Abstract
The possibility that dietary VD3 exposure inhibits endometrial carcinogenesis in an animal model, and modifies the enhanced risk of endometrial carcinoma associated with obesity was investigated. When 4 weeks of age, Pten+/− and wildtype mice were each divided into four treatment groups and fed AIN93G control diet, or AIN93G based diet containing either 25K IU of VD3/kg diet, 58% fat to induce obesity (high fat), or high fat and 25K IU of VD3/kg diet. Mice were kept on these diets until they were sacrificed at week 28. Although VD3 did not affect endometrial cancer risk, it inhibited obesity-induced increase in endometrial lesions. Specifically, high fat diet increased focal glandular hyperplasia with atypia and malignant lesions from 58% in the control diet fed Pten+/− mice to 78% in obese mice. Dietary VD3 decreased the incidence of endometrial pathology in obese Pten+/− mice to 25% (p<0.001). VD3 altered the endometrial expression of 25-hydroxylase (25-OHase), 1α-OHase and vitamin D receptor in the wildtype and Pten+/− mice. Estrogen receptor (ER) -α mRNA levels were higher (p<0.014) and progesterone receptor (PR) protein levels in the luminal epithelium were lower (p<0.04) in the endometrium of control diet fed Pten+/− than wildtype mice, but the expression of these receptors was not affected by the dietary exposures. VD3 reversed the obesity induced increase in osteopontin (p<0.001) and significantly increased E-cadherin expression (p<0.019) in the endometrium of obese in Pten+/− mice. Our data confirm the known association between obesity and endometrial cancer risk. Dietary exposure to VD3 inhibited the carcinogenic effect of obesity on the endometrium; this protective effect was linked to a reduction in the expression of osteopontin and increase in E-cadherin.
INTRODUCTION
Endometrial cancer is the fifth most common cancer among women (1), with approximately 41,200 new cases being diagnosed in the U.S. in 2006 (2). Unopposed exposure to high estrogen levels is the main risk factor for this disease (3). Other risk factors for endometrial cancer include obesity which elevates the risk by 3–5 -fold (4;5). Vitamin D, in contrast, has been proposed to reduce endometrial cancer risk (6;7) as well as the risk of several other cancers (8;9). Vitamin D (VD3) is mainly obtained through synthesis by skin exposed to sunlight, but it can also be obtained via diet. VD3 is converted by 25-hydroxylase (25-OHase) enzyme in the liver to 25(OH)D3, and then by 1α-OHase in the kidney to 1,25(OH)2D3, an active form of VD3. 1,25(OH)2D3 participates in calcium homeostasis and bone metabolism by acting through nuclear vitamin D receptor (VDR). VDR heterodimerizes with retinoid × receptors (RXRs) and this complex potentially mediates the cancer preventive effects of vitamin D (10). Besides the liver and kidney, VD3 metabolizing enzymes are expressed in several other tissues (11;12), including the endometrium (13), and therefore local conversion of VD3 to 25(OH)D3 or 1,25(OH)2D3 could contribute to the actions of this vitamin. Compared to the normal tissue, 25-OHase, 1α-OHase and VDR are found to be over-expressed in several pre-malignant and well to moderately differentiated malignant tissues (14), also in the endometrium (13), and thus the enzymes or VDR might be useful targets to treat endometrial cancer (14).
The effects of vitamin D in preventing cancer may include cell differentiation and apoptosis (15); expression profiling has identified several targets reflective of these actions (16). It also has been proposed that osteopontin and E-cadherin mediate the effects of VD3 (17). Osteopontin is an extracellular matrix glycophosphoprotein implicated in metastasis due to inducing anchorage-independent growth and abrogating adhesion (18). Osteopontin is a well-established target of VDR (19) and its expression also is increased by obesity (20). E-cadherin may mediate the growth inhibitory effects of VD3 by inhibiting β-catenin transcriptional activity (19;21;22), but it is not known whether it’s expression is affected by obesity.
The estrogen receptor (ER) might also be involved in mediating the actions of VD3 on the endometrial cancer. Treatment of MCF-7 human breast cancer cells with 1,25(OH)2D3 reduces ER levels in a dose-dependent manner (23), and suppresses E2-induced increase in progesterone receptor (PR) expression. Further, 1,25(OH)2D3 inhibits MCF-7 cell growth, and decreases the growth-stimulatory effect of 17β-estradiol (E2) on these cells (24). These findings indicate that 1,25(OH)2D3 exerts a direct negative effect on ER gene transcription, and thus the antiproliferative effects of 1,25(OH)2D3 could be partially mediated through their action to down-regulate ER levels and thereby attenuate estrogenic bioresponses (23).
Obesity has an opposite effect on estrogen signaling than VD3. First, obesity is associated with an increase in systemic estrogen levels due to a high level of aromatization of androgens occurring in adipose tissues (25); this is thought to be the key mechanism mediating the effects of obesity on reproductive system cancers, including endometrial cancer. Second, there is some evidence that obese women exhibit higher levels of ER in the endometrium (26) and breast tumors (27) than lean women. The possibility that vitamin D might interact with the effects of obesity on endometrial cancer risk has not been investigated, but based on the observations that vitamin D and obesity have opposing effects on ER signaling, we sought to test the hypothesis that vitamin D intake may prevent the effects of obesity on endometrial cancer risk and the protective effect might occur through ER.
Heterozygous Phosphatase and Tensin Homologue Deleted on Chromosome 10 (Pten)+/− mice were used for this purpose. PTEN is a known tumor suppressor, and frequently mutated or deleted in many cancers, particularly in endometrial cancer (28). Homozygous PTEN deletion is embryonically lethal, but lack of one allele of this gene is sufficient to induce multifocal hyperplasia with atypia and endometrial cancer which is detected between 28 and 52 weeks of age in heterozygous Pten+/− mice (29). It has been proposed that Pten+/− mice represent the most biologically relevant model of human endometrial cancer available (30). Additional benefit of using Pten+/− mice here is that loss of PTEN has been found to activate ERα-dependent pathways that are then suggested to be pivotal for the neoplastic process in these mice (31).
Our results indicate that dietary exposure to 25K IU VD3 for 24 weeks prevented the obesity-induced increase in endometrial pre-malignant and malignant lesions in Pten+/− mice. VD3 also increased bone density, but did not induce any toxicity. The effect of VD3 against obesity-induced increase in endometrial carcinogenesis may be related to inhibition of obesity-induced increase in osteopontin levels and up-regulation of E-cadherin, but it is unlikely to be explained by changes in endometrial expression of ER-α, ER-β or PR in Pten+/− mice.
METHODS
Animals
Heterozygous Pten+/− mice (B6.129-Ptentm1Rps), which are at C57BL/6 background and were obtained from MMHCC at NCI (Frederick, MD), were used. The mouse colony was established by breeding wildtype C57BL/6 female mice with heterozygous Pten+/− male mice. Pten+/− female offspring develop endometrial hyperplasia, some of which progress to adenocarcinomas starting at about 28 weeks of age (32). On the week before weaning at age 21 days, tail samples were obtained and the offspring were genotyped using the primers specified by MMHCC (http://mouse.ncifcrf.gov/protocols.asp?ID=01XH3&pallele=Pten%3Ctm1Rps%3E&prot_no=1).
Mice were housed at the Georgetown University Comparative Medicine Research Facility at appropriate temperature and a standard 12hr light-dark cycle. When not otherwise specified, they were fed pelleted semi-purified American Institute of Nutrition (AIN) 93G diet. All the studies were approved by the Institutional Animal Care and Use Committee.
Post-weaning dietary exposures
Four-week-old female Pten+/− and wildtype mice were each divided to four treatment groups (n=8–12 per group) and fed AIN93G based diet containing either (1) 18% energy from fat and 1K international units (IU) of cholecalciferol/kg diet (=standard AIN93G diet; in this paper this diet is called control diet and cholecalciferol is called VD3), (2) 18% fat and 25K IU of VD3/kg diet, (3) 58% fat to induce obesity (obesity-inducing AIN93G based diet, OID) containing 1.8K IU of VD3/kg diet , and (4) 58% fat and 25K IU of VD3/kg diet. OID contains more VD3 than AIN93G diet, because of an excessive deposition of VD3 in body fat (33) resulting obese individuals to have lower 25(OH)D3 levels (34). The daily adequate allowance of VD3 in humans is 0.4 IU; however, it is not clear what is the recommended daily allowance (RDA) or upper limit (UL) for VD3 (http://ods.od.nih.gov/factsheets/vitamind.asp). Some studies suggest that it is as high as 10,000 IU/day (35). The dose of VD3 used in our study – 25K IU – is 2.5 times higher that the highest dose recommended for humans (35). However, due to metabolic differences between the two species (36), higher VD3 exposure levels in mice are required to achieve the same biological effects seen in humans.
The mice were kept on these diets until they were sacrificed at 28 weeks of age; i.e., a total of 24 weeks. The diets were prepared by Harlan Teklad (Madison, WI). The fat content of the diets were slightly modified from AIN93G diets; all the diets contained 50 g/kg soybean oil (the sole oil in AIN93G diet) and either 20 g/kg (AIN93G) or 300 g/kg feed lard (OID). VD3 was added to fat modified diets.
All mice were weighed once per week using a digital scale to determine changes in body weight development from weaning to 28 weeks of age.
End-points determined at 28 weeks of age
When mice were 28 weeks of age, they were sacrificed to determine the presence of pathological changes in the endometrium. Thus, endometrium was collected and the middle sections of each uterine horn were removed and processed for paraffin blocks for immunohistochemistry and histopathology. The remaining tissue of the two horns were stored in −80 °C for Western blot and real time PCR assays.
Endometrial mRNA expression of 25-hydroxylase (25-OHase), 1α-OHase, 24-OHase, vitamin D receptor (VDR), estrogen receptor (ER)-α, ER-β and progesterone receptor (PR) was determined by real time PCR. Immunohistochemical analysis was used to measure ER-α and PR protein levels separately in the luminal or glandular epithelium and in the endometrial stroma. Pten protein levels were measured using Western blot.
Bone mineral density (BMD) and bone mineral content (BMC) were determined from the carcass using DXA.
Pre-malignant and malignant changes in the endometrium
Changes in endometrial morphology were assessed from histopathological sections processed as paraffin blocks, following the guidelines set by Fyles et al (30). Transverse sections of the uterine horns, and longitudinal sections of the uterocervical junction and ovaries, were examined by a board-certified veterinary pathologist (JMC) blinded to treatment group and genotype. Complex hyperplastic lesions were identified by glandular proliferation and crowding. Cellular atypia was noted in some lesions, consisting of glandular epithelial cell enlargement, loss of normal cellular polarity, and altered nuclear features (dispersed chromatin and prominent nucleoli). Adenocarcinoma was identified by invasion with disruption of the glandular basement membrane.
Endometrial mRNA expression of 25-OHase, 1α-OHase, 24-OHase, VDR, osteopontin, E-cadherin, ER-α, ER-β and PR
Total RNA was extracted from the endometrium of 4–8 Pten+/− and wildtype mice per group, kept on the four different diets. RNA was then cDNA reverse-transcribed from 100 µg/ml of total input RNA using Taqman Reverse Transcription Reagents as described by the manufacturer (Applied Biosystems, Foster City, CA). Next, PCR products were generated from the cDNA samples using the Taqman Universal PCR Master Mix (Applied Biosystems) and Assays-on-Demand (Applied Biosystems) for the appropriate target gene. The 18S Assay-on-Demand (Applied Biosystems) was used as an internal control. All assays were run on 384 well plates so that the cDNA sample from each endometrium was run in triplicate for the target gene and the endogenous control. Real time PCR was performed on an ABI Prism 7900 Sequence Detection System and the results assessed by relative quantitation of gene expression using the ΔΔCT method.
Immunohistochemistry to determine ER-α and PR protein levels in the epithelial and stromal compartments of the endometrium
Five µm paraffin sections, cut transverse, were deparaffinized and rehydrated from xylene through a graded series of ethanol. Antigen retrieval was carried out in a high pH Target retrieval solution (pH 9, Dako S2368, Carpinteria, CA, USA) in a pressure cooker for 20 min, followed by 2 hours cool down in RT. After blocking of endogeneous peroxidases the sections were incubated with monoclonal mouse anti-human ERα (M7047, Dako, Carpinteria, CA, USA, 1:35 dilution) and polyclonal rabbit anti-human PR (A0098, 1:400 dilution) primary antibodies. For negative controls a corresponding IgG was used. The slides were incubated with the primary antibody in +4°C overnight, followed by a secondary antibody and detection using Dako’s EnVison™ Dual Link System HRP DAB+ (K4065, Carpinteria, CA, USA), as instructed by the manufacturer, and counterstained with Harris Hematoxylin (Fisher Scientific, Kalamazoo, MI, USA). To quantify the immunohistochemical staining for ERα and PR, the sections were scored both for the number of positive cells and the intensity of the staining separately in the luminal or glandular epithelium and uterine stroma using a score modified from Allred et al. (37).
Western blot to determine Pten protein levels
The uterine tissue was homogenized, centrifuged and the protein extract collected from the supernatant. 50 µg of protein extract was loaded onto a NuPAGE 12% Bis-Tris gel (Invitrogen Life Technologies, Carlsbad, CA), and gels were run at 150 V. Membranes were then washed with TBST and blocked in 5% milk in TBST for 30 min at room temperature. After blocking, membranes were incubated with antibodies against Pten (1:500 dilution, Cell Signaling Technology, Danvers, MA) overnight at 4°C. Next, membranes were incubated with secondary anti-rabbit IgG or mouse IgG horseradish peroxidase antibodies (1:5000 dilution, Amersham Pharmacia Biotech, Piscataway, NJ) and developed using Super Signal (Pierce, Rockford, IL). Fold differences were calculated by normalization against beta-actin.
Bone density
Bone mineral density (BMD) and bone mineral content (BMC) were determined using dual-energy X-ray absorptiometry (DXA) (GE Lunar Piximus II, Madison, WI). This instrument has been validated for measures of body composition and bone density in mice (38;39). Necropsied carcasses were placed on the specimen tray and scanned a single time.
Data Analysis
Diet-induced changes in body weight were determined using repeated measures analysis of variance (ANOVA). Where appropriate, between-group comparisons were done using Fisher’s LSD method. To determine whether endometrial changes in Pten+/− mice were modified by dietary VD3 and/or high fat exposures, Chi2 test was used. Two-way ANOVA was used to assess treatment effects on Pten expression, VD3 metabolic enzymes, osteopontin, E-cadherin, hormone receptors, body composition and bone characteristics. When the data were not normally distributed, the results were log transformed prior to analysis. Correlations among (a) ER-α, ER-β and PR, and (b) VD3 metabolic enzymes and VDR and endocrine histopathology were assessed using Spearman rank order correlation. Analysis of Covariance ANCOVA was used to determine body weight-independent effects of treatments on bone end-points (40). The analyses were performed using SigmaStat Version 3.0 or SAS JMP Version 5.0. The differences were considered significant if the p-value was less than 0.05. All probabilities were two-tailed.
RESULTS
Effects of OID and VD3 on body weight
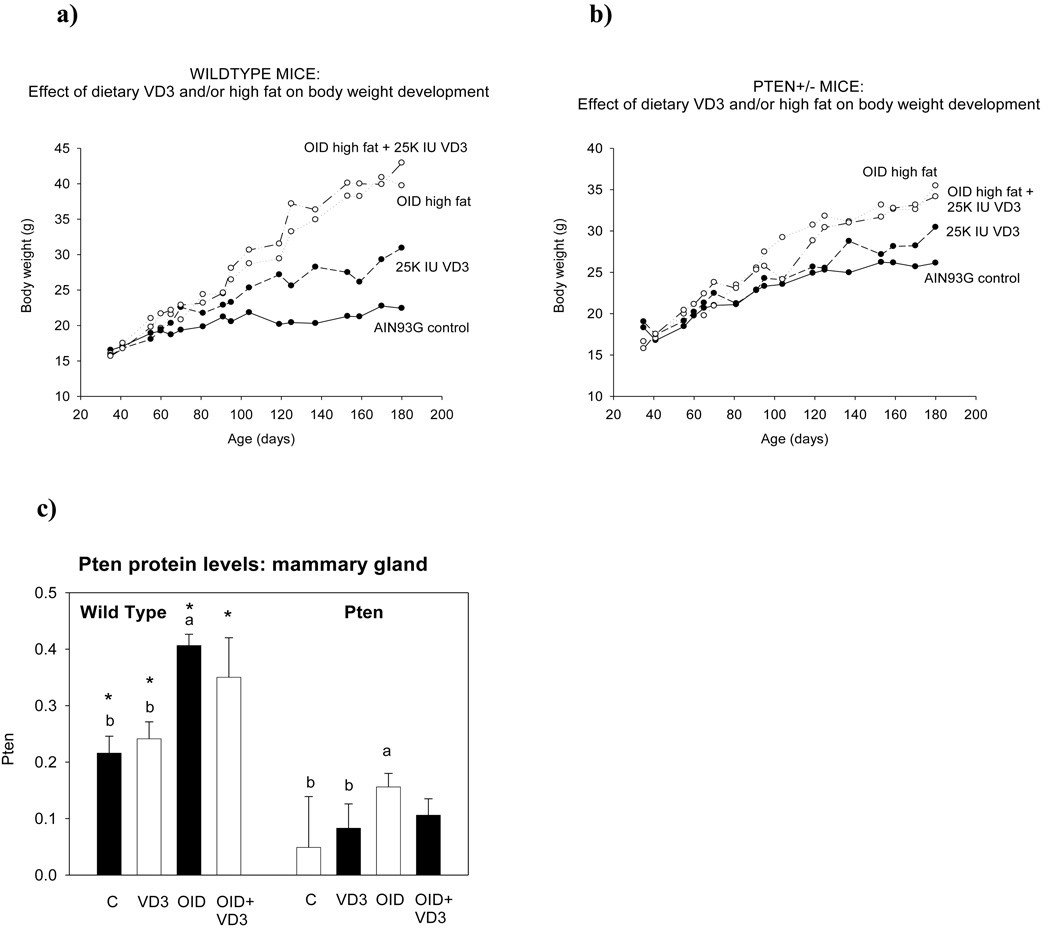
Repeated measures ANOVA revealed a significant increase in body weight over time (p<0.001) and differences in the amount of weight gain among different dietary groups (p<0.001). Exposure to an OID doubled body weights in wildtype mice (p<0.001) (Fig. 1a), and increased them in Pten+/− mice by 36% (p<0.001) (Fig. 1b). Feeding mice a control diet supplemented with 25K IU VD3 increased body weight by 38% in the wildtype (p<0.008) and 17% in the Pten+/− mice (not significant), when compared to the control diet fed mice. Vitamin D3 supplementation did not modify the effects of OID on body weight. No significant differences in weight gain between wildtype and Pten+/− mice were seen, although Pten+/− mice on the AIN93G diet were slightly heavier than wildtype mice throughout the study.
Figure 1.
Changes in body weight between postnatal weeks 4 and 28 in the (a) wildtype and (b) Pten+/− mice fed AIN93G based control diet containing either 18% energy from fat and 1K international units (IU) of cholecalciferol (VD3)/kg feed; vitamin D supplemented control diet containing 25K IU of VD3/kg diet; OID containing 58% fat and 1.8K UI VD3/kg diet; and vitamin D supplemented OID. When compared to control diet fed mice, OID significantly increased body weights in wildtype and Pten+/− mice (p<0.001). Body weights were also elevated in wildtype mice fed VD3 diet (p<0.008) or VD3 supplemented OID (p<0.001). Mean ± SEM of 8–12 mice per group are shown. (c) Pten protein levels, assessed using Western blot, in the mammary gland of 28-week-old wildtype and Pten+/− mice. Bars marked with a different letter are statistically significantly different from each other. Mean ± SEM of 5–7 mice per group are shown.
Since VD3 has been reported to interact with Pten expression (41;42), and such changes could explain the effect of VD3 on endometrial carcinogenesis in the Pten+/− mice, we determined Pten protein levels. Mammary tissues were used for this analysis, because they exhibit less histopathological changes than the endometrium in 28-week-old Pten+/− mice (32); transformed tissue may respond differently to VD3 than normal tissue does (13). As expected, Pten protein expression was significantly lower in the Pten+/− mice than in the wildtype mice (p<0.001). VD3 diet did not impact Pten levels in the Pten+/− or wildtype mice (Fig. 1c), but OID significantly increased the expression of this gene in both genetic backgrounds (p<0.047).
Histopathological changes in the endometrium
The uterine endometrium was examined histologically for evidence of hyperplasia or malignancy. The morphology of lesions was as described previously for this model (30). The findings were characterized as normal, multifocal glandular hyperplasia, (multi)focal glandular hyperplasia with atypia, and endometrial adenocarcinoma. Endometrial hyperplasia with cytologic atypia represents a much greater risk for progression to endometrial cancer than do hyperplasias without cytologic atypia (43). For example, over 50% of women who have atypical hyperplasia at biopsy or curettage are diagnosed with adenocarcinoma in subsequent hysterectomy (44).
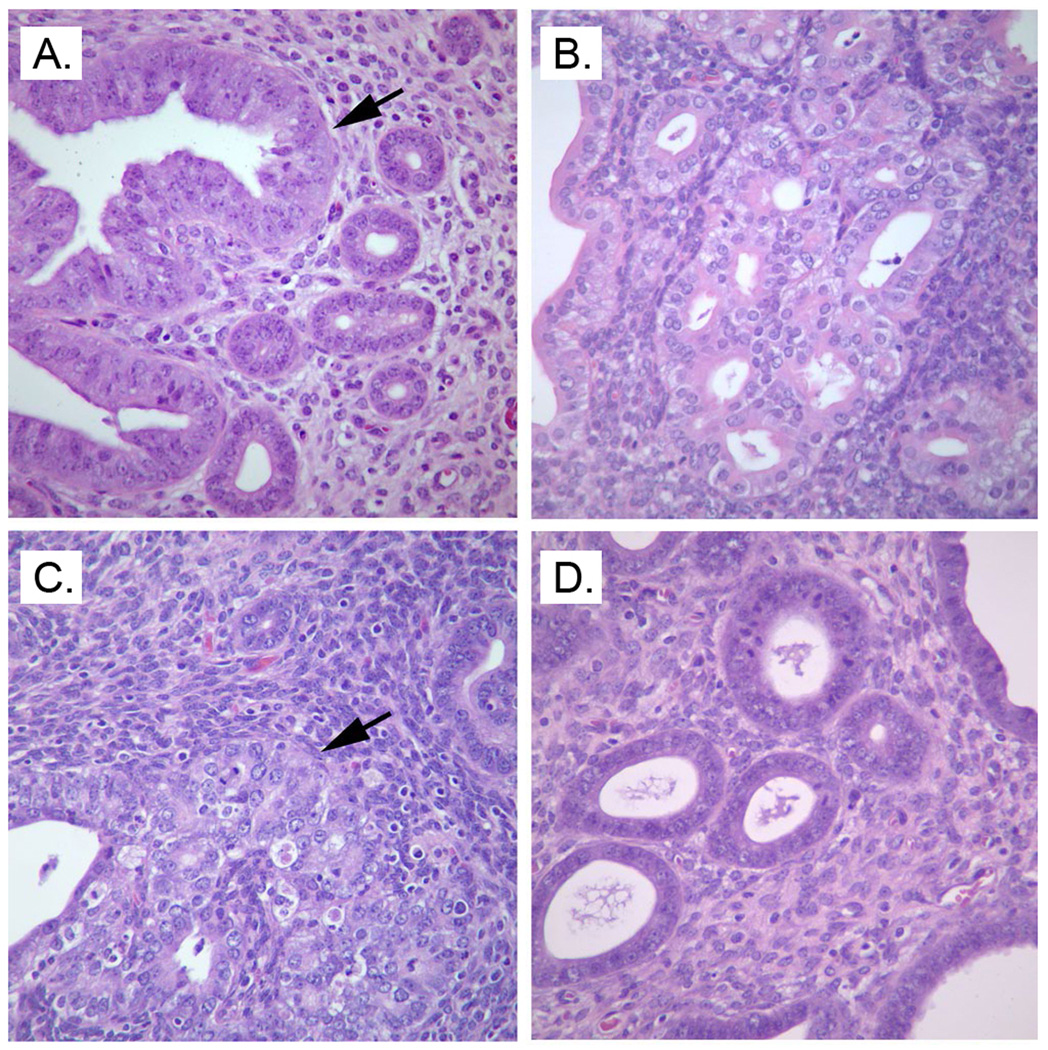
None of the wildtype mice developed pre-malignant lesions, defined as focal or multifocal glandular hyperplasia with atypia, whilst 58% of Pten+/− mice did. Feeding Pten+/− mice an OID increased the pre-malignant and malignant lesions to 78%, with one mouse exhibiting endometrial adenocarcinoma. Dietary exposure to VD3 significantly decreased the incidence of these endometrial lesions in Pten+/− mice fed OID to 25% (Chi2-test: p<0.001) (Table 1a). Figure 2 shows (a) endometrium with multifocal glandular hyperplasia in control diet fed Pten+/− mice, (b) endometrium with (multi)focal glandular hyperplasia and atypia in VD3 supplemented Pten+/− mice, (c) endometrial adenocarcinoma in OID fed Pten+/− mice, and (d) normal endometrial tissue in obese Pten+/− mice supplemented with 25K IU of VD3.
Table 1.
| Table 1a. Effects of VD3 supplementation on pre-malignant and malignant endometrial changes in 28-week-old normal weight and obese Pten+/− and wildtype mice. | |||||
|---|---|---|---|---|---|
| N mice per group |
Normal | Multifocal glandular hyperplasia (MFGH) |
MFGH & atypia [focal/multifocal] |
Endometrial adenocarcinoma |
|
| Genotype | |||||
| WT Control | 11 | 11 (100%) | 0 | 0 | 0 |
| + VD3 | 8 | 8 (100%) | 0 | 0 | 0 |
| High fat | 11 | 11 (100%) | 0 | 0 | 0 |
| + VD3 | 10 | 10 (100%) | 0 | 0 | 0 |
| Pten+/− Control | 12 | 5 (42%) | 0 | 7 (58%) [0/7] | 0 |
| + VD3 | 10 | 3 (30%) | 1 (10%) | 6 (60%) [4/2] | 0 |
| High fat | 9 | 2 (22%) | 0 | 6 (67%) [3/3] | 1 (11%) |
| + VD3 | 8 | 3 (37.5%) | 3 (37.5) | 2 (25%) [0/2] | 0 |
| Table 1b. Expression of vitamin D metabolic enzymes and VDR mRNA in the histopathologically normal endometrium or endometrium containing benign or pre-malignant and malignant changes in 28-week-old Pten+/− mice. Mean and SEM of 3–14 mice per group are shown. | ||||
|---|---|---|---|---|
| Changes in the endometrium |
25-OHase | 1α-OHase | 24-OHase | VDR |
| Normal | 1.59+0.30 | 1.40+0.45 | 0.93+0.23 | 4.33+1.85 |
| Benign lesions | 2.04+0.23 | 1.03+0.46 | 0.31+0.08 | 2.21+0.68 |
| Pre-malignant and malignant lesions |
2.48+0.32 | 1.60+0.36 | 1.39+0.46 | 2.36+0.71 |
Pten+/− mice: Chi2=111.737, df=6, p<0.001
Figure 2.
Mouse endometrium showing (A) endometrium with glandular hyperplasia and atypia in Pten+/− mouse fed the control diet, (B) endometrium with glandular hyperplasia in Pten+/− mouse fed VD3 supplemented diet, (C) endometrial adenocarcinoma in Pten+/− mouse fed the OID, and (D) normal glandular morphology, as in wildtype mice, in Pten+/− mouse fed the OID supplemented with VD3. Endometria were obtained from 28-week-old mice, and sections were stained with hematoxylin and eosin, with 40×objective magnification.
Effects on the expression of 25-OHase, 1α-OHase, 24-OHase and VDR
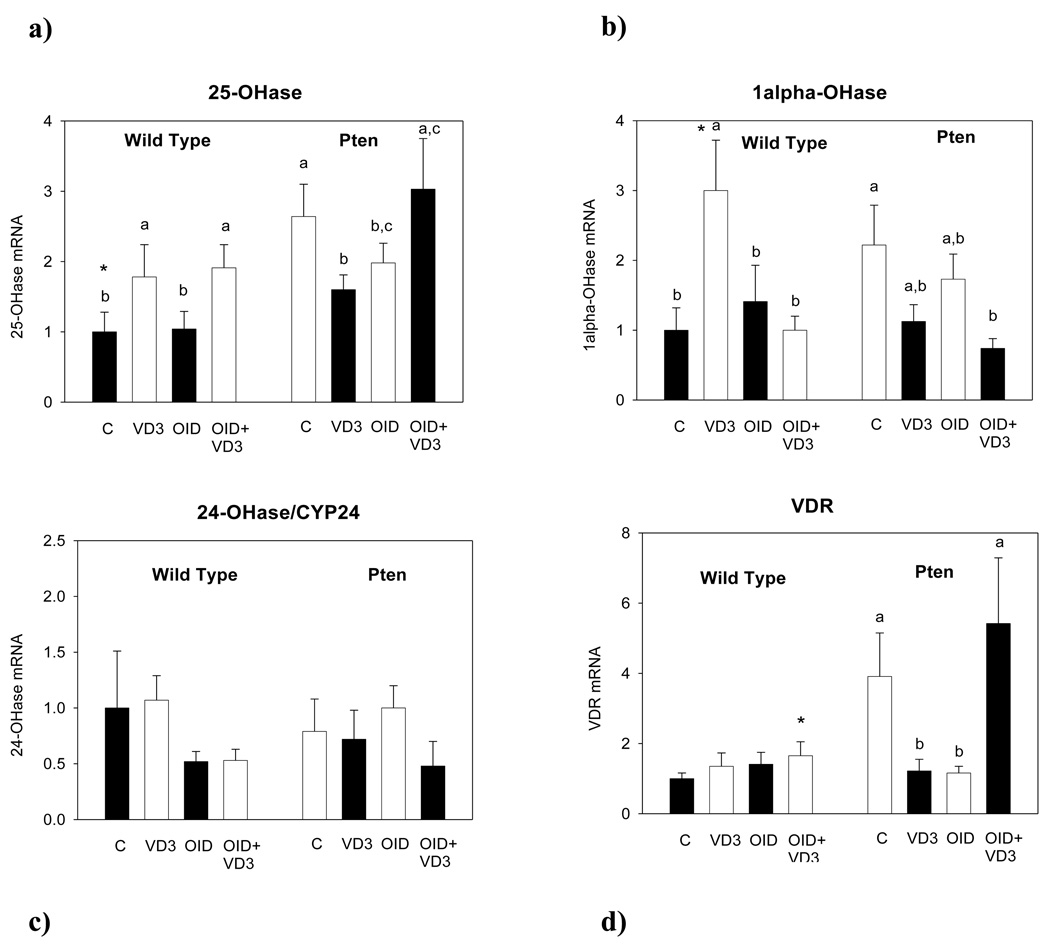
25-OHase expression was significantly higher in the Pten+/− than wildtype mice (p<0.002) (Fig. 3a). Dietary exposures affected 25-OHase expression in the endometrium (p<0.043). VD3 increased 25-OHase expression in the obese wildtype mice (p<0.027). Obese Pten+/− mice supplemented with VD3 expressed higher levels of 25-OHase than normal weight VD3 supplemented mice (p<0.036); however, no significant differences were seen among the control diet fed, VD3 supplemented normal weight and obese Pten+/− mice. (Fig. 3a).
Figure 3.
mRNA expression of VD3 metabolic enzymes (a) 25-hydroxylase (25-OHase), (b) 1α-OHase, (c) 24-OHase, and (d) vitamin D receptor (VDR) in the endometrium of 28-week-old wildtype and Pten+/− mice fed control diet (C), VD3 supplemented control diet (VD3), obesity-inducing diet (OID), or VD3 supplemented OID (OID+ VD3) for 24 weeks. Bars marked with a different letter are statistically significantly different from each other. RT-PCR was used, and data were quantitated using the ΔΔCTmethod and normalized to the control diet fed wildtype group. Mean ± SEM of 5–7 mice per group are shown.
1α-OHase expression was not significantly different between the wildtype and Pten+/− mice (Fig. 3b). However, 1α-OHase expression was increased by VD3 in the normal weight wildtype (p<0.003) but not Pten+/− mice (interaction: p<0.032) (Fig. 3b). No other significant changes were seen.
24-OHase expression was not different between the genotypes or among different dietary groups (Fig. 3c).
VDR expression was higher in the Pten+/− mice than the wildtype mice (p<0.006). VD3 supplementation did not have any effect on endometrial VDR expression in the wildtype mice, but it reduced the expression of this receptor in the normal weight Pten+/− mice (p<0.016) (interaction: p<0.023).
Histopathological changes in the endometrium and expression of VD3 metabolic enzymes or VDR. We also determined whether the presence of benign, pre-malignant or malignant changes in the endometrium of Pten+/− mice affected the expression of VD3 metabolic enzymes or VDR. No significant differences were found in the expression of 25-OHase, 1α-OHase, 24-OHase or VDR among normal, benign lesion, hyperplasia with atypia and cancer (Table 1b), and neither did the expression of VD3 metabolic enzymes or VDR correlate with the degree of transformation of the endometrial tissue.
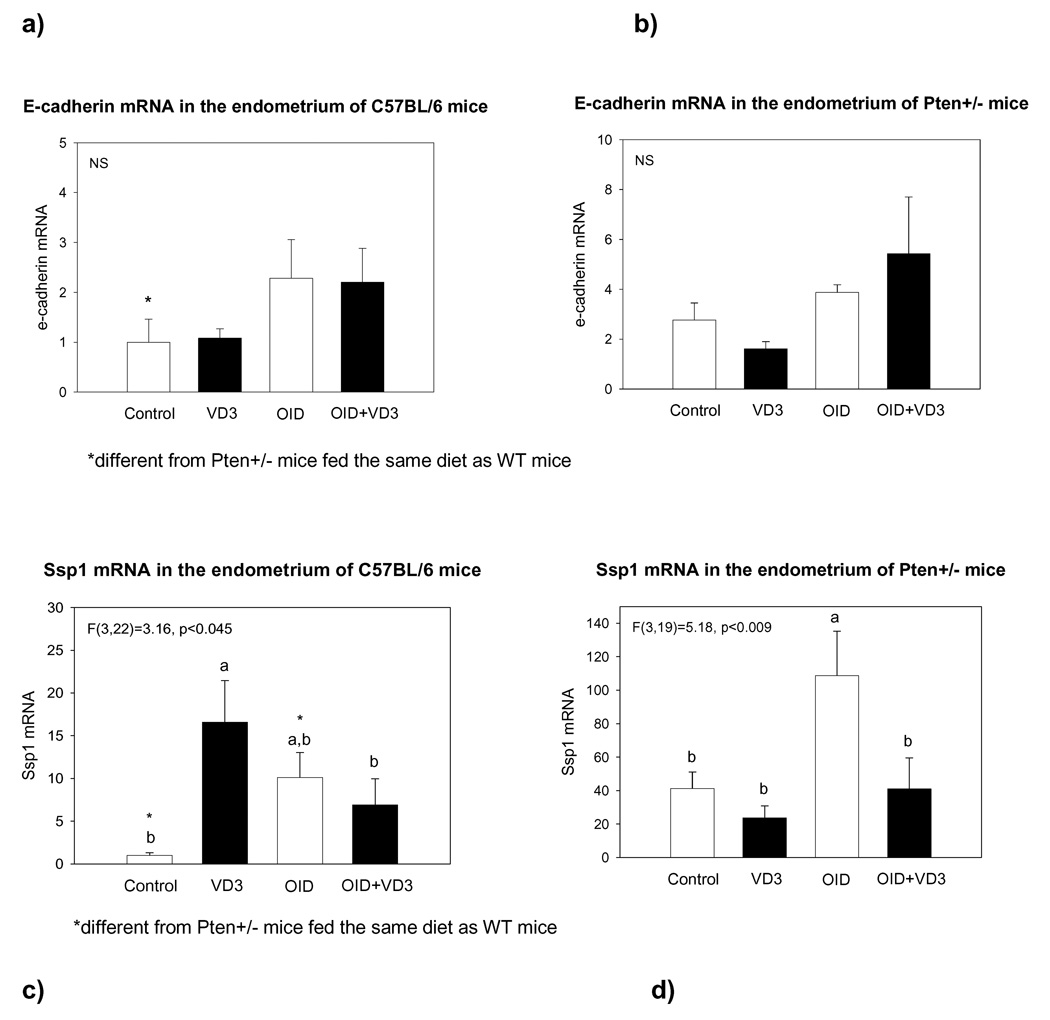
Effects on the expression of E-cadherin and osteopontin mRNA
Both E-cadherin (p<0.004) and osteopontin (p<0.001) levels were significantly higher in the Pten+/− than wildtype mice (Fig. 4). Vitamin D or obesity did not have significant effects on E-cadherin expression in either wildtype or Pten+/− mice, but in both genotypes E-cadherin was significantly higher in VD3 supplemented obese mice than in the control diet fed mice (p<0.019) (Fig. 4a and b).
Figure 4.
mRNA expression in the endometrium of E-cadherin in (a) wildtype and (b) Pten+/− mice, and osteopontin in (c) wildtype and (d) Pten+/− mice which were fed control diet, VD3 supplemented control diet, OID, or VD3 supplemented OID for 24 weeks. E-cadherin levels were higher in the Pten+/− than wildtype mice (p<0.004), and VD3 increased the expression in obese mice (p<0.019). Osteopontin (Ssp1) levels also were significantly higher in Pten+/− and wildtype mice (p<0.001), and VD3 reversed the increase seen in obese mice (p<0.003). RT-PCR was used, and data were quantified using the ΔΔCTmethod and normalized to the control diet fed wildtype group. Bars marked with a different letter are statistically significantly different from each other. Mean ± SEM of 4–8 mice per group are shown.
Dietary exposures affected the expression of osteopontin (p<0.003); however, the effects were different in wildtype and Pten+/− mice (p for interaction<0.002). Osteopontin levels were significantly elevated by VD3 in the wildtype mice (p<0.007), but not in the Pten+/− mice (Fig. 4c and d). Obesity significantly increased osteopontin levels in the Pten+/− mice (p<0.001), but the difference failed to reach significance in the wildtype mice (p<0.11). In both obese wildtype (p<0.049) and Pten+/− mice (p<0.001), VD3 reversed the increase in osteopontin levels.
Effects on the expression of ER-α, ER-β and PR mRNA
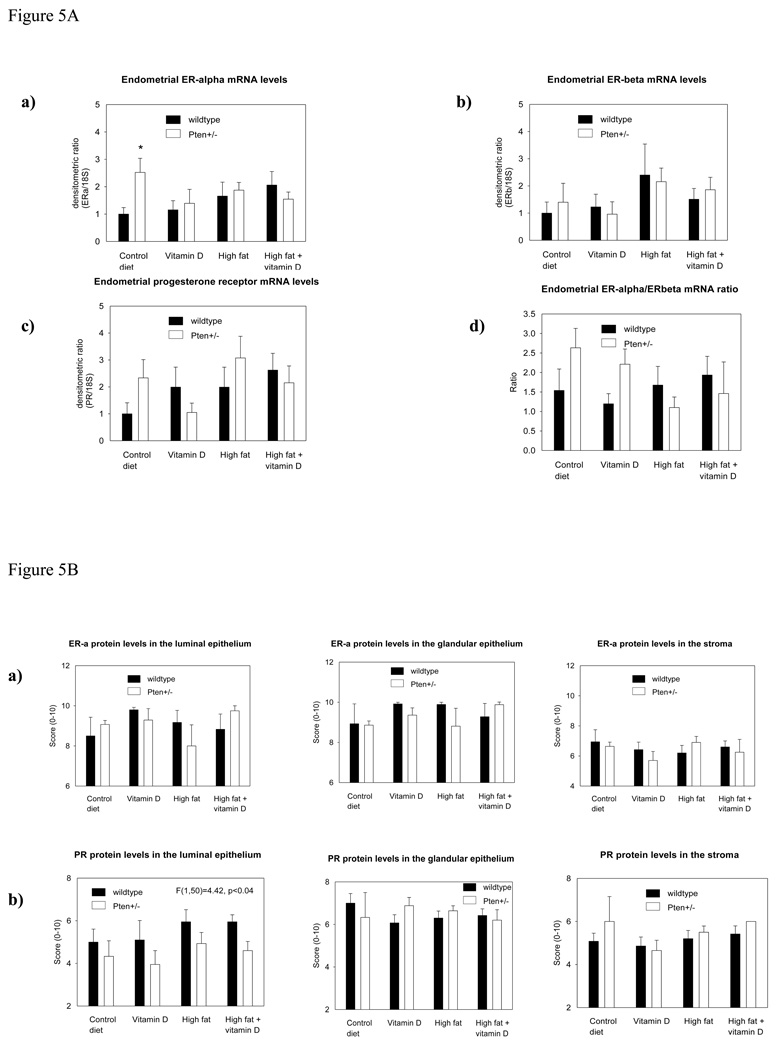
Since Pten+/− mice have been previously reported to express higher levels of ER-α than wildtype mice (30), we first compared the levels of expression in the endometrium between these two groups of mice kept on the control diet. The data indicated that the endometrium of Pten+/− mice expressed significantly elevated levels of ER-α (p<0.014). However, this difference disappeared when mice were fed OID or supplemented with VD3 (Fig. 5Aa). Further, neither ER-β (Fig. 5Ab) nor PR (Fig. 5Ac) mRNA expression was altered among different dietary exposure groups. We also determined ER-α/ ER-β ratio, and it was not affected (Fig. 5Ad)
Figure 5.
Figure 5A. mRNA expression of (a) ER-α, (b) ER-β, (c) PR and (d) ER-α/ER-β ratio in the endometrium of 28-week-old wildtype and Pten+/− mice fed control diet, VD3 supplemented control diet, OID, or VD3 supplemented OID for 24 weeks. ER-α mRNA levels were significantly higher in Pten+/− mice fed the control diet than in the wildtype mice; *p<0.014. No changes among different dietary exposures were seen. Mean ± SEM of 5–7 mice per group are shown.
Figure 5B. Endometrial protein levels of (a) ER-α and (b) PR in the luminal and glandular epithelium and stroma of 28-week-old wildtype and Pten+/− mice. PR expression was significantly lower in the luminal epithelium of Pten+/− mice than of the wildtype mice (p<0.04). Mean ± SEM of 3–10 mice per group are shown.
The level of expression of ER-α and PR is known to be strongly linked to each other, and therefore we compared the expression of the three receptors to each other. Highly significant correlations emerged between ER-α and ER-β (p<0.0001) or PR (p<0.0001), and between PR and ER-β (p<0.0001).
Protein
It is possible that the failure to observe any diet-induced differences in ER or PR expression was due to assessing these receptors in the mRNA obtained from the whole endometrial tissue. To address this possibility, we determined ER-α and PR protein levels by immunohistochemistry which allowed quantitation of these nuclear receptors in the luminal epithelium, glandular epithelium and stroma. ER-α protein levels were not different between the wildtype and Pten+/− mice (Fig. 5Ba). The levels of PR in the luminal epithelium were significantly lower in the Pten+/− mice, in all dietary exposure groups, compared to wildtype mice (p<0.041). No genotype –specific changes were seen in the glandular epithelium (Fig. 5Bb), but in the stroma, PR levels were non-significantly higher in the Pten+/− than wildtype mice (Fig. 5Bc). The latter might explain why PR mRNA levels, determined in the whole uterus, were not altered (Fig. 5Ac).
Bone density
Mice fed VD3 supplemented diet had higher bone mineral density (BMD) than other mice, regardless of genotype (p<0.0015). Adjustment for body weight using analysis of covariance did not alter this result. We also noted that BMD was strongly correlated with body mass in the control diet fed mice (p<0.0001). However, no such correlation was seen in the obese mice (p<0.36); i.e., the increase in body weight in these mice reflected an increase in adipose depot size, while an increase in body weight in control diet fed mice resulted from an increase in lean mass and to a small extent in bone mass. There was a small, but statistically significant interaction between genotype and diet on bone mineral content (BMC). Obese wildtype mice had greater BMC than Pten+/− mice; this difference was not seen in mice fed the control diet (Table 2).
Table 2.
Diet and PTEN effects on weight and bone characteristics of C57BL6 mice.
| N | Mass1 (g) Mean (S.E) |
BMD2 1000*(g/cm2) Mean (S.E.) |
BMC (g)/10 Mean (S.E.) |
||
|---|---|---|---|---|---|
| Genotype | |||||
| WT | Control | 6 | 24.5 (1.9) | 50.07 (0.9) | 4.3 (0.3) |
| w/Vit. D | 5 | 29.0 (1.9) | 55.52 (0.9) | 4.4 (0.3) | |
| High Fat | 5 | 47.9 (1.9) | 51.32 (0.9) | 5.3 (0.3) | |
| w/Vit. D. | 7 | 41.3 (1.6) | 50.00 (0.9) | 5.3 (0.3) | |
| HET | Control | 6 | 25.1 (1.7) | 51.25 (0.9) | 4.5 (0.3) |
| w/Vit. D | 2 | 27.3 (2.9) | 53.50 (1.1) | 5.1 (0.3) | |
| High Fat | 5 | 39.5 (1.9) | 49.58 (0.9) | 4.1 (0.3) | |
| w/Vit. D. | 5 | 37.5 (1.9) | 50.06 (0.9) | 4.4 (0.3) | |
| P (Genotype) | 0.0257 | 0.3750 | 0.1961 | ||
| P (Diet) | <0.0001 | 0.0014 | 0.3857 | ||
| P (Genotype*Diet) | 0.1304 | 0.3120 | 0.027 | ||
Weight after Necropsy, Means are unadjusted.
BMD and BMC reported from GE Lunar Piximus Dual-Energy X-ray Absorptiometer output.
DISCUSSION
Similarly to epidemiological studies in obese women showing an increased endometrial cancer risk (4;5), we found that obesity increased the risk of development of endometrial premalignant and malignant lesions in the Pten+/− mouse model. Dietary supplementation with VD3 inhibited the carcinogenic effect of obesity on the endometrium. The protective effect of VD3 against endometrial cancer in humans remains controversial, although the interactions among VD3, obesity and endometrial cancer have not been studied. Some evidence suggests that women exposed to high levels of VD3 are at a reduced risk of developing endometrial cancer (6;7), but some other studies question the existence of an association (45;46). In our study, VD3 supplementation did not reduce the incidence of pre-malignant endometrial changes in normal weight Pten+/− mice. However, had we assessed the effect of vitamin D at a later time point when more endometrial tumors are present, the findings might have been different. Additional studies on VD3 and endometrial cancer are needed, including animal studies.
Pten+/− mice are an excellent animal model of human endometrial cancer (30), partly because a loss of PTEN is a common event in endometrial cancer in women (28). In the present study, we found that Pten+/− mice exhibit an increased expression of ER-α mRNA in the endometrium, and reduced expression of PR protein in the luminal epithelium. These observations are consistent with the data obtained in women. High ER-α expression in the endometrium is associated with increased endometrial cancer risk (47) and progression (47–50). Further, PR expression, either PR-A or PR-B, is lower in hyperplastic and malignant endometrial tissues than in normal endometrial tissue (51;52). Since ER-α is the predominant ER form in the uterus, ER-β may not play a role in uterine cancer (48;49).
Regardless of the similarity of the changes in ER-α and PR expression in the endometrium of Pten+/− mice and women at high risk of endometrial cancer, there is some controversy as to whether endometrial carcinogenesis in Pten+/− mice is dependent on ER-α and PR. Vilgelm et al. (31) proposed that a loss of Pten results in the activation of ER-α-dependent pathways that are then pivotal for the neoplastic processes occurring in the endometrium of Pten+/− mice. However, Fyles et al. (30) reported that neither ovariectomy nor an exposure to progestin modifies endometrial cancer risk in Pten+/− mice. In accordance with these data, we found no evidence that VD3 intake or an exposure to obesity-inducing OID affected the gene or protein expression of ER-α, ER-β and PR in the Pten+/− or wildtype mice. The reason why OID did not affect steroid receptors may be because this diet does not increase circulating estradiol levels, but it increases leptin levels (53). Thus, our data do not support a role for the three receptors in mediating the endometrial cancer risk increasing effect of an OID or the protective effects of VD3 in obese Pten+/− mice.
Vitamin D up-regulates both osteopontin (19), which promotes anchorage-independent growth, and E-cadherin (19;21;22), which inhibits cell proliferation and invasion. This dual effect may explain why vitamin D has been reported to reduce the incidence and growth of some cancers and possibly increase some (17;19). Pten+/− mice expressed significantly higher levels of both genes than the wildtype mice did. In the uterus of lean wildtype and Pten+/− mice, VD3 did not affect E-cadherin expression, however, in obese mice, VD3 increased the expression of this gene. As expected, VD3 increased osteopontin levels in the wildtype mice; this effect was not seen in the Pten+/− mice. However, VD3 supplementation inhibited the increase in osteopontin mRNA levels in both wildtype and Pten+/− mice. These findings suggest that VD3 may prevent obesity-induced increase in endometrial cancer by up-regulating E-cadherin and down-regulating osteopontin expression.
In accordance with previous studies (54), an OID significantly increased the body weight, and this was seen both in the wildtype and Pten+/− mice. However, the Pten+/− mice gained less weight than the wildtype mice did. One possible explanation for these findings is the role of PTEN in insulin signaling. Insulin controls glucose and lipid metabolism through phosphatidylinositol 3-kinase (PI3K) and serine-threonine kinase AKT. Since PTEN is a negative regulator of the PI3K/AKT pathway, it also inhibits the metabolic effects of insulin (28;55). Down-regulation of PTEN, in turn, reverses insulin resistance in diabetic mice (56). Further, Pten+/− mice, or mice with adipose-tissue specific loss of Pten, exhibit improved systemic glucose tolerance and insulin sensitivity, and decreased fasting insulin levels (57), but no changes in body weight or adiposity (57;58). Thus, the adverse effects of OID on lipid metabolism may have been less severe in the Pten+/− mice than in the wildtype mice. Nevertheless, the increase in body weight in OID fed Pten+/− mice was sufficient to lead to an increased endometrial carcinogenesis.
Vitamin D is reported to up-regulate PTEN expression in cancer cells (42), and therefore VD3 supplementation in our study might have reduced endometrial cancer risk in obese Pten+/− mice by increasing the expression of the remaining Pten allele. PTEN gene also participates in mediating the growth inhibitory actions of vitamin D on cancer cells (41). Consequently, Pten+/− mice might be less sensitive for the effects of VD3 than mice which have both alleles of this gene. In our study, dietary VD3 did not modify Pten expression in the wildtype or Pten+/− mice. However, OID increased Pten protein levels in both genetic backgrounds. This effect is consistent with obesity leading to insulin resistance (59), and high PTEN levels being related to insulin insensitivity (57;60). Pten+/− mice are responsive to vitamin D. In the previous study, the prostates of male Pten+/− mice exposed to 1,25(OH)2D3 via a subcutaneous pump exhibited less high-grade PIN with invasions than mice receiving a placebo (61). However, because PTEN participates in mediating the actions of vitamin D (41), it is possible that the chemopreventive effect of VD3 were underestimated in studies which utilize Pten+/− mice.
We addressed the sensitivity of the Pten+/− mice to VD3 by measuring the expression of enzymes which metabolize VD3 to its biologically active form, 1,25(OH)2D3. If wildtype mice are more sensitive to the actions of VD3 than Pten+/− mice, they are expected to express more significant changes in the expression of VD3 metabolizing enzymes and VDR. Previous studies indicate that vitamin D up-regulates 25-hydroxylase (25-OHase) in the liver where it converts VD3 to 25(OH)D3, but down-regulates 1α -OHase in the kidney where this enzyme converts 25(OH)D3 to 1,25(OH)2D3 (62). VDR is also reported to be down-regulated by VD3 (62). However, vitamin D may not induce similar changes in all the tissues where these enzymes are expressed (63).
We found that both wildtype and Pten+/− mice were affected by VD3, although the responses were slightly different. VD3 increased the expression of 25-OHase in the wildtype and obese Pten+/− mice. This is consistent with the reported effect of VD3 on 25-OHase in the liver (62) and other tissues (64). The expression of 1α-OHase was increased by VD3 in the endometrium of wildtype mice, which is opposite to down-regulation reported in the kidney (62). Consistent with previous findings (62), VD3 reduced the expression of VDR, but this was seen only in the Pten+/− mice. The differences between the wildtype and Pten+/− mice may originate from interactions between VD3 and PTEN (41;42), but they are not reflective of Pten+/− mice being less sensitive to VD3 than the wildtype mice were. Alternatively, the differences may be related to carcinogenic process taking place in the endometrium of the Pten+/− mice. In humans, expression of 25-OHase, 1α-OHase and VDR are found to be higher in several pre-malignant and well to moderately differentiated malignant tissues (14), including the endometrium (13), when compared to the corresponding normal tissue. Reduced expression is seen in poorly differentiated cancers (14). Consistent with these reports, we found that the expression of 25-OHase and VDR were higher in the endometrium of the Pten+/− than wildtype mice. However, when we compared the expression of VDR and vitamin D metabolic enzymes in the normal, benign and pre-malignant/malignant endometrial tissues within Pten+/− mice, no significant differences were observed. These results suggest that although the endometrium of some Pten+/− mice have not undergone histopathological changes by week 28, the fact that it eventually will (32;65) is sufficient to increase the expression of vitamin D metabolic enzymes and VDR. It is possible that changes in vitamin D signaling in the endometrium are predictive of increased endometrial cancer.
A major limitation in using the biologically active form of vitamin D, 1,25(OH)2D3, is its toxicity due to hypercalcemia (66). In contrast, chronic administration of relatively high doses of VD3/cholecalciferol (up to 20K international units, IU) appears safe (67). In our study, there was no indication that dietary exposure to 25K IU VD3 induced toxicity; i.e., weight loss or early death. In fact, we found that an intake of a diet containing 25K IU cholecalciferol for 24 weeks resulted in a higher bone mineral density (BMD), when compared to mice fed a control diet. Obese wildtype mice exhibited the highest bone mineral content (BMC), whilst obese Pten+/− mice exhibited lowest BMC. In Pten+/− mice, BMC was highest in the VD3 supplemented mice. Since BMD and BMC were similar in wildtype and Pten+/− mice fed AIN93G diet, it is not clear why genotype affected the response of consuming VD3 supplemented diet or OID on the bone. Possible explanations include the role of PTEN in insulin signaling (57;60), and interactions between PTEN and vitamin D (41;42) which were already discussed above.
In conclusion, we found that dietary exposure to 25K IU of VD3 prevented the obesity-induced increase in premalignant and malignant endometrial lesions in Pten+/− mice. VD3 did not have any notable hypercalcemic effects, and it increased bone mineral density. Dietary VD3 exposure affected the expression of VD3 metabolic enzymes and VDR in the endometrium, but the effects were different in the wildtype and Pten+/− mice, possibly reflecting the role of these enzymes and VDR as putative treatment targets in the malignant tissue (14) or alternatively caused by the reported interactions between VD3 and PTEN (41;42). However, VD3 did not alter Pten expression in the present study. Although the cancer-risk reducing effects of VD3 might occur via inhibition of ER-α signaling (23;24), and although Pten+/− mice exhibited increased levels of ER-α and reduced levels of PR in the endometrium, there was no evidence of the involvement of these receptors in mediating the protective effects of VD3. Our study suggests that down-regulation of osteopontin and an increase in E-cadherin levels by VD3 in obese Pten+/− mice may explain how this vitamin reduces obesity-promoted endometrial cancer.
Acknowledgement
The authors would like to thank Drs. Salim Shah at Georgetown University and William Helferich at University of Illinois in Urbana for advice regarding dietary vitamin D exposure. The work was funded by grants obtained from the National Cancer Institute (U54 CA100970) and the Department of Defense Telemedicine and Advanced Technology Center, Award Number W81XWH-05-2-0005. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Reference List
- 1.Amant F, Moerman P, Neven P, Timmerman D, Van LE, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. 2006. [Google Scholar]
- 3.McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive factors and risk of endometrial cancer. The Iowa Women's Health Study. Am J Epidemiol. 1996;143:1195–1202. doi: 10.1093/oxfordjournals.aje.a008707. [DOI] [PubMed] [Google Scholar]
- 4.McCullough ML, Patel AV, Patel R, et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev. 2008;17:73–79. doi: 10.1158/1055-9965.EPI-07-2567. [DOI] [PubMed] [Google Scholar]
- 5.Chang SC, Lacey JV, Jr, Brinton LA, et al. Lifetime weight history and al cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2007;16:723–730. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 6.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Is ultraviolet B irradiance inversely associated with incidence rates of endometrial cancer: an ecological study of 107 countries. Prev Med. 2007;45:327–331. doi: 10.1016/j.ypmed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Salazar-Martinez E, Lazcano-Ponce E, Sanchez-Zamorano LM, Gonzalez-Lira G, Escudero-DE Los RP, Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int J Gynecol Cancer. 2005;15:938–945. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 8.Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67:115–127. doi: 10.1017/S0029665108006964. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 10.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102:156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Segersten U, Holm PK, Bjorklund P, et al. 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1alpha-hydroxylated vitamin D analogue. Breast Cancer Res. 2005;7:R980–R986. doi: 10.1186/bcr1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 13.Agic A, Xu H, Altgassen C, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 2007;14:486–497. doi: 10.1177/1933719107304565. [DOI] [PubMed] [Google Scholar]
- 14.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 15.Welsh J. Targets of vitamin D receptor signaling in the mammary gland. J Bone Miner Res. 2007;22 Suppl 2:V86–V90. doi: 10.1359/jbmr.07s204. [DOI] [PubMed] [Google Scholar]
- 16.Wood RJ, Tchack L, Angelo G, Pratt RE, Sonna LA. DNA microarray analysis of vitamin D-induced gene expression in a human colon carcinoma cell line. Physiol Genomics. 2004;17:122–129. doi: 10.1152/physiolgenomics.00002.2003. [DOI] [PubMed] [Google Scholar]
- 17.Campbell FC, Xu H, El Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissuespecific growth control. Biochem Pharmacol. 2010;79:1–9. doi: 10.1016/j.bcp.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurisetty VV, Johnston PG, Johnston N, et al. RAN GTPase is an effector of the invasive/metastatic phenotype induced by osteopontin. Oncogene. 2008;27:7139–7149. doi: 10.1038/onc.2008.325. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, McCann M, Zhang Z, et al. Vitamin D receptor modulates the neoplastic phenotype through antagonistic growth regulatory signals. Mol Carcinog. 2009;48:758–772. doi: 10.1002/mc.20520. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Ambrosi J, Catalan V, Ramirez B, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab. 2007;92:3719–3727. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 21.Ordonez-Moran P, Larriba MJ, Palmer HG, et al. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697–710. doi: 10.1083/jcb.200803020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S, Islam MN, Dakshanamurthy S, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–3379. [PubMed] [Google Scholar]
- 24.Nolan E, Donepudi M, VanWeelden K, Flanagan L, Welsh J. Dissociation of vitamin D3 and anti-estrogen mediated growth regulation in MCF-7 breast cancer cells. Mol Cell Biochem. 1998;188:13–20. [PubMed] [Google Scholar]
- 25.Kirschner MA, Schneider G, Ertel NH, Worten E. Obesity, Androgens, Estrogens, and Cancer Risk. Cancer Res. 1982;42:3281S–3285S. [PubMed] [Google Scholar]
- 26.Moran C, Garcia-Hernandez E, Cortes MA, Calzada L, Salazar L, Bermudez JA. Estradiol and progesterone endometrial receptors and body fat distribution in obese women. Gynecol Obstet Invest. 1996;42:117–119. doi: 10.1159/000291916. [DOI] [PubMed] [Google Scholar]
- 27.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 28.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 29.Vollmer G. Endometrial cancer: experimental models useful for studies on molecular aspects of endometrial cancer and carcinogenesis. Endocr Relat Cancer. 2003;10:23–42. doi: 10.1677/erc.0.0100023. [DOI] [PubMed] [Google Scholar]
- 30.Fyles A, Wood G, Li M, et al. Neither ovariectomy nor progestin treatment prevents endometrial neoplasia in pten+/− mice. Gynecol Oncol. 2008;108:395–401. doi: 10.1016/j.ygyno.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Vilgelm A, Lian Z, Wang H, et al. Akt-mediated phosphorylation and activation of estrogen receptor alpha is required for endometrial neoplastic transformation in Pten+/−mice. Cancer Res. 2006;66:3375–3380. doi: 10.1158/0008-5472.CAN-05-4019. [DOI] [PubMed] [Google Scholar]
- 32.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- 33.Blum M, Dallal GE, wson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27:274–279. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 35.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 36.ICH. Guidance for Industry S1C(R2) Dose Selection for Carcinogenicity Studies. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) September, Revision 1. 2008. Ref Type: Report. [Google Scholar]
- 37.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 38.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 39.Berrigan D, Lavigne JA, Perkins SN, Nagy TR, Barrett JC, Hursting SD. Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In Vivo. 2005;19:667–674. [PubMed] [Google Scholar]
- 40.Packard GC, Boradman TJ. The misuse of ratios, indexes, and percentages in ecophysiological research. Physiol Zool. 2005;61:1–9. [Google Scholar]
- 41.Liu W, Asa SL, Ezzat S. 1alpha,25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol Endocrinol. 2005;19:2349–2357. doi: 10.1210/me.2005-0117. [DOI] [PubMed] [Google Scholar]
- 42.Hisatake J, O'Kelly J, Uskokovic MR, Tomoyasu S, Koeffler HP. Novel vitamin D(3) analog, 21-(3-methyl-3-hydroxy-butyl)-19-nor D(3), that modulates cell growth, differentiation, apoptosis, cell cycle, and induction of PTEN in leukemic cells. Blood. 2001;97:2427–2433. doi: 10.1182/blood.v97.8.2427. [DOI] [PubMed] [Google Scholar]
- 43.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A longterm study of "untreated" hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 44.Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma. Gynecol Oncol. 1999;73:170–171. doi: 10.1006/gyno.1998.5328. [DOI] [PubMed] [Google Scholar]
- 45.McCullough ML, Bandera EV, Moore DF, Kushi LH. Vitamin D and calcium intake in relation to risk of endometrial cancer: a systematic review of the literature. Prev Med. 2008;46:298–302. doi: 10.1016/j.ypmed.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negri E, La Vecchia C, Franceschi S, Levi F, Parazzini F. Intake of selected micronutrients and the risk of endometrial carcinoma. Cancer. 1996;77:917–923. doi: 10.1002/(sici)1097-0142(19960301)77:5<917::aid-cncr17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Tan DS, Lambros MB, Marchio C, Reis-Filho JS. ESR1 amplification in endometrial carcinomas: hope or hyperbole? J Pathol. 2008;216:271–274. doi: 10.1002/path.2432. [DOI] [PubMed] [Google Scholar]
- 48.Shabani N, Kuhn C, Kunze S, et al. Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur J Cancer. 2007;43:2434–2444. doi: 10.1016/j.ejca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Shabani N, Mylonas I, Jeschke U, et al. Expression of estrogen receptors alpha and beta, and progesterone receptors A and B in human mucinous carcinoma of the endometrium. Anticancer Res. 2007;27:2027–2033. [PubMed] [Google Scholar]
- 50.Mylonas I, Jeschke U, Shabani N, et al. Normal and malignant human endometrium express immunohistochemically estrogen receptor alpha (ER-alpha), estrogen receptor beta (ER-beta) and progesterone receptor (PR) Anticancer Res. 2005;25:1679–1686. [PubMed] [Google Scholar]
- 51.Arnett-Mansfield RL, deFazio A, Wain GV, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001;61:4576–4582. [PubMed] [Google Scholar]
- 52.Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma--new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54:667–679. doi: 10.1507/endocrj.kr-114. [DOI] [PubMed] [Google Scholar]
- 53.de Assis S, Galam K, Hilakivi-Clarke L. High birth weight increases mammary tumorigenesis in rats. Int J Cancer. 2006;119:1537–1546. doi: 10.1002/ijc.21936. [DOI] [PubMed] [Google Scholar]
- 54.Ray A, Nkhata KJ, Grande JP, Cleary MP. Diet-induced obesity and mammary tumor development in relation to estrogen receptor status. Cancer Lett. 2007 doi: 10.1016/j.canlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Butler M, McKay RA, Popoff IJ, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 57.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498–2510. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong JT, Kim PT, Peacock JW, et al. Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia. 2007;50:395–403. doi: 10.1007/s00125-006-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 60.Elghazi L, Bernal-Mizrachi E. Akt and PTEN: beta-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20:243–251. doi: 10.1016/j.tem.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banach-Petrosky W, Ouyang X, Gao H, et al. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3.1;Pten mutant mice. Clin Cancer Res. 2006;12:5895–5901. doi: 10.1158/1078-0432.CCR-06-1039. [DOI] [PubMed] [Google Scholar]
- 62.Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29:388–396. doi: 10.1016/j.mam.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Byrne ME, Chang E, et al. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26:2567–2572. [PubMed] [Google Scholar]
- 65.Suzuki A, Nakano T, Mak TW, Sasaki T. Portrait of PTEN: messages from mutant mice. Cancer Sci. 2008;99:209–213. doi: 10.1111/j.1349-7006.2007.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vieth R. Vitamin D and cancer mini-symposium: the risk of additional vitamin D. Ann Epidemiol. 2009;19:441–445. doi: 10.1016/j.annepidem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Fleet JC, Gliniak C, Zhang Z, et al. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr. 2008;138:1114–1120. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]