat. Neuroscience. 1990;38:447–456. doi: 10.1016/0306-4522(90)90041-2. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Marton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J. Neurosci. 2008a;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 2008b;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, Burger C, Sullivan LF, Muzyczka N, Lewin AS, Mandel RJ. rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson's disease. Exp. Neurol. 2007;207:289–301. doi: 10.1016/j.expneurol.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994;117:877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Limousin-Dowsey P, Ashby P, Pollak P, Brown P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson's disease. Brain. 2001;124:378–388. doi: 10.1093/brain/124.2.378. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. NeuroReport. 2005;16:1877–1881. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Metz GA, Tse A, Ballerman M, Smith LK, Fouad K. The unilateral 6-OHDA rat model of Parkinson's disease revisited: an electromyographic and behavioural analysis. Eur. J. Neurosci. 2005;22:735–744. doi: 10.1111/j.1460-9568.2005.04238.x. [DOI] [PubMed] [Google Scholar]
- Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 6-0HDA-lesioned rats: effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27:278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Murer MG, Tseng KY, Kasanetz F, Belluscio M, Riquelme LA. Brain oscillations, medium spiny neurons, and dopamine. Cell. Mol. Neurobiol. 2002;22:611–632. doi: 10.1023/A:1021840504342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J. Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Poloskey SL, Bergstrom DA, Walters JR. Parafascicular thalamic nucleus activity in a rat model of Parkinson's disease. Exp. Neurol. 2009;217:269–281. doi: 10.1016/j.expneurol.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Poloskey SL, Flanagan KK, Eisenhofer G, Bergstrom DA, Walters JR. Dopamine lesion-induced changes in subthalamic nucleus activity are not associated with alterations in firing rate or pattern in layer V neurons of the anterior cingulate cortex in anesthetized rats. Eur. J. Neurosci. 2007;26:1925–1939. doi: 10.1111/j.1460-9568.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando: Academic Press; 1986. [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. NeuroReport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Kuhn AA, Trottenberg T, Schneider GH, Kupsch A, Brown P. Elevations in local gamma activity are accompanied by changes in the firing rate and information coding capacity of neurons in the region of the subthalamic nucleus in Parkinson's disease. Exp. Neurol. 2006;202:271–279. doi: 10.1016/j.expneurol.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Bianchi A, Chiesa V, Baselli G, Caputo E, Tamma F, Rampini P, Egidi M, Locatelli M, Barbieri S, Scarlato G. Movement-related modulation of neural activity in human basal ganglia and its L-DOPA dependency: recordings from deep brain stimulation electrodes in patients with Parkinson's disease. Neurol. Sci. 2002;23:S101–S102. doi: 10.1007/s100720200089. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp. Neurol. 2004;189:369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp. Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, Bergman H. Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J. Neurosci. 2001;21:RC128 1–RC128 5. doi: 10.1523/JNEUROSCI.21-03-j0006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J. Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfs A, Nikkhah G, Rosenthal C, Rundfeldt C, Brandis A, Samii M, Loscher W. Hemispheric asymmetries in spontaneous firing characteristics of substantia nigra pars reticulata neurons following a unilateral 6-hydroxydopamine lesion of the rat nigrostriatal pathway. Brain Res. 1997;761:352–356. doi: 10.1016/s0006-8993(97)00475-7. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Walters JR. Nigrostriatal lesion and dopamine agonists affect firing patterns of rodent entopeduncular nucleus neurons. J. Neurophysiol. 2002;88:487–496. doi: 10.1152/jn.00844.2001. [DOI] [PubMed] [Google Scholar]
- Sanderson P, Mavoungou R, Albe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neurosci. Lett. 1986;67:25–30. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Tillerson J. Intervention strategies for degeneration of dopamine neurons in parkinsonism: optimizing behavioral assessment of outcome. In: Emerich D, Dean R, Sanberg P, editors. Central Nervous System Diseases. Totowa: Humana Press Inc; 1999. pp. 131–151. [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Sharott A, Moll CKE, Engler G, Denker M, Grun S, Engel AK. Different subtypes of striatal neurons are selectively modulated by cortical oscillations. J. Neurosci. 2009;29:4571–4585. doi: 10.1523/JNEUROSCI.5097-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Neural responses in multiple basal ganglia regions during spontaneous and treadmill locomotion tasks in rats. Exp. Brain Res. 2004;157:303–314. doi: 10.1007/s00221-004-1844-y. [DOI] [PubMed] [Google Scholar]
- Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB. J. 2003;17:1820–1830. doi: 10.1096/fj.03-0163com. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Tierney PL, Bergstrom DA, Soucy AL, Itoga CA, Allers KA, Ruskin DN, Walters JR. Single unit activity and local field potentials in paired recordings from subthalamic nucleus and globus pallidus neurons in awake rats: effect of alterations in dopamine receptor stimulation. Soc. Neurosci. Abstr. 2003;2003 Online. Program No. 273.5. [Google Scholar]
- Tort ABL, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. USA. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottenberg T, Fogelson N, Kuhn AA, Kivi A, Kupsch A, Schneider GH, Brown P. Subthalamic gamma activity in patients with Parkinson's disease. Exp. Neurol. 2006;200:56–65. doi: 10.1016/j.expneurol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kargieman L, Gacio S, Riquelme LA, Murer MG. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. Eur. J. Neurosci. 2005;22:2579–2586. doi: 10.1111/j.1460-9568.2005.04456.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Pazo JH, Murer MG, Riquelme LA. Subthalamic nucleus lesions reduce low frequency oscillatory firing of substantia nigra pars reticulata neurons in a rat model of Parkinson's disease. Brain Res. 2001a;904:93–103. doi: 10.1016/s0006-8993(01)02489-1. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J. Neurosci. 2001b;21:6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MAA, Redish AD. Low and high gamma oscillations in rat ventral striatum have distinct relationships to behavior, reward, and spiking activity on a learned spatial decision task. Front. Integr. Neurosci. 2009;3:1–19. doi: 10.3389/neuro.07.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Bergstrom DA. Basal ganglia network synchronization in animal models of Parkinson's disease. In: Tseng K-Y, editor. Cortico-Subcortical Dynamics in Parkinson's Disease. New York: Humana Press; 2009. pp. 117–142. [Google Scholar]
- Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–776. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Tierney PL, Bergstrom DA. Oscillatory activity and synchronization in the basal ganglia network in rodent models of Parkinson's disease. In: Groenewegen HJ, Voorn P, Berendse HW, Mulder AB, Cools AR, editors. The Basal Ganglia IX. New York: Springer Press; 2009. pp. 443–459. [Google Scholar]
- Weinberger M, Mahant N, Hutchison W, Lozano A, Moro E, Hodaie M, Lang A, Dostrovsky J. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J. Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. 3. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp. Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp. Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- Williams D, Kuhn A, Kupsch A, Tijssen M, van Bruggen G, Speelman H, Hotton G, Yarrow K, Brown P. Behavioural cues are associated with modulations of synchronous oscillations in the human subthalamic nucleus. Brain. 2003;126:1975–1985. doi: 10.1093/brain/awg194. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Williams D, Kuhn A, Kupsch A, Tijssen M, van Bruggen G, Speelman H, Hotton G, Loukas C, Brown P. The relationship between oscillatory activity and motor reaction time in the parkinsonian subthalamic nucleus. Eur. J. Neurosci. 2005;21:249–258. doi: 10.1111/j.1460-9568.2004.03817.x. [DOI] [PubMed] [Google Scholar]
- Zold CL, Ballion B, Riquelme LA, Gonon F, Murer MG. Nigrostriatal lesion induces D2-modulated phase-locked activity in the basal ganglia of rats. Eur. J. Neurosci. 2007;25:2131–2144. doi: 10.1111/j.1460-9568.2007.05475.x. [DOI] [PubMed] [Google Scholar]