Abstract
The mechanism(s) by which iron in blood is transported across the blood-brain barrier (BBB) remains controversial. Here we have examined the first step of this trans-cellular pathway, namely the mechanism(s) of iron uptake into human brain microvascular endothelial cells (hBMVEC). We show that hBMVEC actively reduce non-transferrin bound FeIII (NTBI) and transferrin-bound FeIII (TBI); this activity is associated with one or more ferrireductases. Efficient, exo-cytoplasmic ferri-reduction from TBI is dependent upon transferrin receptor (TfR), also. Blocking holo-Tf binding with an anti-TfR antibody significantly decreases the reduction of iron from transferrin by hBMVEC, suggesting that holo-Tf needs to bind to TfR in order for efficient reduction to occur. Ferri-reduction from TBI significantly decreases when hBMVEC are pre-treated with PtII, an inhibitor of cell surface reductase activity. Uptake of 59Fe from 59Fe-Tf by endothelial cells is inhibited by 50% when ferrozine is added to solution; in contrast, no inhibition occurs when cells are alkalinized with NH4Cl. This indicates that the iron reduced from holo-transferrin at the plasma membrane accounts for at least 50% of the iron uptake observed. hBMVEC-dependent reduction and uptake of NTBI utilizes a PtII-insensitive reductase. Reductase-independent uptake of FeII by hBMVEC is inhibited up to 50% by ZnII and/or MnII by a saturable process suggesting that redundant FeII transporters exist in the hBMVEC plasma membrane. These results are the first to demonstrate multiple mechanism(s) of TBI and NTBI reduction and uptake by endothelial cells (EC) of the BBB.
Keywords: Blood-brain barrier, Iron, Neurodegeneration, Transferrin, Dcytb, STEAP2
Introduction
Iron, while required for several normal physiological processes, can be duplicitous in nature. Iron’s innate ability to accept and donate electrons is vital for cellular processes such as mitochondrial energy generation, myelination, neurotransmission, oxygen transport, and cellular division (Salvador 2010; Madsen and Gitlin 2007). A disruption in the normal redox chemistry of brain iron can be detrimental to normal function. Improper iron regulation by cells of the brain has been linked to neurological disorders such as Alzheimer’s and Parkinson’s disease (Madsen and Gitlin 2007; Rivera-Mancía et al. 2010). To fully understand the pathology of these neurological diseases, we need to fully understand the mechanisms of iron transport into the brain, the cellular distribution/regulation of iron in the brain parenchyma and the mechanism(s) of iron efflux out of the central nervous system (CNS). Here, we focus in on the first of these steps; iron uptake by the capillary endothelial cells (EC) of the blood brain barrier (BBB).
The BBB is a unique entity which consists of microvascular endothelial cells (MVEC), glial cells, and pericytes all of which are thought to act as a single unit to protect the brain from harmful polar molecules. The endothelial cells of the BBB possess tight-junctions while lacking the fenestrations common to EC in the periphery; therefore, solutes in the blood must be transported transcellulary into the brain (Rouault and Cooperman 2006; Abbott et al. 2006).
Iron in the blood is coordinated to small molecule ligands such as citrate (Königsberger et al. 2000) (non-transferrin bound iron, NTBI) or to the iron transport protein Tf (transferrin bound iron, TBI). For endothelial cells to acquire iron from either TBI or NTBI, a luminal uptake mechanism must exist. Typically, uptake of TBI occurs when holo-Tf binds its receptor, followed by the release and reductase- dependent reduction of TBI in the endosome; this FeII is then exported from the endosome through a divalent cation transporter such as divalent metal transporter 1 (DMT1) (Rouault and Cooperman 2006; Garrick 2011). Previous studies have demonstrated the expression and co-localization of the iron-related transport proteins DMT1 and TfR in the brain microvasculature (Burdo et al. 2001; Yang et al. 2010; Siddappa et al. 2002), although the expression of DMT1 is controversial (Moos et al. 2007). A thorough examination of the divalent cations which act either as substrate or competitor of DMT1-mediated transport, has been reported (Garrick et al. 2006). Such cations include but may not be limited to MnII, CdII, FeII, PbII, CoII, NiII, ZnII, and possibly CuI and/or CuII.
Recent reports indicate that transcripts expressing the ferrireductases duodenal cytochrome b (Dcytb) and six-transmembrane epithelial antigen of the prostate 2 (STEAP2) exist in the brain (Ohgami et al. 2006; Tulpule et al. 2010). Both of these ferrireductases catalyze the transport of electrons from cytosolic reductants [ascorbate or NAD(P)H] to extracellular or endosomal FeIII (Ohgami et al. 2006; Atanasova et al. 2004). However, there are no literature reports demonstrating expression or functional reductase activity of either Dcytb or STEAP2 in brain MVEC (BMVEC).
To investigate the uptake mechanism(s) associated with EC accumulation of Fe from TBI and NTBI, we used monolayers of a human brain microvascular endothelial cell line (hBMVEC). Using this model system we examined the expression of iron-related transport proteins and their roles in iron reduction and uptake. We distinguish between TBI and NTBI reduction and assess the divalent metal ion uptake kinetic properties of the divalent cation transporter(s) in hBMVEC.
Materials and Methods
Cell culture
hBMVEC were a generous gift from Dr. Supriya Mahajan at the Center for Innovation, Buffalo, NY. hBMVEC and Caco-2 cells were cultured in RPMI 1640 containing 1X MEM amino acids, 1X MEM vitamins (Mediatech Inc, Manassas, VA), 10% FBS (Gemini Bio-products, West Sacramento, CA), 10% NuSerum (BD Biosciences, Bedford, MA), 25 mM HEPES (Thermo Fisher Scientific, Waltham, MA), penicillin (100 U/mL), streptomycin (100 U/mL), 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO), 1 mM sodium pyruvate, heparin (5 U/mL) (Alfa Aesar, Ward Hill, MA), and NaHCO3, pH 7.4. Cells were incubated in a humidified incubator at 37°C, 5% CO2. The culture medium was replaced every other day and cells were passaged weekly. All experiments were performed in 24-well tissue culture dishes unless otherwise specified. Experiments were performed between passage 4 and 8 when cells reached approximately 90–95% confluency.
Indirect Immunofluorescence
All indirect immunofluorescence incubations involved gentle agitation. Monolayers were washed 3× with 1 ml of phosphate-buffered saline (PBS) solution. The cells were then fixed for 10 min at room temperature (RT) with 3.7% formaldehyde, and washed 4x with PBS (5 min/wash). Non-specific binding sites were blocked with 3% Bovine Serum Albumin (BSA) (EMD Chemicals, Darmstadt, Germany) in PBS at RT for 90 min. Cells were then incubated with primary antibody (1:1000 dilution of goat polyclonal anti-human TfR antibody (R&D Systems, Minneapolis, MN), mouse monoclonal anti-human VWF antibody, or goat polyclonal anti-STEAP2 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA)) in 3% BSA in PBS over-night (O/N) at 4°C. Cultures were then washed 3x with PBS, after which cells were incubated with secondary antibody (1:3000 dilution of Alexa Fluor 488 donkey anti-goat IgG (Life Technologies, Grand Island, NY) (TfR & STEAP2), or Cy5 conjugated rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) (VWF)) in 3% BSA in PBS at RT for 1 h in the dark. The cells were then washed 3× with PBS in the dark at RT, after which they were imaged. Images were obtained using a Zeiss Axio Observer inverted microscope, 40× magnification and Zeiss AxioVision software (Zeiss, Thornwood, NY). The captured images were adjusted for brightness and contrast using Adobe Photoshop 7.0 software.
Immunoblots
Membrane proteins from Caco-2 and hBMVEC were separated from the cellular protein pool as follows. Cells were removed from 100 mm tissue culture dishes, washed 2x with PBS, and were pelleted. The cell pellet was resuspended with membrane purification buffer (50 mM Tris, 150 mM NaCl, pH 7.4) in a 5:1 ratio of buffer to pellet. Protease inhibitors were added to the cell suspension and the cells were cracked open by 3 rapid freeze-thaw cycles in liquid nitrogen and ice, respectively. The cell suspension was then centrifuged at 14,000xg for 30 min at 4°C. The supernatant was removed leaving only membranes and membrane proteins in the pellet. This fraction was resuspended with a 1:1 ratio of mammalian lysis buffer plus protease inhibitors (100 mM HEPES, pH 7.4, 0.5% (v/v) IGEPAL) to pellet and was stored O/N at 4°C. For whole cell lysates, confluent monolayers of cells were lysed with mammalian lysis buffer plus protease inhibitors for 1h 37°C 5%CO2 110rpm. Protein content was quantified and equal amounts of protein were run in each well of a 10% SDS-PAGE for 20 min at 109 V followed by 180 V until the front ran off the gel. Proteins were transferred onto a PVDF membrane at 0.15 amps for 75 min using a semi-dry transfer apparatus. The PVDF membranes were blocked with 5% BSA in TBST for 1 h at 4°C. Membranes were then incubated with primary antibody (1:10,000 dilution of mouse monoclonal anti-DMT1 antibody (Acris Antibodies, San Diego, CA), 1:5,000 dilution of goat polyclonal anti-STEAP2 antibody, or 1:5,000 dilution of rabbit polyclonal anti-human Dcytb antibody (Acris Antibodies, San Diego, CA)) in TBST O/N at 4°C. The anti-DMT1 antibody is specific for exon 2 so it is not isoform-specific. Membranes were washed for 1 h then incubated with secondary antibody (0.3 μl/ml of goat anti-rabbit IgG-HRP or donkey anti-goat IgG-HRP (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in TBST 3% BSA for 1 h at RT. Membranes were washed for 1 h with TBST and then processed in a dark room using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Waltham, MA) as per instructed. Films were scanned and the captured images were adjusted for brightness and contrast using Adobe Photoshop 7.0 software.
PtII Cytotoxicity Assay
All assays were performed using CytoTox 96 Non-Radioactive Cytotoxicity Assay from Promega (Promega Corporation, Madison, WI) as per instructions. Briefly, experiments were performed in triplicate under conditions mimicking the ferrozine assay. After a 3.5h incubation, the supernatants were collected and assayed for LDH content. Percent cytotoxicity is calculated by dividing experimental LDH levels by total LDH times one hundred.
Colorimetric-based ferrozine assay for FeII
All assays were performed with a total working volume of 150 μl shaking at 100 rpm in a 37°C 5% CO2 humidified incubator. We used a physiological incubation buffer (PIB) for all assays (25 mM MOPS, 25 mM MES, 5.4 mM KCl, 5 mM glucose, 140 mM NaCl, 1.8 mM CaCl2, 800 μM MgCl2, pH 7.0). Final working concentrations of reagents (unless otherwise specified) were as follows: 10 μg/ml TfR-Ab, 25 μM Holo-Tf (EMD Chemicals, Darmstadt, Germany), 100 μM potassium tetrachloroplatinateII (PtII) (Alfa Aesar, Ward Hill, MA), 100 μM FeCl3 (Thermo Fisher Scientific, Waltham, MA), and 250 μM Citrate, 1 mM ferrozine, 250 μM apo-Tf from Sigma-Aldrich, St. Louis, MO. Each assay contained ferrozine and citrate (unless noted otherwise), plus the reagents indicated in the respective graph. All pre-incubations were conducted for 30 min after which the cells were washed with PBS and assay reagents were added and incubated for 3h. After the 3h incubation the PIB was collected and 50 μl was transferred to a 96-well plate. Absorbance readings were taken at 562 nm using a FLUOstar Omega spectrophotometer (BMG Labtech, Cary, NC) corrected for a blank of PIB containing identical reagents and incubation procedures minus cells. Cells were washed, lysed, and their protein content quantified. Experimental absorbance readings were extrapolated onto a ferrozine-FeII standard curve was used to convert experimental absorbance reading into the amount of FeII present in solution. These values were normalized for protein content.
59Fe-Transferrin loading procedure
The loading of 59Fe onto Tf was performed as previously described (Burdo et al. 2003) with modifications. Briefly, using Tf-loading buffer (0.1 M HEPES, pH 7.5, 0.15 M NaCl), 20 mM NaHCO3, 21.3 mM NTA, 44 μM 59Fe, and 20 μM apo-Tf we did the following. 59Fe was incubated with Tf-loading buffer containing NaHCO3 and NTA for 5 min at RT after which apo-Tf was added and incubated for 2 h at RT. This 59Fe-Tf solution containing non-Tf bound 59Fe was buffer exchanged using a Nanosep 10K MWCO Omega spin column (PALL Corporation, Port Washington, NY) 3× 10 min at 5,500 rpm. This procedure yielded a 59Fe-Tf purity of >95%.
59Fe uptake assays
All assays were performed in a 24-well tissue culture dish with a total working volume of 150 μl. Assay conditions were 1 h at 37°C 5% CO2 shaking 120 rpm. Control assays, accounting for cell-associated 59Fe, were performed at 4°C 120 rpm. The radionuclide 59FeCl3 was purchased from Perkin-Elmer, Waltham, MA. In all assays 1.0 μM 59Fe was used regardless of its speciation, unless indicated otherwise. Reagent concentrations, where applicable, are as follows: 1 mM ferrozine, 100 μM PtII, 7.5 mM NH4Cl, and 250 μM citrate. Citrate was added to all uptake and kinetic assays. Cells were pre-incubated with all assay reagents excluding the 59Fe substrate for 30 min after which 59Fe or 59Fe-Tf were added. Each experiment was stopped after 1 h via consecutive washes with ice cold quench buffer (62.5 mM Tris, 12.8 mM EDTA (Thermo Fisher Scientific, Waltham, MA), 37.5 mM succinic acid (Sigma-Aldrich, St. Louis, MO). Cell monolayers were then lysed with 200 μl mammalian lysis buffer; 100 μl of this lysate were assayed for 59Fe using a Wallac γ counter. The remaining 100 μl was used for quantification of protein content. Counts were converted to pmol 59Fe using a 59Fe standard curve. Total 59Fe content/replicate was normalized for protein concentration. Each datum point from our 59Fe-saturation or Mn/Zn competition assays was taken after 1 h incubation. For our saturation and competition experiments 59Fe was kept in a reduced state by incubation with 2 mM ascorbate throughout the assay. This was done to make 59Fe uptake reductase independent.
Quantification of protein concentration
The protein content of Caco-2 and hBMVEC were determined according to the method described by Marion M. Bradford (Bradford 1976) using BSA as the standard.
Results
Identification of iron transport proteins in cultured hBMVEC
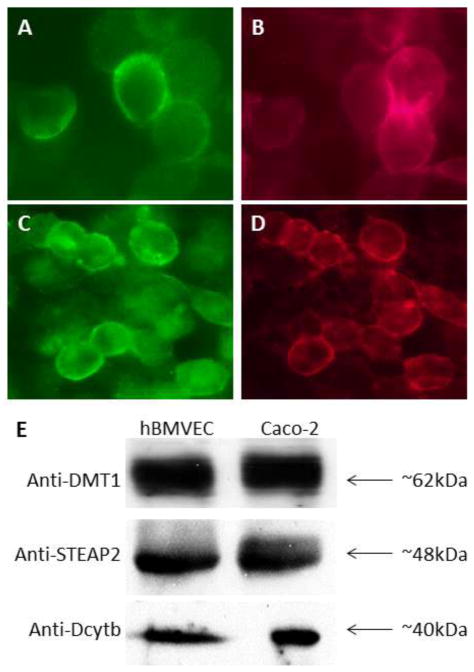
To begin our analysis of the iron-related proteins expressed by hBMVEC we confirmed the expression of both DMT1 and TfR. Using an immunoblot containing proteins purified from the membranes of both Caco-2 and hBMVEC, we probed for DMT1 with an antibody that is specific for exon 2 and found a strong band at ~62kDa (Figure 1e). Using indirect immunofluorescence, we found that TfR in hBMVEC was plasma membrane-associated (Figure 1a); our relatively low-resolution epifluorescence images precluded our visualizing endosomal TfR. Von-Willebrand factor (VWF), an endothelial cell-specific protein, was used as a marker for hBMVEC (Figure 1b).
Fig. 1. TfR, DMT1, STEAP2, and Dcytb expression in hBMVEC.
Indirect immunofluorescence was carried out using hBMVEC monolayers. Co-immunofluorescence yields localization of both TfR and VWF to the plasma membrane (a & b, respectively) (40× magnification). The same is true for STEAP2 and VWF (c and d, respectively) (40X magnification). Immunoblot’s were carried out with proteins purified from the membranes (DMT1) or from whole cell lysates (STEAP2 & Dcytb) of the respective cell types as indicated. Antibodies used are as indicated to the left of the blots and size in kDa to the right.
Next, we examined the expression of ferrireductases in hBMVEC. Probing for Dcytb via immunoblot, we identified Dcytb immunoreactive protein of ~40kDa in both hBMVEC and Caco-2 cells as positive control (Figure 1e). Strong bands were also identified at ~48kDa when probed with an anti-STEAP2 antibody (Figure 1e). We used indirect immunofluorescence to probe the sub-cellular localization of STEAP2 in hBMVEC and found the protein to localize to the plasma membrane (Figure 1c); again our relatively low-resolution epifluorescence images precluded our visualizing endosomal STEAP2. Plasma membrane localization of STEAP2 occurs in cells expressing the endothelial cell-specific marker VWF (Figure 1d). Full-length western blots displayed little to no background when using antibodies from Figure 1e, indicating a high degree of specificity (Supplementary Figure 1). These data present novel evidence for the expression of the ferrireductases STEAP2 and Dcytb in hBMVEC.
Reduction of iron from Tf is dependent upon citrate and TfR
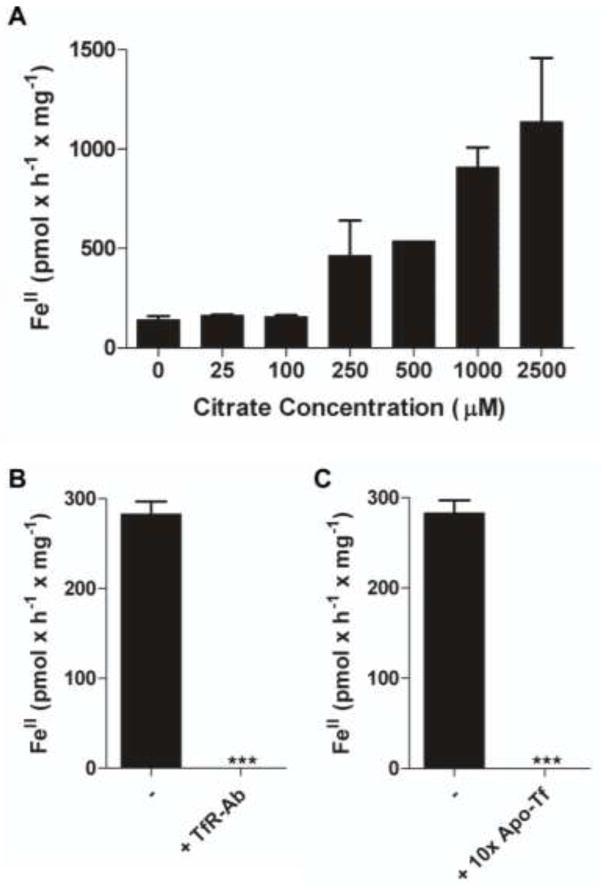
Monolayers of hBMVEC were incubated with holo-Tf, ferrozine, and increasing amounts of citrate. Increasing the citrate concentration yielded an increased reduction of iron from Tf as measured by the formation of ferrozine-FeII complex (Figure 2a). The inhibition of Tf, TfR interactions at the hBMVEC surface by either anti-TfR antibody or 10-fold excess of apo-Tf significantly inhibited the reduction of iron from Tf (Figure 2b and 2c, respectively). These data show that hBMVEC can reduce iron from Tf and this hBMVEC-dependent reduction is dependent upon citrate concentration and the interaction of Tf with endogenous TfR.
Fig. 2. Reduction of TBI is dependent upon citrate concentration and Tf-TfR interaction.
The hBMVEC- dependent formation of FeII species were quantified using colorimetric ferrozine assays. Each sample was blank corrected with a minus hBMVEC control and normalized for protein concentration. Monolayers of hBMVEC were incubated with TBI, ferrozine, and either increasing concentrations of citrate (a), anti-TfR antibody (b), or 10-fold apo-Tf (250 μM) (c). (b and c) contained a constant citrate concentration. n = 3 for each result in each panel. Data are mean ± standard deviation (SD). ***P-value < 0.0001 as analyzed by the paired t-test.
Cell-surface ferrireductases are required for the hBMVEC-dependent reduction of TBI and NTBI
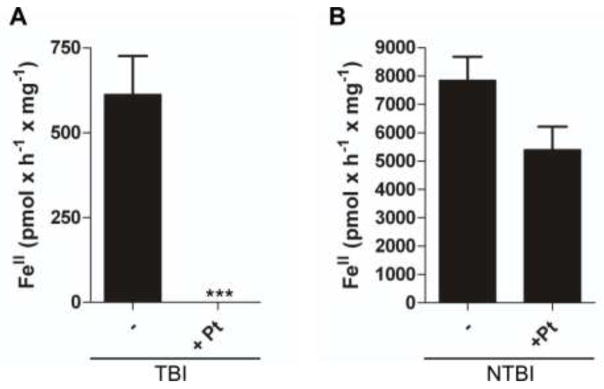
We used PtII, an inhibitor of pyridine nucleotide-dependent reductases (Armarego and Ohnishi 1987) including cell-surface ferrireductases (Eide et al. 1992), to assess the relative contributions of ferrireductases in mediating the reduction of TBI and NTBI. Dose-response experiments indicated that 100 μM PtII exihibited minimal cytotoxicity in hBMVEC monolayers (Supplementary Table 1) while displaying maximal effectiveness in inhibition of ferrireduction by hBMVEC (data not shown). The addition of 100 μM PtII to the hBMVEC monolayer significantly inhibited reduction of iron from Tf (Figure 3a). In contrast, the addition of 100 μM PtII did not significantly inhibit the reduction of NTBI (Figure 3b).
Fig. 3. Reduction of TBI and NTBI is dependent upon hBMVEC-surface ferrireductases.
Colorimetric ferrozine assays were used to quantify the formation of FeII species in solution. Each sample was blank corrected with a minus hBMVEC control and normalized for protein concentration. Potassium tetrachloroplatinateII (100 μM) was used to inhibit reductase activity by hBMVEC. Substrates were either TBI (a) or NTBI (b). Values are mean ± SD (a, n =12; b, n=3). ***P-value < 0.0001 as analyzed by the paired t-test.
hBMVEC can accumulate 59Fe from TBI and NTBI
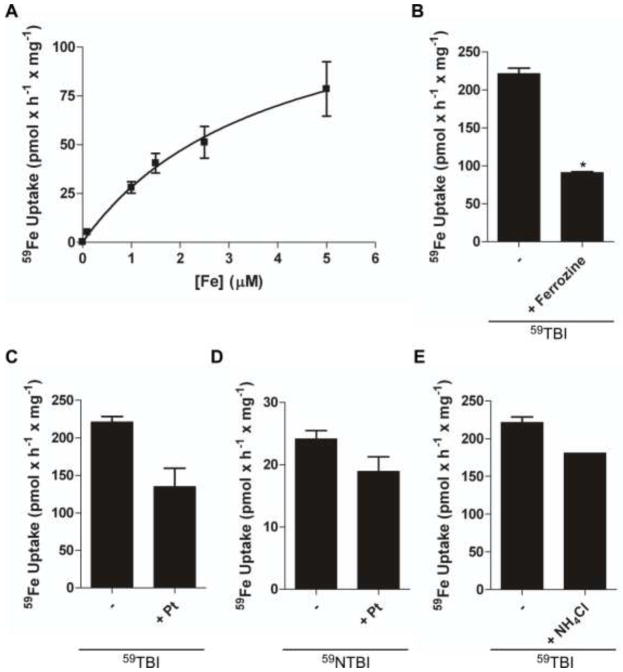
FeII released and reduced from Tf or exogenous ligand (e.g. citrate) is likely substrate for hBMVEC divalent cation transporter(s) at the plasma membrane. We monitored the uptake of iron into hBMVEC through the use of the radionuclide 59Fe using either 59Fe-NTBI (59NTBI) or 59Fe-TBI (59TBI). Monolayers of hBMVEC accumulate 59Fe with 59FeII-citrate as substrate (reductase-independent uptake conducted in the presence of 2 mM dihydroascorbate). Under these conditions, hBMVEC accumulate iron with a KM = 3.9 ± 1.1 μM (Figure 4a). Using 59TBI as substrate without added Asc (reductase-dependent) we tested the postulate that a FeII transporter is involved in TBI uptake. We used ferrozine to specifically inhibit accumulation of 59FeII from 59TBI. Ferrozine will chelate any free ferrous iron thus preventing the translocation into the cell of 59FeII released from 59Fe-Tf by ferrireduction. In this assay, ferrozine inhibited reductase-dependent iron uptake by approx. 50% (Figure 4b).
Fig. 4. Both TBI and NTBI are substrate for endosomal-independent hBMVEC iron uptake.
A γ counter was used to quantify the amount of 59Fe uptake by hBMVEC monolayers. The kinetics of 59FeII-citrate plus Asc accumulation by hBMVEC are shown (a). Data are shown representing hBMVEC accumulation of 59Fe from 59Fe-Tf in the presence ferrozine (b) or PtII (c). 59NTBI uptake in the presence of PtII was also monitored (d). Effect of hBMVEC-alkalinization via NH4Cl on 59TBI accumulation was quantified and compared to control (e). n = 3 for each result in each panel. The Michaelis-Menten equation was used to calculate the curve (a). Data are mean ± SD. *P-value < 0.01 as analyzed by the unpaired t-test.
PtII was used to correlate ferrireductase inhibition with iron uptake inhibition. Uptake of 59TBI by hBMVEC was quantified in the presence of PtII (Figure 4c). The remaining cell-associated 59Fe in Figures 4b and 4c can be attributed to residual 59TBI-TfR interactions, the quantities of which are comparable to 4°C controls (141.1 ± 10.5 pmol × h-1 × mg-1; data not shown). PtII had no effect on 59FeII uptake when 59NTBI was used as substrate (Figure 4d) suggesting that a cell-surface PtII-insensitive ferrireductase is involved in 59NTBI uptake by hBMVEC. The data from Figure 4d parallel our reductase assay results given in Figure 3b.
Acidification is not required for the accumulation of TBI by hBMVEC
To assess the contribution made by canonical TfR cycling we performed alkalinization assays. Alkalinization assays using NH4Cl inhibit proton accumulation in the endosomes, thus minimizing the proton-dependent mobilization of TBI and subsequent accumulation of iron. The data from the NH4Cl alkalinization assay are depicted in Figure 4e; the results demonstrate that hBMVEC uptake of 59Fe from 59TBI is not dependent on endosomal acidification and, therefore, likely not strongly dependent on canonical Tf-TfR cycling.
Iron accumulation by hBMVEC can be partially inhibited by MnII and ZnII
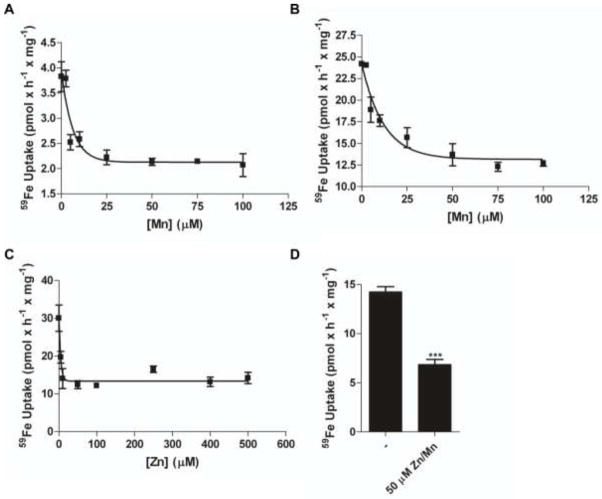
Divalent cation transporters often transport several different metal ions which can compete with one another for uptake (Garrick et al. 2006). Therefore, we used MnII and ZnII in an attempt to inhibit 59FeII translocation in a reductase-independent protocol (+Asc). MnII inhibited 59FeII uptake into hBMVEC by ~50% at both 0.1 μM and 1.0 μM 59FeII concentrations; IC50 = 4.3 ± 1.2 μM and 8.4 ± 2.5 μM (Figure 5a and 5b, respectively). ZnII also inhibited 59FeII accumulation by ~50% with an IC50 = 3.1 ± 0.7 μM (Figure 5c). By quantifying this competition in the presence of both divalent cations we tested the hypothesis that two separate divalent cation transporters were functioning in hBMVEC, one specific for MnII, the other specific for ZnII. However, inclusion of MnII and ZnII together at 50 μM each in the uptake buffer resulted in the equivalent 50% inhibition of 59FeII accumulation seen with either metal ion alone (Figure 5d).
Fig. 5. MnII and ZnII partially inhibit the uptake of ferrous iron by hBMVEC.
A γ counter was used to quantify the amount of 59FeII-citrate plus Asc uptake by hBMVEC monolayers. ManganeseII inhibition of 59FeII-citrate plus Asc accumulation using either 0.1 μM 59Fe (a) or 1.0 μM 59Fe (b) as substrate. ZincII inhibition of 1.0 μM 59FeII-citrate plus Asc is shown (c). Combined ZnII and MnII inhibition of 1.0 μM 59FeII- citrate plus Asc is shown (d). n = 3 for each result in each panel with the exception of (d) where n = 6. Curves were obtained using a one phase exponential decay equation. Data are mean ± SD. ***P-value < 0.0001 as analyzed by the paired t-test.
Discussion
The identification of the major iron transport proteins in hBMVEC is required for an understanding of the mechanisms regulating TBI and NTBI uptake at the BBB. Both TfR and DMT1 have been identified in rat brain microvessels by indirect immunofluorescence (Burdo et al. 2001). These workers also showed TfR and DMT1 co-localization in this tissue. Figure 1 corroborates this report regarding the expression of both TfR and DMT1 by the brain microvasculature. Further investigation using confocal micrscopy would provide the necessary resolution to localize these proteins to specific hBMVEC compartments although our lower-resolution images do indicate their expression at the PM.
The reduction of FeIII in Tf, and thus Fe dissociation, requires an increase in the reduction potential (E°) of FeIII in the FeIII-Tf complex which is <-500 mV at pH 7 (Kraiter et al. 1998). The E° of TBI increases when 1) Tf associates with TfR, 2) an exogenous ligand is presented in solution (i.e. citrate), or 3) when the pH of the solution becomes more acidic. For example, upon Tf binding to TfR, the FeIII potential is increased by >200 mV (Dhungana et al. 2004). Electron transfer from endogenous reductants (i.e. NADH) to TBI are thermodynamically favorable when the E° of TBI becomes more positive than the E° of the endogenous reductants (Dhungana et al. 2004). Cell-surface ferrireductases channel these reducing equivalents from intracellular reductants through the plasma membrane to TBI and NTBI. In relation to the STEAP family of metalloreductases, Steap2 is highly expressed in the brain and co-localizes with TfR; these observations make Steap2 a likely hBMVEC ferrireductase candidate (Ohgami et al. 2006). Given the presence of STEAP2 (Ohgami et al. 2006) as well as Dcytb (Tulpule et al. 2010) transcripts in the brain, we assessed the expression of these two ferrireductases in hBMVEC specifically. The immunoblots (Figure 1e) indicated that both Dcytb and STEAP2 are present in these cells. Redundancy in function is not unexpected for the BBB; there is evidence for such redundancy in Steap3 K/O mice which retain residual erythroid reductase activity (Ohgami et al. 2005). We demonstrated also a plasma membrane localization of Steap2 in hBMVEC (Figure 1c), indicating its potential to act as a cell-surface ferrireductase in this cell type. Further investigation using confocal microscopy would be beneficial in identifying Steap2 in endosomal compartments.
Our data demonstrated that in the presence of citrate hBMVEC can reduce Tf-bound FeIII at neutral pH. This result is consistent with literature data indicating that both high citrate concentrations and Tf-TfR interactions increase the E° of Tf-associated FeIII (Weaver et al. 2010; Dhungana et al. 2004; Bates et al. 1967). Citrate is a physiologically relevant ligand in the blood with reported concentrations ranging from 90 μM (Bradbury 1997; Gaasch et al. 2007) to 110 μM (Wolcott and Boyer 1948; Königsberger et al. 2000). As Tf in the blood, at a concentration of 25 μM, is only 30% saturated with FeIII the physiological ratio of Tf:citrate is roughly 1:13 (Vincent and Love 2011). Upon titration with 250 uM citrate (Tf:citrate ratio of 1:10) a significant increase in the reduction of TBI by hBMVEC was observed (Figure 2a). At physiological concentrations the exogenous ligand citrate likely increases the E° of TBI allowing iron to be reduced from Tf by our hBMVEC (Figure 2a). In addition, the data in Figure 2b and 2c are consistent with observations that the Tf, TfR interaction alters the coordination of the FeIII in such a way as to raise its E° allowing for reduction (Dhungana et al. 2004).
Next, we wanted to investigate the hypothesis that cell-surface ferrireductases are actively reducing TBI and/or NTBI. The Kaplan lab demonstrated that Fre1, a cell-surface ferrireductase found in Saccharomyces cerevisiae, is inhibited by PtII (Eide et al. 1992): PtII had previously been demonstrated to inhibit pyridine-nucleotide reductases likely by reaction with protein cysteine thiol(s) (Armarego and Ohnishi 1987). We extended these observations regarding PtII to our mammalian cell culture system and demonstrated that PtII effectively inhibited cell-surface reduction of TBI (Figure 3a). In contrast, PtII had no effect on NTBI reduction by hBMVEC (Figure 3b). We propose that in hBMVEC a PtII-sensitive cell- surface ferrireductase is required for the reduction of TBI while a PtII-insensitive one is responsible for FeIII reduction (and release) in NTBI. This apparent difference in reductase sensitivity to PtII is under current investigation; note, however, that while Dcytb and the Steap proteins share ferrireductase activity, they are neither genetic nor structural homologs.
Since TBI and NTBI appear reduced at the cell-surface of hBMVEC, we hypothesized that the substrate for hBMVEC iron accumulation would be FeII. To test this we conducted a reductase-independent NTBI 59FeII-uptake analysis with hBMVEC monolayers (Figure 4a). Our results indicate that hBMVEC accumulate 59FeII with a KM value ~3.9 μM similar to the value for DMT1 expressed in HEK293 cells, ~3 μM (Garrick MD 2006). To investigate whether FeII reduced from Tf was acting as substrate for hBMVEC iron uptake we incubated hBMVEC with 59TBI and ferrozine. Our results showed that ferrozine significantly inhibited the uptake of 59FeII from TBI into hBMVEC (Figure 4b), suggesting that FeII reduced from Tf is substrate for uptake at the PM of hBMVEC. We interpret residual 59Fe accumulation by hBMVEC in the presence of ferrozine as “cell-associated iron”; that is, 59FeIII most probably in complex with Tf-TfR. Further examination of this inference is ongoing.
We sought to correlate the PtII inhibition of FeIII reductase activity with an inhibition of 59FeIII uptake. Unlike in our reductase assays, we detected no significant difference in the uptake of either 59TBI or 59NTBI when PtII was present (Figure 4c & 4d, respectively). The relative effectiveness of PtII inhibition on iron uptake differs from what we see regarding the PtII effect on reduction (Figure 3a). The residual 59Fe accounted for in the presence of PtII is consistent with what is seen when ferrozine is present (Figure 4b). Taken together, when “cell-associated” 59Fe is accounted for, these data indicate that PtII inhibits 59TBI uptake in hBMVEC. We must assume that “cell-associated” 59Fe also exists in our controls resulting in significantly less 59Fe uptake in comparison to FeII produced from reduction by hBMVEC. Thus, we infer from our data that FeIII reduction is not the rate-limiting step in iron accumulation by hBMVEC. This inference is consistent with the fact that overall FeIII reduction in 3 h was 3.5-fold greater than the corresponding 59Fe-uptake in the same time period, or 2-fold greater if not accounting for residual “cell-associated” 59Fe.
In canonical Tf-TfR endosomal cycling, the dissociation and reduction of iron from Tf is partially mediated by endosomal acidification. Furthermore, transport of FeII through DMT1 has a pH optimum of 5.5 and involves an inward proton current that is coupled to iron translocation (Gunshin et al. 1997). With these facts in mind, we performed an alkalinization assay to determine if acidification contributes to the accumulation of iron by hBMVEC. The data in Figure 4e demonstrate that an alkaline environment is not inhibitory to accumulation of iron from Tf indicating that cell-surface and/or endosomal acidification does not contribute the 59Fe accumulation we observe. We conclude that canonical Tf-TfR endosomal cycling does not make a quantitatively significant contribution to the 59TBI uptake in this cell system as this mechanism is enhanced by endosomal acidification (Steere et al.; Steere et al. 2010; Byrne et al. 2010).
DMT1 has a broad substrate range which includes FeII, MnII, and ZnII; reciprocally, MnII and ZnII can inhibit FeII transport by DMT1 (Gunshin et al. 1997; Garrick et al. 2006; Roth et al. 2002). We assessed the activity of MnII and ZnII as inhibitors of 59FeII uptake by hBMVEC. We demonstrated that the separate and simultaneous competition with each metal inhibited the accumulation of iron by hBMVEC by ~50% (Figure 5a-d), that is, both ions inhibited but their actions were neither additive nor synergistic. We propose, therefore, that at least two divalent cation transporters are expressed at the hBMVEC PM: a FeII, MnII, and ZnII transporter and a FeII-specific one that is insensitive to MnII or ZnII. We calculated an IC50 for each cation to provide an estimate of the kinetic KM they might have as substrates. This IC50 for MnII was 8.4 ± 2.5 μM whereas the value for ZnII was 3.1 ± 0.7 μM (Figure 5e-f). Application of the Cheng-Prusoff equation to these IC50 values (Biochem Pharm 1973; Cer et al 2009) provided estimates for KI values; these were: Mn, 8.4 μM; Zn, 3.1 μM. These values do not correlate with KM values for MnII and ZnII in the uptake of these divalent metal ions by DMT1; the KM value for MnII is ~1 μM (Garrick, 2006) while this value for ZnII is ~42 μM (Iyengar 2009). Therefore, we suggest a FeII transporter other than DMT1 contributes significantly to ferrous iron uptake by hBMVEC. One candidate for this activity is Zip14 which has been identified at the mRNA level in the CNS (Bishop et al. 2010; Girijashanker et al. 2008). We are currently testing this hypothesis.
Supplementary Material
15 μg (Dcytb & STEAP2) or 25 μg (DMT1) of protein from whole cell lysates (Dcytb & STEAP2) or membrane proteins (DMT1) of either hBMVEC or Caco-2 cells were subjected to SDS-PAGE and immunobloting by respective antibodies as indicated. Representative ladder is diagrammed to the left of blots and indicates size in kDa.
Acknowledgments
We thank Dr. Supriya Mahajan for her generous gift of hBMVEC. This work was supported by a grant from the National Institute of Health and a fellowship from the American Heart Association.
Abbreviations
- Asc
Ascorbic acid
- BBB
Blood-brain barrier
- BMVEC
Brain microvascular endothelial cells
- CNS
Central nervous system
- Dcytb
Duodenal cytochrome b
- DMT1
Divalent metal transporter 1
- EC
Endothelial Cells
- E°
Electrochemical potential
- hBMVEC
Human brain microvascular endothelial cells
- MVEC
Microvascular endothelial cells
- NTBI
Non-transferrin bound iron
- STEAP2
Six-transmembrane epithelial antigen of the prostate 2
- TBI
Transferrin-bound iron
- Tf
Transferrin
- TfR
Transferrin receptor
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7 (1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Armarego WLF, Ohnishi A. Inactivation of dihydropteridine reductase (human brain) by platinum (II) complexes. European Journal of Biochemistry. 1987;164 (2):403–409. doi: 10.1111/j.1432-1033.1987.tb11072.x. [DOI] [PubMed] [Google Scholar]
- Atanasova B, Mudway IS, Laftah AH, Latunde-Dada GO, Mckie AT, Peters TJ, Tzatchev KN, Simpson RJ. Duodenal Ascorbate Levels Are Changed in Mice with Altered Iron Metabolism. The Journal of Nutrition. 2004;134 (3):501–505. doi: 10.1093/jn/134.3.501. [DOI] [PubMed] [Google Scholar]
- Bates GW, Billups C, Saltman P. The Kinetics and Mechanism of Iron(III) Exchange between Chelates and Transferrin. Journal of Biological Chemistry. 1967;242 (12):2810–2815. [PubMed] [Google Scholar]
- Bishop G, Scheiber I, Dringen R, Robinson S. Synergistic accumulation of iron and zinc by cultured astrocytes. Journal of Neural Transmission. 2010;117 (7):809–817. doi: 10.1007/s00702-010-0420-9. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB. Transport of Iron in the Blood-Brain-Cerebrospinal Fluid System. Journal of Neurochemistry. 1997;69 (2):443–454. doi: 10.1046/j.1471–4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72 (1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burdo J, Menzies S, Simpson I, Garrick L, Garrick M, Dolan K, Haile D, Beard J, Connor J. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. Journal of Neuroscience Research. 2001;66 (6):1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Antonetti DA, Wolpert EB, Connor JR. Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience. 2003;121 (4):883–890. doi: 10.1016/s0306-4522(03)00590-6. [DOI] [PubMed] [Google Scholar]
- Byrne SL, Chasteen ND, Steere AN, Mason AB. The Unique Kinetics of Iron Release from Transferrin: The Role of Receptor, Lobe–Lobe Interactions, and Salt at Endosomal pH. Journal of Molecular Biology. 2010;396 (1):130–140. doi: 10.1016/j.jmb.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhungana S, Taboy CH, Zak O, Larvie M, Crumbliss AL, Aisen P. Redox Properties of Human Transferrin Bound to Its Receptor†. Biochemistry. 2004;43 (1):205–209. doi: 10.1021/bi0353631. [DOI] [PubMed] [Google Scholar]
- Eide D, Davis-Kaplan S, Jordan I, Sipe D, Kaplan J. Regulation of iron uptake in Saccharomyces cerevisiae. The ferrireductase and Fe(II) transporter are regulated independently. Journal of Biological Chemistry. 1992;267 (29):20774–20781. [PubMed] [Google Scholar]
- Gaasch J, Lockman P, Geldenhuys W, Allen D, Van der Schyf C. Brain Iron Toxicity: Differential Responses of Astrocytes, Neurons, and Endothelial Cells. Neurochemical Research. 2007;32 (7):1196–1208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- Garrick M. Human iron transporters. Genes & Nutrition. 2011;6 (1):45–54. doi: 10.1007/s12263-010-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick MDKH, Vargas F, Singleton S, Zhao L, Smith JJ, Paradkar P, Roth JA, Garrick LM. Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochemical Journal. 2006;398 (3):539–545. doi: 10.1042/BJ20051987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick MD, Singleton ST, Vargas F, Kuo H-C, Zhao L, Knopfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM. DMT1: Which metals does it transport? Biological Research. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 Gene Encodes ZIP14, A Metal/Bicarbonate Symporter: Similarities to the ZIP8 Transporter. Molecular Pharmacology. 2008;73 (5):1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388 (6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Königsberger L-C, Königsberger E, May PM, Hefter GT. Complexation of iron(III) and iron(II) by citrate. Implications for iron speciation in blood plasma. Journal of Inorganic Biochemistry. 2000;78 (3):175–184. doi: 10.1016/s0162-0134(99)00222-6. [DOI] [PubMed] [Google Scholar]
- Kraiter DC, Zak O, Aisen P, Crumbliss AL. A Determination of the Reduction Potentials for Diferric and C- and N-Lobe Monoferric Transferrins at Endosomal pH (5.8) Inorganic Chemistry. 1998;37 (5):964–968. doi: 10.1021/ic970644g. [DOI] [Google Scholar]
- Madsen E, Gitlin JD. Copper and Iron Disorders of the Brain. Annual Review of Neuroscience. 2007;30 (1):317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- Moos T, Nielsen TR, Skjørringe T, Morgan EH. Iron trafficking inside the brain. Journal of Neurochemistry. 2007;103 (5):1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37 (11):1264–1269. doi: 10.1038/ng1658. http://www.nature.com/ng/journal/v37/n11/suppinfo/ng1658_S1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108 (4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Mancía S, Pérez-Neri I, Ríos C, Tristán-López L, Rivera-Espinosa L, Montes S. The transition metals copper and iron in neurodegenerative diseases. Chemico-Biological Interactions. 2010;186 (2):184–199. doi: 10.1016/j.cbi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Dolan KG, Lis A, Garrick MD. Effect of the iron chelator desferrioxamine on manganese-induced toxicity of rat pheochromocytoma (PC12) cells. Journal of Neuroscience Research. 2002;68 (1):76–83. doi: 10.1002/jnr.10207. [DOI] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain Iron Metabolism. Seminars in Pediatric Neurology. 2006;13 (3):142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Salvador GA. Iron in neuronal function and dysfunction. BioFactors. 2010;36 (2):103–110. doi: 10.1002/biof.80. [DOI] [PubMed] [Google Scholar]
- Siddappa AJM, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. Journal of Neuroscience Research. 2002;68 (6):761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- Steere A, Byrne S, Chasteen N, Smith V, MacGillivray R, Mason A. Evidence that His349 acts as a pH-inducible switch to accelerate receptor-mediated iron release from the C-lobe of human transferrin. Journal of Biological Inorganic Chemistry. 2010;15 (8):1341–1352. doi: 10.1007/s00775-010-0694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AN, Byrne SL, Chasteen ND, Mason AB. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochimica et Biophysica Acta (BBA) - General Subjects (0) doi: 10.1016/j.bbagen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulpule K, Robinson SR, Bishop GM, Dringen R. Uptake of ferrous iron by cultured rat astrocytes. Journal of Neuroscience Research. 2010;88 (3):563–571. doi: 10.1002/jnr.22217. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Love S. The binding and transport of alternative metals by transferrin. Biochimica et Biophysica Acta (BBA) - General Subjects (0) 2011 doi: 10.1016/j.bbagen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Weaver KD, Gabričević M, Anderson DS, Adhikari P, Mietzner TA, Crumbliss AL. Role of Citrate and Phosphate Anions in the Mechanism of Iron(III) Sequestration by Ferric Binding Protein: Kinetic Studies of the Formation of the Holoprotein of Wild-Type FbpA and Its Engineered Mutants. Biochemistry. 2010;49 (29):6021–6032. doi: 10.1021/bi902231c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott GH, Boyer PD. A Colorimetric Method for the Determination of Citric Acid in Blood and Plasma. Journal of Biological Chemistry. 1948;172 (2):729–736. [PubMed] [Google Scholar]
- Yang W, Jung K, Lee M, Lee Y, Nakagawa S, Niwa M, Cho S, Kim D. Transient Expression of Iron Transport Proteins in the Capillary of the Developing Rat Brain. Cellular and Molecular Neurobiology. 2010:1–7. doi: 10.1007/s10571-010-9558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
15 μg (Dcytb & STEAP2) or 25 μg (DMT1) of protein from whole cell lysates (Dcytb & STEAP2) or membrane proteins (DMT1) of either hBMVEC or Caco-2 cells were subjected to SDS-PAGE and immunobloting by respective antibodies as indicated. Representative ladder is diagrammed to the left of blots and indicates size in kDa.