Abstract
Childhood-onset schizophrenia (COS) is a rare severe form of schizophrenia that may have greater salient genetic risk. Despite evidence for high heritability, conclusive genetic causes of schizophrenia remain elusive. Recent genomic technologies in concert with large case-control cohorts, have led to several associations of highly penetrant rare copy number variants (CNVs) and schizophrenia. We previously reported two patients with COS who carried a microduplication disrupting the PXDN and MYT1L genes at 2p25.3. This is a significantly higher rate of duplications within our COS population (N=92) when compared to 2,026 healthy controls (p=0.002). As a replication, we report a meta-analysis of four recently published studies that together provide strong evidence for association between variably-sized microduplications involving the MYT1L gene and schizophrenia. None have reported this separately. Altogether, among 5,325 cases and 9,279 controls, 10 microduplications were observed; 9 in cases and 1 in a control (OR=15.7, p=0.001). Further, the 2% rate observed in our COS patients is also significantly higher than the rate in adult onset cases (0.14%, OR=16.6, p=0.01). This report adds to the growing body of literature implicating rare CNVs as risk factors for schizophrenia, and that some risk CNVs are more common among extreme early-onset cases.
Keywords: CNV, childhood-onset schizophrenia, 2p25.3, MYT1L, PXDN, 1M Illumina SNP microarray
INTRODUCTION
Schizophrenia is a chronic and severe disorder that affects ~1% of the population and ranks among the top 10 causes of disability worldwide(Tandon et al. 2009). Childhood-onset schizophrenia (COS), a rare and severe form of the condition exhibits a prevalence of less than 1 in 30,000(McKenna et al. 1994). Previous work has established the phenotypic and neurobiologic continuity between COS and the more typical adult onset form of the disorder (AOS)(Nicolson and Rapoport 1999; Rapoport et al. 1999). For 20 years, the child psychiatry branch of the National Institutes of Mental Health has studied COS to understand the etiology and neurobiology of the illness.
Recently, high density microarrays have allowed us to perform genome-wide assessments of previously unknown submicroscopic genomic deletions and duplications. These technologies have identified many rare Copy Number Variants (CNVs) that impact known genes in the COS population(Walsh et al. 2008). Since 2008, several studies have provided substantial evidence that there is a greater burden of CNVs in schizophrenia. For example, The International Schizophrenia Consortium (ISC) conducted one of the largest studies with 3,391 cases and 3,181 controls from seven European populations; and found cases had a 1.15 fold excess of rare CNVs which increased to 1.67 fold for deletions >500 Mb(Consortium 2008). Also, the Wellcome Case Control Consortium (WCCC) assessed the involvement of rare (<1%) CNVs in 471 cases of schizophrenia and 2,792 controls and determined large CNVs (>1 Mb) were 2.26 times more common in cases (p=0.00027)(Kirov et al. 2009). Lastly, CNV burden has drawn attention from the most recurrent CNV deletions (1q21.1, 3q29, 15q11, 15q13.3, and 22q11.2) and duplications (7q36.3(VIPR2) and 16p11.2)) which are now considered to be strong risk factors for schizophrenia(Levinson et al. 2011; Magri et al. 2010).
In 2008, Vrijenhoek et al. first reported two schizophrenia patients with microduplications that disrupted MYT1L at 2p25.3. We subsequently reported two COS patients also carrying microduplications impacting the MYT1L and PXDN genes(Addington and Rapoport 2009). Contrary to other recurrent CNVs associated with schizophrenia, this region does not contain an obvious mechanism for non-allelic homologous recombination (NAHR), i.e. low copy flanking repeats(Ingason et al. 2011). Given that this was the only recurrent CNV besides 22q11.2 and 16p11.2 observed in the COS cohort, we tested the hypothesis that microduplications in 2p25.3 are associated with schizophrenia through a meta-analysis of public datasets.
MATERIALS AND METHODS
Five published case-control studies were combined from publicly available validated CNV calls. Cohorts, ancestry and microarray platforms are detailed in Table 1. Published .bed files from large population cohorts were collected from each study to examine CNVs at 2p25.3. We performed association tests by comparing the number of variants reported in cases versus controls using Fisher’s exact test.
Table 1.
| Study | cases n | # of dups. | % | controls n | # of dups. | % | size (kb) | UCSC Build HG18 | Odds Ratio | p-val |
|---|---|---|---|---|---|---|---|---|---|---|
| NIMH COS1 | 92 | 2 | 2.17% | 2026*2 | 0 | 0.00% | 143-216 | chr2:1618945-1835426 | NA | 0.002 |
| chr2:1713636-1857129 | ||||||||||
|
| ||||||||||
| other studies | ||||||||||
|
| ||||||||||
| Vrijenhoek et al 20083 | 806 | 2 | 0.25% | 706 | 0 | 0.00% | 967-3800 | chr2:859616-1826716 | NA | 0.5 |
| chr2:1400000-4500000 | ||||||||||
| ISC 20084 | 3391 | 3 | 0.09% | 3181 | 1 | 0.03% | 103-238 | chr2:1712998-1815909 | 2.8 | 0.63 |
| chr2:1712998-1922382 | ||||||||||
| chr2:1716437-1826717 | ||||||||||
| Kirov et al 20095 | 471 | 1 | 0.21% | 2792 | 0 | 0.00% | 484 | chr2:1785886-2269413 | NA | 0.14 |
| Ikeda et al 20106 | 575 | 1 | 0.17% | 564 | 0 | 0.00% | 855 | chr2:987335-1842013 | NA | 1 |
|
| ||||||||||
| Totals | ||||||||||
|
| ||||||||||
| total other studies | 5233 | 7 | 0.13% | 7253 | 1 | 0.01% | 9.7 | 0.01 | ||
| total all 5 studies | 5325 | 9 | 0.17% | 9279 | 1 | 0.01% | 15.7 | 0.001 | ||
|
| ||||||||||
| COS vs. other studies | ||||||||||
|
| ||||||||||
| COS vs. AOS7 | 92 | 2 | 2.17% | 5233 | 7 | 0.13% | 16.6 | 0.01 | ||
Affymetrix 500K arrays and Agilent 185K or 244K CGH (for validation); mixed ancestry; Walsh et al. 2008
Illumina HumanHap 550 arrays; 2/3 Caucasian, 1/3 African-American ancestry; controls from Children’s Hospital Of Philadelphia (CHOP) CNV database (cnv.chop.edu)
Affymetrix 250K or Illumina HumanHap550v3 arrays; Dutch ancestry from GROUP (Genetic Risk and Outcome I Psychosis) Consortium
Affymetrix 5.0 or 6.0 arrays; 7 populations of European ancestry
Affymetrix 500K arrays; WTCCC (Wellcome Trust Case Control Consortium)
Affymetrix 5.0 arrays, Japanese ancestry
COS=childhood onset schizophrenia; AOS=adult onset schizophrenia
RESULTS
The number of CNVs affecting the coding region of the MYT1L gene in cases and controls are listed (Table 1). We compared our NIMH COS patient sample to the 2,026 healthy children sample published by Shaikh et al 2009(Shaikh et al. 2009). Comparing CNVs in the 2p25.3 region, there was a significant excess in the COS sample (p=0.002). While no single study from the published literature revealed a trend for association with duplications in this region, in aggregate, there was a clear excess of duplications in subjects with schizophrenia compared to controls (OR=9.7, p=0.01). Combining all 5 studies, a total of 10 duplications (9 cases; 1 control, Figure 1), and zero deletions were reported (OR=15.7, p=0.001). Further, COS subjects had a significantly higher rate of duplications when compared to AOS subjects (OR= 16.6, p=0.01). The microduplications associated with schizophrenia varied in size from 103 kb to 3.8 Mb, though the minimal region of overlap was in the 1,800,000 to 1,900,000 region of 2p25.3 (UCSC hg18), disrupting the 3′ end of MYT1L. Lastly, all but one of the microduplications observed in the subjects with schizophrenia also impacted the neighboring PXDN gene. Interestingly, a qPCR analysis revealed significant over-expression of MYT1L exon junction 21/22 in patient COS534 (carrying the most restricted duplication), but no differential expression for patient COS1358 versus unaffected controls. Neither patient demonstrated detectable expression for the remaining exons (MYT1L 22/23; 22/24; PXDN exon 1) after 40 cycles under optimized conditions (Supplementary table 1).
Figure 1.
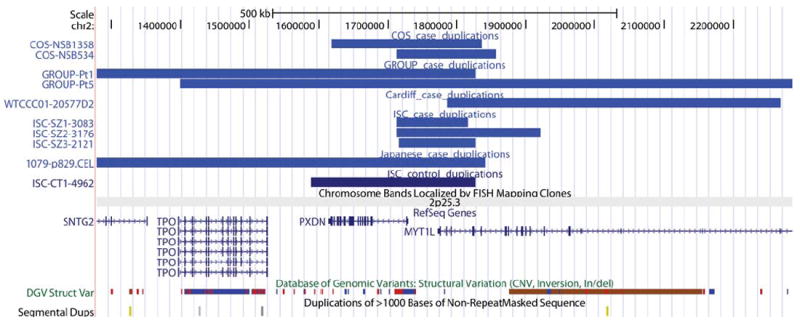
Combination of all 5 studies, a total of 10 duplications (9 cases; 1 control), and zero deletions were reported (OR=15.7, p=0.001).
In terms of inheritance patterns, patient COS-NSB1358 inherited the microduplication from his unaffected mother, but was not transmitted to a healthy sibling. Inheritance for COS-NSB534 was undeterminable as the mother lacked the CNV, and the father was unavailable.
DISCUSSION
We report that microduplications disrupting the MYT1L gene confer a clear increase in risk for schizophrenia. This study provides strong evidence for an association between schizophrenia and MYT1L, whose increase is reported here for the first time and was made possible by meta-analysis of four published studies. The overall rate of 0.17% for this duplication in patients with schizophrenia is comparable with rates observed for other rare CNVs associated with high risk (i.e. 1q21.1 and 22q11.2)(Kirov 2010). In addition, our data suggests even greater prevalence of this microduplication in COS patients as compared to other published large population studies which include adult onset cases (OR=16.6). This increased rate of specific rare CNVs concurs with work from our group on 22q11.2 deletions(Sporn et al. 2004) and 16p11.2 duplications(Addington and Rapoport 2009; McCarthy et al. 2009), which suggests more salient genetic influences in COS compared to AOS(Addington and Rapoport 2009).
The reported microduplications varied in size and breakpoint, likely due to the absence of flanking segmental duplications. Thus nonallelic homologous recombination is the unlikely CNV promoting mechanism for 2p25.3 (though common for other recurrent CNVs associated with neuro-developmental disorder risks (Carvalho et al. 2010; Lupski and Stankiewicz 2005)). Aside from the association with deletions in NRXN1, the affected region is also unique because the duplications affect only 2 genes (MYT1L and PXDN), limiting the number of implicated risk genes. In contrast, other schizophrenia-associated CNV regions contain as many as 28 (16p11.2 duplication) or 40 genes (22q11.2 deletion), making identification of the most relevant genes difficult. However, inference about the functional impact of duplications is more challenging than deletions because of the unknown location and orientation of the duplicated DNA. Thus long-range epistatic effects on gene dosage and aberrant transcription are implicated but not confirmed.
MYT1L is known to be involved in proliferation and differentiation of oligodendrocytes, thus disruption of the coding region could lead to aberrant transcriptional activity throughout the CNS(Kim et al. 1997). Work within our group suggests COS patients exhibit up to 2.2% slower white matter growth rates per year than healthy controls; thereby relating abnormalities in oligodendrocyte function to potential deficits in schizophrenia(Gogtay et al. 2008). Law et al. suggest that MYT1L family genes may regulate Neuregulin1 and modulate synaptic development and function(Law et al. 2006). Work by Vierbuchen et al. also demonstrate that MYT1L, along with ASCL1 and BRN2, can convert mouse fibroblasts directly into functional neurons in vitro(Vierbuchen et al. 2010). The authors hypothesize that changes in transcriptional activity could lead to genome-wide alterations of epigenetic mechanisms affecting DNA methylation and histone modifications. Thus, there is a clear putative role for MYT1L in affecting neuronal cell identity, differentiation and plasticity. The PXDN gene is an extracellular matrix-associated peroxidase thought to affect peroxide driven oxidations, phagocytosis and immune defense. Concurrently, much literature suggests the presence of oxidative stress playing an important role in the physiopathology of schizophrenia(Do et al. 2009), therefore PXDN may be a potential risk gene as well.
In conclusion, current studies suggest that the 2p25.3 locus gives rise to diverse neurodevelopmental phenotypes(Buizer-Voskamp et al. 2011). Further resequencing efforts will be critical in elucidating the role of this gene as a risk factor for schizophrenia. Our findings provide convincing evidence for the role of microduplications involving MYT1L that provide further insight into the genetic basis of schizophrenia.
Supplementary Material
Acknowledgments
YL carried out the newly obtained data acquisition, analyzed the CNV data, performed the gene expression analyses and drafted the manuscript. AM carried out the publicly available data acquisition and prepared the patient histories. RL prepared the lymphoblastoid cell lines and cultured them for RNA extraction. JLR heavily participated in the design of the study and supervised revisions of the manuscript. NG collected the clinical covariates for the study and obtained the assents/consents of the patients and their families. AMA conceived of the study and its design, supervised all analyses, and led the drafting of the manuscript. All authors read and approved the final manuscript.
References
- Addington AM, Rapoport JL. The genetics of childhood-onset schizophrenia: when madness strikes the prepubescent. Curr Psychiatry Rep. 2009;11(2):156–61. doi: 10.1007/s11920-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buizer-Voskamp JE, Muntjewerff JW, Strengman E, Sabatti C, Stefansson H, Vorstman JA, et al. Genome-wide analysis shows increased frequency of copy number variation deletions in dutch schizophrenia patients. Biol Psychiatry. 2011;70(7):655–62. doi: 10.1016/j.biopsych.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, Zhang F, Lupski JR. Evolution in health and medicine Sackler colloquium: Genomic disorders: a window into human gene and genome evolution. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1765–71. doi: 10.1073/pnas.0906222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–41. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19(2):220–30. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, et al. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proc Natl Acad Sci U S A. 2008;105(41):15979–84. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingason A, Rujescu D, Cichon S, Sigurdsson E, Sigmundsson T, Pietilainen OP, et al. Copy number variations of chromosome 16p13.1 region associated with schizophrenia. Mol Psychiatry. 2011;16(1):17–25. doi: 10.1038/mp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Armstrong RC, v Agoston D, Robinsky A, Wiese C, Nagle J, et al. Myelin transcription factor 1 (Myt1) of the oligodendrocyte lineage, along with a closely related CCHC zinc finger, is expressed in developing neurons in the mammalian central nervous system. J Neurosci Res. 1997;50(2):272–90. doi: 10.1002/(SICI)1097-4547(19971015)50:2<272::AID-JNR16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kirov G. The role of copy number variation in schizophrenia. Expert Rev Neurother. 2010;10(1):25–32. doi: 10.1586/ern.09.133. [DOI] [PubMed] [Google Scholar]
- Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, Holmans P, et al. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18(8):1497–503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5’ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103(17):6747–52. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168(3):302–16. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Stankiewicz P. Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005;1(6):e49. doi: 10.1371/journal.pgen.0010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Sacchetti E, Traversa M, Valsecchi P, Gardella R, Bonvicini C, et al. New copy number variations in schizophrenia. PLoS One. 2010;5(10):e13422. doi: 10.1371/journal.pone.0013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41(11):1223–7. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33(5):636–44. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46(10):1418–28. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56(7):649–54. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, et al. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res. 2009;19(9):1682–90. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn A, Addington A, Reiss AL, Dean M, Gogtay N, Potocnik U, et al. 22q11 deletion syndrome in childhood onset schizophrenia: an update. Mol Psychiatry. 2004;9(3):225–6. doi: 10.1038/sj.mp.4001477. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–43. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


