Abstract
Because half of spinal cord injuries occur at the cervical level, we evaluated the therapeutic effect of erythropoietin (EPO) delivered by direct injection of a non-replicating herpes simplex virus (HSV) -based vector coding for EPO (vEPO) in a model of cervical hemicord contusion at C7. One hour after SCI, either vEPO or control vector carrying a reporter gene (vC) was injected into the cord above and below the lesion. Animals injected with vEPO showed a statistically significant improvement in the ipsilateral forelimb function, as measured by open field evaluation of motor performance, paw reaching in the cylinder test and misplacement in grid walk. The was also significant improvement of hindlimb motor function in BBB score and gait analysis using a computer-based system (Noldus Catwalk) compared to control vector-injected animals. The size of the injury-induced cavity and the residual inflammatory/necrotic area in the spinal cord were significantly reduced at the site of injury in vEPO treated animals compared to control vector-injected animals. Microtubule associate protein tau, phosphorylated and non-phosphorylated neurofilament protein (NFH), and the synaptic proteins synaptophysin and PSD95 were all significantly increased in the spinal cord of vEPO treated animals compared to control vector injected animals. These data suggest that gene transfer of EPO after cervical spinal cord injury by minimizing the injury size and enhancing tissue sparing preserves large axons and promote synaptogenesis.
Introduction
Acute traumatic spinal cord injury (SCI) results in devastating loss of neurological function below the level of injury, and while animal studies have frequently examined thoracic or lumbar models, almost 50% of human SCI occurs at the cervical level (Jackson et al., 2004). For these patients, even modest recovery of upper extremity function would have a major impact on the quality of life (Anderson, 2004), but effective pharmacologic treatment is lacking (Georgios K, 2009). Although administration of glucocorticosteroids early after injury may improve recovery (Brines M, 2006), it may also contribute to deleterious effects on early mortality and morbidity (2002).
Erythropoietin (EPO), a cytokine initially characterized for its effects on erythropoiesis has been demonstrated to have remarkable tissue-protective activity in preclinical models of neuronal, retinal, cardiac, and renal ischemic injury (Brines et al., 2004; Grasso, 2004; Grasso et al., 2004). We have previously demonstrated that expression of human EPO achieved by gene transfer using a non-replicating herpes simplex virus (HSV)-based vector has protective effects in rodent models of Parkinson’s disease (Puskovic et al., 2006) in the central nervous system, and can protect against the progression of neuropathy in mice with diabetes (Chattopadhyay et al., 2009).
Injury to the spinal cord after contusion occurs in two phases: an acute phase reflects acute mechanical damage, and a later secondary phase (Zai et al., 2005) that involves a complex cascade of molecular events including disturbances of ionic homeostasis, local edema, focal hemorrhage, excitotoxicity, effects of the presence of free radicals and free fatty acids, and activation of an inflammatory response. In the current study we investigated the effects of EPO delivered by injection of a non-replicating HSV-based vector expressing rat EPO (vEPO) one hour after acute asymmetric contusion of the cervical spinal cord. We found that vector-mediated expression of EPO reduced tissue damage and improved forelimb and hindlimb motor function, and that the improvement in behavioral measures coincided with increase levels of axonal cytoskeletal proteins and synaptic proteins in the cervical spinal cord as compared to animals treated with control vector.
Materials and Methods
Vector Construct
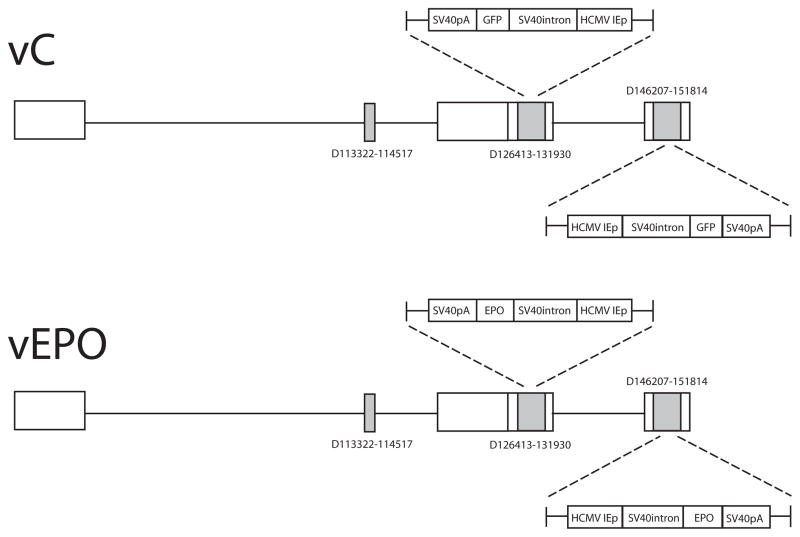
Full length rat EPO was amplified from a cDNA library prepared from total RNA extracted from rat lung, cloned into BamH1-EcoR1 cut HCMV-polyA/SASB3–16 and co-transfected with the nonreplicating HSV recombinant UL41E1G6 on 7b cells, a complementing cell line derived from African green monkey kidney (Vero) cells modified to provide the essential ICP4 and ICP27 gene products in trans. Clones were selected by identification of clear plaques, purified by limiting dilution and the EPO insert confirmed by PCR followed by DNA sequencing. The clone which expressed the highest levels of EPO on infection of 293 cells (designated vEPO) was propagated to high titer (4 × 1010 pfu/ml), and used in the experiments. Control vector vC is identical to vEPO except that a reporter gene for green fluorescent protein (GFP) or Lac Z was inserted in the expression cassette in place of the EPO gene (Fig. 1). These backbone of these non-replicating HSV constructs also differ from the non-replicating HSV recombinants used in the previous studies in that vector is defective in expression of 4 immediate early gene products and the transgene expression cassette has been placed into the ICP4 locus; similar to the HSV recombinant expressing preproenkephalin that is currently in clinical trial in patients with intractable pain from cancer (Wolfe et al., 2009).
Fig. 1.
Animal Studies
Female Sprague–Dawley rats weighing 225–250 g were housed one to four per cage approximately 7 days prior to the beginning of the study, with free access to food and water and maintained on a 12:12, light:dark schedule at 21° C and 60% humidity. All housing conditions and experimental procedures were carried out in accordance with approved institutional animal care and use protocols. Under isoflurane anesthesia a C6–8 vertebral laminectomy was performed, exposing the C7 spinal cord segment without opening of the dura, and 160 Kdyne blunt force trauma was applied asymmetrically to the right dorsum of C7 spinal cord using a 1.5 mm diameter probe controlled by a computerized impactor device (Infinite Horizons, Precision Systems and Instrumentation, Lexington, KY). One hour after injury 1 μl containing 4×1010 plaque forming units (pfu) of either vEPO or vC was injected above the lesion, and an additional 1 μl injected below the lesion on right side of the spinal cord at a rate of 0.5 μl/min (total injected volume 2 μl). Sham animals underwent the same spinal cord exposure but no injury or injection was performed. For the behavioral analysis, there were 8 animals in each SCI group (vEPO and vC) and 4 sham controls. At the conclusion of the behavioral experiments (8 weeks after injury), animals were perfused through the heart with 100 ml of phosphate-buffered saline (PBS) followed by 500 ml of 4% paraformaldehyde in PBS. The spinal cord was removed, postfixed at 4°C overnight, and cryoprotected with 30% sucrose in PBS. For protein analysis, 2 and 8 weeks after injury animals were perfused through the heart with 100 ml of PBS and spinal cord removed and quickly frozen in dry-ice.
Behavioral assessment
Forelimb motor function was evaluated using the following tests:
Open-field testing
The open-field environment consisted of a circular plexiglas enclosure 95 cm in diameter, 40 cm high with an anti-skid floor. Rats were tested in pairs in a shadowy light to encourage locomotor activity. Animals that remained stationary for a period longer than 20 sec were picked up and placed at the center of the open-field arena to reinitiate locomotion. All rats were scored over a time window of a maximum duration of 4min, although the observation period was sometimes extended to make sure that the animals’ abilities were not underestimated.
Limb-use asymmetry test (Cylinder test)
Forelimb use during explorative activity was analyzed by putting rats in a transparent cylinder (20 cm diameter and 30 cm height) for 5 min or 20 touchs during the trial. A mirror was placed behind the cylinder at an angle to enable the rater to record forelimb movements when the animal was turned away from the rater. The cylindrical shape encourages vertical exploration of the walls with the forelimbs as well as landing activity.
Measurement of forelimb placing
Forelimb placing asymmetry was scored using the vibrissae-elicited forelimb placing test (Schallert et al., 2000). Animals were held by their torsos allowing forelimbs to hang free. While holding the animal, the experimenter made gentle up and down movements in space prior to place testing, which facilitated muscle relaxation and eliminated any struggling movements. Independent testing of each forelimb was induced by brushing the respective vibrissae on the edge of a table top once per trial for 10 trials. Intact animals place the forelimb of both sides quickly onto the countertop. Animals with unilateral damage, depending on the lesion site, will show varying degrees of impaired limb placing ability, while still placing the unimpaired limb reliably. A score of one was given each time the rat placed its forelimb on the edge of the tabletop in response to the vibrissae stimulation. Percent unsuccessful placing responses were determined (number incorrect ×10) for impaired forelimb and non-impaired responses. Typically, animals place successfully on 100% of the trials with the unimpaired limb; therefore, only the responses of the impaired limb are shown.
Gridwalk
The grid walk test was initially developed to assess the ability of the animal to precisely control hind paw placement (Bresnahan et al. 1987; Kunkel-Bagden et al. 1992). We used a runway with regularly spaced horizontal bars 1.0 cm apart. Errors were coded when the animal misplaced either the forepaw or the hindpaw, such that the paw fell through the space in the grid, up to a maximum of 20 missteps. Animals that did not display at least frequent stepping in the BBB test were unable to cross the walkway and were assigned the maximum score.
Hindlimb motor function was evaluated using the following tests:
Basso–Beattie–Bresnahan (BBB) test
Hindlimb motor behavior was assessed once a week after injury for 5 weeks. Animals were trained to walk in an open area and the hindlimb movement rated on a 21-point scale, with a score of 0 corresponding to no observable hind limb movement and a score of 21 given when plantar stepping toe clearance, trunk stability, coordinated limb movement, and an erect tail was present (Basso et al., 1995).
‘CatWalk' automated gait analysis
The animals traverse a walkway (plexiglass walls, spaced 8 cm apart) with a glass floor (100 × 15 × 0.6 cm3) (length × width × thickness) located in a darkened room. Light from an otherwise completely encased white fluorescent tube (length 110 cm, 30 W) enters the distal (from the observer) long edge of this glass floor. Sufficiently far from the edge, it strikes the surface below the critical angle and is entirely internally reflected. Only at those points where a paw touches the glass, light exits the floor and scatters at the paw, illuminating the points of contact only. Via a mirror, the corridor’s floor is monitored by a Pulnix TM-765E CCD camera (Pulnix Inc., UK) equipped with a wide angle objective (Cosimar 8.5 mm).
Primary cell culture
Primary spinal cord neurons from E17 rat pups were plated on 12 well plates coated with poly-D-lysine, at a concentration of 1.5×106 cells per well and cultured in Neurobasal media containing B27, Glutamax I, Albumax II, and penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). At 14 days in vitro (DIV) vectors vEPO or vC at a multiplicity of infection (MOI) of 2 were added to the well for 2 hrs. Two and three days after infection cell lysates of spinal cord neurons and their culture media were collected for Western blot analysis and ELISA determination of transgene expression. Each experiment was repeated three times.
Western Blot
10 mm in length of spinal cords around epicenter were dissected and homogenized in lysis buffer containing 50 mM Tris, 10 mM NaCl, 1% Nonidet P-40, 0.02% NaN3, protease inhibitor, and phosphatase inhibitor mixtures (Sigma) at pH 7.4. Cultured E17 spinal cord neurons were collected in the same lysis buffer after being dislodged from the culture plates with a cell scraper. Cell lysates and tissue homogenates were sonicated and centrifuged at 15,000 × g for 10 min at 4 at 15,000ere so DRG cell cultures was centrifuged at 1,000 × g for 10 min at 4 °C. Protein concentration in tissue homogenates and cell lysates was determined using the BCA assay (Pierce) and spectrophotometry (AD340; Beckman Coulter). Aliquots containing 20 μg of protein were dissolved in Laemmli buffer and boiled at 95 vC for 5 min.
Proteins were separated on 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and then transferred onto a polyvinylidene diflouride membrane (Millipore, Medford, MA, USA). Immunoblots were probed with primary antibody to anti-HA (Sigma, Saint Louis, MO, USA), anti-synaptophysin (Millipore Corporation, Billerica, MA, USA), anti-PSD-95 (Millipore Corporation, Billerica, MA, USA), anti-phosphorylated neurofilament H and tau (SMI31 Covance Corporation, Emeryville, CA, USA), anti-nonphosphorylated neurofilament H (SMI32 Covance Corporation, Emeryville, CA, USA), anti-GAPDH (Millipore Corporation, Billerica, MA, USA), or anti-β-actin (Santa Cruz Biotechnology), then incubated with horseradish peroxidase-conjugated secondary antibody, followed by enhanced chemiluminescence detection (Amersham Biosciences, Arlington Heights, IL, USA). Chemiluminescence detection values were used to quantitate the Western blot results, and the value of the protein of interest normalized to the appropriate internal control. Each in vitro experiment was repeated four times and each animal experiment represented the results of samples from eight different animals. Data are presented as mean ± SEM.
Enzyme-linked Immunosorbent Assay
The amount of EPO released in vitro of produced in vivo was determined using a commercially available ELISA kit (R&D System). Each of the experiments was repeated four times.
Semi-quantitative RT-PCR
Total RNA was isolated from rat spinal cord via Trizol (Invitrogen, Carlsbad, CA, USA). cDNA prepared from mRNA isolated from rat spinal cord was amplified using following primer sets: b-actin-forward (5′-CAG TTC GCC ATG GAT GAC GAT ATC-3′) and b-actin-reverse (5′-CAC GCT CGG TCA GGA TCT TCA TG-3′) for β-actin, EPO-forward (5′-CCG GAA TTC GCC AGG CGC GGA GAT G-3′) and EPO-reverse (5′-CGG GAT CCT CAA GCG TAA TCT GGA ACA TC-3′) for EPO. Amplification was carried out by denaturation at 94° C for 5 min followed by 28 cycles (94° C for 30 sec, 68° C for 3 min, and 1 cycle 68° C for 8 min) using a GeneAmp PCR 2700 (Applied Biosystems, Foster City, CA). Each in vitro experiment was repeated four times and each animal experiment represents the results of samples from five different animals. Data are presented as mean ± SEM.
Measurement of lesion cavity
At 8 weeks post-injury, cervical spinal cord was removed, post-fixed and cryoprotected and sectioned using a cryostat (Leica). Twenty μm serial sections of spinal cord were thaw-mounted onto cold Superfrost glass slides (Fisher Scientific), heated at 37° C and stained with hematoxylin–eosin following standard protocol. Five serial sections were selected every 10 sections at the epicenter of the lesion. The area of the cavity containing tissue damage was determined from images captured using a Nikon Eclipse E1000 microscope equipped with a Plan Apo2X/0.1 lens using Metamorph 7.0 software (Molecular Devices, Palo Alto, CA).
Immunohistochemistry
Similar serial cryosections through the epicenter of the lesion, as described above, from 8 week post-injury cervical spinal cords of vEPO and vC treated animals were examined for CD-45 expression using a monoclonal antibody (1:1000; Invitrogen) followed by complementary secondary tagged fluorescent antibody (Alexa Fluor 488; Invitrogen).
Statistical analysis
The statistical significance of the difference between groups for the in vitro and in vivo western results was determined by one-way ANOVA (SPSS 12.0) using Bonferonni’s correction for the multiple post hoc analyses. Parametric statistics using the general linear model for repeated measures were used to identify significant effects of treatment conditions on the behavioral analysis of motor function (SPSS 18.0). The statistical significance of the difference between groups for the neurite length measurements was determined using the Student’s t-test. All results were expressed as mean ± SEM with p < 0.05 considered significant.
Results
Characterization of the EPO-expressing HSV-vector vEPO
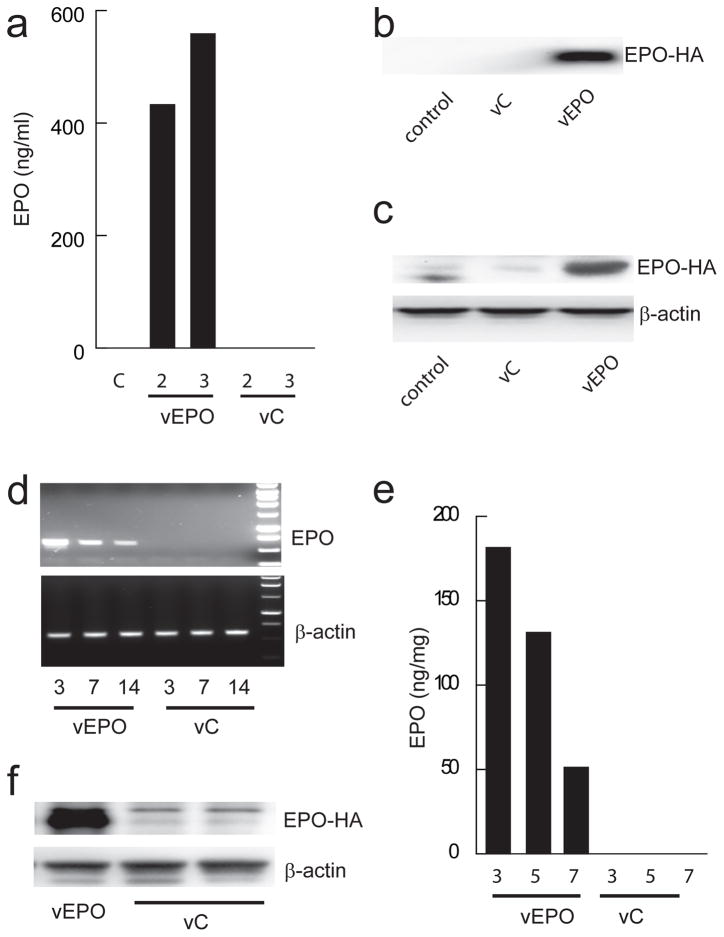
Spinal cord neurons in vitro transfected with vEPO (MOI 1) showed expression of HA tagged EPO protein in cell lysate detected by Western blot at 72 hours post-infection using anti-HA antibody to detect only the transgene (Fig. 2a). Release of EPO from the cells transfected with vEPO at 48 hrs after transfection was demonstrated in culture media by Western blot (Fig. 2b) and quantitated by ELISA (Fig. 2c). No transgene EPO was detected under basal conditions or from cells transfected with vC. In vivo, injection of 2 μL of vEPO into the cervical spinal cord resulted in robust EPO mRNA expression that was sustained for more than 14 days (Fig. 2d). The expression of HA-tagged EPO protein was confirmed by Western blot (Fig. 2f).
Fig. 2.
Intraspinal injection of vEPO reduces post-traumatic cavity and inflammatory response
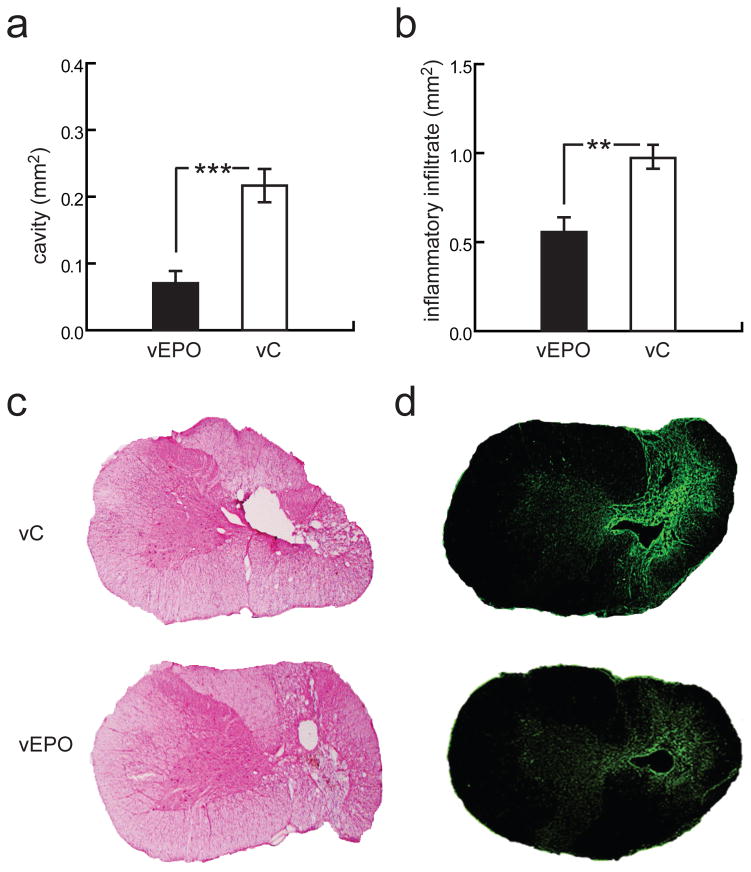
SCI injury was induced by asymmetric contusion on the right dorsal spinal cord at C7 level. The degree of tissue injury and tissue sparing resulting from vector-mediated EPO expression was determined by analysis of H&E stained serial sections of spinal cord. Animals treated with vEPO had significantly reduced post-traumatic cavity area within the spinal cord demonstrating decreased tissue damage as compared with animals treated with vC (vEPO = 0.07 ± 0.03 mm2 and vC = 0.22 ± 0.04 mm2; p < 0.001) (Fig. 3a, c). The area of inflammatory infiltrate was determined by immunohistochemistry using an anti CD-45 (leukocyte common antigen) monoclonal antibody. Animals treated with vEPO had significantly reduced residual area of post-traumatic inflammatory response as compared with animals treated with vC (vEPO = 0.56 ± 0.16 mm2 and vC = 0.97 ± 0.12 mm2; p < 0.01) (Fig. 3b, d).
Fig. 3.
Animals treated with vEPO show improved forelimb motor function after SCI
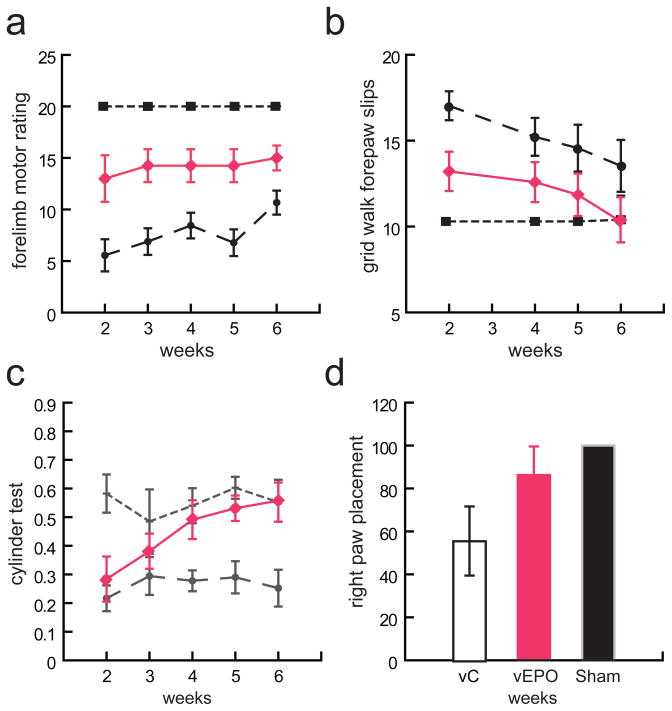
Right forelimb function was assessed by modified forelimb motor rating scale, forepaw slips in the gridwalk, paw reaching and preference in the cylinder test, and in the paw placement test. Animals injected intraspinally vEPO 1 h after SCI showed a significantly higher forelimb rating score, beginning at 2 week after injury and persisting over the course of 6 weeks post-injury compared with the animals injected with control vector (Fig. 4a). A significant improvement in forelimb stepping accuracy in the grid walk test which was seen already at 2 weeks in animals treated with vEPO compared to control (Fig. 4b), Both VEPO and vC treated improved over 6 weeks of testing but vEPO treated animals performed persistenly better that vC treated animals. Analysis of paw reaching in cylinder test was also markedly improved and at 4, 5 and 6 weeks the forelimb performance was similar to sham animals (Fig. 4c). However the paw placement determined at 6 weeks after injury although improved in vEPO treated animals did not reached statistical significance as the test produced significant variability within treatment cohorts (Fig. 4d).
Fig. 4.
Intraspinal injection of vEPO improves hind limb locomotor function after SCI
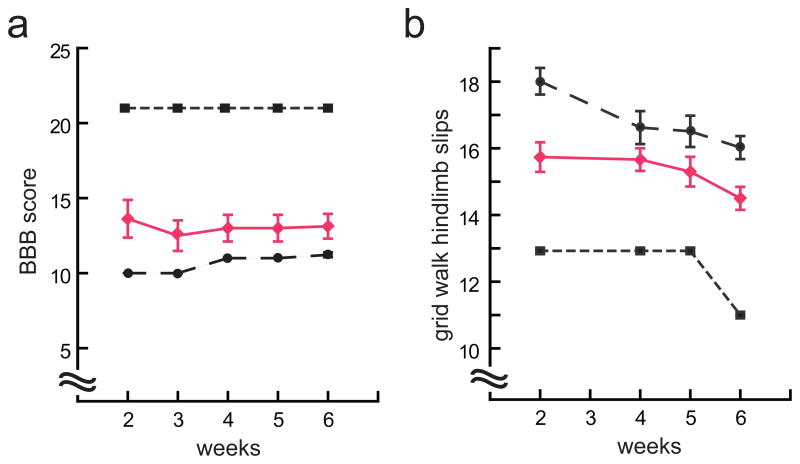
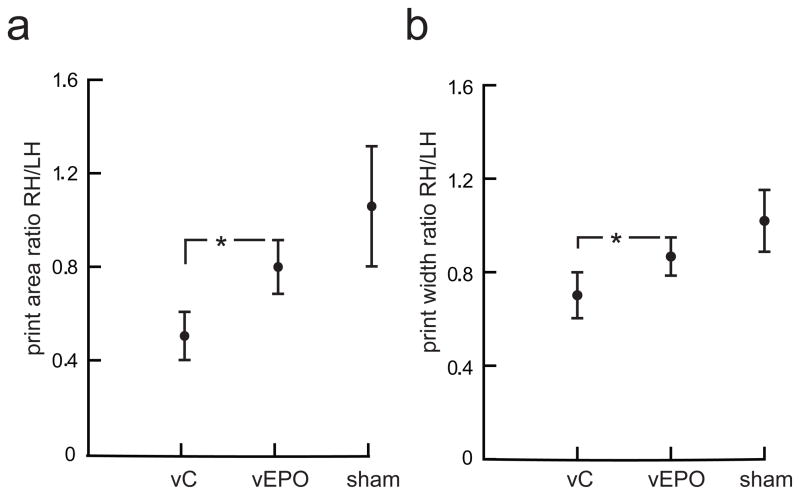
In addition to improvement in forelimb function, significant improvement in hindlimb function was observed in animals injected with vEPO compared with the animals injected with control vector. Beginning at 2 weeks after injury, animals injected with showed significantly higher hind limb BBB score that persisted over the course of 6 weeks post-injury compared with animals injected with control vector (Fig. 5a). A significant improvement in right hindlimb stepping accuracy in the grid walk test was seen beginning at 2 weeks and persisting up to 6 weeks (Fig. 5b). Hindpaw print area and hindpaw width, ratios determined by computerized digital gait instrument (Catwalk, Noldus) at 7 weeks after spinal cord injury were similarly improved in vEPO-injected compared to control vector-injected animals (Fig. 6).
Fig. 5.
Fig. 6.
Biochemical measures of recovery
Neurofilaments (NF), composed of NF-L, NF-M and NF-H subunits, are the most abundant structural components in large diameter myelinated axons (Lee and Cleveland, 1996). Unlike other intermediate filaments, neurofilaments have characteristic “side arms” extending from the filament backbone that form bridges between filaments and which sequences are rich in serine and threonine residues. These KSP repeats in the carboxy-tails of NF-H and NF-M are recognized in their phosphorylated or dephosphorylated states by the antibodies SMI 31 and SMI 32 respectively.
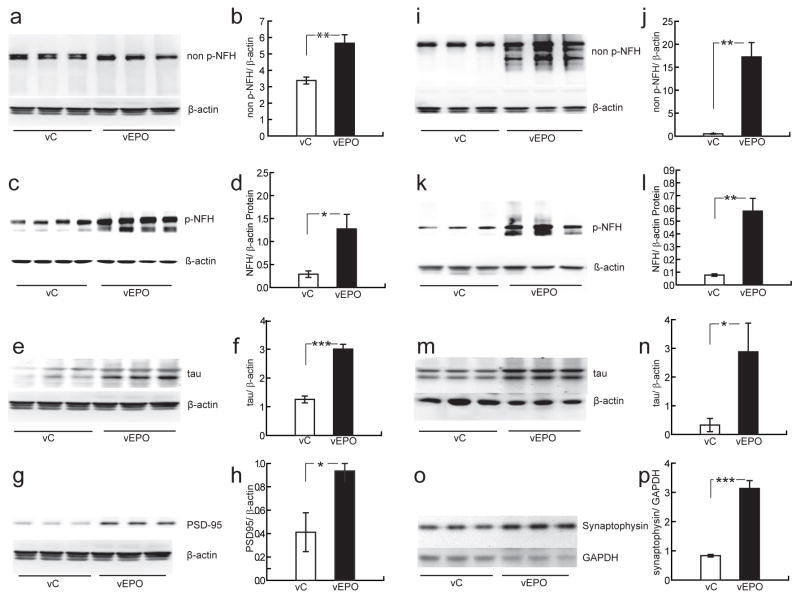
The expression of cytoskeletal and synaptic proteins were evaluated in cervical spinal cords at the site of injury of animals sacrificed either 2 weeks or 8 weeks post injury. vEPO treated animals had significantly higher levels of cytoskeletal proteins known to be most abundant in large myelinated axons including non phosphorylated and phosphorylated neurofilament H subunit and phosphorylated microtubule associated protein Tau, at 2 and 8 weeks post-injury compared with vC (Fig. 7). Increased synaptic proteins PSD95, scaffolding protein anchoring glutamate receptors to the postsynaptic site, and synaptophysin, associated with synaptic vesicles at the presynaptic site, were observed at 2 weeks and 8 weeks respectively suggesting enhanced synaptic connectivity in vEPO treated animals (Fig. 7).
Fig. 7.
Discussion
We have used extensive behavioral testing of forelimb function to demonstrate that vector-mediated delivery of EPO shortly after cervical spinal cord injury results in substantial improvement in forelimb motor behavior. Upper extremity and hand weakness remains a critical problem for patients with cervical injuries as limitations in activities of daily living and ability to care for oneself become severely curtailed. We have also observed a mild improvement in hindlimb locomotor function. The improvement induced by vEPO occurred early after treatment, suggesting that by minimizing the injury burden during the acute phase may underlie the early recovery of function that later translates in better preservation of spinal cord tissue or enhanced tissue sparing. The use of EPO after acute injury has been supported by the fact that in spinal cord EPO receptor (EPOR) is highly expressed in capillaries, especially in the white matter, in glial and neuronal elements including motor neurons of the ventral horn and during development or after injury synthesis of EPO lags temporally behind expression of EPOR (Bernaudin et al., 1999; Celik et al., 2002). The biological roles play by EPO in the nervous system following injury include preservation of vascular integrity, decrease bleeding time and neovascularization, suppression of mononuclear cells infiltration and cytokine production, as well as prevention apoptotic cell death (Brines and Cerami, 2005).
Neurofilament L, M and H subunits are prominent components of the axonal cytoskeleton and the number increase with axon caliber. Phosphorylated carboxy-tails of neurofilament H has been demonstrated to contribute to the expansion of large diameter axons as they are most highly present in the internodal axolemma of normal large myelinated axons and are reduced or lacking in small myelinated and unmyelinated fibers (Hoffman et al., 1984; Mata et al., 1992; Elder et al., 1998). The biochemical data in the current report supports the conclusion that vEPO preserves large myelinated fibers, as indicated by phosphorylated cytoskeletal proteins at times that are presumed too early for substantial regeneration of large caliber axons and most likely represents reduce early damage and enhanced tissue sparing along the white matter tracts outlining the injury site.
The effect of vEPO on synaptic proteins may suggest enhanced synaptic connectivity within the gray matter near the lesion site as sprouting and short axon growth has been previously reported within similar time frame following SCI with different treatment modalities. In particular EPO has been shown to enhance axon and dendrite growth in hippocampal neurons during early stages of development and promoted outgrowth of dopaminergic neurons projecting to the striatum (Puskovic et al., 2006; Ransome and Turnley, 2008).
Administration of recombinant EPO (rEPO) has been used in animal models of acute thoracic SCI with significant success (Kwon et al.) that has led to the initiation of clinical trials (Siren et al., 2009). Our studies are the first to: 1) demonstrate the beneficial effects of EPO in cervical spinal cord injury; and 2) use direct application of an EPO expressing vector instead of systemic delivery. The importance of enhancing EPO expression at the injury site is the avoidance of complications resulting from systemic delivery including thrombocytosis, erythrocytosis, hypertension and others that may pose clinical risk and hamper recovery. While recombinant EPO administered systemically has a half life of hours and removal occurs via hepatic and renal clearance, vEPO has prolonged expression, with an initial peak of expression within the first 24h and decreasing but still detectable at 8 weeks post SCI and vector delivery. This time course may be important because pro-inflammatory cytokines induced EPOR expression and the up-regulation of EPO receptor expression may persists several weeks following SCI (Nagai et al., 2001; Grasso et al., 2005). The current results add to the body of literature supporting that early and sustained delivery of erythropoietin following SCI may have important contributions towards clinical recovery.
The therapeutic window of opportunity for EPO following cervical SCI has not been established neither its potential effects on long distance axonal regeneration. These parameters are under consideration as we have recently published therapeutic applications of an HSV vector expressing EPO under a control of a regulatable expression cassette (Wu et al.). A similar HSV vector with a regulatable expression cassette using the latency associated HSV promoter (LAT) for long term expression of EPO is currently being tested in animal models of chronic disease and may be an appropriate therapeutic tool for cervical SCI.
References
- Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50:S63–72. doi: 10.1097/00006123-200203001-00013. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Walter C, Mata M, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009;132:879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Friedrich VL, Jr, Kang C, Bosco P, Gourov A, Tu PH, Zhang B, Lee VM, Lazzarini RA. Requirement of heavy neurofilament subunit in the development of axons with large calibers. J Cell Biol. 1998;143:195–205. doi: 10.1083/jcb.143.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G. An overview of new pharmacological treatments for cerebrovascular dysfunction after experimental subarachnoid hemorrhage. Brain Res Brain Res Rev. 2004;44:49–63. doi: 10.1016/j.brainresrev.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10:93–98. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Passalacqua M, Morabito A, Buemi M, Macri B, Brines ML, Tomasello F. Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery. 2005;56:821–827. doi: 10.1227/01.neu.0000156493.00904.7e. discussion 821–827. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Griffin JW, Price DL. Control of axonal caliber by neurofilament transport. Journal of Cell Biology J1 - JCB. 1984;99:705–714. doi: 10.1083/jcb.99.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AB, Dijkers M, Devivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil. 2004;85:1740–1748. doi: 10.1016/j.apmr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Kwon SY, Won SC, Han JW, Shin YJ, Lyu CJ. Feasibility of sequential high-dose chemotherapy in advanced pediatric solid tumors. Pediatr Hematol Oncol. 27:1–12. doi: 10.3109/14992020903352226. [DOI] [PubMed] [Google Scholar]
- Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- Mata M, Kupina N, Fink DJ. Phosphorylation-dependent neurofilament epitopes are reduced at the node of Ranvier. Journal of Neurocytology. 1992;21:199–210. doi: 10.1007/BF01194978. [DOI] [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- Puskovic V, Wechuck J, Krisky D, Collins J, Glorioso JC, Fink DJ, Mata M. HSV-mediated delivery of erythropoietin restores dopaminergic function in MPTP-treated mice. Mol Ther. 2006 doi: 10.1016/j.ymthe.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci. 2008;38:537–547. doi: 10.1016/j.mcn.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009;6:108–127. doi: 10.1016/j.nurt.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Mata M, Fink DJ. A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther. 2009 doi: 10.1038/gt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Mata M, Fink DJ. Prevention of Diabetic Neuropathy by Regulatable Expression of HSV-Mediated Erythropoietin. Mol Ther. doi: 10.1038/mt.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai LJ, Yoo S, Wrathall JR. Increased growth factor expression and cell proliferation after contusive spinal cord injury. Brain Res. 2005;1052:147–155. doi: 10.1016/j.brainres.2005.05.071. [DOI] [PubMed] [Google Scholar]