Abstract
Hematopoietic cell transplantation (HCT) offers potentially curative therapy for patients with myelodysplastic syndromes (MDS). However, as most patients with MDS are in the 7th or 8th decade of life, only few of these were transplanted in the past, using high-dose conditioning regimens. The development of reduced-intensity conditioning has allowed to offer HCT also to older patients and those with clinically relevant comorbid conditions. Dependent upon disease status and the type of clonal chromosomal abnormalities present at the time of HCT, some 25%–75% of patients will be cured of their disease and survive long-term. Recent results with HLA matched unrelated donors are comparable to those with HLA genotypically identical siblings. The increasing use of cord blood and HLA haploidentical donors is expected to make HCT available to a growing number of patients. However, post-transplant relapse and graft-versus-host disease remain problems requiring further investigation.
Keywords: Allogeneic hematopoietic cell transplantation, myelodysplastic syndrome, conditioning regimens, disease stage and prognosis, relapse, patient age, comorbid conditions, donors and sources of stem cells
1. INTRODUCTION
As described elsewhere in this issue, myelodysplastic syndromes (MDS) are clonal diseases of hematopoietic stem/progenitor cells with great heterogeneity of presentation and prognosis. [1] About one-third of patients will progress to chronic myelomonocytic leukemia (CMML) or acute myeloid leukemia (AML), while the remaining patients show progressive peripheral blood cytopenias that may prove fatal due to intervening infections or hemorrhage [2]. New therapeutic options, such as hypomethylating agents or immunomodulatory drugs improve hematologic parameters and reduce transfusion requirements for variable periods of time [3].
The only currently available treatment strategy with proven curative potential is allogeneic hematopoietic cell transplantation (HCT), with long-term survival probabilities ranging from 25%–75% [4]. However, there is considerable bias in the selection of patients for HCT, based on age, disease stage, comorbid conditions, and possibly other factors. This is illustrated by the striking age differences reported between the overall population of patients with MDS and patients treated with non-transplant therapy and those who ultimately receive HCT [3,5]. The fact that the median age of patients diagnosed with MDS is in the 7th decade has prevented many of these patients from being considered for HCT until quite recently. The development of reduced/low intensity conditioning (RIC) regimens has made HCT available for many older or medically infirm patients. Limitations related to donor availability have been eased by the inclusion of HLA-haploidentical donors and the use of cord blood. However, numerous questions remain unanswered, such as optimum timing of HCT and proper integration with novel non-transplant modalities.
2. WHO SHOULD BE CONSIDERED FOR ALLOGENEIC HCT?
In principle, any patient with MDS should at least be informed about the possibility of HCT early in the disease course. This does not mean that patients should be offered HCT indiscriminately as we discussed in a recent perspective [6]. Rather, the pros and cons must be discussed, and a treatment plan should be developed on which HCT may be the up-front recommendation or an option “down the road”. Certainly in patients with “advanced” MDS HCT should figure high on the menu of treatment options. In a retrospective registry-based analysis Cutler and collaborators showed that up-front HCT was associated with a superior life expectancy among patients with intermediate-2 and high-risk MDS based on IPSS (International Prognostic Scoring System) criteria [7]. Patients with low risk disease, in contrast, had the best outlook if HCT was delayed until disease progression. For patients in the intermediate-1 risk group there was only a slight advantage of delaying HCT. The IPSS, however, does not take into consideration patient age, comorbid conditions and clinical determinants such as transfusion dependence [8,9]
Since age has been a major factor determining the decision about allogeneic HCT, younger patients are likely to come to HCT sooner than older patients. Kuendgen et al. [10] presented registry data on 232 patients less than 50 years of age, and 2,496 patients more than 50 years of age, and showed that 42% of the younger patients underwent HCT compared to 8% of the older cohort. However, improvement of supportive care and, importantly, the use of RIC for HCT (see below) have substantially improved HCT outcome among older patients, and currently there is no clear chronologic upper age limit. In fact, in a retrospective multicenter analysis of 1,333 MDS patients 50 years or older, the 4-year estimated treatment-related mortality (TRM) was 36% in the 50–60 year old cohort, and 39% in patients older than 60 years [11]. In a CIBMTR analysis of 1,080 patients with MDS undergoing RIC HCT between 1995 and 2005, similarly, age did not impact TRM, relapse, relapse-free survival (RFS), or overall survival (OS) [12]. It is important to note, however, that no prospective comparison is available, and enrollment of patients into past trials was subject to considerable selection bias on the basis of biologic rather than chronologic age.
The availability of non-HCT modalities, in particular hypomethylating agents, which may delay disease progression, has generated a potential decision dilemma: should patients continue on those therapies until they no longer respond or, alternatively, should patients go to HCT when they achieve their best response? The decision may well depend upon the individual patient. However, there is mounting evidence that HCT at the time when patients “fail” is associated with inferior outcome. This question by itself is reason enough for patients to have an early consultation to discuss the possibility of HCT. Recent publications analyzing factors that predict the probability of response to hypomethylating therapy should help in formulating recommendations [13].
3. DISEASE STAGE AND POST-HCT PROGNOSIS
It follows from the above discussion that “timing” of HCT remains a central question. Implied in this question are the importance of disease duration, disease progression, development of treatment resistance and acquisition of co-morbid conditions, among others.
An American Society of Blood and Marrow Transplantation (ASBMT) consensus paper [14] recommended early HCT for patients with IPSS intermediate-2 or high-risk at diagnosis (as suggested by the earlier report by Cutler et al). It also recommended HCT for selected patients with lower risk disease at diagnosis who have poor prognostic features, for example, older age, refractory cytopenias, or transfusion dependence. The impact of transfusion dependence is considered in the WPSS and was shown by Alessandrino et al. to be reflected in inferior outcome after HCT) [15]. It is expected that incorporation of newer prognostic tools such as flow cytometry or molecular markers will improve our ability to select high-risk patients for HCT. We have shown, for example, that among patients considered to have low-risk disease by IPSS criteria the presence of aberrant marrow blasts clearly identifies patients with more aggressive or resistant disease and a higher probability of relapse after HCT [16].
An earlier study [17] showed the probability of 5-year RFS in patients with RA/RARS, RAEB, RAEB-T and secondary AML (sAML) to be 52%, 34%, 19% and 26%, respectively, strongly supporting the concept that HCT should be carried out at the earliest point possible. However, as discussed above, patients at different stages of MDS might have superior life expectancies if HCT is delayed. Secondly, some patients have advanced disease already at the time of diagnosis. And then there are all those other factors, including age and comorbid conditions, that will need to be considered.
A study of 118 patients <18 years of age undergoing unrelated donor HCT showed an 8-year RFS of 65% for RCMD compared to 48% and 28% for RAEB and RAEB-T respectively [18]. In another series of 374 patients with low-risk MDS (median age 39 years), the 4-year OS was 52%. Older age and disease duration of >12 months were associated with inferior survival [19]. Studies at our own center indicate that post-HCT RFS correlates inversely with IPSS classification [20], with RFS in the range of 75% for patients with low-risk MDS and approximately 30% for patients with high-risk disease. The difference was due almost exclusively to relapse. Basically all recent analyses indicate that the patient’s karyotype is a stronger predictor for post-HCT outcome than the myeloblast count. For example, an analysis by the EBMT Group showed 5-year relapse probabilities of 34%, 35% and 57%, respectively, and TRM of 33%, 42% and 46%, respectively, among patients with good, intermediate and poor risk cytogenetics by IPSS [14]. An analysis by Armand et al. in 476 patients [21] supports those data, which were further validated a in a multi-center analysis based on “transplantation-specific” cytogenetics grouping for MDS patients, which uses only two risk groups, good/intermediate versus poor risk [22].
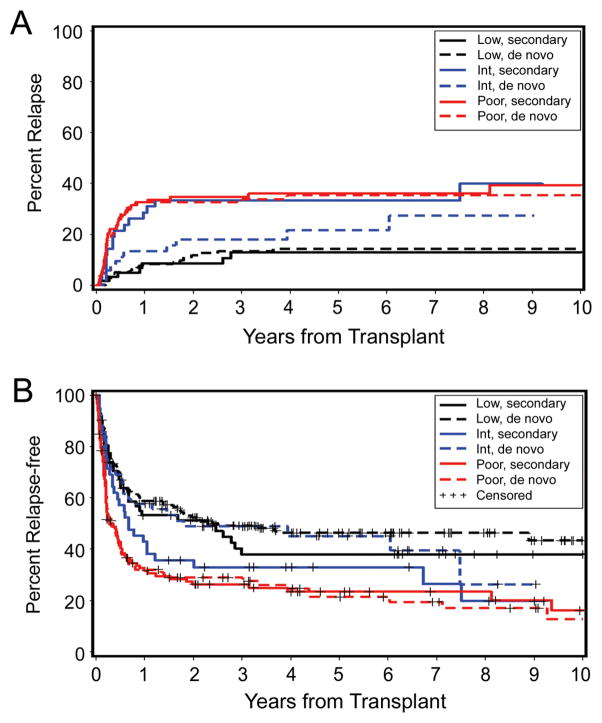
The European Blood and Marrow Transplantation (EBMT) Group [23] analyzed the post-transplant outcome of 692 patients with MDS according to their cytogenetic status. Univariate analysis showed 5-year OS and RFS rates in the intermediate and high-risk groups of 40% vs. 35%, and 31% vs. 21%, respectively. In support of the central importance of the karyotype, a recent analysis [24] showed that patients with secondary MDS had a post-HCT prognosis comparable to patients with de novo MDS once the data were adjusted for cytogenetic risk (Figure 1). The overwhelming impact of cytogenetics will be given greater weight in a modified IPSS scheme.
Figure 1. Impact of cytogenetic risk category on HCT outcome in patients with de novo and with secondary MDS/tAML.
A) Probability of relapse, B) Probability of relapse-free survival, based on cytogenetic risk as defined by IPSS [75]. This research was originally published in Blood. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. C. Chang, B.E. Storer, B.L. Scott, E.M. Bryant, H.M. Shulman, M.E. Flowers, B.M. Sandmaier, R.P. Witherspoon, R.A. Nash, J.E. Sanders, A. Bedalov, J.A. Hansen, B.E. Clurman, R. Storb, F.R. Appelbaum, H. Joachim Deeg, Blood 2007; 110: 1379–1387. © the American Society of Hematology.
Additional novel genetic factors are being studied to determine their role as possible predictors of transplant outcomes [25].
4. OTHER RISK FACTORS
Numerous studies have shown that the presence of comorbid conditions impacts overall treatment success, including outcome of HCT. Several scoring systems have been developed, including the HCT-specific co-morbidity index (HCT-CI) [26,27] and a risk score based primarily on pulmonary function [28]. With rare exceptions studies show that an elevated risk score, be it due to a prior solid tumor, the presence of cardiac or pulmonary (or other organ) insufficiency, metabolic and psychiatric disorders, among others, significantly interfere with long-term HCT success. For example, we showed recently in a cohort of 85 patients with CMML transplanted from related or unrelated donors, that those with an HCT-CI score of 0–2 had a RFS of 53% compared to 27% among those with scores of 3 or greater [29]. The impact of comorbidity is observed with both high-dose conditioning and RIC [26,30].
High levels of pre-HCT serum ferritin (e.g. > 1000 μg/L ) have been shown in multiple studies to correlate with inferior OS after HCT [31–33], possibly irrespective of blood transfusion burden. Some consequences of iron overload in the HCT setting may include increased risk of blood stream infections, invasive fungal infections and sinusoidal obstruction syndrome [34,35]. However, ferritin levels may reflect processes other than iron overload, and it is unclear if iron chelation will minimize the identified risks in MDS patients [36]. Prospective clinical trials are not available, but are needed to clarify this issue [37].
Bone marrow fibrosis in patients with MDS is associated with more rapid disease progression. Scott et al. showed that the presence of marrow fibrosis also adversely impacted transplant outcome, mostly in patients with advanced MDS [38]. This was recently confirmed by Kröger et al. in an analysis of 721 patients [39].
Finally, a significant correlation between DR15 and tumor necrosis factor (TNF) polymorphisms at position −308 among patients with MDS has been observed [40]. The TNF-308 AG genotype conferred an increased risk of TRM compared to the GG genotype. Conversely, the TNF-863 AA genotype was correlated with decreased overall mortality and TRM compared with the CC genotype.
5. PRE-TRANSPLANT AND ADJUVANT THERAPY
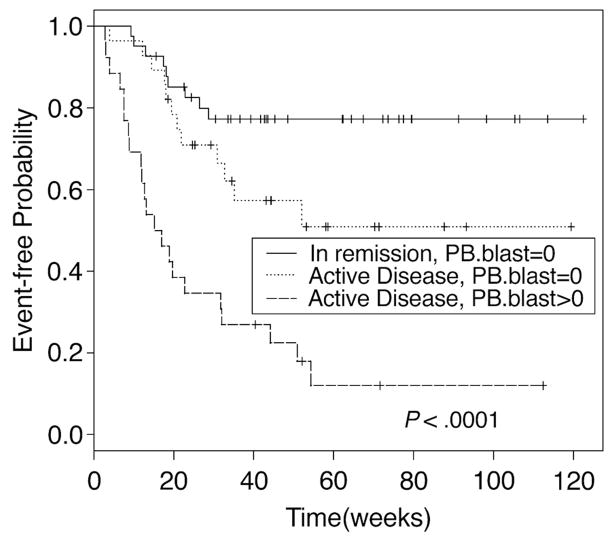
In the absence of randomized prospective trials, the value of induction chemotherapy or other treatment prior to allogeneic HCT remains undetermined. Al-Ali et al. [41] analyzed the outcome of 593 patients with MDS/sAML. There were 167 patients (28%) who received HCT without prior chemotherapy. The remaining patients received either an autologous (n=290) or an URD (n=136) HCT while in first complete remission. OS at 3 years was 40% for patients not given chemotherapy, and 41% and 50%, respectively for the two treated groups (p=0.01) The corresponding figures for RFS were 34%, 28% and 44%, respectively (p= 0.03). TRM in the three groups was 49%, 17%, and 38% (P<0.001), and relapse incidence was 30%, 24% and 62%, respectively (P<0.001). De Lima et al, in a study including patients with MDS and AML, suggested that the depths of response to pre-HCT therapy also affected post-HCT outcome (Figure 2) [42]. These data point to a benefit of pre-HCT debulking; however, patients who achieved remissions were transplanted at that time, whereas those who did not, were likely not to come to HCT. Similar results were reported by others [43].
Figure 2. Relapse-free survival by disease status and circulating blasts.
HCT results in 74 patients with AML and 22 patients with MDS. Patients had high risk disease or were given pre-HCT chemotherapy or both. At HCT patients were either in complete remission without blasts in peripheral blood (PB) or had active disease in the marrow without or with circulating blasts. This research was originally published in Blood. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. M. de Lima, D. Couriel, P.F. Thall, X Wang, T. Madden. R. Jones, E.J. Shpall, M. Shahjahan, B. Pierre, S. Giralt, M. Korbling, J.A. Russell, R.E. Champlin, B.S. Andersson, Blood 2004; 104: 857–864. © the American Society of Hematology.
When patients were transplanted while not in remission [44], the incidence of NRM was significantly higher than among patients who were in remission (48.9 vs. 18.8%, P=0.014), and the relapse incidence did not differ significantly (35.0 vs 26.2%, P=0.44).
Warlick et al [45] observed a 1-year cumulative incidence of relapse of 18% in 84 patients who achieved complete remissions, compared to 35% among those transplanted with active disease. Similar observations have been reported by others [46,47]. While HCT in remission appears beneficial to post-HCT outcome, remissions are not achieved consistently, and it is likely that remission induction selects “responsive” patients. In the absence of controlled data, the decision to use pre-HCT induction therapy should be made on an individual basis.
Many patients with MDS will come to HCT after or during treatment with the hypomethylating agents azacitidine or decitabine. It is unclear to what extent treatment with hypomethylating agents before HCT impacts peri-and post-HCT events. The Moffitt Cancer Center group reported the effect of pre-HCT azacitidine in 30 patients who received a median of four cycles of the drug, as compared to 24 untreated patients [48], and showed 1-year estimates of OS, RFS and relapse of 47%, 41% and 20% for azacitidine treated patients, and 60%, 51% and 32%, respectively, for untreated patients. In a retrospective analysis of 17 high-risk MDS patients (median age 55 years) who underwent allogeneic HCT after therapy with decitabine at MD Anderson Cancer Center, 11 were alive in remission at a median follow-up of 12 months [49]. We believe that if patients do receive hypomethylating therapy and respond, they should be transplanted at that time (assuming they are HCT candidates), rather than waiting until they lose their response. Whether hypomethylation has a place in post-HCT management in patients who are at high risk for relapse is currently being investigated.
Additional strategies include preemptive donor lymphocyte infusion (DLI) [50] and various vaccination or cellular immunotherapy strategies [51,52].
6. DONORS OTHER THAN HLA-IDENTICAL SIBLINGS
Although HLA-identical related donors have remained the first choice for most transplant teams, the numbers of HCT from HLA-matched URDs has grown rapidly, and the use of HLA haploidentical related donors or unrelated cord blood is expanding. Currently the data in patients with MDS are still limited [53].
An earlier analysis from the National Marrow Donor Program (NMDP) [54] including 510 unrelated donor HCT for MDS showed a probability of RFS at 2 years of 29%, and 26% at 4 years. The 2-year cumulative incidence of TRM was 54%. A study from our Center [20] involved 109 patients with MDS conditioned with busulfan (BU) targeted to plasma concentrations of 800 to 900 ng/mL plus cyclophosphamide (CY), and transplanted from related (n=45) or URD (n=64). RFS at 3 years was 56% for related and 59% for unrelated recipients. The relapse incidence was 16% for related and 11% for unrelated recipients. NRM at 100 days (3 years) was 12% (28%) for related and 13% (30%) for unrelated recipients. These data established that results with URDs selected on the basis of high resolution HLA matching, were comparable to those with HLA-matched siblings.
Sato et al [55], reporting on 33 MDS patients transplanted with cord blood, showed 5-year TRM and relapse rates of 14% and 16%, respectively, with a 5-year RFS of 70%. Parikh and collaborators [56] reported on 23 pediatric MDS patients (median age 11 years) treated with TBI-based preparative regimens and transplanted with 4/6 or 5/6 HLA antigen matched cord blood. One and 3-year RFS probabilities were 70% and 61%, respectively. The grafts contained a median of 4.0 × 107 total nucleated cells/kg, and the cumulative incidence of neutrophil and platelet engraftment was 91% and 70%, respectively. A more recent study from Minnesota [57] in patients aged 55 years or older compared results with HLA matched related donors prepared with RIC (n=47) to those with cord blood (n=43); 23% of patients who received cord blood had MDS. The 3-year RFS and OS for matched related and cord blood recipients were 30% vs. 34%, (P=0.98) and 43% vs. 34% (P=0.57), respectively. The incidences of grades II–IV acute GVHD and TRM at 180 days were comparable, but cord blood recipients had less chronic GVHD (17% vs. 40% at 1 year). At present, most centers give preference to an allele-level matched URD over cord blood. However, it is likely that the use of cord blood will increase further. The selection algorithms for less well matched donors are highly center-specific and beyond the scope of this review.
HLA-haploidentical (i.e., mismatched related donor) HCT have been used less often in patients with MDS than in other diseases, in part because of concern about engraftment as many of these patients have not received cytotoxic therapy before coming to HCT (see above). De Witte et al. reported an analysis of 1378 transplants carried out for patients with MDS on behalf of the EBMT group, including 91 patients transplanted from HLA non-identical related donors [58]. The 3-year survival was 28%, and TRM was 66%, i.e. higher than in any other type of HCT. Among 28 patients undergoing T-cell-depleted HLA-haploidentical HCT after conditioning with fludarabine, melphalan, thiotepa and ATG at the MD Anderson Cancer Center, patients with bone marrow blasts <15% had a higher survival probability than patients with more advanced disease (42% versus 0%) [59]. Day-100 and 1-year TRM was 9% and 32%, respectively. Chen et al. [60] reported on 36 patients with high-risk MDS who underwent HLA mismatched HCT including HLA haploidentical transplants with G-CSF-primed bone marrow and G-CSF-mobilized peripheral blood progenitor cells, and showed a 2-year RFS of 65%. Patients transplanted within 7 months of diagnosis had better RFS than those transplanted later (89% versus 43%). The use of HLA haploidentical donors is expanding rapidly. .
7. SELECTION OF STEM CELL SOURCE: BONE MARROW VERSUS PERIPHERAL BLOOD GRAFTS
G-CSF-mobilized peripheral blood progenitor cells (PBPC) provide more rapid engraftment than marrow, but convey a higher risk of GVHD, particularly in its chronic form. Retrospective analyses have suggested PBPC to be a better stem cell source for MDS patients, resulting in superior survival, in patients undergoing related donor HCT [61,62]. Survival benefit was associated with faster engraftment, lower TRM, increased chronic GVHD rates, and lower relapse rates. Guardiola and collaborators studied retrospectively 234 MDS patients and compared outcomes after PBPC and marrow HCT. Significantly improved 2-year TRM, and RFS (50% versus 39%) were observed with PBPC [63]. The results of a completed randomized CTN study comparing marrow and PBPC as sources of stem cells in the URD setting are currently being analyzed.
8. THE SPECTRUM OF CONDITIONING REGIMEN INTENSITY
Total body irradiation (TBI)-based high-dose conditioning regimens, the “classic” among conditioning regimens, have been effective, but also associated with considerable acute and delayed complications [54]. BU combined with CY or fludarabine provides an excellent alternative to TBI and has been extensively investigated [42,64]. RIC regimens are frequently considered for MDS patients, given the older age range of such patients. The possibilities of conditioning regimens now comprise a wide spectrum, including regimens such as fludarabine plus CY, or fludarabine plus 200 cGy TBI, “standard” CY/TBI, BU/Cy or fludarabine plus melphalan, to name just a few [65,66].
In a retrospective study of 836 patients, including 215 conditioned with RIC, and 621 patients conditioned with high-dose regimens, the 3-year relapse incidence was significantly increased with RIC, whereas TRM was significantly decreased [67]. Parker et al. [68] showed that RIC and sibling or URD HCT was associated with significantly shorter duration of marrow aplasia, less mucositis and infections, less need for parenteral nutrition, less acute and chronic GVHD, and lower early TRM (9% versus 31%) when compared to high dose conditioning for MDS. The 2-year actuarial OS and RFS were 48% and 39%, respectively, in the RIC group, and 44% and 44%, respectively, in the high-dose group, while TRM was 31% and 50%, respectively. With URD, OS was superior in the RIC arm (49% versus 34%) [68]. Others have indicated similar outcomes after either high-dose or RIC transplants [69].
Shimoni et al [70] reported on 112 consecutive patients with AML/MDS transplanted over a 5-year period. Fifty-eight had active disease at HCT (defined as >10% marrow blasts) and 54 were in remission. Forty-five patients were given high-dose conditioning (intravenous BU/CY). Sixty-seven patients who were not eligible for high-dose regimens were given RIC with fludarabine and intravenous BU at a dose of 6.4 mg/kg (FB2; n=41) or other “reduced-toxicity” regimens using fludarabine and intravenous BU at 12.8 mg/kg (FB4; n=26). Five-year OS rates were 59%, 50% and 60% after BU/CY, FB4 and FB2, respectively. However, when the analysis was limited to patients with active disease, the corresponding figures for 5-year OS were 42%, 19% and 0%.
In a recent study comparing RIC (n=39) with a high-dose regimen (n=39) in patients with AML and MDS aged 40–60 years, the median OS and RFS were 31.0 months, and 20.7 months, respectively, with no significant difference between the two cohorts [71]. The 3-year TRM and relapse rates were 28% and 25%, respectively. Only disease risk (but not conditioning) was significantly associated with OS, RFS and cumulative incidence of relapse.
A prospective randomized trial comparing high-dose with RIC regimens in patients with MDS or AML to be transplanted from related or unrelated donors has recently been initiated in the USA (CTN 0901). One would assume that the major trade-off with a RIC regimen would be more relapses, whereas TRM might be decreased.
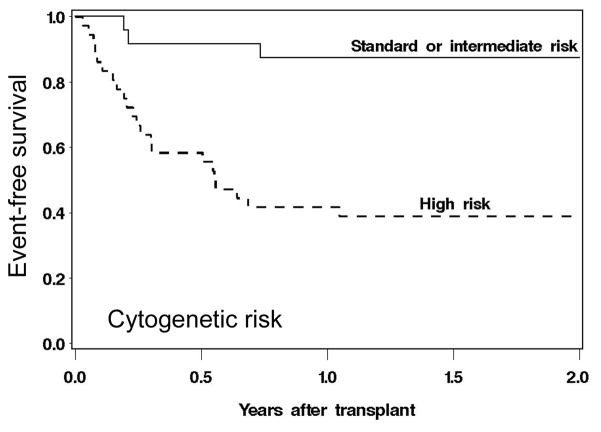
At this time, one should presumably use more intensive (higher dose) regimens in patients with high-risk disease, and adjust downward only as needed, primarily based on the patient’s comorbid conditions. There may be “intermediate intensity” regimens, such as combinations of fludarabine and treosulfan, which in a recent trial were very well tolerated (NRM at 2 years <10 %) and at least in patients with MDS or AML without high risk cytogenetics, resulted in a 2-year RFS of > 80% (Figure 3) [72].
Figure 3. Relapse-free survival in patients with MDS or AML conditioned with fludarabine plus treosulfan and transplanted from HLA matched related or unrelated donors.
Shown are the results based on cytogenetic risk (per IPSS criteria for MDS, and per cooperative group criteria for AML). Reprinted from Biology of Blood and Marrow Transplantation. Conditioning with Treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. E.R. Nemecek, K.A. Guthrie, M.L. Sorror, B.L. Wood, K.C. Doney, R.A. Hilger, B.L. Scott, T.J. Kovacsovics, R.T. Maziarz, A.E. Woolfrey, A. Bedelov, J.E. Sanders, J.M. Pagel, E.J. Sickle, R. Witherspoon, M.E. Flowers, F.R. Appelbaum, H. Joachim Deeg, Biology of Blood and Marrow Transplantation 2011; 17:341–350. © 2011, with permission from Elsevier.
9. SUMMARY AND CONCLUSIONS
HCT is currently the only treatment modality with proven curative potential in patients with MDS. With the development of RIC regimens, HCT can be offered to patients in the 7th and even 8th decade of life, an important fact in view of the age distribution of MDS. Further, the availability of unrelated donors (and the ability to select suitable individuals on the basis of DNA sequencing of HLA), the establishment of cord blood as a source of stem cells, and, most recently, the renewed interest in the use of HLA-haploidentical donors will allow to offer HCT to a rapidly growing number of patients. Dependent upon disease stage and characteristics, some 25% to 75% of transplanted patients will be cured of their MDS. While a proportion of patients may suffer from chronic medical problems after HCT, more than 70% report their quality of life as being very good to excellent by 2 years after HCT. However, despite all progress, with some patients now followed for more than 25 years after successful HCT, relapse of MDS and GVHD remain important hurdles. While recent research suggests that it may be possible to separate experimentally a GVL effect from the reactions that cause GvHD [73,74], clinically this remains to be proven. Immunotherapy or radioimmunotherapy may further improve HCT outcome. With the availability of approved drugs for the non-transplant treatment of MDS, many patients come to HCT at a more advanced stage and probably with more resistant disease than was the case in the past. Future studies will have to incorporate those agents into the overall management of patients with MDS, including HCT.
Acknowledgments
We thank Helen Crawford and Bonnie Larson for help with manuscript preparation. This work was supported by research funding from the National Institutes of Health, Bethesda, MD grants P01HL036444, P01CA018029, and P30CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. Additionally, F.X is also supported by funds from SHDC12010202.
Supported by: HL036444, CA018029, CA015704; F.X is supported by funds from SHDC12010202, Shanghai, PRC
ABBREVIATIONS
- AML
acute myeloid leukemia
- ASBMT
American Society for Blood and Marrow Transplantation
- ATG
anti-thymocyte globulin
- BU
busulfan
- CIBMTR
Center for International Blood and Marrow Transplant Research
- CMML
chronic myelomonocytic leukemia
- CTN
Clinical Trials Network
- CY
cyclophosphamide
- DLI
donor lymphocyte infusion
- EBMT
European Blood and Marrow Transplantation
- FB4 (FB2)
fludarabine + Bu given for 4 (2) days
- G-CSF
granulocyte colony stimulating factor
- GVHD
graft-versus-host disease
- GVL
graft-versus-leukemia (effect)
- HCT
hematopoietic cell transplantation
- HCT-CI
HCT comorbidity index
- HLA
human leukocyte antigen
- IPSS
International Prognostic Scoring System
- MDS
myelodysplastic syndromes
- OS
overall survival
- PBPC
peripheral blood progenitor cells
- RFS
relapse-free survival
- RIC
reduced-intensity conditioning
- TBI
total body irradiation
- TRM
treatment-related mortality
- URD
unrelated donor
- WHO
World Health Organization
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases (Review) Nature Reviews Cancer. 2007;7:118–29. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PL. Myelodysplastic syndrome. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, editors. Hematology: Basic Principles and Practice. New York: Churchill Livingstone; 2000. pp. 1106–29. [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartenstein M, Deeg HJ. Hematopoietic stem cell transplantation for MDS. Hematol Oncol Clin North Am. 2010;24:407–22. doi: 10.1016/j.hoc.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram W, Lim ZY, Mufti GJ. Allogeneic transplantation for myelodysplastic syndrome (MDS) (Review) Blood Rev. 2007;21:61–71. doi: 10.1016/j.blre.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ, Sandmaier BM. Who is fit for allogeneic transplantation? Blood. 2010;116 :4762–70. doi: 10.1182/blood-2010-07-259358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 8.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuendgen A, Strupp C, Aivado M, et al. Myelodysplastic syndromes in patients younger than age 50. J Clin Oncol. 2006;24:5358–65. doi: 10.1200/JCO.2006.07.5598. [DOI] [PubMed] [Google Scholar]
- 11.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–11. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 12.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–11. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 14.Oliansky DM, Antin JH, Bennett JM, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transplant. 2009;15:137–72. doi: 10.1016/j.bbmt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112:895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 16.Scott BL, Wells DA, Loken MR, Myerson D, Leisenring WM, Deeg HJ. Validation of a flow cytometric scoring system as a prognostic indicator for posttransplantation outcome in patients with myelodysplastic syndrome. Blood. 2008;112:2681–6. doi: 10.1182/blood-2008-05-153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runde V, de Witte T, Arnold R, et al. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: early transplantation is associated with improved outcome. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:255–61. doi: 10.1038/sj.bmt.1701084. [DOI] [PubMed] [Google Scholar]
- 18.Woodard P, Carpenter PA, Davies SM, et al. Unrelated donor bone marrow transplantation for myelodysplastic syndrome in children. Biol Blood Marrow Transplant. 2011;17:723–8. doi: 10.1016/j.bbmt.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Witte T, Brand R, van Biezen A, et al. Allogeneic stem cell transplantation for patients with refractory anaemia with matched related and unrelated donors: delay of the transplant is associated with inferior survival. Br J Haematol. 2009;146:627–36. doi: 10.1111/j.1365-2141.2009.07809.x. [DOI] [PubMed] [Google Scholar]
- 20.Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–7. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–64. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armand P, Deeg HJ, Kim HT, et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone Marrow Transplant. 2010;45:877–85. doi: 10.1038/bmt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onida F, Brand R, van Biezen A, et al. Impact of cytogenetics on outcome of patients with MDS or secondary AML undergoing allogeneic HSCT from HLA-identical siblings: a retrospective analysis of the EBMT-CLWP. Blood. 2006;108:750aq. #2653. [Google Scholar]
- 24.Upton A, McCune JS, Kirby KA, et al. Fluconazole coadministration concurrent with cyclophosphamide conditioning may reduce regimen-related toxicity postmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:760–4. doi: 10.1016/j.bbmt.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittelman M, Oster HS, Hoffman M, Neumann D. The lower risk MDS patient at risk of rapid progression (Review) Leuk Res. 2010;34:1551–5. doi: 10.1016/j.leukres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 27.Boehm A, Sperr WR, Leitner G, et al. Comorbidity predicts survival in myelodysplastic syndromes or secondary acute myeloid leukaemia after allogeneic stem cell transplantation. Eur J Clin Invest. 2008;38:945–52. doi: 10.1111/j.1365-2362.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 28.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144:407–14. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 29.Eissa H, Gooley TA, Sorror ML, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: relapse-free survival is determined by karyotype and comorbidities. Biol Blood Marrow Transplant. 2011;17:908–15. doi: 10.1016/j.bbmt.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J-H, Lee J-H, Lim S-N, et al. Allogeneic hematopoietic cell transplantion for myelodysplastic syndrome: prognostic significance of pre-transplant IPSS score and comorbidity. Bone Marrow Transplant. 2010;45:450–7. doi: 10.1038/bmt.2009.190. [DOI] [PubMed] [Google Scholar]
- 31.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–8. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146:310–6. doi: 10.1111/j.1365-2141.2009.07774.x. [DOI] [PubMed] [Google Scholar]
- 33.Platzbecker U, Bornhäuser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14:1217–25. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maradei SC, Maiolino A, de Azevedo AM, Colares M, Bouzas LF, Nucci M. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood. 2009;114:1270–5. doi: 10.1182/blood-2009-03-212282. [DOI] [PubMed] [Google Scholar]
- 35.Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110:1303–6. doi: 10.1002/cncr.22909. [DOI] [PubMed] [Google Scholar]
- 36.Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008;14:790–4. doi: 10.1016/j.bbmt.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Pullarkat V. Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? (Review) Blood. 2009;114:5251–5. doi: 10.1182/blood-2009-07-234062. [DOI] [PubMed] [Google Scholar]
- 38.Scott BL, Storer BE, Greene JE, Hackman RC, Appelbaum FR, Deeg HJ. Marrow fibrosis as a risk factor for post-transplant outcome in patients with advanced MDS or AML with multilineage dysplasia. Biol Blood Marrow Transplant. 2007;13:345–54. doi: 10.1016/j.bbmt.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Kröger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–70. doi: 10.1182/blood-2009-07-234880. [DOI] [PubMed] [Google Scholar]
- 40.Newell LF, Gooley T, Hansen JA, Stirewalt DL, Petersdorf EW, Deeg HJ. Tumor necrosis factor polymorphism affects transplantation outcome in patients with myelodysplastic syndrome but not in those with chronic myelogenous leukemia, independent of the presence of HLA-DR15. Biol Blood Marrow Transplant. 2010;16:1700–6. doi: 10.1016/j.bbmt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Ali HK, Brand R, van Biezen A, et al. A retrospective comparison of autologous and unrelated donor hematopoietic cell transplantation in myelodysplastic syndrome and secondary acute myeloid leukemia: a report on behalf of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Leukemia. 2007;21:1945–51. doi: 10.1038/sj.leu.2404774. [DOI] [PubMed] [Google Scholar]
- 42.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous buslfan and fludarabine: clinical and pharmacokinetic results of a myeloabltive, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–64. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 43.Nakai K, Kanda Y, Fukuhara S, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19:396–401. doi: 10.1038/sj.leu.2403640. [DOI] [PubMed] [Google Scholar]
- 44.Scott BL, Storer B, Loken M, Storb R, Appelbaum FR, Deeg HJ. Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant. 2005;11:65–73. doi: 10.1016/j.bbmt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Warlick ED, Cioc A, DeFor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–8. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13:454–62. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–68. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Field T, Perkins J, Huang Y, et al. 5-azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. :9999. doi: 10.1038/bmt.2009.134. prepublished online 22 June 2009. [DOI] [PubMed] [Google Scholar]
- 49.De Padua SL, de Lima M, Kantarjian H, et al. Feasibility of allo-SCT after hypomethylating therapy with decitabine for myelodysplastic syndrome. Bone Marrow Transplant. 2009;43:839–43. doi: 10.1038/bmt.2008.400. [DOI] [PubMed] [Google Scholar]
- 50.Kolb HJ, Schmid C, Tischer J, et al. Allogeneic stem cell transplantation for MDS and sAML following reduced intensity conditioning and preemptive donor lymphocyte transfusion. Blood. 2006;108:101a. #324. [Google Scholar]
- 51.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–42. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren EH. The human graft-versus-tumor response - and how to exploit it. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. Oxford, UK: Wiley-Blackwell; 2009. pp. 232–47. [Google Scholar]
- 53.Robin M, Sanz G, Ionescu I, et al. Unrelated cord blood transplantation (UCBT) in adults with MDS or secondary acute myeloblastic leukemia (sAML): a survey on behalf of Eurocord and CLWP of EBMT. Blood. 2009;114:493. doi: 10.1038/leu.2010.219. #1198. [DOI] [PubMed] [Google Scholar]
- 54.Castro-Malaspina H, Harris RE, Gajewski J, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99 :1943–51. doi: 10.1182/blood.v99.6.1943. [DOI] [PubMed] [Google Scholar]
- 55.Sato A, Ooi J, Takahashi S, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with advanced myelodysplastic syndromes. Bone Marrow Transplant. 2011;46:257–61. doi: 10.1038/bmt.2010.91. [DOI] [PubMed] [Google Scholar]
- 56.Parikh SH, Mendizabal A, Martin PL, et al. Unrelated donor umbilical cord blood transplantation in pediatric myelodysplastic syndrome: a single-center experience. Biol Blood Marrow Transplant. 2009;15:948–55. doi: 10.1016/j.bbmt.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–9. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Witte T, Hermans J, Vossen J, et al. Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110:620–30. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 59.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45:429–36. doi: 10.1038/bmt.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Liu K, Xu L, et al. HLA-mismatched hematopoietic SCT without in vitro T-cell depletion for myelodysplastic syndrome. Bone Marrow Transplant. 2010;45:1333–9. doi: 10.1038/bmt.2009.351. [DOI] [PubMed] [Google Scholar]
- 61.del Canizo MC, Martinez C, Conde E, et al. Peripheral blood is safer than bone marrow as a source of hematopoietic progenitors in patients with myelodysplastic syndromes who receive an allogeneic transplantation. Results from the Spanish registry. Bone Marrow Transplant. 2003;32:987–92. doi: 10.1038/sj.bmt.1704246. [DOI] [PubMed] [Google Scholar]
- 62.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–31. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 63.Guardiola P, Runde V, Bacigalupo A, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99:4370–8. doi: 10.1182/blood.v99.12.4370. [DOI] [PubMed] [Google Scholar]
- 64.Russell JA, Duan Q, Chaudhry MA, et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant. 2008;14:888–95. doi: 10.1016/j.bbmt.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Deeg HJ, Maris MB, Scott BL, Warren EH. Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia. 2006;20:1701–5. doi: 10.1038/sj.leu.2404327. [DOI] [PubMed] [Google Scholar]
- 66.Deeg HJ. Optimization of transplant regimens for patients with myelodysplastic syndromes (MDS) In: Berliner N, Lee SJ, Linenberger M, Vogelsang GB, editors. Hematology 2005: American Society of Hematology Education Program Book. Washington, DC: American Society of Hematology; 2005. pp. 167–73. [DOI] [PubMed] [Google Scholar]
- 67.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–46. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 68.Parker JE, Shafi T, Pagliuca A, et al. Allogeneic stem cell transplantation in the myelodysplastic syndromes: interim results of outcome following reduced-intensity conditioning compared with standard preparative regimens. Br J Haematol. 2002;119:144–54. doi: 10.1046/j.1365-2141.2002.03796.x. [DOI] [PubMed] [Google Scholar]
- 69.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–35. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 70.Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia. 2010;24:1050–2. doi: 10.1038/leu.2010.12. [DOI] [PubMed] [Google Scholar]
- 71.Khabori MA, El-Emary M, Xu W, et al. Impact of intensity of conditioning therapy in patients aged 40–60 years with AML/myelodysplastic sydrome undergoing allogeneic transplantation. Bone Marrow Transplant. :9999. doi: 10.1038/bmt.2010.164. prepublished online July 12, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:341–50. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawase T, Matsuo K, Kashiwase K, et al. HLA mismatch combinations associated with decreased risk of relapse: implications for the molecular mechanism. Blood. 2009;113:2851–8. doi: 10.1182/blood-2008-08-171934. [DOI] [PubMed] [Google Scholar]
- 74.Michalek J, Collins RH, Durrani HP, et al. Definitive separation of graft-versus-leukemia-and graft-versus-host-specific CD4+ T cells by virtue of their receptor beta loci sequences. PNAS. 2003;100:1180–4. doi: 10.1073/pnas.0337543100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [erratum appears in Blood 1998 Feb 1;91(3):1100] [PubMed] [Google Scholar]