Abstract
Invasiveness is one of the key features of aggressive prostate cancer, however our understanding of the precise mechanisms effecting invasion remains limited. The ceramide hydrolyzing enzyme acid ceramidase (AC), overexpressed in most prostate tumors, causes an aggressive and invasive phenotype through downstream effectors that have not yet been well characterized. Here we demonstrate that AC, through generation of sphingosine-1-phosphate (S1P), promotes Ets1 nuclear expression and binding to the promoter region of matrix-degrading protease cathepsin B. Through confocal microscopy and flow cytometry, we found that AC overexpression promotes pericellular localization of cathepsin B and its translocation to the outer leaflet of the cell membrane. AC overexpressing cells have an increased abundance of cathepsin B-enriched invasive structures and enhanced ability to invade through a collagen matrix, but not in the presence of an inhibitor of cathepsin B. In human prostate tissues, AC and cathepsin B overexpression were strongly associated and may relate to poor cancer outcome. These results demonstrate a novel pathway by which AC, through S1P, promotes an invasive phenotype in prostate cancer by causing overexpression and secretion of cathepsin B through activation and nuclear expression of Ets1. As prostate cancer prognosis is dramatically worse when invasion has occurred, this study provides critical insight into the progression towards lethal prostate cancer.
Keywords: Prostate cancer, acid ceramidase, cathepsin B, invasion, tumor metastasis
Introduction
Prostate cancer is the second leading cause of cancer death in males in the United States1. Despite favorable cure rates for localized disease with surgery and/or radiotherapy, treatment of invasive and metastatic disease usually relies on cytotoxic chemotherapies and androgen withdrawal. These treatments have debilitating side effects and are rarely curative2. With the stark reduction in prognosis once prostate cancer has become invasive, research focusing on understanding mechanisms of prostate tumor invasion are of critical importance.
The lysosomal cysteine protease cathepsin B plays an important role in physiological protein turnover and processing, making cathepsin B an important contributor to diverse processes including autophagy, antigen presentation, and activating cleavage-induced signaling cascades3–5. Cathepsin B is deregulated in a number of human malignancies, including prostate cancer6. It has been observed that increased cathepsin B protein levels, pericellular localization and secretion are thought to contribute to prostate cancer invasion and metastasis with cathepsin B acting both directly and indirectly on extracellular matrix remodeling and degradation7. While an increasing body of research explains the mechanisms by which elevated expression and secretion of cathepsin B contribute to prostate cancer metastasis and invasion, the mechanisms by which cathepsin B is overexpressed and secreted remain poorly understood.
Our group has observed that AC, a lysosomal enzyme that deacylates ceramide, forming sphingosine, is overexpressed in most prostate tumors compared to normal prostate tissue8. Importantly, we have shown that AC mediates an aggressive phenotype in prostate cancer cells including promotion of invasion, migration, proliferation, and resistance to therapy9, 10. In the present study, we explored the relationship between AC and cathepsin B in prostate cancer.
Materials and Methods
Cell lines and reagents
DU145, PC3, Dupro (American Type Culture Collection, Manassas, VA), and PPC1 (a gift from Dr. Yi Lu at the University of Tennessee)11 were cultured in RPMI 1640 containing 10% bovine growth serum (HyClone), 100 μg/ml streptomycin, and 100 units/ml penicillin (HyClone). Cell lines were validated in February 2011 by STR profiling using the PowerPlex1.2 marker set. CA074me, CA074 (Calbiochem, San Diego, CA), SKI (2-(p-Hydroxyanilino)-4-(p-chlorophenyl) thiazole, Thermo Fisher), LCL38512 were pre-incubated with cells for 2 h before exposure to treatments. Pertussis toxin (Sigma Aldrich) was incubated with cells 2 hours prior to additional treatment. W146 (Cayman Chemical) was pre-incubated with cells 15 minutes prior to additional treatment with S1P. SEW 2871 (Tocris) was applied in the cell culture medium for one hour prior to analysis. AC cDNA was purchased from Origene (Rockville, MD) and Ad-AC (adenovirus expressing AC and GFP) and Ad-GFP (empty vector control) were developed by Vector Biolabs (Philadelphia, PA). The short hairpin sequence (5′CCGGGCTGTTATTGACAGCGATATACTCGAGTATATCGCTGTCAATAACAGCTTTTT-3′) obtained from Open Biosystems (Huntsville, AL) was validated and developed into an adenoviral delivery vector (Ad-shAC) by Vector Biolabs.
AC overexpressing cell lines
DU145-AC-EGFP and DU145-EGFP have been described13. PPC1, PC3, DU145 and Dupro cells were transfected with a pEF6/V5-His-TOPO plasmid (Invitrogen) containing lacZ-V5 or AC-V5, and stable clones were obtained by long-term culture with 5 μg/ml blasticidin (Invitrogen) to generate PPC1-LacZV5, PPC1-ACV5, PC3-LacZV5, PC3-ACV5, DU145-LacZV5 and DU145-ACV5.
Adenovirus infection
1 ×106 cells were infected in suspension in 3ml serum free RPMI 1640 with Ad-AC, Ad-shAC or Ad-GFP at multiplicity of infection (MOI) 50 unless otherwise specified. Cells were then plated on 60mm culture dishes. After 6 hours, 3ml of complete media was added to the infected cells. Infection was verified after 24 hours by GFP expression.
Conditioned media
Infected cells were grown for 24 hours in complete media then serum-starved for 18 hours. Media was centrifuged at 500 then 3000g prior to passing through Millipore UltraFree 100K (Burlington, MA) concentrators.
Immunoblotting
Western blotting was performed as previously described with 50μg of total protein from cell lysate or 30μl of concentrated media14. NuPAGE Bis-Tris 4–12% precast gels were utilized (Novex). Antibodies include: Mouse anti-human CatB (Oncogene Research Products; San Diego, CA), mouse anti-human AC (Pharmingen; San Diego, CA), rabbit anti-human CatD (Santa Cruz Biotechnology; Santa Cruz, CA) or goat anti-human CatL (Santa Cruz).
Confocal microscopy
Cells grown on 8-well chamber slides (Becton-Dickinson, Franklin Lakes, NY) were fixed in 3.7% paraformaldehyde, permeabilized with methanol, and blocked with 1.5% bovine serum albumin in PBS, prior to incubation with CatB primary antibodies in 1.5% BSA/PBS. Bound primary antibodies were detected with Alexa Fluor 555–conjugated goat anti-mouse secondary antibodies (Invitrogen). Confocal microscopy was performed using a Leica TCS SP2 AOBS confocal microscope.
Live cell CatB Assay
Cells were infected with Ad-AC or Ad-GFP. After 24 hours the CatB Detection Kit (Calbiochem) was used according to manufacturer instructions.
RNA Isolation and real-time PCR
Total RNA was isolated using the RNAeasy kit (Qiagen, Chatsworth, CA). Transcripts of CatB, CatD and CatL were assayed by two-step RT-PCR protocol (Ambion, Austin, TX) according to manufacturer instructions. Real-time PCR was performed by using iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). Primers were obtained from www.realtimeprimers.com. CatD forward 5′-TGGAGGACCTGATTGCCAAA-3′, reverse 5′-AGCAGTTTGCAGTGGATGGA-3′; CatL forward 5′-TCTTGTTGGGCTTTTAGTGC-3′, reverse 5′-AGGCCTCCATTATCCTGAAC-3′; CatB forward 5′-GAG ACC AAG TCC TGG CTA CA -3′, reverse 5′-CAT TCC TGC GTC TCT GTC TT -3′. The mRNA levels were acquired of three independent experiments from the value of threshold cycle (Ct) of the real-time PCR using the ΔΔCt method and normalized against the housekeeping gene β-actin.
Migration and invasion
PPC1 cells were infected with Ad-AC or Ad-GFP. After 24 hours, migration and invasion assays were performed as previously described10.
S1P treatment
Cells were plated in 5% serum containing RPMI 1640 and incubated overnight. The next day, PPC1 cells were pretreated with 5% serum containing RPMI 1640 in the absence or presence of 100 ng/ml pertussis toxin. After two hours pre-treatment, S1P was added in 5% medium for 2 hours (unless indicated otherwise). Cells were collected and cell fractionation was carried out using the NE-PER Nuclear/Cytoplasmic isolation kit (Thermo-Fisher).
Chromatin immunoprecipitation
Chromatin was prepared using the chromatin immunoprecipitation (ChIP) assay kit (Millipore #17-295) per manufacturer’s instructions, and immunoprecipitated using anti-Ets1 (Santa Cruz 55581). In silico identification of consensus binding sites for Ets1 on the CTSB promoter was performed using Transcription Element Search System (TESS, University of Pennsylvania). Primers designed for promoter enrichment analysis of the putative Ets1 binding site at −418 bp (accession AF086639) using the following primers: forward 5′-TGG CTG TCA GGA TAT GAC TAG GGT -3′, reverse 5′-TTA TCC AGG CTG TGG CGA CAG TAA -3′. Experimental cell cultures are fixed with formaldehyde (1.0% for 20 mins at 37° C), washed in ice-cold PBS, and sheared using a Branson Digital Sonifier (model 450) at 30% amplitude for 10 secs. on ice. Protein-DNA complexes are immunoprecipitated using 5.0 μg of antibody. Upon of reverse-crosslinking immunoprecipates, four microliters from 0.2 ml of eluted DNA was used in each real-time reaction, which was conducted using an iCycler (Bio-rad) with iQ SYBR Green Supermix (Bio-rad) per the manufacturer’s instructions. Primers were used at a concentration of 250nM. Cycling conditions were as follows: pre-incubation, 50° C for 10 min, 95° C for 3 min., followed by 50 cycles of denaturation at 95° C, 30 sec.; annealing/extension at 60° C, 45 sec.; and data acquisition at the end of each extension. Melting curve and data analysis was carried out per the manufacturer’s recommendations using the accompanying software (Bio-Rad).
Preparation of tumor tissue microarray
27 formalin-fixed paraffin-embedded prostate carcinomas were obtained from the Hollings Cancer Center Tissue Biorepository (Medical University of South Carolina). All tissues were obtained in accordance with an Institutional Review Board approved protocol (#426). One-millimeter tissue cylinders were punched from representative tumor areas of a “donor” tissue block and brought into different recipient paraffin blocks each containing 27 individual samples. Three tissue cores were sampled from each tumor, and one core was sampled from patient matched adjacent normal tissue. Four-micrometer thick sections of the TMA were cut and processed for immunohistochemistry.
Immunohistochemistry
TMA Slides were deparaffinized by heating to 65 °C and immersing in xylene and rehydrated through an alcohol gradient. Antigen retrieval was performed by immersing the slides in diluted Target Retrieval Solution (Dako, Carpinteria, California) heated to 90 °C for 30 min in a water bath. Sections were cooled to room temperature and washed with PBS prior to quenching endogenous peroxidase activity with a peroxide/methanol bath for 30 min. Slides were washed with PBS and then blocked with a blocking solution (DAKO protein-free serum block). The primary antibodies AC (1:50, Santa Cruz Biotechnology) and CatB (1:500, Calbiochem) were diluted with DAKO antibody diluent, added to the slides and incubated at 4°C for overnight. A biotinylated link antibody plus streptavidin biotin peroxidase kit (DAKO LSAB+ System-HRP) was then utilized along with a DAB chromagen and peroxide substrate to detect the bound antibody complexes. The slides were counterstained with hematoxylin and dehydrated through graded alcohols to xylene. A pathologist, blinded to tissue identity, scored three areas per tissue core and evaluated them using the following scale: 0, no staining of any cells; 1, faint staining; 2, moderate intensity staining; and 3–5, intense staining.
Statistics
Experiments were performed in triplicate unless otherwise stated and are representative of a minimum of three independent experiments. Results are expressed as the mean +/− SD unless otherwise stated. Statistical significance was determined using the methods indicated for each figure.
Results
Acid ceramidase induces Ets1 promoter binding and transcription of cathepsin B
Initial investigation of the relationship between AC and cathepsin B revealed that stable overexpession of AC was frequently accompanied by increased expression of the 32 and 25 kDa (active) bands of cathepsin B (Fig. 1A). To determine whether increased cathepsin B protein expression reflected increased cathepsin B mRNA, RT-PCR analysis was performed. AC overexpressing cell lines were observed to have more abundant cathepsin B mRNA than control cells (Fig. 1B). Protein stability studies with cyclohexamide revealed no change in cathepsin B protein stability in the presence of higher levels of AC (Supp. Fig. 1). Similar analyses of cathepsins D and L showed no upregulation of these related proteins (Supp. Fig 2), suggesting a specific transcriptional upregulation of cathepsin B when AC is overexpressed.
Figure 1. Acid ceramidase causes transcriptional upregulation of cathepsin B through Ets1 activation.
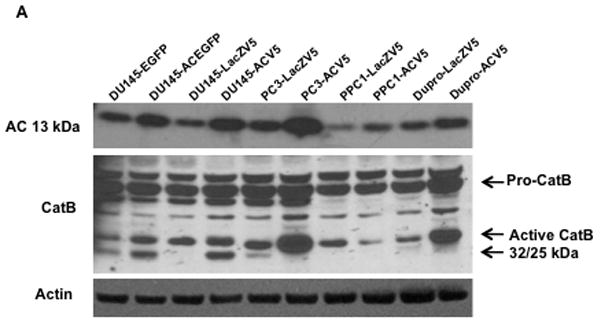
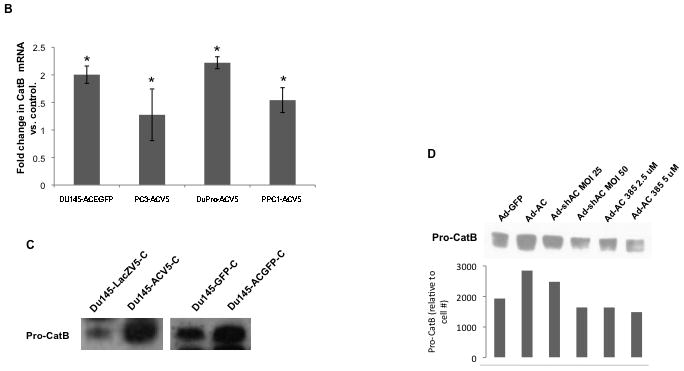
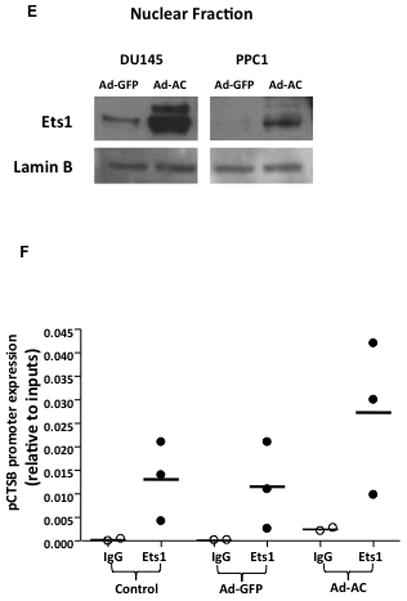
DU145, PC3, PPC1, and Dupro prostate cancer cell lines were generated using a plasmid coding for AC or empty vector. Western blot analysis (A) and RT-PCR (B) were performed on these cell lines. mRNA expression is normalized to empty vector expression. Conditioned media from DU145 AC-overexpressing cell lines were concentrated and protein expression was compared to control cells (C). Conditioned media of cells infected with Ad-GFP (MOI 50), Ad-AC (MOI 50), Ad-shAC, or Ad-AC (MOI 50) with the AC inhibitor LCL385 were collected and analyzed by western blotting (D) ImageJ densitometry values were normalized by treatment-specific cell number to obtain the column graph depicting relative cathepsin B as detected on the immunoblot. Nuclei of DU145 and PPC1 cells infected with Ad-GFP or Ad-AC were isolated and subjected to Western blotting for Ets1 expression (E). Chromatin immunoprecipitation of uninfected, Ad-GFP, or Ad-AC infected PPC1 cells demonstrates Ets1 binding to the cathepsin B promoter (F). *p<.05 student’s t-test.
We were intrigued to find that AC overexpression promoted increased transcription of cathepsin B and increased protein expression of its active bands, but not the 43 kDa proform of this enzyme. This led us to analyze conditioned culture medium which revealed that pro-cathepsin B is secreted by AC overexpressing cells (Fig. 1C and Supp. Fig. 3 and 7). Secretion of pro-cathepsin B was reduced by knockdown of AC with adenoviral delivery of a short hairpin targeting AC (ASAH1) and by treatment with the AC inhibitor LCL 385 (Figure 1D and Supp. Fig. 4). Analysis of protein expression in nuclear isolates revealed that AC overexpressing cells have elevated Ets1 in the nucleus compared to control cells (Figure 1E). ChIP analysis of Ets1 binding to the promoter region of the cathepsin B gene, CTSB, confirmed Ets1 is a bona fide cis-binding protein upstream of CSTB. Furthermore, forced over-expression of AC via adenoviral vector transduction, compared with uninfected or GFP-infected controls, demonstrated increased Ets1 binding to the CTSB promoter (Figure 1F). These results implicate Ets1 as a transcriptional mediator of AC-induced cathepsin B overexpression.
Sphingosine 1-phosphate drives transcription and secretion of cathepsin B
Acid ceramidase deacylates ceramide to generate a fatty acid and sphingosine. Sphingosine can be phosphorylated by sphingosine kinase to form the potent signaling lipid S1P. Transient overexpression of AC in PPC1 cells using adenoviral delivery causes a significant increase in S1P (Figure 2A). Due to the pleiotropic signaling functions of S1P, we investigated its role in AC-regulated cathepsin B transcription and secretion. Treatment of cells with exogenous S1P caused a dose-dependent secretion of pro-cathepsin B with significant secretion even at low-nanomolar concentrations of S1P (Figure 2B). Conversely, treating AC-overexpressing cells with the sphingosine kinase inhibitor SKI reduced pro-cathepsin B secretion in a dose-dependent manner (Figure 2C). To determine whether S1P impacts Ets1 promoter binding and therefore contributes to transcriptional upregulation of cathepsin B, we analyzed the expression of Ets1 in the nucleus after S1P treatment. Interestingly, S1P promoted an increase in nuclear Ets1, an effect that was dampened by pretreatment with pertussis toxin (PTX), which interferes with S1P receptor (S1PR) signaling by inactivating S1PR-associated G-proteins (Fig. 2D and Supp. Fig. 5–6). Further exploration of S1P receptor signaling in this phenomenon revealed that pretreatment of S1P stimulated cells with W146, a specific antagonist of S1PR1, blocks S1P-mediated nuclear enrichment of Ets1 (Figure 2E). Additionally, treatment with the specific S1PR1 agonist SEW 2871 promoted Ets1 nuclear accumulation, mimicking treatment with S1P. Other S1PR antagonists (JTE013 and VPC23019) did not prevent S1P from causing Ets1 to move into the nucleus (data not shown). This result suggests that S1P mediates nuclear translocation of Ets1 through activation S1PR1. Exogenous S1P treatment greatly increased cathepsin B promoter binding by Ets1 in a ChIP assay (Figure 2F), solidifying S1P as a mediator of AC-induced Ets1 promotion of cathepsin B expression.
Figure 2. S1P drives AC-induced cathepsin B transcription and expression.
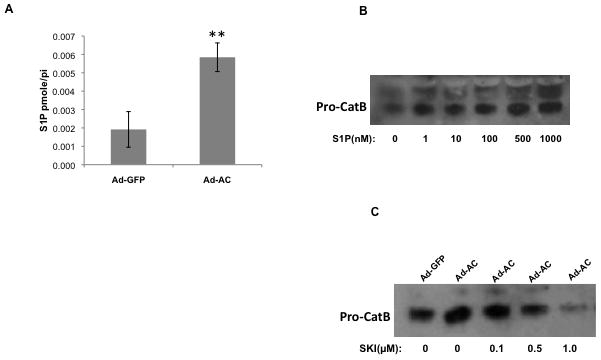
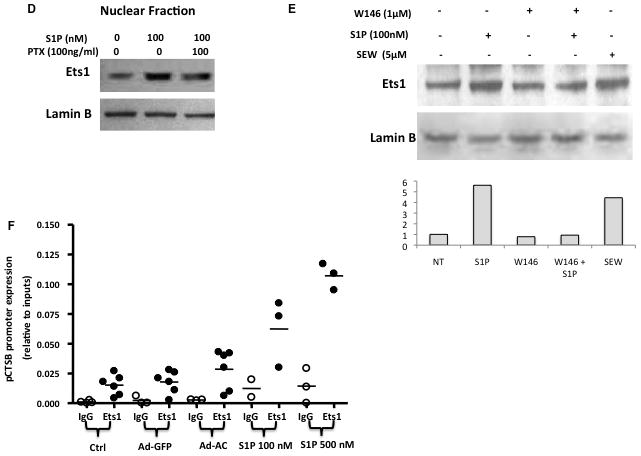
Ad-AC infected cells were collected and S1P concentration was analyzed by LC-MS (A). Naïve PPC1 cells were stimulated with exogenous S1P at the indicated concentrations and the conditioned media were analyzed by Western blotting (B). Cathepsin B secretion was analyzed in the conditioned medium of Ad-GFP or Ad-AC infected cells treated with the indicated concentrations of the sphingosine kinase inhibitor SKI two hours prior to infection (C). The nuclear fractions of PPC1 cells stimulated with either S1P or S1P plus pertussis toxin were collected and analyzed by Western blotting (D). The nuclear fractions of PPC1 cells stimulated with S1P, W146, S1P plus W146, or SEW 2871 were collected and analyzed by Western blotting. ImageJ was used to determine Ets1 band intensity normalized to Lamin B, and these values were normalized to the untreated lane (E). Chromatin immunoprecipitation of control, Ad-GFP, Ad-AC infected PPC1 cells, or S1P stimulated PPC1 cells was utilized to determine Ets1 binding to the cathepsin B promoter under these conditions (F).
Acid ceramidase causes pericellular redistribution of cathepsin B
Compared to Ad-GFP infected cells, which demonstrate a typical punctate lysosomal distribution of cathepsin B (Figure 3A), Ad-AC infected cells have strong cathepsin B staining at the cell periphery (Figure 3B). Treatment of Ad-AC infected cells with either Ad-shAC (Figure 3C) or the AC inhibitor LCL385 (Figure 3D) interfered with the pericelluar redistribution of cathepsin B caused by AC overexpression. Ad-AC infected cells treated with a fluorogenic cathepsin B substrate had significantly greater fluorescence than Ad-GFP control cells as well as an increased fluorescence towards the cell periphery and membrane (Figure 3E–F). These results demonstrate that AC promotes pericellular and membrane association of cathepsin B and that in AC overexpressing cells, cathepsin B activity is elevated and concentrated at the cell membrane.
Figure 3. Active cathepsin B localizes to the cell membrane in AC overexpressing cells.
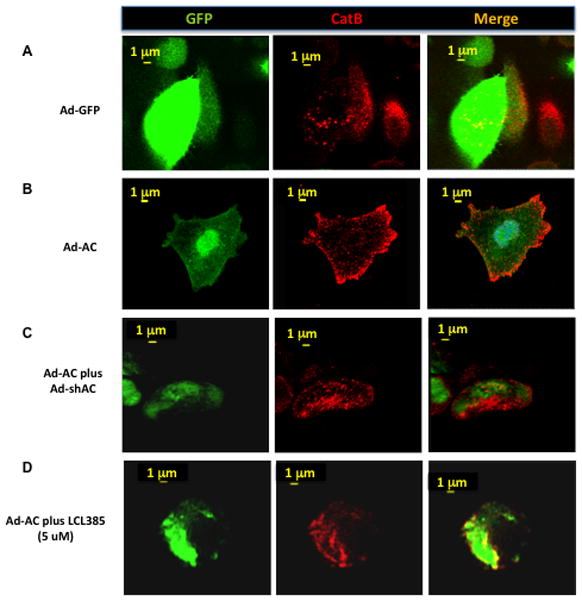
We performed confocal microscopy on PPC1 cells infected for 24 hours with Ad-GFP (A) or Ad-AC(B) at MOI 50. Green fluorescence represents the GFP tag of the adenoviruses and indicates successful infection. Red fluorescence represents immunostaining for cathepsin B(A–D) or cathespin B activity (E and F). In C, PPC1 cells were co-infected with Ad-AC and Ad-shAC. PPC1 cells were infected with Ad-AC and simultaneously treated with the AC inhibitor LCL385 (D). PPC1 cells were infected with Ad-GFP (E) or Ad-AC (F) and treated with a fluorogenic cathepsin B substrate.
Cathepsin B associates with the outer leaflet of the cell membrane
Access of cathepsin B to the extracellular matrix supports cancer cell invasiveness. In order to determine whether AC-induced pericellular/membrane association of cathepsin B enhances cathepsin B expression on the outer leaflet of the cell membrane, we evaluated cell surface expression of cathepsin B. PPC1 cells were collected using either proteolytic trypsin or non-proteolytic Cell Stripper and subjected to FACS analysis for cell surface expression of cathepsin B (Figure 4A). AC-overexpressing cells collected using Cell Stripper had 17-fold higher fluorescence intensity than cells collected with trypsin. Because the antibodies in this experiment must access cathepsin B in non-permabilized cells and because proteolytic cell collection with trypsin, but not non-proteolytic Cell Stripper, neutralizes cathepsin B detection by FACS, we can conclude that cathepsin B is present on the outer leaflet of the cell membrane and thus can access the extracellular matrix.
Figure 4. AC overexpression promotes cathepsin B outer membrane association.
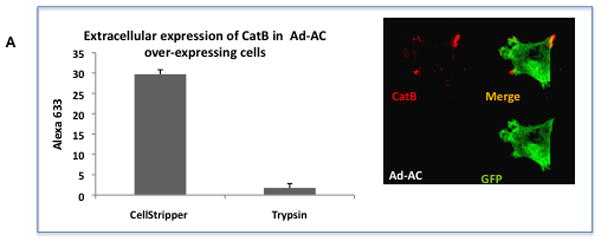
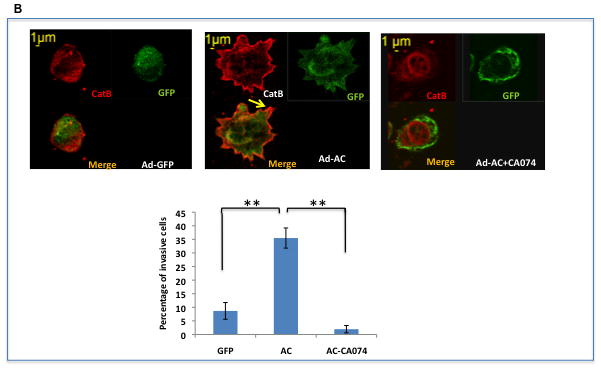
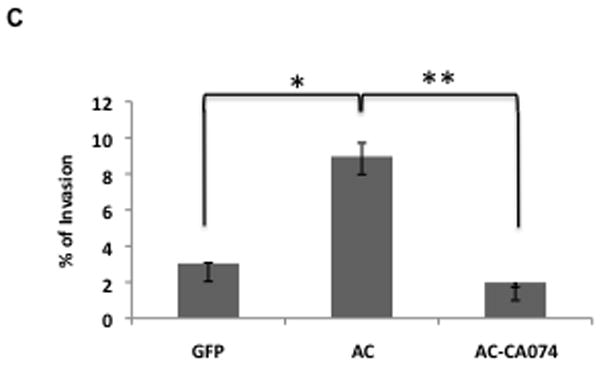
A: Ad-AC infected PPC1 cells were collected with non-proteolytic Cell Stripper or Trypsin and analyzed by FACS for cell surface expression of cathepsin B. The confocal image shows a non-permeabilized Ad-AC infected PPC1 cell immunostained for cathepsin B. B: Ad-GFP or Ad-AC infected PPC1 cells were plated on an artificial matrix in the presence or absence of 1μM CA074 and immunostained for cathepsin B (red). One hundred cells from each treatment were counted for the presence of cathepsin B-rich invasive structures as indicated with the arrow. C: Cells were infected as indicated and plated in collagen coated transwells with or without CA074. Invasion through the matrix was analyzed after 24 hours.
We sought to determine the functional implications of outer membrane associated cathepsin B in tumor invasion. As predicted by the translocation of the matrix degrading protease to the outer surface of the cell, confocal microscopy of AC overexpressing cells plated on an artificial collagen matrix shows an increase in invasive structures rich in cathepsin B compared to control cells (Figure 4B). Treatment with the cathepsin B inhibitor CA074 starkly reduces the appearance of these structures. Similarly, Ad-AC infected cells invaded through a collagen matrix more than Ad-GFP infected cells, but not when CA074 was used (Figure 4C). These results demonstrate an important role for cathepsin B in AC-induced tumor cell invasiveness. Importantly, overexpression of cathepsin B in certain contexts has been associated with induction of apoptosis, however we found no evidence that AC-induced cathepsin B expression induces apoptosis (Supp. Fig. 8).
Acid ceramidase and cathepsin B are upregulated in the same patient tumor tissues
Our previous work has definitively shown that AC is overexpressed in most patient tumor tissue compared to benign adjacent tissue. To determine whether cathepsin B is also overexpressed in prostate cancer, we immunostained tissue microarray slides for AC and cathepsin B. For each tissue, the benign score was subtracted from the tumor score and the results for each tumor for AC and cathepsin B are plotted in Figure 5A. Of the 27 patients, 17 (62%) had significantly increased AC level and 19 (70%) had significantly increased cathespin B levels when compared to normal adjacent tissue (significance defined as tumor score – benign score > 3 standard deviations). Application of Fisher’s Exact Test to the data demonstrate that the association between expression of AC and cathepsin B is highly significant (r= 0.7091, p<0.0001). These results provide convincing evidence that cathepsin B and AC overexpression are correlated, consistent with our data that S1P generated by AC overexpression induces cathepsin B. Additionally, we find that a greater proportion (0.70, versus the 0.52 for all tumors) of tumors that highly overexpress both AC and cathepsin B (tumor score – benign score> 4 standard deviations above benign) were either invasive at the time of resection or eventually recurred as evidenced by prostate specific antigen rebound. While our sample size is insufficient to conclude that this is not due to chance, the data suggest that high levels of AC and cathepsin B overexpression may predict aggressive prostate cancer and thus may guide physicians in determining whether or not to treat aggressively. Figure 5B is a representative tissue section stained for AC, CatB, and IgG (control). Note the strong brown staining for AC and CatB in the tumor tissue compared to the normal adjacent tissue.
Figure 5. Cathepsin B and AC are upregulated in prostate tumor tissues.
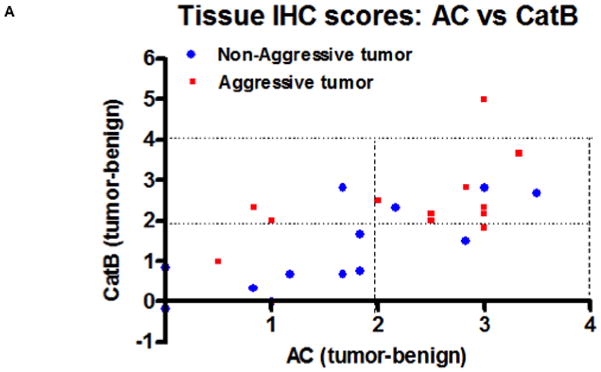
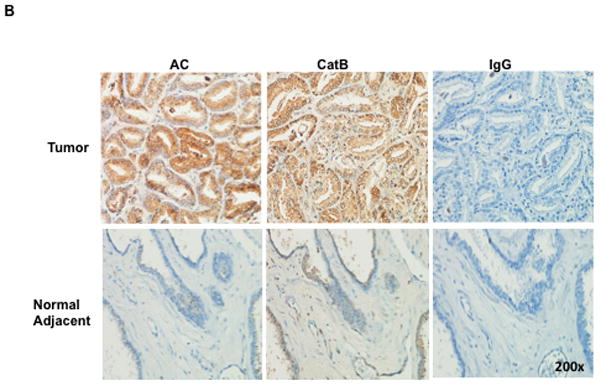
A tissue microarray was assembled composed of patient matched tumor and normal adjacent tissue and immunostained for cathepsin B and AC. A blinded pathologist scored the tissues from 1 to 5 for strength of staining. For each patient, tumor score minus benign score was plotted for cathepsin B (ordinate) and AC (abscissa) (A). Red squares are indicative of tumors that were invasive at the time of resection or later recurred by post-surgical rebound of PSA levels. B: A single patient tumor and normal adjacent tissue slide showing representative AC and cathepsin B staining (brown).
Discussion
A great deal of evidence now supports bioactive sphingolipids as key players in multiple aspects of cancer biology. By its metabolism of ceramide, acid ceramidase has the potential to promote cancer by reducing the pro-apoptotic lipid ceramide and favoring generation of S1P, which has demonstrated roles in preventing apoptosis as well as promoting inflammation and cell invasion 15–18. Our previous work supports an oncogenic role for AC in prostate cancer, including promoting prostate cancer cell invasion9, 19, 20. In this study we investigated the mechanism of AC-induced cell invasion and discovered a prominent role for AC and S1P in matrix degradation by upregulation, pericellular localization, and secretion of cathepsin B.
Our initial investigation revealed that AC overexpression in both stable cell lines and in transiently transduced cells overexpressed cathepsin B at the protein level and had elevated cathepsin B mRNA by RT-PCR. Since we found that AC did not affect cathepsin B protein stability, we concluded that AC-induced cathepsin B upregulation was due either to increased transcription or post-transcriptional mRNA stability. It is reported in the literature that Ets1 is a key transcription factor for cathepsin B, a finding supported by the presence of three proposed Ets1 binding sites in the cathepsin B promoter region21, 22. Our studies reveal that both AC and S1P promote both elevated nuclear Ets1 and increased Ets1 binding to the cathepsin B promoter by chromatin immunoprecipitaiton.
Cathepsin B has been implicated as a contributor to cell death including apoptosis and lethal autophagy23. Thus, it was important for us to determine whether AC-induced cathepsin B was also a contributor to cell death. Supplementary Figure 8 demonstrates that while cathepsin B appears to be an important factor in ceramide-induced apoptosis, neither a reduction in cell viability nor an increase in apoptosis seems to be associated with overexpression of AC. These data are in keeping with our observations that AC promotes pericellular localization and membrane association of cathepsin B, which is associated with increased malignancy and invasion 24.
Essential for new achievements in preventing morbidity and mortality associated with prostate cancer, and all solid cancers for that matter, will be a more comprehensive understanding of what promotes cancer invasion and metastasis, since exceptional cure rates are available to patients with localized disease whereas dismal prognoses accompany invasive and metastatic disease. In this study, we showed that cathepsin B is pericellularly redistributed when AC is overexpressed. Pericellular redistribution of cathepsin B is associated with cancer cell invasion, presumably by degradation of the extracellular matrix. Importantly, we demonstrate here that cathepsin B is actually localized to the outer leaflet of the cell membrane as flow cytometric analysis and confocal microscopy of non-permeabilized cells revealed antibody recognition of cathepsin B on the cell surface but not when first treated with trypsin. This finding is key as it demonstrates that cathepsin B is not only pericellular in distribution, but has direct access to the extracellular matrix where its role as a protease can contribute to tumor microenvironment remodeling and tumor cell invasion. AC overexpressing cells had distinct cathepsin B-rich invasive structures present with strong cathepsin B staining at the leading edge when plated on a synthetic matrix. These invasive structures were not present when cells were incubated with a specific cathepsin B inhibitor, strongly supporting a role for cathepsin B matrix degradation as a factor in AC-overexpression induced cell invasiveness.
These findings complement a growing body of literature asserting the role cathepsin B plays in extracellular matrix degradation and invasion of tumor cells. It is known that cancer cells express cathepsin B on the cell surface and secrete it into the extracellular space. Bellezza, et al, found increased cathepsin B and procathepsin B on the surface of secreted prostatomes from LNCaP and PC-3 compared to prostatomes from healthy ejaculate25. This study is of particular interest, as most prostate cancers have elevated AC8, thus we may speculate that high levels of AC in those cell lines drives upregulation and secretion of cathepsin B, as descried in this study. Extracellular cathepsin B not only exhibits an invasive phenotype in vitro, as in this study, but also in animal models. In a metastatic model of colon cancer, cathepsin B activity was detected at the cell membrane, and treatment with an inhibitor of extracellular cathepsin B inhibited formation of liver metastases26, hightlighting the importance of cathepsin B, particularly its extracellular form, in invasion and metastasis. Interestingly, a recent study demonstrated that invasion of prostate cancer cells in response to hepatocyte growth factor was incumbent upon anterograde lysosome trafficking and was accompanied by secretion of cathepsin B27. The present study elucidates a novel mechanism by which cathepsin B is upregulated and secreted, and therefore adds knowledge of how prostate cancer may progress from localized to invasive.
Our findings that AC induces important changes in the regulation of cathepsin B are made relevant to the actual disease process by the finding that AC and cathepsin B are co-upregulated in prostate cancer tissues. Our studies lead us to propose that a contributing mechanism of cathepsin B upregulation in these patients is due to AC upregulation promoting local S1P secretion and transcriptional upregulation of cathepsin B through Ets1. While localized prostate cancer is slow to progress and frequently cured, our study points to AC upregulation as an important mediator of prostate cancer invasion and perhaps metastasis, the defining features of deadly prostate cancer. As such, AC and cathepsin B may be important contributors to the pathogenesis and progression of deadly prostate cancer, and each represents an opportunity for further study and possibly intervention.
Supplementary Material
Acknowledgments
Financial support: This work was supported by NIH/NCI PO1 CA97132, Grant 1R24CA82933, NIH, C06 RR015455, The National Centers for Research Resources UL1RR029882 and the MUSC Lipidomics Core Facility.
Grant support
This work was supported by the National Institute of Health (5P01CA097132-07) and the Department of Defense (PCTA: PC101962). The project described was also supported by Award Number UL1RR029882 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Imaging facilities for this research were supported, in part, by Cancer Center Support Grant P30 CA138313 to the Hollings Cancer Center, Medical University of South Carolina
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Stavridi F, Karapanagiotou EM, Syrigos KN. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat Rev. 36:122–30. doi: 10.1016/j.ctrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Katunuma N, Matsunaga Y, Saibara T. Mechanism and regulation of antigen processing by cathepsin B. Adv Enzyme Regul. 1994;34:145–58. doi: 10.1016/0065-2571(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 4.Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jaattela M. Cathepsin B Acts as a Dominant Execution Protease in Tumor Cell Apoptosis Induced by Tumor Necrosis Factor. J Cell Biol. 2001;153:999–1010. doi: 10.1083/jcb.153.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha SD, Ham B, Mogridge J, Saftig P, Lin S, Kim SO. Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm. J Biol Chem. 2010;285:2120–9. doi: 10.1074/jbc.M109.065813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez PL, Farre X, Nadal A, Fernandez E, Peiro N, Sloane BF, Shi GP, Chapman HA, Campo E, Cardesa A. Expression of cathepsins B and S in the progression of prostate carcinoma. International Journal of Cancer. 2001;95:51–5. doi: 10.1002/1097-0215(20010120)95:1<51::aid-ijc1009>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Yan S, Sloane BF. Molecular regulation of human cathepsin B: implication in pathologies. Biol Chem. 2003;384:845–54. doi: 10.1515/BC.2003.095. [DOI] [PubMed] [Google Scholar]
- 8.Norris JS, Bielawska A, Day T, El-Zawahri A, Elojeimy S, Hannun Y, Holman D, Hyer M, Landon C, Lowe S, Dong JY, McKillop J, et al. Combined therapeutic use of AdGFPFasL and small molecule inhibitors of ceramide metabolism in prostate and head and neck cancers: a status report. Cancer Gene Ther. 2006;13:1045–51. doi: 10.1038/sj.cgt.7700965. [DOI] [PubMed] [Google Scholar]
- 9.Mahdy AE, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, Bai A, Gault CR, McPherson AS, Garcia N, Beckham TH, Saad A, et al. Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol Ther. 2009;17:430–8. doi: 10.1038/mt.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, et al. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6:1455–60. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- 11.Brothman AR, Lesho LJ, Somers KD, Wright GL, Jr, Merchant DJ. Phenotypic and cytogenetic characterization of a cell line derived from primary prostatic carcinoma. Int J Cancer. 1989;44:898–903. doi: 10.1002/ijc.2910440525. [DOI] [PubMed] [Google Scholar]
- 12.Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, Elojeimy S, Rembiesa B, Pierce J, Norris JS, Hannun YA. Novel analogs of D-e-MAPP and B13. Part 2: Signature effects on bioactive sphingolipids. Bioorganic & Medicinal Chemistry. 2008;16:1032–45. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad AF, Meacham WD, Bai A, Anelli V, Mahdy EM, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, Hannun YA, et al. The Functional Effects of Acid Ceramidase Over-Expression in Prostate Cancer Progression and Resistance to Chemotherapy. Cancer Biology & Therapy. 2007;6:1455–60. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Elojeimy S, El-Zawahry AM, Holman DH, Bielawska A, Bielawski J, Rubinchik S, Guo GW, Dong JY, Keane T, Hannun YA, Tavassoli M, et al. Modulation of ceramide metabolism enhances viral protein apoptin’s cytotoxicity in prostate cancer. Mol Ther. 2006;14:637–46. doi: 10.1016/j.ymthe.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, Upson JJ, Smith CD. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53:997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller AV, Alvarez SE, Spiegel S, Lebman DA. Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Molecular and cellular biology. 2008;28:4142–51. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KS, Kim M-K, Lee HY, Kim SD, Lee SY, Kim JM, Ryu SH, Bae Y-S. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochemical and Biophysical Research Communications. 2007;356:239–44. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]
- 18.Young N, Pearl DK, Van Brocklyn JR. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol Cancer Res. 2009;7:23–32. doi: 10.1158/1541-7786.MCR-08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saad AF, Meacham WD, Bai A, Anelli V, Mahdy EM, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, Hannun YA, et al. The Functional Effects of Acid Ceramidase Over-Expression in Prostate Cancer Progression and Resistance to Chemotherapy. Cancer Biology & Therapy. 2007;6:1455–60. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- 20.Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, Meacham WD, Mahdy AEM, Saad AF, Turner LS, Cheng JA, Day T, et al. Role of Acid Ceramidase in Resistance to FasL: Therapeutic Approaches Based on Acid Ceramidase Inhibitors and FasL Gene Therapy. Mol Ther. 2007;15:1259–63. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- 21.Yan S, Berquin IM, Troen BR, Sloane BF. Transcription of human cathepsin B is mediated by Sp1 and Ets family factors in glioma. DNA Cell Biol. 2000;19:79–91. doi: 10.1089/104454900314591. [DOI] [PubMed] [Google Scholar]
- 22.Sloane BF, Yan S, Podgorski I, Linebaugh BE, Cher ML, Mai J, Cavallo-Medved D, Sameni M, Dosescu J, Moin K. Cathepsin B and tumor proteolysis: contribution of the tumor microenvironment. Seminars in Cancer Biology. 2005;15:149–57. doi: 10.1016/j.semcancer.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka S. Cathepsin B facilitates autophagy-mediated apoptosis in SPARC overexpressed primitive neuroectodermal tumor cells. Cell Death Differ. 17:1529–39. doi: 10.1038/cdd.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH Affects Distribution and Secretion of Cathepsin B in Malignant Cells. Cancer Res. 1994;54:6517–25. [PubMed] [Google Scholar]
- 25.Bellezza I, Aisa MC, Palazzo R, Costanzi E, Mearini E, Minelli A. Extracellular matrix degrading enzymes at the prostasome surface. Prostate Cancer Prostatic Dis. 2005;8:344–8. doi: 10.1038/sj.pcan.4500828. [DOI] [PubMed] [Google Scholar]
- 26.Van Noorden CJ, Jonges TG, Van Marle J, Bissell ER, Griffini P, Jans M, Snel J, Smith RE. Heterogeneous suppression of experimentally induced colon cancer metastasis in rat liver lobes by inhibition of extracellular cathepsin B. Clin Exp Metastasis. 1998;16:159–67. doi: 10.1023/a:1006524321335. [DOI] [PubMed] [Google Scholar]
- 27.Steffan JJ, Williams BC, Welbourne T, Cardelli JA. HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+-H+ exchangers. J Cell Sci. 2010;123:1151–9. doi: 10.1242/jcs.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


