Abstract
Peptides are receiving increased attention as therapeutic agents, due to their high binding specificity and versatility to be modified as targeting or carrier molecules. Particularly, peptides with anti-angiogenic activity are of high interest due to their applicability to a wide range of cancers. In this study we investigate the biological activity of two novel antiangiogenic peptides in pre-clinical glioma models. One peptide SP2000 is derived from collagen IV and the other peptide SP3019 belongs to the CXC family. We previously characterized the capacity of SP2000 and SP3019 to inhibit multiple biological endpoints linked to angiogenesis in human endothelial cells in several assays. Here we report additional studies using endothelial cells and focus on the activity of these peptides against human glioma cell growth, migration and adhesion in vitro and growth as tumor xenografts in vivo. We found that SP2000 completely inhibits migration of the glioma cells at 50 μM and SP3019 produced 50% inhibition at 100 μM. Their relative anti-adhesion activities were similar with SP2000 and SP3019 generating 50% adhesion inhibition at 4.9 ± 0.82 μM and 21.3 ± 5.92 μM respectively. In vivo glioma growth inhibition was 63 % for SP2000 and 76% for SP3019 after 2 weeks of administration at daily doses of 10mg/kg and 20 mg/kg, respectively. The direct activity of these peptides against glioma cells in conjunction with their anti-angiogenic activities warrants their further development as either stand-alone agents or in combination with standard cytotoxic or emerging targeted therapies in malignant brain tumors.
Keywords: Angiogenesis, cancer therapy, endothelial cell, glioblastoma, proliferation, migration, adhesion
Introduction
A recent study by National Cancer Institute predicts that the societal costs attributed to brain cancer will increase by 0.83 billion dollars by the year 2020 [1]. This increase in cost is predicted in spite of new medical and therapeutic discoveries, thus supporting the urgent need for more cost effective and efficacious therapies. Peptides are receiving increased attention as therapeutic agents, due to their high binding specificity and versatility to be modified as targeting or carrier molecules [2–4]. Peptides are being increasingly used in therapeutic applications for multiple diseases including cancer. One example under development for multiple cancers is Cilengitide, an RGD pentapeptide that inhibits ανβ3 and ανβ5 integrins [5, 6]. Peptides with anti-angiogenic activity [7, 8] are of particular interest due to the role for angiogenesis as a process central to the development and malignant progression of multiple malignancies [9]. Glioblastoma multiforme (GBM), is one of the most common and aggressive brain tumors with a dismal median survival of 12–15 months. Invasive gliomas such as GBM are characterized by insidious infiltration of tumor cells into surrounding brain and robust tumor angiogenesis that together cause tumor growth, morbidity and death [10, 11]. The current standard of care for GBM consists of surgical resection of primary tumor, concurrent adjuvant chemotherapy and radiation followed by additional adjuvant chemotherapy. Bevacizumab, an antibody against vascular endothelial growth factor (VEGF) received expedited approved by FDA (May 2009) for treatment of patients with progressive disease following prior therapy. Several studies have demonstrated that Bevacizumab leads to an extension of progression free survival without obviously prolonging the overall survival. The limitations of Bevacizumab and other emerging anti-angiogenic strategies may relate in part to the compensatory induction of an invasive glioma phenotype and growth pattern. Thus more effective therapies using agents with multiple mechanisms of action (e.g. anti-angiogenic + anti-invasion) are needed [9, 12].
In this study we investigate the application of two anti-angiogenic peptides belonging to different classes in application to glioma therapy. Targeting the disease from different perspectives, angiogenic and tumorigenic, along with inducing these effects via different receptors and thus affecting different signaling and molecular pathways could lead to an effective multimodal therapeutic approach.
Chemokines are cytokines involved in directed migration of leukocytes and are classified in four major classes, CXC, CC, C and CX3C. Amongst these, chemokines belonging to the CXC family have been shown to be involved in tumor angiogenesis [13, 14]. We previously showed that peptides derived from this family are strongly active in inhibiting the migration and proliferation of human umbilical vein endothelial cells (HUVEC) [15, 16] and also are capable of inhibiting tumor growth in a breast cancer xenograft model [17].
The other peptide that we investigate in this study is derived from collagen IV and it interacts with integrins, specifically with the αVβ3 and αVβ1 integrins which are overexpressed on both tumor endothelial and cancer cells [16, 18]. We previously characterized the effect of this peptide on endothelial cells and found that it is capable of strongly inhibiting endothelial cell proliferation and also inhibits migration (80% inhibition at 30 μg/ml) [19, 20]. This peptide and a homologous mimetic peptide were shown to inhibit tumor growth in breast cancer [17, 21] and lung cancer [22] xenograft models.
In this study we focus on the characterization of the activity of the peptides on glioma cells and test their activity in vivo in glioma xenografts using U87 cells.
Materials and Methods
Cell culture
Human umbilical vein endothelial cells (HUVEC) were purchased from Lonza and were grown under the manufacturer’s recommendation using Endothelial Basal Media (EBM-2) supplemented with the Bullet Kit (EGM-2, Lonza). Cells of passages 2–7 were used for experiments. U87 cells were originally purchased from American Type Culture Collection (ATCC) and maintained in Minimum Essential Medium w/ Earle Salts and L-glutamine (MEM 1X; Mediatech, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco), 2 mM Sodium Pyruvate (Gibco), 0.1 mM MEM-Non-essential Amino Acids (Gibco) and penicillin-streptomycin (Gibco). Cells were grown at 37°C in a humidified incubator with 5% CO2.
Peptide synthesis
The peptides were synthesized using solid phase synthesis and were supplied as TFA salts with an amidated C terminus by Abgent (San Diego, CA). The purity of the peptides was > 95% and the supplier provided product characterization (MALDI-TOF and HPLC traces) as proof of MW and purity accuracy. The 20-mer collagen IV derived peptide which we call SP2000 (sequence LRRFSTMPFMFCNINNVCNF) was solubilized in 5% DMSO and water due to its hydrophobic profile, while the 24-mer CXCL1 chemokine derived peptide which we call SP3019 (sequence NGRKACLNPASPIVKKIIEKMLNS) was solubilized in PBS. The pH of solubilized peptides was found to be around pH 7. For all experiments the DMSO percentage was maintained at non-toxic threshold (determined by toxicity curves of DMSO on cells) with a final DMSO percentage (< 0.2%) which was used as control in all experiments.
Proliferation assays
Colorimetric based proliferation assay using WST-1 (Roche) proliferation reagent was used to assess the anti-proliferative effect. 2000 cells/well were plated in 96-well plates and allowed to adhere overnight. On the next day, the medium was exchanged with serum-containing medium containing either peptide or the solubilization vehicle for the control. Three days later the medium with peptides was replaced with serum-free EBM-2 media containing WST-1 reagent, and incubated for four hours as per manufacturer’s protocol. The change in color is due to the formazan dye which is the result of the cleavage of the tetrazolium salt WST-1 by the mitochondrial succinate-tetrazolium reductase. Absorbance measurements at 450 nm were made with Victor V fluorescence plate reader (Perkin Elmer, MA). Dose response curves of percent live cells (in comparison to untreated cells but incubated in complete media with 0.2% DMSO) were created. Assays were performed in at least two independent replicates and each replicate was performed using three experimental replicates.
Migration assay
Migration inhibition was investigated using the Oris Pro Migration assay (Platypus Technologies, CMA 1.101). Briefly, 40,000 cells/well in complete medium were added to a 96 well plates containing well stoppers to block migration of cells to the center region of the wells. Cells were allowed to adhere for 4 hours, after which the stoppers were removed and fully supplemented media with or without experimental peptide was added to the wells. After 18 hours cells were stained with calcein AM (0.5μg/ml) (Invitrogen, CA) and the cells that migrated to the center of the well were quantified by reading fluorescence using a Victor V plate reader (Perkin Elmer, MA) and also imaged using a Nikon microscope (Eclipse T-100); images were acquired with the CCD Sensicam mounted on a Nikon microscope (Cooke Company, MI). The detection of the cells that migrated into the previously restricted region is possible due to the addition of a detection mask at the bottom of the plate, which obstructs from measurement cells that did not migrate.
Adhesion assay
The adhesion inhibition activity of the peptides in cellular adhesion was assessed using the RT-CIM technology [23, 24]. 25,000 cells/well were plated in 16-wells E-plates (Roche) in the presence or absence of the peptide. The adhesion was monitored over time (3 hours) by measuring changes in electrical impedance, which is a direct measure of the cells adhering on the electrodes. IC50 curves were created for each cell type and peptide. Assays were performed in at least two independent replicates and each replicate was performed using two experimental duplicates.
Tube formation assay
The compounds were tested for their ability to inhibit tube formation, an in vitro correlate of angiogenesis; the protocol was described by Arnaoutova et al. [25, 26] and it consists of plating HUVEC on top of basement membrane extract and after incubation at 37°C the cells naturally rearrange themselves in a network of tubes. Briefly, 50 μL/well of Matrigel (BD Biosciences) was plated in a cold 96-well plate and incubated at 37°C for 30 min for polymerization. 15,000 cells/well were added to the top of the gel and incubated in complete media in the presence or absence of peptide for 19 hours. Images were captured using the CCD Sensicam mounted on a Nikon microscope (Eclipse T-100). Assays were performed in at least two independent replicates and each replicate was performed using three experimental replicates and one image of a randomly chosen field was acquired per well.
Tumor xenografts
Animals were housed and treated according to the approved animal protocol of the Institutional Care and Use Committee at Johns Hopkins Medical Institution (JHMI). Glioma xenografts were generated as previously described [27]. Female 6- to 8- week-nude mice (National Cancer Institute, Frederick, MD) were anesthetized by i.p. injection of ketamine (100mg/kg) and xylazine (5 mg/kg). Subcutaneous xenografts were generated by injecting 4 × 106 of cells in 0.05 mL of media s.c. in the dorsal flank of nude mice. When tumors reached ≈ 100 mm3, the mice were randomly divided into groups (n = 5 per group) and treatment commenced. Peptides were administered once per day (for 14 days) intraperitoneally (i.p.) at doses of 10mg/kg for SP2000 and 20mg/kg for SP3019; these dose were based on our previous work [17]. Tumor volumes were estimated by measuring two dimensions [length (a) and width (b)] and calculating volume as V = ab2 / 2.
Data analysis
Statistical significance (p < 0.05) within each experiment using the independent Student’s t-test and ANOVA accompanied by Dunnett’s test if we were comparing different sets of data to one group.
Results
Proliferation
Previous work from our laboratory has shown that both peptides (SP2000 and SP3019) inhibit the proliferation of HUVEC cells almost completely at 40 μg/ml [15, 19]. In this study we analyzed the effect of each peptide on the proliferation of human glioma cells. The results are depicted in Figure 1 and show that the peptides had little effect on U87 cancer cell proliferation.
Figure 1. Proliferation activity.
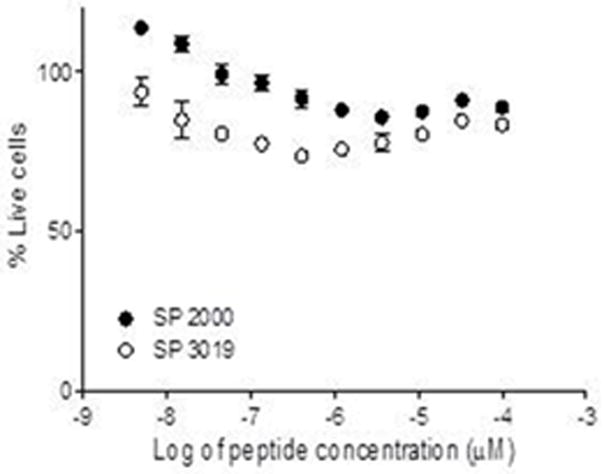
The activity of the peptides on U87 glioma cell proliferation. The cells were incubated in the presence of peptides and the % of live cells was calculated in comparison to cells incubated with media containing the solubility vehicle. Error bars depict STD
Migration
The capacities of these peptides to inhibit endothelial cell migration was previously characterized. SP2000 and SP3019 inhibited HUVEC migration by 80% at 30 μg/ml and 50% at 20 μg/ml, respectively [15, 19]. We analyzed the potential activity of the peptides on U87 glioma cell migration using the Platypus assay. Cells were exposed to peptides for 18 hours and allowed to migrate into a previously restricted area. The results represented in Figure 2 illustrate strong inhibition of glioma cell migration. The SP2000 peptide displayed very strong inhibitory activity (completely inhibits migration of the glioma cells at 50 μM) consistent with its molecular target integrins which are critical to cellular migration [16]. SP3019 was substantially less potent in this assay, displaying 50% inhibition at 100 μM. The quantification of the migration was performed by measuring fluorescence intensity of the calcein labeled cells and it is depicted in panel (J) of Figure 2; the negative values are a result of the subtraction of the background. In some cases the alignment of stoppers is not quite exact thus allowing some cells to enter the occlusion zone leading to negative measurements when the average of the background is subtracted from the experimental value. However the pictures captured show that the treatment and the background conditions are very similar thus indicating no migration in the case of the treatment.
Figure 2.
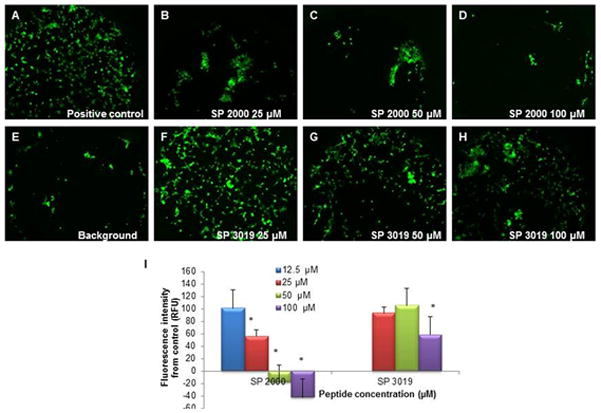
Migration activity. The inhibitory activity of the peptides on the migration of the glioma cells U87. Panel (A) illustrates the positive control (untreated cells); panels (B) through (D) depict the inhibition activity of the SP2000 at several concentrations, Panel (E) illustrates a typical background measurement (image of the well with the stopper removed immediately preceding the imaging) and panels (F) through (H) demonstrate the inhibitory activity of SP3019 peptide. Panel (I) illustrates the quantification of the images. The negative values are due to the subtraction of the background values which can vary significantly between the wells depending on the alignment of the stopper in the well. Error bars depict STD. * indicates statistical significance (p < 0.05) from the control.
Adhesion
The strong inhibitory activity of SP2000 against both HUVEC and U87 cell migration predicted that it would likely also inhibit HUVEC and glioma cell adhesion. Thus we tested these peptides using the RT-CIM adhesion assay and the results are presented in Figure 3. The SP2000 peptide was effective at inhibiting the adhesion of both cell types as depicted by the IC50; SP 2000 1.7 ± 0.67 μM on HUVEC and 4.9 ± 0.82 μM on U87 cells. SP3019 displayed a lower potency with IC50 of 23.59 ± 15.5μM on HUVEC and 21.3 ± 5.92 μM on U87 cells.
Figure 3.
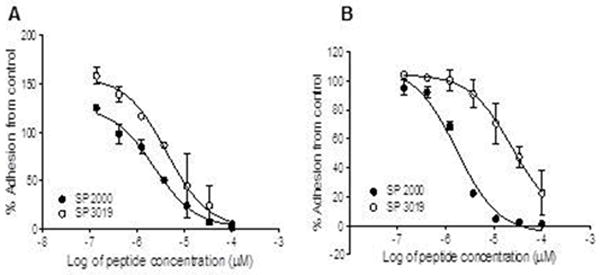
Inhibition of adhesion. Panel (A) illustrates the inhibition activity on the adhesion of HUVEC and panel (B) demonstrates the activity of both peptides at inhibiting the adhesion of U87 glioma cells. Error bars depict STD.
Tube formation
Tube formation is a typical comprehensive in vitro assay to assess angiogenesis. This assay incorporates the adhesion and migration dynamics and thus it should give a more realistic measurement of the activity of compounds in vivo where the interplay between all effects is important in suppressing tumor growth. Figure 4 shows that both peptides are capable of inhibiting tube formation of HUVEC when plated on Matrigel extract. We have tested the activity of the peptides at high concentration 100 μM because at this concentration the effects of the peptides are drastic, leading to complete inhibition of the tube formation thus not requiring quantification of the effect, which can be subjective and difficult to interpret.
Figure 4.
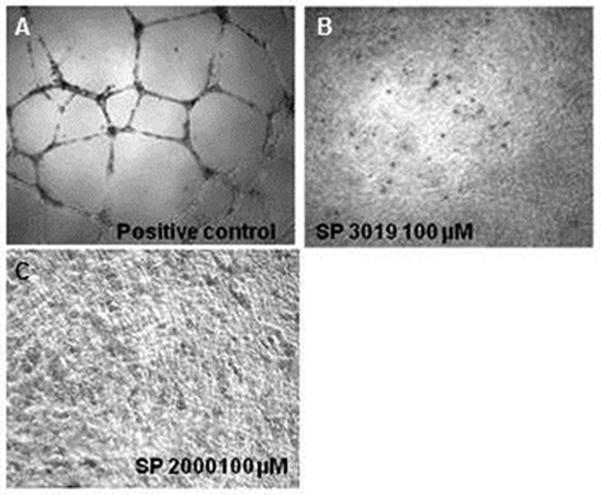
Tube formation inhibition. Panel (A) illustrates the positive control, HUVEC cell on Matrigel in complete media without any peptide treatment; panel (B) the inhibition of tube formation with SP3019; and panel (C) with SP2000.
In vivo tumor growth suppression
Based on the in vitro peptide activities on both endothelial and tumor cells we tested their therapeutic potential using the U87 human glioma xenograft model. Immune deficient Nu/nu mice received U87 cells by subcutaneous injection and daily treatment with peptides began after tumors reached a volume of approximately 100 mm3. Peptides were administered intraperitoneally (i.p) at 10 mg/kg for SP2000 and 20mg/kg for SP3019 based on our previous experiments with other types of cancer [17, 21, 22]. Both peptides were active in inhibiting tumor growth, as illustrated in Figure 5. Tumor volumes in the treated groups were significantly different from the control group starting with day 3 and continued throughout the experiment (p < 0.05). At day 14 the tumors in the treated groups displayed a size reduction of 62.5 % and 75.7% for SP2000 and SP3019 respectively. Treatment with scrambled peptide was tested for SP3019 in this study and for SP2000 in a previous study [17]; the results were not statistically different from the control but statistically different from the treatment groups.
Figure 5.
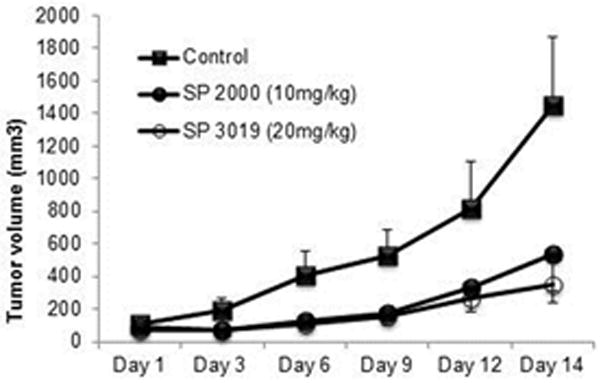
In vivo tumor xenograft inhibition. Tumor volume of subcutaneous glioma xenograft measured every third day. Squares depict the control group which received i.p. injection of the solubility vehicle (PBS), solid circle represents the tumor growth of the group treated with daily i.p. injections of SP2000 and the open circles of tumors treated with SP3019. Error bars depict STD.
Discussion
Glioblastoma multiforme (GBM) or gliomas account for approximately 40% of malignant brain tumors and there are 10,000 GBM cases diagnosed annually with median survival time of 15 months and 5-year survival of less than 5% (National Cancer Institute). GBM are highly vascularized tumors which makes tumor vasculature a primary therapeutic target. A milieu of molecules that play a role in GBM tumorigenesis and angiogenesis have been identified and some of them have been investigated as drug targets [28]. Among the molecules that serve as primary targets for anti-angiogenic therapy are vascular endothelial growth factors (VEGF) and their receptors VEGFRs; platelet derived growth factor (PDGF) and its receptors; matrix metalloproteinase, especially MMP2 and MMP9; integrins αvβ3 and αvβ5, transcription factor hypoxia-inducible factor 1α, and chemokines and their receptors [28]. The peptides investigated in this study target two of the above categories, the integrin and chemokines families. We have shown that both peptides are active at inhibiting proliferation, migration, and adhesion and tube formation of endothelial cells and also possess activity at inhibiting adhesion and migration of tumor cells. The collagen derived peptide, SP2000, is quite strong at inhibiting migration of U87 cells in vitro. One of the reasons gliomas are so difficult to treat is because the tumor cells are highly migratory and at the time of surgical resection many cells have already disseminated from the primary tumor giving rise to secondary tumors at a later point [29]. Thus therapeutic approaches that inhibit migration of tumor cells hold promise for not only being effective at inhibiting primary tumor growth but also to reduce the development of anatomically distant secondary tumors. In vitro tumor spheroids have been shown to be characterized by two regions: core and rim regions. Cells in the core are categorized as proliferative while cells in the rim are highly migratory. These two cell phenotypes also exhibit differential sensitivity to conventional chemotherapeutic treatment, with the cells in the core region being responsive while the rim cells are not, indicating that migratory phenotype might be responsible for the low therapeutic response seen in clinic [29, 30]. Thus the peptides presented here exhibit a high activity at inhibiting migration, so perhaps as therapeutic agents in conjunction with chemotherapy they could change the migratory phenotype to a proliferating profile thus increasing the sensitivity to chemotherapy. This in conjunction with their activity on reducing tumor vasculature could result in a considerable change in overall response to therapy.
In vivo tumor growth inhibition was strong, 62.5 % and 75.7% reduction in size for SP2000 and SP3019 respectively. These activities correlated well with the in vitro activity and it may be the result of a dual targeting approach: endothelial and tumor cells. Previously we demonstrated that treatment with SP2000 peptide leads to a decrease in vasculature and in proliferative cells, both mouse and human in comparison with control, while the SP3019 peptide led to a decrease in microvascular density but an increase in proliferation of mouse cells, which could be due to a stimulation of macrophages [17]. Nude and SCID are immunocomprismized mice with a loss in either T or both T and B cells; however they carry normal or even higher levels of macrophages and natural killer (NK) cells [31]. The previous results indicating an increase in proliferation of mouse cells coupled with a higher CD31 staining present in this study (results not shown) are indicative of stimulation of macrophages and NK cells (both cells types express the CD31 marker). Thus treatment with the peptide derived from chemokines could also bring a multimodality approach by stimulating infiltration of immune cells into the tumors which could lead to tumor growth suppression [32, 33]; these aspects warrant further investigation.
In conclusion, in this study we present the novel application of two peptides; one derived from collagen IV (SP2000) and the other derived from the CXCL1 chemokine (SP3019) to glioma growth inhibition. The peptides demonstrate strong activity in in vitro screening which translated to strong tumor growth inhibition in glioma xenograft. These peptides show multimodal potential which warrants them for further study in combination with standard chemotherapeutic approaches.
Acknowledgments
The authors thank all members of the laboratory for insightful discussions and review of the manuscript. We thank Dr. Emmanouil D. Karagiannis for his participation in preliminary experiments for this study. We are grateful to ACEA Biosciences for the use of the Personal RT-CIS system which allowed us to perform migration and adhesion experiments and in particular to Dr. Yama Abassi for detailed technical discussions. Dr. Popel serves as the Chief Scientific Officer of AsclepiX Therapeutics, LLC. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. This work was supported by the National Institutes of Health grants R21 CA131931 and R01 CA138264.
References
- 1.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhutia SK, Maiti TK. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008;26:210–217. doi: 10.1016/j.tibtech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 4.Reardon DA, Neyns B, Weller M, Tonn JC, Nabors LB, Stupp R. Cilengitide: an RGD pentapeptide alphanubeta3 and alphanubeta5 integrin inhibitor in development for glioblastoma and other malignancies. Future Oncol. 2011;7:339–354. doi: 10.2217/fon.11.8. [DOI] [PubMed] [Google Scholar]
- 5.Nabors LB, Fiveash JB, Markert JM, Kekan MS, Gillespie GY, Huang Z, et al. A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma. Arch Neurol. 2010;67:313–319. doi: 10.1001/archneurol.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reardon DA, Turner S, Peters KB, Desjardins A, Gururangan S, Sampson JH, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9:414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs. 2009;12:779–784. [PubMed] [Google Scholar]
- 8.Rosca EV, Koskimaki JE, Pandey NB, Rivera CG, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Current Pharmaceutical Biotechnology. 2011;12:1101–1116. doi: 10.2174/138920111796117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5:610–620. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 10.Nicholas MK, Lukas RV, Chmura S, Yamini B, Lesniak M, Pytel P. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol. 2011;38:243–253. doi: 10.1053/j.seminoncol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Lim SK, Llaguno SR, McKay RM, Parada LF. Glioblastoma multiforme: a perspective on recent findings in human cancer and mouse models. BMB Rep. 2011;44:158–164. doi: 10.5483/BMBRep.2011.44.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahluwalia MS, Gladson CL. Progress on antiangiogenic therapy for patients with malignant glioma. J Oncol. 2010;2010:689018. doi: 10.1155/2010/689018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Karagiannis ED, Popel AS. Novel anti-angiogenic peptides derived from ELR-containing CXC chemokines. J Cell Biochem. 2008;104:1356–1363. doi: 10.1002/jcb.21712. [DOI] [PubMed] [Google Scholar]
- 16.Karagiannis ED, Popel AS. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc Natl Acad Sci USA. 2008;105:13775–13780. doi: 10.1073/pnas.0803241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koskimaki JE, Karagiannis ED, Rosca EV, Vesuna F, Winnard PT, Jr, Raman V, et al. Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts. Neoplasia. 2009;11:1285–1291. doi: 10.1593/neo.09620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabatabai G, Weller M, Nabors B, Picard M, Reardon D, Mikkelsen T, et al. Targeting integrins in malignant glioma. Target Oncol. 2010;5:175–181. doi: 10.1007/s11523-010-0156-3. [DOI] [PubMed] [Google Scholar]
- 19.Karagiannis ED, Popel AS. Identification of novel short peptides derived from the alpha 4, alpha 5, and alpha 6 fibrils of type IV collagen with anti-angiogenic properties. Biochem Biophys Res Commun. 2007;354:434–439. doi: 10.1016/j.bbrc.2006.12.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera CG, Rosca EV, Pandey NB, Koskimaki JE, Bader JS, Popel AS. Novel peptide-specific quantitative structure-activity relationship (QSAR) analysis applied to collagen IV peptides with antiangiogenic activity. J Med Chem. 2011;54:6492–6500. doi: 10.1021/jm200114f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosca EV, Koskimaki JE, Pandey NB, Wolff AC, Popel AS. Development of a biomimetic peptide derived from collagen IV with anti-angiogenic activity in breast cancer. Cancer Biol Ther. 2011;12:808–817. doi: 10.4161/cbt.12.9.17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koskimaki JE, Karagiannis ED, Tang BC, Hammers H, Watkins DN, Pili R, et al. Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model. BMC Cancer. 2010;10:29. doi: 10.1186/1471-2407-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keogh RJ. New technology for investigating trophoblast function. Placenta. 2010;31:347–350. doi: 10.1016/j.placenta.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Rahim S, Uren A. A real-time electrical impedance based technique to measure invasion of endothelial cell monolayer by cancer cells. J Vis Exp. 2011 doi: 10.3791/2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 26.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5:628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 27.Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, et al. EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther. 2009;8:1751–1760. doi: 10.1158/1535-7163.MCT-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstner ER, Duda DG, di Tomaso E, Sorensen G, Jain RK, Batchelor TT. Antiangiogenic agents for the treatment of glioblastoma. Expert Opin Investig Drugs. 2007;16:1895–1908. doi: 10.1517/13543784.16.12.1895. [DOI] [PubMed] [Google Scholar]
- 29.Demuth T, Rennert JL, Hoelzinger DB, Reavie LB, Nakada M, Beaudry C, et al. Glioma cells on the run - the migratory transcriptome of 10 human glioma cell lines. BMC Genomics. 2008;9:54. doi: 10.1186/1471-2164-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Demuth T, Ross KR, et al. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J Neurooncol. 2001;53:161–176. doi: 10.1023/a:1012253317934. [DOI] [PubMed] [Google Scholar]
- 31.Christianson SW, Greiner DL, Schweitzer IB, Gott B, Beamer GL, Schweitzer PA, et al. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol. 1996;171:186–199. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 32.Cao MY, Lee Y, Feng N, Li H, Du C, Miao D, et al. NK cell activation and tumor infiltration are involved in the antitumor mechanism of Virulizin. Cancer Immunol Immunother. 2005;54:229–242. doi: 10.1007/s00262-004-0582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielawska-Pohl A, Blesson S, Benlalam H, Trenado A, Opolon P, Bawa O, et al. The anti-angiogenic activity of IL-12 is increased in iNOS−/− mice and involves NK cells. J Mol Med. 2010;88:775–784. doi: 10.1007/s00109-010-0620-7. [DOI] [PubMed] [Google Scholar]


