Abstract
Background
Defibrillation therapy for atrial fibrillation (AF) and flutter (AFl) is limited by pain induced by high-energy shocks. Thus, lowering the defibrillation energy for AFl/AF is desirable.
Objective
In this study we apply low voltage multiple shock defibrillation therapy in a rabbit model of atrial tachyarrhythmias comparing its efficacy to single shocks and antitachycardia pacing (ATP).
Methods
Optical mapping was performed in Langendorff-perfused rabbit hearts (n=18). Acetylcholine (7±5–17±16 μM) was administered to promote sustained AFl and AF, respectively. Single and multiple monophasic shocks were applied within 1 or 2 cycle lengths (CLs) of the arrhythmia.
Results
We observed AFl (CL=83±15 ms, n=17) and AF (CL=50±8 ms, n=11). ATP had a success rate of 66.7% in the case of AFl, but no success with AF (n=9). Low voltage multiple shocks had 100% success for both arrhythmias. Multiple low voltage shocks terminated AFl at 0.86±0.73 V/cm (within 1 CL) and 0.28±0.13 V/cm (within 2 CLs), as compared to single shocks at 2.12±1.31 V/cm (p<0.001) and AF at 3.46±3 V/cm (within 1 CL), as compared to single shocks at 6.83±3.12 V/cm (p=0.06). No ventricular arrhythmias were induced. Optical mapping revealed that termination of AFl was achieved by a properly timed, local shock-induced wave which collides with the arrhythmia wavefront, whereas AF required the majority of atrial tissue to be excited and reset for termination.
Conclusion
Low voltage multiple shock therapy terminates AFl and AF with different mechanisms and thresholds based on spatiotemporal characteristics of the arrhythmias.
Keywords: atrial flutter, atrial fibrillation, optical mapping, defibrillation, antitachycardia pacing
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia in the world, affecting over 2.2 million people in the United States. A recent population study estimates that by the year 2050, over 12 million Americans may be affected.1 Complications of AF include thromboembolic stroke, congestive heart failure, and increased mortality.2, 3 In addition, AF leads to more hospitalizations than any other arrhythmia.4 Treatment strategies are designed to reduce the frequency and duration of arrhythmia episodes, as it is known that AF begets AF.5
AF management strategies include antitachycardia pacing (ATP) and cardioversion. ATP delivers a train of stimuli coordinated with the arrhythmia cycle length (CL) that attempts to disrupt the circuit(s) maintaining AF or AFl, however its success rates are around, or less than, 60%.6 External cardioversion boasts success rates of up to 97%,7 but requires high shock energies and sedation of the patient. On the other hand, the use of intracardiac shocks, by the implantable atrial defibrillator (IAD),8 requires less energy and patient sedation.9 Although initial clinical trials have shown that the IAD has a high specificity and sensitivity to AF and delivers safe and effective shocks,10 it has not gained widespread acceptance because the energy for successful endocardial cardioversion still exceeds the pain threshold.11 Thus, lowering the defibrillation energy is highly desirable.
In a recent study, we applied a method for low voltage termination of ventricular tachycardia in a rabbit whole heart model and found a significant reduction in the defibrillation threshold.12 In this study, we aim to apply this therapy to a rabbit model of AFl and AF. However, important distinctions between AFl and AF must be considered.13 AFl is defined by a macro-reentrant circuit, which can rotate around an anatomic or functional, line of block. Major anatomical structures, including the region between the vena cavae and the pulmonary veins, are usually involved in defining reentry.14 AF, on the other hand, involves multiple reentrant circuits defined primarily by local excitability and refractory periods. In this study, we hypothesize that low voltage multiple shock therapy will terminate anatomically-defined AFl with higher efficacy than functionally-defined AF, due to its ability to efficiently penetrate the longer excitable gap associated with AFl and, consequently, upin reentry.
Methods
Experimental Preparation
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Washington University. Experiments were performed in vitro on hearts obtained from New Zealand white rabbits (n=18) of both sexes. The isolation procedure has been described previously.15 The whole heart was placed posterior side up in a temperature-controlled bath (Radnoti, Monrovia, CA) at 37°C and Langendorff-perfused under constant pressure (60 mmHg) with oxygenated Tyrode’s solution. The excitation-contraction uncoupler blebbistatin15 (10 μM, Tocris Biosciences, Ellisville, MO) and the voltage-sensitive dye, di-4-ANEPPS (10 μM, Molecular Probes, Eugene, OR) were added to the perfusate.
Optical Mapping and Defibrillation Setup
During a 20–30 minute equilibration period, bipolar electrodes, which could be switched between pacing and sensing, were placed on the left atrial appendage (LAA), right atrial appendage (RAA), and right atrial (RA) free wall. Shocks were applied from two stainless steel mesh electrodes placed 10 cm apart connected to the pulse generator as previously described.16 Optical mapping was performed with a 100x100 pixel MiCAM Ultima-L CMOS camera (SciMedia USA).
Experimental Protocol
After optimization of the optical signals, the intrinsic activity of each preparation was recorded. Pacing thresholds and effective refractory periods (ERP) from all locations were determined. Far field stimulation thresholds as a result of shocks were also determined for both polarities (n=7).
Programmed stimulation was applied to initiate an arrhythmia by a Master-8 programmable stimulator (A.M.P.I, Jerusalem) and a custom-written stimulation interface. Programmed stimulation consisted of either an S1–S2 protocol or burst pacing. If arrhythmia initiation was unsuccessful, incremental concentrations of acetylcholine chloride (ACh, 1–100 μM, Sigma-Aldrich, St. Louis, MO) were added until a sustained arrhythmia was induced.17 A sustained arrhythmia was defined as lasting at least 2–3 minutes, although if not terminated, the arrhythmias would persist on the order of an hour. Single or multiple (3–8) 5–10 ms monophasic shocks were applied equally spaced out within either one or two CLs of the arrhythmia (n=18), after the average CL of each arrhythmia episode was calculated. In addition, we also applied ATP consisting of 8 pulses at 50–100% of the arrhythmia CL from either the RAA or lower RA (n=9). If the arrhythmia was terminated by either the shock or ATP, a new arrhythmia was immediately reinitiated as described above. Success was defined as termination of the arrhythmia within three seconds of therapy application.
Data Analysis
A custom-designed Matlab-based computer program, described previously,14 was used to analyze experimental data offline. Activation maps were constructed from activation times calculated from the dV/dtmax of each pixel. Quantitative data are expressed as mean±standard deviation. The Student’s T Test was used to determine statistical significance (p<0.05).
Results
Initiation of Arrhythmias
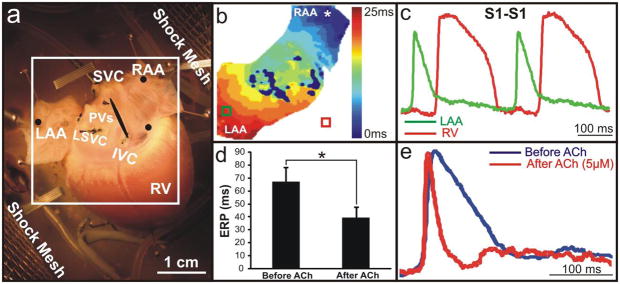
Figure 1a shows an optical mapping field of view encompassing the posterior side of the atria, including both the RAA and LAA. Figure 1b shows an example activation map while pacing the RAA (CL=300 ms), with the corresponding OAPs shown in Figure 1c. Upon addition of an ACh concentration needed for the induction of a sustained arrhythmia, the atrial ERP was reduced from 67±11 ms to 40±8 ms (p<0.001) (Figure 1d). Figure 1e illustrates the significant shortening of the action potential duration, establishing the functional substrate necessary for inducing and maintaining AFl/AF.
Figure 1. Experimental Setup.
(a) Photograph of a typical preparation with shock meshes labeled, located 10 cm apart. The black circles represent the locations of pacing/sensing electrodes. (b,c) Typical activation map and optical action potentials (OAPs) during pacing of the RAA (300 ms). (The deep blue areas in the center represent pixels that have been excluded from analysis due to noise from sutures and connective tissue.) (d) Average ERP before and after ACh. (e) Representative OAPs from the RAA before and after the addition of 5 μM ACh reflecting a 57% decrease in the action potential duration.
Upon addition of 7±5 μM ACh, we induced AFl maintained by a single macroreentrant circuit (CL=83±15 ms, n=17) rotating around the anatomic zone of conduction block between the superior and inferior vena cava (Figure 2a, Online Movie 1). Figure 2b and Online Movie 2 show that AF was maintained by multiple reentry circuits (CL=50±8 ms, n=11) with an average ACh concentration of 17±16 μM. Dominant frequency analysis reflected the major rotors maintaining each arrhythmia as illustrated in Figures 2c and 2d. In only one heart out of 18 were we unable to induce any sustained arrhythmias even with the addition of 500 μM ACh. Additionally, in three preparations we were able to initiate AFl before the addition of ACh, although the arrhythmias were sustained enough in only two preparations in which we performed limited defibrillation testing.
Figure 2. Typical Atrial Flutter and Fibrillation.
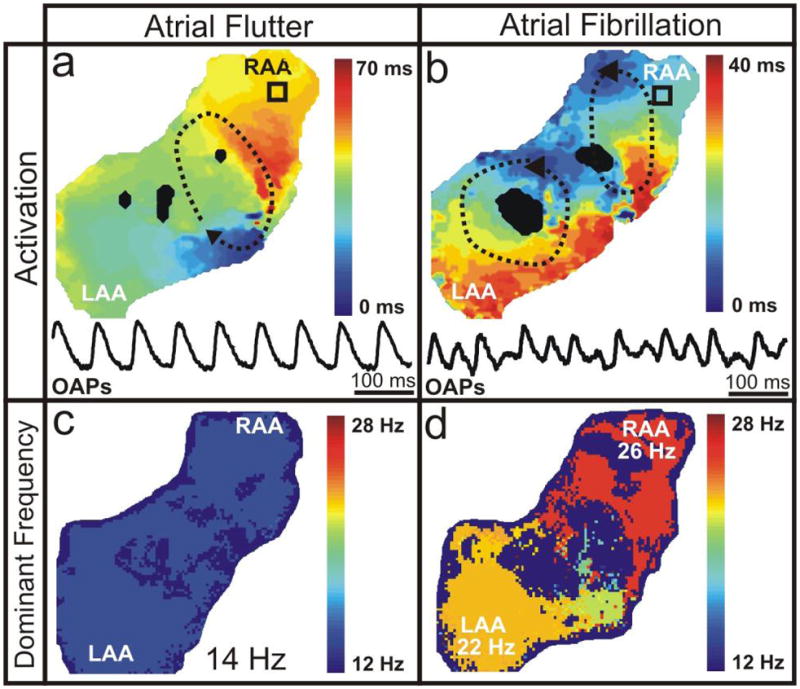
(a,b) Typical activation maps, representative of one arrhythmia cycle, and OAPs (as indicated by the black squares) of AFl and AF, respectively. Dashed black lines with arrows show the location and direction of the reentrant circuits. (c,d) Frequency distribution for typical examples of AFl and AF, respectively.
Application of ATP
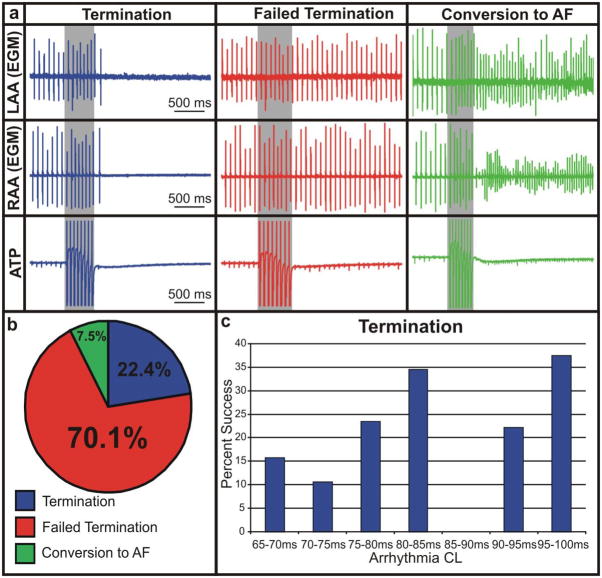
ATP was applied in nine hearts from an electrode placed on either the RAA or lower RA, similar to clinical RA pacing leads. We applied 107 rounds of ATP from both locations consisting of 8 pulses at 50–100% of the arrhythmia CL (Figure 3). Figure 3b shows that 22.4% of ATP applications to AFl were successful in terminating the arrhythmia. However, 70.1% of applications had no effect on the arrhythmia and 7.5% of applications converted AFl to AF. Therapy applied at 80% of the arrhythmia CL had the highest likelihood of success (13/34 applications resulted in termination, p=0.0006), whereas ATP at 90% of the arrhythmia CL resulted in the highest incidence of failed termination (38/41 applications had no effect, p=0.0008). As shown in Figure 3c, ATP was most successful in arrhythmias with longer average CLs (80–85ms and 95–100ms). Overall, ATP successfully terminated arrhythmias in 6 out of 9 preparations (66.7%). Furthermore, the outcome of ATP was not dependent on electrode location and, as expected, ATP did not terminate any episodes of AF.
Figure 3. Application of ATP to AFl.
(a) Representative electrograms of termination, failed termination, and conversion to AF upon application of ATP (8 pulses, 50–100% of arrhythmia CL) to AFl from the RAA and lower RA. (b) Distribution of outcome during application of ATP to AFl. (c) Percentage of therapy success as a function of CL.
Far Field Excitation Threshold
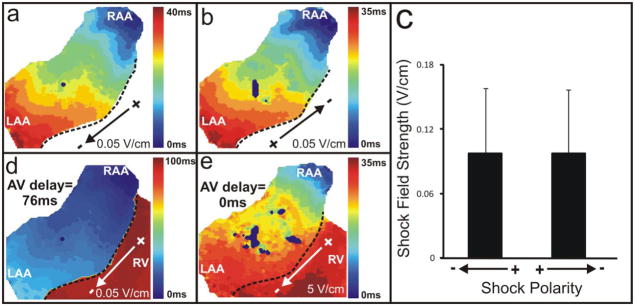
Prior to arrhythmia induction, we applied single shocks to determine the atrial far field excitation threshold (n=7). Figures 4a and 4b show that shocks of opposite polarities resulted in identical atrial activation patterns. Four out of seven preparations, such as that illustrated, demonstrated activation patterns originating from the RAA, however, in two preparations the earliest activation began from the LAA, and in one preparation activity originated from the lower RA. We observed no difference in the atrial far field excitation threshold when comparing opposite polarity shocks (0.097±0.06 V/cm for both) (Figure 4c). Lower voltage shocks (i.e. 0.05 V/cm) resulted in physiological activation of the ventricles via the AV node and conduction system, whereas higher voltage shocks (i.e. 5 V/cm) resulted in simultaneous activation of both the atria and ventricles (Figures 4d and 4e). The ventricular far field excitation threshold was 0.43±0.13 V/cm.
Figure 4. Far Field Excitation.
(a,b) Activation maps resulting from opposite polarity shocks at a field strength of ±0.05 V/cm. The dotted black line demarcates the AV groove. (c) Average shock field strength of both polarities (0.097±0.06 V/cm). (d) Activation of the ventricles at a physiological AV delay (76 ms) due to far field excitation of the atria with a shock field strength of 0.05 V/cm. (e) Simultaneous activation of the atria and ventricles with a higher voltage shock (5 V/cm).
In the preparation illustrated in both Figures 2 and 4, the location of earliest activation (RAA, Figures 4a and 4b) corresponded with the location of the dominant rotor maintaining AF (RAA, Figure 2d). This, however, was not representative of all experiments. The dominant rotor corresponded to the site of earliest activation via far field shocks in only 50% of the preparations in which both the threshold was measured and AF was induced.
AFl Termination by Low Voltage Multiple Shock Therapy
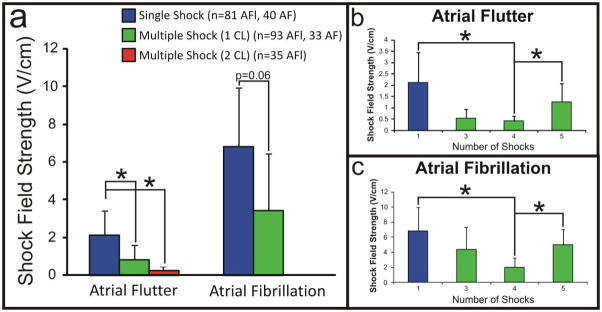
The efficacy of low voltage multiple shock therapy was tested during AFl and AF, and then compared to both ATP and the application of single, phase-independent shocks. In the case of AFl, we applied a total of 405 trials of low voltage multiple shock therapy: 302 trials were delivered within 1 CL of the arrhythmia and 103 trials were delivered within 2 CLs. Figure 5a shows that the defibrillation threshold was significantly decreased during the trials in which we applied multiple shocks within 1 CL (0.86±0.73 V/cm) and 2 CLs (0.28±0.13 V/cm), as compared to single shocks (2.12±1.31 V/cm). Despite the significantly decreased defibrillation threshold for multiple shocks delivered over 2 CLs, this therapy resulted in an increased rate of conversion of AFl to sustained AF when compared to multiple shocks delivered over 1 CL (16.5% versus 14.2%, p=0.3). Single shocks resulted in AF in 6.4% of therapy applications. Additionally, 54.3% of terminations in the case of multiple shocks delivered over 2 CLs were preceded by a short run of AF. Overall, immediate termination of AFl occurred in 63% of cases, whereas 37% of terminations were preceded by a 1.46±0.8 s run of AF. For multiple shocks delivered within 1 CL, 4 shocks resulted in the lowest defibrillation threshold (0.41±0.22 V/cm), as compared to 3 (0.53±0.41 V/cm) and 5 (1.26±0.82 V/cm) shocks (Figure 5b). Defibrillation of AFl was successful by multiple shocks in 100% of the preparations in which the arrhythmia was induced.
Figure 5. Defibrillation Thresholds.
(a) Defibrillation thresholds for single shocks, multiple shocks delivered within 1 CL, and multiple shocks delivered within 2 CL. (b,c) Defibrillation thresholds for single shocks and multiple shocks (3–5) delivered within 1 arrhythmia CL for AFl and AF, respectively.
AF Termination by Low Voltage Multiple Shock Therapy
In the case of AF, we applied a total of 233 trials of low voltage multiple shock therapy delivered within one CL of the arrhythmia. Once again, defibrillation of AF by multiple shocks was successful in 100% of preparations and the defibrillation threshold was decreased (p=0.06) during the trials in which we applied multiple shocks within one CL (3.46±3 V/cm), as compared to the application of single shocks (6.18±3.12 V/cm) as shown in Figure 5a. For multiple shocks delivered within 1 CL, 4 shocks again resulted in the lowest defibrillation threshold (1.97±1.25 V/cm), as compared to 3 (4.38±2.92 V/cm) and 5 (4.97±2.08 V/cm) shocks in this arrhythmia model (Figure 5c).
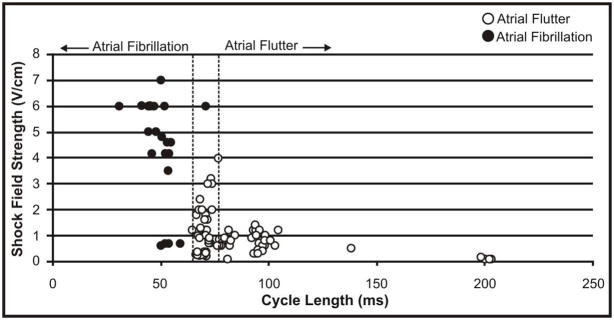
Figure 6 shows the average CLs of AFl and AF, which were successfully defibrillated during the course of the study, as a function of defibrillation threshold. There is a trend that AFl with longer CLs, require lower shock strengths for defibrillation, however the trend for defibrillation of AF is not as clear.
Figure 6.
Defibrillation Threshold as a Function of Arrhythmia CL.
Mechanisms of Termination
Optical data obtained during the application of multiple shocks to AFl and AF revealed that the mechanism of termination relies on the recruitment of a sufficient number of secondary sources of excitation, resulting from virtual electrodes, to effectively terminate all reentrant circuits involved in the arrhythmia. Due to the fact that the maintenance of AFl and AF require different numbers of reentrant circuits, the energy needed to create a sufficient amount of secondary sources varies between the arrhythmias. In addition to the field strength of the shock, the timing of the shock is also important.18
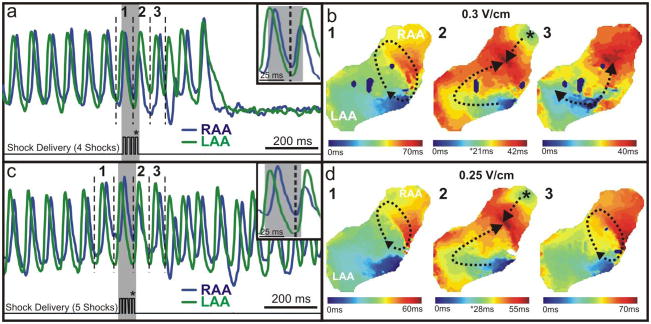
The termination of AFl was achieved through unpinning of the arrhythmia wavefront by a properly timed shock with excitation arising from the location of earliest activation corresponding to the region of highest anatomical heterogeneity.19 In the example of successful termination shown in Figure 7a and 7b and Online Movie 3, the train of multiple shocks was delivered approximately 7 ms earlier than the unsuccessful shock (Figure 7c and 7d, Online Movie 4) in relation to the rotating arrhythmia wavefront.
Figure 7. Application of Multiple Shocks to AFl.
(a) In this example of successful arrhythmia termination, four 10 ms shocks (0.3 V/cm) were applied during one CL of AFl (70 ms). OAPs are shown from the RAA and LAA before and after termination. (b) Activation maps reconstructed from the dV/dtmax of the OAPs denoted by 1–3 in panel (a). (c) In this example of unsuccessful arrhythmia termination, five 10 ms shocks (0.25 V/cm) were applied during one CL of the same arrhythmia. (d) Activation maps reconstructed from the OAPs denoted 1–3 in panel (c). The asterisks in panels (b,d) represent the shocks indicated in panels (a,c).
The termination of AF was achieved by higher strength shocks able to excite and, consequently, recruit enough secondary sources to disrupt the multiple rotors maintaining the arrhythmia. As shown in Figure 8 and Online Movies 5 and 6, successful and unsuccessful terminations of AF relied on the amount of tissue depolarized by one or more shocks in the delivered therapy. In a dramatic example of successful termination of AF, as shown in Figure 8a and Online Movie 5, the first and fourth shocks (10 V/cm) in the series recruited atrial tissue in opposing areas as can be seen at 750 and 800 ms. In this preparation, a shock of <10 V/cm was not sufficient to defibrillate sustained AF. In the example of unsuccessful termination of AF, as shown in Figure 8b and Online Movie 6, from a different preparation, the shocks delivered at 2 V/cm were not strong enough to engage a sufficient amount of atrial tissue and create enough secondary sources for termination. Consequently, the arrhythmia continued to persist post shock.20 Subsequent multiple shock therapy applications in this preparation terminated AF at 6 V/cm.
Figure 8. Application of Multiple Shocks to AF.
(a) In this example of successful arrhythmia termination, four 10 ms shocks (10 V/cm) were applied during one CL of AF (55 ms). OAPs are shown from the RAA and LAA before and after termination by the shocks shown. Arrhythmia wavefronts, reconstructed from the dV/dtmax, before, during, and after shock application. (b) In this example of unsuccessful termination, three 10 ms shocks (2 V/cm) were applied during one CL (50 ms). Arrhythmia wavefronts before, during, and after shock application. Arrhythmia wavefront frames indicated by red lettering indicate those reconstructed during shock application.
Therapy Safety
During the application of a total of 638 rounds of low voltage multiple shock therapy in 17 preparations, no episodes of ventricular flutter or fibrillation were induced. In addition, there was no evidence of atrial electroporation as the majority of shocks were delivered below the atrial electroporation threshold (9.2–13.6 V/cm).22
Discussion
In the present study, a new method of low voltage multiple shock therapy was tested in a rabbit model of atrial tachyarrhythmias. We found a significant reduction in the defibrillation thresholds for both AFl and AF, as compared to single phase-independent shocks and success rates of 100%, as opposed to 66.7% and 0% with ATP for AFl and AF, respectively. Furthermore, four shocks delivered within one CL exhibited the lowest defibrillation threshold for both arrhythmias.
The low success rate of ATP in our study, as compared to the 100% success rate of multiple shock therapy, can be explained by the inability of local excitation to invade the excitable gap of reentry when the pacing electrode is not located close to the core of reentry. On the other hand, multiple shock therapy, based on the virtual electrode polarization (VEP) hypothesis,23 uses far field electrical stimuli to produce excitation in larger areas of tissue to destabilize and terminate reentrant tachyarrhythmias.18 VEP predicts that, in response to an external electric field, opposite sides of functional and anatomical heterogeneities will experience either hyperpolarization or depolarization.18, 24 The depolarized region of the heterogeneity can then become a secondary source of excitation and collide with the existing reentry resulting in termination.
All shocks delivered in this study were monophasic, chosen because of the results of our previous investigation of ventricular tachycardia in a rabbit model of infarction.12 In that study, it was reported that the use of monophasic shocks not only reinforced the VEP response of the tissue, but also terminated ventricular tachycardia with significantly lower energies than multiple biphasic shocks. More importantly, it was found that the second phase of biphasic shocks reversed the VEP effect of the first phase, preventing maintenance of the secondary source of excitation responsible for colliding with and terminating the arrhythmia wavefront.
Arrhythmia Organization and Defibrillation
The spatiotemporal organization of AFl/AF is important to defibrillation studies. Several different animal models of the arrhythmia, based on human states of the disease, have been developed, reflecting differences in such characteristics as dominant frequency, organization, and electrical/structural remodeling.17, 25–27 Additionally, Everett, et al recently showed that higher defibrillation thresholds are directly related to arrhythmia models with higher dominant AF frequencies.28
In our study, with more anatomically-defined AFl and more functionally-defined AF, we reported increased defibrillation thresholds for AF as opposed to AFl. The mechanisms of defibrillation, as revealed by our optical mapping studies, show that the defibrillation of AFl/AF relies on the ability of the delivered therapy to infiltrate the excitable gap of reentry. In the case of AFl, the long excitable gap associated with a single macroreentrant circuit was easily penetrated by a properly timed shock originating from the region of highest heterogeneity (Figure 7). AF, on the other hand, exhibits multiple smaller reentrant circuits with higher dominant frequencies and, consequently, smaller excitable gaps. Multiple shock therapy, in this case, required larger voltage shocks in order to excite a sufficient amount of tissue to disrupt the multiple rotors maintaining that arrhythmia (Figure 8).
Patient Pain Perception
Studies investigating patient pain perception have revealed that internal shocks >0.1 J are generally perceived as uncomfortable, although there is a wide variation in perception perhaps due to varying autonomic tone, the presence of drugs, and location of the shock electrodes.11, 29 Additionally, the majority of patients are unable to distinguish the difference between shocks delivered higher than this threshold.11 During clinical studies in which several intracardiac shocks were delivered about 5 minutes apart, patients perceived later shocks in the series to be more painful than the initial one regardless of the energy delivered.30 Clinical testing will need to be performed to determine how a train of multiple shocks delivered in quick succession will be perceived, as the overall energy delivered may exceed that of a single shock.
Safety
In our study in the intact heart, we induced no episodes of ventricular tachyarrhythmias. As AFl/AF ranged from 50–200 ms in our experimental rabbit arrhythmia model, we have the ability to deliver low voltage multiple shock therapy safely and effectively within the ventricular refractory period without causing additional arrhythmias.21 Human AFl/AF has been reported to range from 120 to 200 ms,31, 32 while the ventricular refractory period is ≥200 ms.33 Moreover, the AFl defibrillation threshold with multiple shocks delivered within two CLs was only 0.28±0.13 V/cm and thus about 1.5 times less than the ventricular excitation threshold (0.43±0.13 V/cm). Consequently, additional in vivo experiments will need to be performed to evaluate this therapy in humans as low strength atrial defibrillation shocks have been shown to excite the ventricles34 and, depending on the arrhythmia, may fall within the ventricular refractory period.
A recent study by Fenton, et al proposed and tested a therapy termed “far-field antifibrillation pacing” in a coronary-perfused canine RA/RV preparation.35 Far field shocks were applied at the CL of the arrhythmia and, although no episodes of ventricular arrhythmias were reported, unlike in our study, shocks could coincide with the T wave to induce ventricular arrhythmias in the whole heart.34 Additionally, in this study, defibrillation thresholds were reported for a mixture of atrial tachyarrhythmias making comparison between our studies difficult. As we have shown in this study, AFl and AF have distinct spatiotemporal characteristics resulting in varying defibrillation strengths and mechanisms.
Conclusion
Our findings indicate that low voltage multiple shock therapy has the potential to serve as a useful treatment for atrial tachyarrhythmias such as AFl and AF. In this study, we have shown that multiple shocks delivered over 1 or 2 CLs significantly reduced the defibrillation threshold of AFl and AF, although multiple shocks delivered over 2 CLs resulted in an increased conversion of AFl to AF. We achieved defibrillation success in all preparations and induced no ventricular arrhythmias. Based on this study, we conclude that low voltage multiple shock therapy terminates AFl with higher efficacy than AF due to the unique spatiotemporal characteristics of the arrhythmias.
Supplementary Material
Acknowledgments
This project was supported by the AHA BGIA 0860047Z (VVF) and NIH R01 HL67322 (IRE).
Abbreviations
- AF
atrial fibrillation
- AFl
atrial flutter
- ATP
antitachycardia pacing
- CL
cycle length
- LAA
left atrial appendage
- RAA
right atrial appendage
- RA
right atrium
- ACh
acetylcholine chloride
- ERP
effective refractory period
- VEP
virtual electrode polarization
- OAP
optical action potential
Footnotes
Conflicts of Interest: IRE is a member of the Board of Directors and owns stock in Cardialen, Inc. CMA, CMR, IRE, and VVF are co-inventors on the patent entitled “Method and Device for Three-Stage Atrial Cardioversion Therapy”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Crandall MA, Horne BD, Day JD, et al. Atrial fibrillation significantly increases total mortality and stroke risk beyond that conveyed by the CHADS2 risk factors. Pacing Clin Electrophysiol. 2009;32(8):981–986. doi: 10.1111/j.1540-8159.2009.02427.x. [DOI] [PubMed] [Google Scholar]
- 3.Seiler J, Stevenson WG. Atrial fibrillation in congestive heart failure. Cardiol Rev. 2010;18(1):38–50. doi: 10.1097/CRD.0b013e3181c21cff. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin. 2009;27(1):13–24. vii. doi: 10.1016/j.ccl.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 6.Gillis AM, Koehler J, Morck M, Mehra R, Hettrick DA. High atrial antitachycardia pacing therapy efficacy is associated with a reduction in atrial tachyarrhythmia burden in a subset of patients with sinus node dysfunction and paroxysmal atrial fibrillation. Heart Rhythm. 2005;2(8):791–796. doi: 10.1016/j.hrthm.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Koster RW, Dorian P, Chapman FW, Schmitt PW, O’Grady SG, Walker RG. A randomized trial comparing monophasic and biphasic waveform shocks for external cardioversion of atrial fibrillation. Am Heart J. 2004;147(5):e20. doi: 10.1016/j.ahj.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Dosdall DJ, Ideker RE. Intracardiac atrial defibrillation. Heart Rhythm. 2007;4(3 Suppl):S51–56. doi: 10.1016/j.hrthm.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alt E, Ammer R, Schmitt C, et al. A comparison of treatment of atrial fibrillation with low-energy intracardiac cardioversion and conventional external cardioversion. Eur Heart J. 1997;18(11):1796–1804. doi: 10.1093/oxfordjournals.eurheartj.a015175. [DOI] [PubMed] [Google Scholar]
- 10.Schoels W, Swerdlow CD, Jung W, Stein KM, Seidl K, Haffajee CJ. Worldwide clinical experience with a new dual-chamber implantable cardioverter defibrillator system. J Cardiovasc Electrophysiol. 2001;12(5):521–528. doi: 10.1046/j.1540-8167.2001.00521.x. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell AR, Spurrell PA, Boodhoo LE, Sulke N. Long-term care of the patient with the atrial defibrillator. Am Heart J. 2004;147(2):210–217. doi: 10.1016/j.ahj.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Ripplinger CM, Lou Q, Efimov IR. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm. 2009;6(7):1020–1027. doi: 10.1016/j.hrthm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51(8):779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 14.Fedorov VV, Chang R, Glukhov AV, et al. Complex Interactions Between the Sinoatrial Node and Atrium During Reentrant Arrhythmias in the Canine Heart. Circulation. 2010;122:782–789. doi: 10.1161/CIRCULATIONAHA.109.935288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedorov VV, Lozinsky IT, Sosunov EA, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4(5):619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 16.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 2009;6(1):87–97. doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43(3):483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Ripplinger CM, Krinsky VI, Nikolski VP, Efimov IR. Mechanisms of unpinning and termination of ventricular tachycardia. Am J Physiol Heart Circ Physiol. 2006;291(1):H184–192. doi: 10.1152/ajpheart.01300.2005. [DOI] [PubMed] [Google Scholar]
- 19.Trayanova N, Skouibine K, Aguel F. The role of cardiac tissue structure in defibrillation. Chaos. 1998;8(1):221–233. doi: 10.1063/1.166299. [DOI] [PubMed] [Google Scholar]
- 20.Gray RA, Ayers G, Jalife J. Video imaging of atrial defibrillation in the sheep heart. Circulation. 1997;95(4):1038–1047. doi: 10.1161/01.cir.95.4.1038. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Li L, Nikolski V, Wallick DW, Efimov IR. Shock-induced arrhythmogenesis is enhanced by 2,3-butanedione monoxime compared with cytochalasin D. Am J Physiol Heart Circ Physiol. 2004;286(1):H310–318. doi: 10.1152/ajpheart.00092.2003. [DOI] [PubMed] [Google Scholar]
- 22.Fedorov VV, Kostecki G, Hemphill M, Efimov IR. Atria are more susceptible to electroporation than ventricles: implications for atrial stunning, shock-induced arrhythmia and defibrillation failure. Heart Rhythm. 2008;5(4):593–604. doi: 10.1016/j.hrthm.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efimov I, Ripplinger CM. Virtual electrode hypothesis of defibrillation. Heart Rhythm. 2006;3(9):1100–1102. doi: 10.1016/j.hrthm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Takagi S, Pumir A, Pazo D, Efimov I, Nikolski V, Krinsky V. Unpinning and removal of a rotating wave in cardiac muscle. Phys Rev Lett. 2004;93(5):058101. doi: 10.1103/PhysRevLett.93.058101. [DOI] [PubMed] [Google Scholar]
- 25.Katsouras G, Sakabe M, Comtois P, et al. Differences in atrial fibrillation properties under vagal nerve stimulation versus atrial tachycardia remodeling. Heart Rhythm. 2009;6(10):1465–1472. doi: 10.1016/j.hrthm.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 27.Verheule S, Wilson E, Everett Tt, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107(20):2615–2622. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everett THt, Wilson EE, Olgin JE. Effects of atrial fibrillation substrate and spatiotemporal organization on atrial defibrillation thresholds. Heart Rhythm. 2007;4(8):1048–1056. doi: 10.1016/j.hrthm.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Ladwig KH, Marten-Mittag B, Lehmann G, Gundel H, Simon H, Alt E. Absence of an impact of emotional distress on the perception of intracardiac shock discharges. Int J Behav Med. 2003;10(1):56–65. doi: 10.1207/s15327558ijbm1001_05. [DOI] [PubMed] [Google Scholar]
- 30.Steinhaus DM, Cardinal DS, Mongeon L, Musley SK, Foley L, Corrigan SL. Internal defibrillation: pain perception of low energy shocks. Pacing Clin Electrophysiol. 2002;25(7):1090–1093. doi: 10.1046/j.1460-9592.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 31.Haissaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109(24):3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 32.Kim AM, Olgin JE, Everett THt. Role of atrial substrate and spatiotemporal organization in atrial fibrillation. Heart Rhythm. 2009;6(8 Suppl):S1–7. doi: 10.1016/j.hrthm.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Kong TQ, Jr, Goldberger JJ, Parker M, Wang T, Kadish AH. Circadian variation in human ventricular refractoriness. Circulation. 1995;92(6):1507–1516. doi: 10.1161/01.cir.92.6.1507. [DOI] [PubMed] [Google Scholar]
- 34.Gray RA, Jalife J. Effects of atrial defibrillation shocks on the ventricles in isolated sheep hearts. Circulation. 1998;97(16):1613–1622. doi: 10.1161/01.cir.97.16.1613. [DOI] [PubMed] [Google Scholar]
- 35.Fenton FH, Luther S, Cherry EM, et al. Termination of atrial fibrillation using pulsed low-energy far-field stimulation. Circulation. 2009;120(6):467–476. doi: 10.1161/CIRCULATIONAHA.108.825091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.