Abstract
The potential to deliver nanoparticles directly into the targeted cells is important in the therapeutic applications for infectious diseases. The possibility of therapeutic agent being attached to the nanoparticles by chemical modification has provided a novel drug delivery option. Interestingly, the discovery of carbon nanotubes and graphene has given an excellent imaging and therapeutic agent for the biomedical applications. In spite of continuous advancement in pharmaceutical drug delivery viz. micelles, vesicles and liquid crystals etc. during the past decades, their prohibitive production has limited their use. Nanomaterials with their properties of biodegradation, equal biodistribution, mass production and long time storage make them attractive alternative for future biomedical applications. Nanoparticles surface functionalized with specific biomolecules based drug delivery has driven new direction for modulating the pharmacokinetics and pharmacodynamics, biorecognition; and increasing the efficacy of targeted drugs. These new strategies are likely to minimize drug degradation and loss, increase drug availability, and opens up new vistas for drug delivery.
Keywords: Nanoparticle, infectious diseases, drug delivery, therapeutic, nanotherapy
Introduction
Infectious diseases are caused by the presence and growth of pathogenic biological agents like bacteria, fungi parasites etc. in the host organism and characterized by presence of clinical symptoms. According to global infectious disease statistics, leishmaniasis is endemic in 98 countries, one of the world’s most neglected diseases, affecting largely the poorest of the poor, mainly in 85 developing countries; 350 million people are considered at risk of contracting leishmaniasis, and some 2 million new cases occur yearly1. Malaria almost eradicated 30 years ago, is on the rise again, affecting more than 500 million people annually, causing 1–3 million deaths. Cholera, a disease endemic in the Indian subcontinent, Russia and sub-Saharan Africa, is spread mostly through contaminated drinking water and unsanitary conditions. An estimated of 200,000 cases are reported by the World Health Organization (WHO) annually. Tuberculosis causes nearly 2 million deaths every year, and WHO estimates that nearly 1 million people will be infected between 2000 and 2020 if more effective procedures are not adopted. Typhoid fever causes an estimated death of 600,000 annually, out of 12–17 million cases. Measles has seen a drastic reduction in countries where a vaccine is already available, but it is still prevalent in developing countries. Rotavirus, the most common cause of viral gastroenteritis worldwide kills more than 600,000 children year each, mostly in developing countries. Recent years have seen dengue outbreaks all over Asia and Africa, and according to a WHO estimate, the annual incidence of dengue is 50 million. Several influenza epidemics in the 20th century caused millions of death worldwide, including the worst epidemic; the Spanish influenza outbreak that killed more than 500,000 people in 19182.
At the beginning of the 20th century, infectious diseases were the leading cause of death worldwide. In developing countries, most deaths from infectious diseases occur among children and young adults leading to the loss of healthy and productive life. Infection caused by bacteria, virus or protozoa can lead to malfunction or miscommunication, leading to life threatening diseases. These infectious agents are nanometer in size, their shape and size make them compatible for transportation to specific biological compartments. Nanotherapy aims to use the chemical and physical characteristics of nanomaterial for the treatment of infectious disease at molecular level3.
The key advantages of nanoparticle in biomedical applications are that they can be easily transported tagged to the diagnostic and therapeutic drug or biomolecules, due to their unique chemical and physical properties, for targeted cells. Nanoparticles are similar in scale to cells and tissue systems, so they can be engineered to have different sizes and outer surface properties. The unique chemical and physical property of nanoparticles makes it suitable in the diagnosis, imaging and therapeutic application for the infectious diseases. The attachment of biomolecules like antibodies, proteins and drugs with nanoparticles for targeted delivery to specific cells and tissue system has yielded new treatment options. The nanoparticles have reduced toxicity and time release of drug in circulatory system because of their specific and targeted nature. These characteristics make them a modern generation drug delivery vehicle, diagnostic device and monitoring agents, which has proposed to bring it very close to the concept of magic bullets.
The current resolution in the biological research is strongly linked to the availability of nanoparticles that enable its manipulation with biomolecules to study the biological processes at the molecular level using state-of-the-art fluorescent nanoparticle imaging techniques. It is possible to control the characteristics of drugs, including solubility, controlled release and specific site-targeted delivery4. The nanoparticles are produced by chemical modifications, therefore for any biomedical application they can be modified as per their possible environmental requirements. Most of the nanoparticles are soluble in organic and aqueous solvent therefore they can be functionalized for biomedical application. Use of nanotechnology in immunization, design and delivery of drugs, has been explored a promising alternative to current antibiotic based therapy4, 5. Nanomaterials are able to cross biological membranes and gain access to cells, tissues and organs that larger-sized particles normally cannot. They can gain access to the bloodstream following inhalation or ingestion, and some can even penetrate the skin. Nanoscale drug delivery systems also have the ability to improve the pharmacokinetics and increase biodistribution of therapeutic agents to target organs, which will result in improved efficacy6. However it remains to be determined how potential toxicity issues will impact the use of nanotechnology in the control of infectious diseases. This review introduces various challenges in controlling infectious diseases, encompassing diagnosis of resistance and nanotherapy for infectious diseases.
Nanoparticle functionalization and attachment with biomolecules
Synthesized nanoparticles are often not suitable for biological applications because of their surface characteristics. Surface functionalization of nanoparticle is usually required for improving their aqueous dispersibility and biocompatibility, and obtaining appropriate surface functional groups for bioconjugation. Surface attachment with biomolecules such as proteins, antibodies and DNA is usually carried out using conventional bioconjugation methods7. Biomolecules such as proteins/peptides, lipids, carbohydrates, and nucleic acids have huge potential for the antimicrobial application after attachment with nanoparticles8. Using proper functionalization procedures, it is possible to design a stable and highly active biomolecule nanoparticle hybrid system for many biological applications, such as antimicrobial agents. Physicochemical properties of nanoparticles, interface/linking agents and selective biomolecules play a vital role in dictating the antimicrobial activity8. The recognition and capture of biomolecules at solid surfaces has numerous bioanalytical applications in bio- and immunosensor diagnostic devices9. For efficient immobilization, the maximum biochemical activity and minimum nonspecific interactions must be achieved. Using a system that couples proteins with nanoparticles, the efficiency of the immobilization can be increased.
Rational selection of therapeutically active bioactive molecules can increase the biological application against infectious diseases. Biomolecules can be attached to nanoparticles through either physical adsorption or chemical covalent coupling reactions8. In physical adsorption, hydrophobic and electrostatic interactions between biomolecules and nanoparticles have shown superior activity over the interaction between nanoparticles However physical adsorption of biomolecules with nanoparticles has some limitations like; (i) distribution of biomolecules on nanoparticles (ii) desorption of biomolecules over nanoparticles (iii) orientation of biomolecules over nanoparticles. Covalent chemical modification takes place by functionalization of nanoparticles with amine, sulphide or carboxyl groups. This method avoids the physical adsorption limitations, so it is more preferable for the attachment of biomolecules to nanoaparticles8.
Nanotechnology assisted diagnosis of Infectious diseases
The rapid and sensitive detection of pathogenic agent is extremely important for the control of infectious diseases. The unique electrical, physical, chemical, luminescent, magnetic and catalytic properties of nanomaterials enable fast, sensitive and cost effective diagnosis as well as rapid determination of susceptibility for the treatment of infectious diseases when nanoparticles are attached with biomolecules. Anti-antibody conjugated nanoparticles are useful for the tracking of diseased path at molecular level in infectious diseases10. mChip (mobile micro fluidic chip for immunoassay on protein markers) deserves a mention here as it served a miniaturized enzyme linked immunosorbent assay (ELISA) in resource limited settings, it performed equally to lab based gold standard immunoassays, with versatility in the type of blood samples (whole blood, plasma or serum). In remote setting of developing countries, mChip have high sensitivities for both HIV and syphilis and potentially infected patients can be diagnosed immediately, and their samples can be confirmed by test with greater specificity10.
Nanotechnology for the improvement of infectious diseases vaccination
The nano-engineering of vaccines allows the creation of better adjuvants and vaccine delivery systems. The effectiveness of a vaccine is measured by its ability to interact with, and stimulate, the immune system. Advancement of nanoparticle has made possible its use as novel adjuvants and colloidal vaccine carrier to immunize the infected animals. New generation nanoparticle adjuvant are designed to minimize their side effects, prolong the immune response, and concurrently stimulate humoral, cellular, and mucosal immune responses11. Calcium phosphate nanoparticle can be used as potent adjuvant than alum. It induces high titers of immunoglobulin G2a antibody which facilitated a high percentage of protection against HSV-2 infection, without inflammation at the site of administration12. The size, physical and chemical property, surface charge of nanoparticle makes it more suitable for the enhancement of mononuclear phagocytic system, stimulation of antigen presenting cells for the activation of immune system13. Nanoparticles have several benefits over bacteria and viruses for vaccine delivery because of their size in micrometer and nanometer range14. Oral delivery of nanoparticles for the development of vaccine makes it stable in gastrointestinal tract, protective for encapsulated substances and able to modulate physicochemical properties15. In vivo studies on mice have shown protection from influenza A virus in viral pneumonitis after single intranasal immunization and it does not require cold chain for storage16.
Role of Nanotechnology in the treatment of infectious diseases
Nanoparticles or drugs attached with nanoparticles can assist the natural key components of immune system (antibodies and cytokines) by simultaneously fighting with infectious agents at molecular level to overcome the effect of disease. Nanosized silver, zinc and graphene and their compounds have been explored to inactivate microorganisms17,18. A wide range of drugs or biomolecules can be effectively administered using nanocarrier like metal nanoparticles, liposome or carbon based nanomaterials. Some type of water soluble or insoluble drugs can be conjugated on or inside nanoparticles, and are useful for nanotherapy. Key pharmacokinetic characteristics of antibiotics, including improved solubility, controlled release, and specific site-targeted delivery, can be achieved by employing appropriate nanocarriers4. Nanoemulsions are submicron sized emulsions that are under extensive investigation as drug carriers for improving the delivery of therapeutic agents. Nanoemulsions are thermodynamically stable isotropic system in which two immiscible liquid (water and oil) are mixed to form a single phase by means of an appropriate surfactants. The droplet sizes fall typically in the range of 20–200 nm and show narrow size distributions. Nanoemulsion has great promise for the future of cosmetics, diagnostics and drug therapies for drugs like amphotericin B 19.
Effective antimicrobial activity of nanomaterials
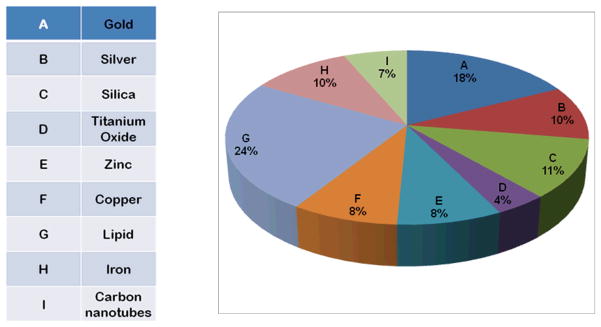
High surface area to volume ratio, unique physico-chemical property and surface charges present over nanomaterial makes them suitable for effective antimicrobial action20. Antibacterial nanoparticles consist of metal and metal oxides, carbon based nanomaterials and nanoemulsions. The antibacterial activity of silver nanoparticle has been shown to depend on dose and is more pronounced against gram negative than gram positive bacteria. Naturally occurring bacteria are independent of acquisition of resistant activity for nanoparticles. Although the detailed antimicrobial mechanisms of nanomaterials is a matter of active research, from whatever little is known, the nanomaterial exert antimicrobial activity via photocatalytic production of reactive oxygen species that damage cellular and viral components, compromising the bacterial cell wall/membrane, interruption of energy transduction, inhibition of enzyme activity and DNA synthesis20, 21. The applications of different nanoparticles have been summarized in Table-1 and been described in detail in next section. Figure 1 provides a glimpse of the nanoparticles of different types which have been used in last 10 years in some way in field of infectious diseases.
Figure 1.
Pie chart showing the percentage of published articles referring to the application of different nanoparticles in infectious diseases during the period from 1992 to 2011.
Most important among the liposomal antimicrobial agents is the liposomal form of amphotericin B. Food and Drug Administrative (FDA) indicates its use in aspergillosis, candidiasis, and cryptococosis including cryptococcal meningitis. It also has been used as an empiric therapy for presumed fungal infection in patients of febrile neutropenia. Besides being an excellent anti fungal agent it has leishmanicidal activity. Liposomal amphotericin B (AmBisome; Gilead Sciences, USA) is licensed in several European countries and USA for primary treatment of visceral leishmaniasis. In Indian subcontinent single dose of AmBisome has shown 96% cure rate in human visceral leishmaniasis22.
Gold nanoparticles
The unique chemical and physical properties of gold nanoparticles allow them to be used for transporting and unloading the pharmaceuticals23. Cells can uptake gold nanoparticles without cytotoxic effects24. Gold nanoparticles are nowadays widely used in immunohistochemistry to identify protein-protein interactions25. These have also been utilized as nontoxic drug carriers for selective drug delivery. It has been reported that using Polyethylene glycol coated colloidal gold nanoparticles (PT-cAu-TNF-α) with an incorporated TNF (tumor necrosis factor)-α can delay tumor growth and enhance tumor thermal therapy in mice when they are administered intravenously proper dosage. Gold nanoparticles covered with quaternary ammonium groups, as antimicrobial agents26 interact with plasmid DNA through electrostatic interactions, resulting in effective protection of DNA from enzymatic digestion27. Gold nanoparticles functionalized with covalently attached oligonucleotides activate genes related with innate immune system and pathways in human peripheral blood mononuclear cells28. Noncovalent encapsulation of drugs into gold nanoparticle monolayer provides direct release of unmodified drugs to target cells. This strategy relies on the use of ligands to generate hydrophobic pockets into the nanoparticle monolayer29. This has been utilized for treatment of amyloidogenesis where peptide-gold nanoparticles were selectively attached to b-amyloid protein (Ab; amyloidogenic aggregates) and irradiated with microwave. This treatment produced dramatic effects on the Ab aggregates, inhibiting both the amyloidogenesis and the restoration of the amyloidogenic potential. This approach offers a new strategy to inhibit, locally and remotely, the amyloidogenic process, which could have application in Alzheimer’s disease therapy30.
Silver nanoparticles
Silver nanoparticles have been used in antibacterial activity, burn wound treatment and dental work in form of silver nitrate or silver sulfadiazine31. Silver nanoparticles have been shown to inhibit Pseudomonas aeruginosa and E. coli bacteria by blocking the respiratory chain and cell cycle division step32. Silver nanoparticles interact simultaneously with sulphur containing proteins of bacterial membranes and phosphorous containing compounds like DNA to inhibit replication. The nanoparticles anchor and penetrate the bacterial cell wall, modulate the cellular signaling by dephosphorylting putative key peptide substrates on tyrosine residues33. The bactericidal effect of silver has also been attributed to inactivation of enzyme phosphomannose isomerase that catalyses the conversion of mannose 6 phosphate to fructose 6 phospahate which is an important intermediate of glycolysis, the most common pathway in bacteria sugar catabolism33. Silver nanoparticles have been studied particularly in context of HIV virus. The former interact and kills HIV-1 virus via preferential binding to the gp120 glycoprotein knobs 34. The mechanism of antiviral action of the silver nanoparticles as well as the inhibition of transmission of HIV-1 infection in human cervix organ culture has also been described in detail 35. The silver nanoparticles because of their ability to come to close proximity to heme, tryptophan, and amide as well aromatic amine residues induces conformational changes in haemoglobin which becomes unfolded through the increment of β-sheet structure. The silver nanoparticles–Hb can form charge-transfer complexes where the Hb-heme along with the silver nanoparticles are involved in the electron transfer mechanism and form Hb–silver nanoparticles assembled structure. This electron transfer mechanism has been found to be dependent on the size of silver particle36.
Silica Nanoparticles
Silica nanoparticles provide biocompatible solid support for enzyme immobilization. The immobilized enzyme molecules on the nanoparticle surface have shown excellent enzymatic activity37. Ultrafine silica nanoparticles, functionalized with amino groups, have been shown to bind and protect plasmid DNA from enzymatic digestion and make effective cell transfection in vitro38. Dense silica nanoparticles serve as an uptake-enhancing component by physical concentration at the cell surface; enhanced transfection due to the particles is seen with almost every transfection reagent tested with little toxicity39. Mesoporous silica nanoparticle is a promising material for biomedical applications, such as delivering drugs or biological molecules (siRNA or DNA), to the target cells or tissues. With positive-charge functionalization on their surface, mesoporous silica nanoparticles have already been used as vectors for siRNA delivery in in vitro experiments. Nevertheless, such siRNA packaging strategy avoid utilizing the mesopores and consequently hinders further modifications on the delivery vehicle surface40. For CN-recognition, a series of bisindolyl compounds 1–3 were prepared, and their chromodosimetric color changes toward anions were investigated. Nucleophilic addition of the cyanide ion to the meso position of the bisindolyl group gave rise to breaking of the double bond conjugation, thereby inducing spectroscopic changes in the compound. Mesoporous silica nanoparticles also gave color changes from deep orange to yellow in response to the cyanide ions41. Silica nanoparticles can be served as a good platform for aptamer hybridization for detection of ATP using hoechst33258 as the signal reporter, because of their DNA-protection property, easy separation, facile surface modification, and good biocompatibility42.
Titanium dioxide nanoparticles
Titanium Dioxide avidly binds lipopolysaccharide with bridging calcium cations, and the complex induces marked proinflammatory signalling in primary human mononuclear phagocytes43. Photoactivated TiO2 nanoparticles have been shown to kill in vitro either bacteria or tumor cells in culture following UV irradiation, by generation of reactive oxygen species; the killing was highly effective although devoid of specificity44. Titanium dioxide nanoparticles have significant effects on regulating genes involved in circadian rhythms, kinase activity, vesicular transport and immune response45. Exposure to TiO2 nanoparticles could affect synaptic plasticity in offspring’s hippocampal dentate gyrus area in vivo, which indicates that developmental brains, especially in lactation, are susceptible to TiO2 nanoparticles exposure46.
Zinc oxide nanoparticles
Nanoparticles of ZnO enriched with poly vinylferrocenium have significantly higher antibacterial effects on Staphylococcus aureus than five other metal oxide nanoparticles 47. The potential application of ZnO nanoparticles as a bacteriostatic agent in visible light may have future applications in the development of derivative agents to control the spread and infection of a variety of bacterial strains47). Cellular responses like apoptosis in the presence of ZnO nanoparticles require p53 as the molecular master switch towards programmed cell death also suggest that in cells without robust p53, protective response can be triggered towards carcinogenesis when stimulated by DNA damage inducing agents like ZnO nanoparticles48.
Copper nanoparticles
CuO nanoparticles in suspension generated by thermal plasma technology showed activity against a range of bacterial pathogens, including meticillin-resistant Staphylococcus aureus and Escherichia coli, with minimum bactericidal concentrations ranging from 100 μg/mL to 5000 μg/mL. CuO nanoparticles incorporated into polymers suggest release of ions, which is required for optimum killing of bacteria49. The antibacterial activity investigation of zero valent copper nanoparticles (size-12 nm) have shown that interaction of copper nanoparticles with E. coli resulted in the formation of cavities/pits in the bacterial cell wall through scanning electron microscopic analysis50.
Lipid nanoparticles
The in vivo applicability of liposomal delivery system depends on its routes of administration, oral, intravenous, subcutaneous, dermal, transdermal, intraperitoneal, intramuscular or inhalation through the bronchial track. All these pathways have specific characteristics and limitations. The liposomes, when administered orally, can survive stomach digestion, but are lysed by the lipolytic enzymes in the intestine. It has been shown that they can protect the entrapped materials from such degradation and hence, this route is used for liposomes in oral vaccination. When injected intravenously, liposomes are rapidly cleared from the blood and absorbed mainly by the phagocyte cells of the reticuloendothelial system. Liposomes injected through subcutaneous, transdermal or intramuscular routes may remain in the in circulation longer. Thus, it may act as a depot of drugs and facilitate the slow release of the entrapped materials from the vesicles. Encouraging results have been found of liposomal drugs in the treatment of a wide spectrum of diseases in experimental animals and in human. These could include treatment of skin and eye diseases, antimicrobial and anticancer therapy, metal chelation, enzyme and hormone replacement therapy, vaccine and diagnostic imaging, etc.
Liposome-encapsulated antimonials were found to be 700 times more active than unencapsulated drug, thus confirming the potential of liposomal systems in the treatment of leishmaniasis51. It was found that apolipoprotein E (ApoE) acts as an endogenous targeting ligand and plays a major role in the plasma clearance and hepatic uptake of ionizable lipid nanoparticles. ApoE binds to ionizable lipid nanoparticles and mediates their uptake through hepatic receptors, such as low-density lipoprotein receptors52.
Iron Nanoparticles
Ligand-targeted micro particles of iron oxide are widely used 53 in magnetic resonance imaging (MRI) of blood vessels in experimental models of acute vascular inflammation, ischemia-reperfusion injury, atherosclerosis and ischemic stroke. The synthetic super paramagnetic iron oxide nanoparticles posses a spherical shape and good super paramagnetic behavior, highly biocompatible characteristics, and have been used in the study of the migratory behavior and bio distribution of dendritic cells in vivo54. The current and potential usefulness as contrast agents for magnetic resonance imaging and nuclear magnetic resonance the ultra small super magnetic iron oxide gained importance55. In MRI guided ultrasound-enhanced brain drug delivery super paramagnetic iron oxide play an important role as theranostic agent56. They offer unique properties for cell tracking by magnetic resonance imaging in cellular immunotherapy, subcellular packaging of magnetic nanoparticles 57. The particles have also been used to validate tumor targeting, wherein the human serum albumin coated iron oxide nanoparticle with doxorubicin is used, so they can assist in the translocation even accumulation of doxorubicin in the nucleus58. The bactericidal effect of Zero-valent iron nanoparticles (nano-Fe0) was a unique property of nano-Fe0, which was not observed in other types of iron-based compounds59. Unlike the gold nanoparticle, the iron oxide nanoparticles have an inhibitory effect on E. coli in a concentration dependant manner. However the phase contrast microscopic study clearly demonstrated that the effect of both Fe3O4 and Au nanoparticle extended up to the level of cell division which was evident as the abrupt increase in bacterial cell length60.
Carbon Nanotubes
The functionalized carbon nanotubes have several advantages when used as a nanovector for therapeutic molecules. Within nanomaterial family, carbon nanotubes have emerged as a new alternative and efficient tool for transporting and translocating therapeutic molecules. Carbon nanotubes can be functionalized with bioactive peptides, proteins, nucleic acids and drugs, and used to deliver their cargos to cells and organs61. One of the unique characteristic of carbon nanotubes is the network formation of carbon atoms in the nanometer scale, allowing the creation of nano-channels via cellular membranes62. The functional groups of carbon nanotubes when modified with therapeutic molecules are stably attached to the nanotube backbone and therefore avoid the risk of macromolecule desorption. The chemical synthesis of functionalized carbon nanotubes-amphotericin B involves covalent coupling rather than biological molecules, which makes it cheaper to make than existing liposomal amphotericin B. The intracellular nature of L. donovani makes it suitable for targeted drug delivery by functionalized carbon nanotubes. Both in vitro and in vivo experiments demonstrated a marked increase in the efficacy of functionalized carbon nanotubes-amphotericin B compared with amphotericin B63. Because of their favorable pharmacokinetic and toxic profiles the polyethylene glycol modified carbon nanotubes are a promising type of nonviral delivery systems64. They are potential drug carriers in targeted delivery which is utilized for the diagnosis and treatment of central nervous system disorders and infectious diseases65.
Conclusions
In this review we have made an attempt to describe a variety of biofunctionalized nanoparticles, which provides widespread opportunities in detection, treatment and vaccination of infectious diseases by functionally attached biomolecules. The physical and chemical characteristic of the nanoparticles can be used as a tool for the treatment of infectious diseases. Therapeutically active bioactive molecules have shown increased biological efficacy against infectious diseases, when they are attached with nanovectors. There are many foreseen promises using nanoparticles but these particles have limitations like water solubility, drug resistance and toxic effects, which may be overcome by the development of nanomedicine using biomolecule conjugated nanoparticles in coming future. The toxicological issues associated with understanding and elimination of the fateof nanocarriers can be possibility solved using drug carriers madefrom natural polymers.
Table 1.
| Drug delivery systems | Stage of development | Examples of application | References |
|---|---|---|---|
|
| |||
| Nanoemulsions | Preclinical | Amphotericin B | 19 |
| Paclitaxel | 66 | ||
| Dexamethasone | 67 | ||
| Benzathine, penicillin G | 68 | ||
| Aciclovir | 69 | ||
| Camptothecin | 70 | ||
|
| |||
| Liposomes | Marketed | Amphotericin | 22, 71 |
| Daunorubicin | 72 | ||
| Doxorubicin | 73 | ||
|
| |||
| Drug nanocrystals | Preclinical | Amphotericin B | 74, 75 |
| Etoposide, camptothecin, paclitaxel | 76 | ||
|
| |||
| Lipid-based nanoparticles | Preclinical | Doxoribicin | 77 |
| Camptothecin | 78 | ||
|
| |||
| Polymer-based nanoparticles | Preclinical | Tamoxifen | 79 |
| Cyclosporin-A | 80 | ||
| Theophylline | 81 | ||
|
| |||
| Albumin nanoparticles | Marketed | Paclitaxel oligonucleotides |
82 83, 84 |
|
| |||
| Dendrimers | Preclinical | Indometacin | 85 |
| 5-fluorouracil | 86 | ||
| Antisense Oligonucleotides | 87 | ||
|
| |||
| Carbon Nanotubes | Preclinical | Amphotericin B | 63 |
Acknowledgments
This study was supported by NIAID, NIH TMRC Grant No. 1P50AI074321 for the financial support. Author Vijay Kumar Prajapati is thankful to ICMR, New Delhi, India for providing Senior Research Fellowship.
References
- 1.Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, G; 22–26 March 2010. [Google Scholar]
- 2.http://www.infoplease.com/ipa/A0903696.
- 3.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nat Rev Microbiol. 2004;2(3):231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 4.Allaker RP, Ren G. Trans R Soc Trop Med Hyg. 2008;102(1):1–2. doi: 10.1016/j.trstmh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PW, Stapleton PD, Paul Luzio J. Drug Discov Today. 2002;7(21):1086–1091. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- 6.Moghimi SM, Szebeni J. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 7.Veerapandian M, Yun K. Appl Microbiol Biotechnol. 90(5):1655–1667. doi: 10.1007/s00253-011-3291-6. [DOI] [PubMed] [Google Scholar]
- 8.Tallury P, Malhotra A, Byrne LM, Santra S. Adv Drug Deliv Rev. 62(4–5):424–437. doi: 10.1016/j.addr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yam CM, Pradier CM, Salmain M, Fischer-Durand N, Jaouen G. J Colloid Interface Sci. 2002;245(1):204–207. doi: 10.1006/jcis.2001.7981. [DOI] [PubMed] [Google Scholar]
- 10.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. Nat Med. 17(8):1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 11.Feng ZQ, Zhong SG, Li YH, Li YQ, Qiu ZN, Wang ZM, Li J, Dong L, Guan XH. Chin Med J (Engl) 2004;117(1):83–87. [PubMed] [Google Scholar]
- 12.HQ, ARM, SLJ, CWB, TM, SJDB Clin Diagn Lab Immunol. 2000;7(6):899–903. doi: 10.1128/cdli.7.6.899-903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice-Ficht AC, Arenas-Gamboa AM, Kahl-McDonagh MM, Ficht TA. Curr Opin Microbiol. 13(1):106–112. doi: 10.1016/j.mib.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Peek LJ, Middaugh CR, Berkland C. Adv Drug Deliv Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. J Control Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Myc A, Kukowska-Latallo JF, Bielinska AU, Cao P, Myc PP, Janczak K, Sturm TR, Grabinski MS, Landers JJ, Young KS, Chang J, Hamouda T, Olszewski MA, Baker JR., Jr Vaccine. 2003;21(25–26):3801–3814. doi: 10.1016/s0264-410x(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C. ACS Nano. 4(7):4317–4323. doi: 10.1021/nn101097v. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Zheng X, Yan D, Yin G, Liao X, Kang Y, Yao Y, Huang D, Hao B. Langmuir. 2008;24(8):4140–4144. doi: 10.1021/la7035949. [DOI] [PubMed] [Google Scholar]
- 19.Brime B, Frutos P, Bringas P, Nieto A, Ballesteros MP, Frutos G. J Antimicrob Chemother. 2003;52(1):103–109. doi: 10.1093/jac/dkg266. [DOI] [PubMed] [Google Scholar]
- 20.Weir E, Lawlor A, Whelan A, Regan F. Analyst. 2008;133(7):835–845. doi: 10.1039/b715532h. [DOI] [PubMed] [Google Scholar]
- 21.Pal S, Tak YK, Song JM. Appl Environ Microbiol. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. N Engl J Med. 362(6):504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv Drug Deliv Rev. 2008;60(11):1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 25.Salata O. J Nanobiotechnology. 2004;2(1):3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin S, Ye K. Biotechnol Prog. 2007;23(1):32–41. doi: 10.1021/bp060348j. [DOI] [PubMed] [Google Scholar]
- 27.Han G, Ghosh P, Rotello VM. Adv Exp Med Biol. 2007;620:48–56. doi: 10.1007/978-0-387-76713-0_4. [DOI] [PubMed] [Google Scholar]
- 28.Kim EY, Schulz R, Swantek P, Kunstman K, Malim MH, Wolinsky SM. Gene Ther. doi: 10.1038/gt.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana S, Bajaj A, Mout R, Rotello VM. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyleen Araya IO, Bastus Neus G, Guerrero Simón, Puntes Víctor F, Giralt Ernest, Kogan Marcelo J. Nanoscale Res Lett. 2008;3:435–443. [Google Scholar]
- 31.Chopra I. J Antimicrob Chemother. 2007;59(4):587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 32.Klasen HJ. Burns. 2000;26(2):117–130. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 33.Grangeasse C, Obadia B, Mijakovic I, Deutscher J, Cozzone AJ, Doublet P. J Biol Chem. 2003;278(41):39323–39329. doi: 10.1074/jbc.M305134200. [DOI] [PubMed] [Google Scholar]
- 34.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ. J Nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lara HH, Garza-Trevino EN, Ixtepan-Turrent L, Singh DK. J Nanobiotechnology. 9:30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahato M, Pal P, Tah B, Ghosh M, Talapatra GB. Colloids Surf B Biointerfaces. 88(1):141–149. doi: 10.1016/j.colsurfb.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Qhobosheane M, Santra S, Zhang P, Tan W. Analyst. 2001;126(8):1274–1278. doi: 10.1039/b101489g. [DOI] [PubMed] [Google Scholar]
- 38.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Proc Natl Acad Sci U S A. 2005;102(32):11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo D, Han E, Belcheva N, Saltzman WM. J Control Release. 2004;95(2):333–341. doi: 10.1016/j.jconrel.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Xie QR, Zhang J, Xia W, Gu H. Biomaterials. 32(35):9546–9556. doi: 10.1016/j.biomaterials.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 41.Kim HJ, Lee H, Lee JH, Choi DH, Jung JH, Kim JS. Chem Commun (Camb) 47(39):10918–10920. doi: 10.1039/c1cc13481g. [DOI] [PubMed] [Google Scholar]
- 42.Cai L, Chen ZZ, Dong XM, Tang HW, Pang DW. Biosens Bioelectron. 29(1):46–52. doi: 10.1016/j.bios.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 43.Ashwood P, Thompson RP, Powell JJ. Exp Biol Med (Maywood) 2007;232(1):107–117. [PubMed] [Google Scholar]
- 44.Elvira G, Moreno B, Del Valle I, Garcia-Sanz JA, Canillas M, Chinarro E, Jurado JR, Silva A. J Biomater Appl. doi: 10.1177/0885328210393294. [DOI] [PubMed] [Google Scholar]
- 45.Jovanovic B, Ji T, Palic D. Ecotoxicol Environ Saf. 74(6):1518–1525. doi: 10.1016/j.ecoenv.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Gao X, Yin S, Tang M, Chen J, Yang Z, Zhang W, Chen L, Yang B, Li Z, Zha Y, Ruan D, Wang M. Biol Trace Elem Res. doi: 10.1007/s12011-011-8990-4. [DOI] [PubMed] [Google Scholar]
- 47.Jones N, Ray B, Ranjit KT, Manna AC. FEMS Microbiol Lett. 2008;279(1):71– 76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 48.Ng KW, Khoo SP, Heng BC, Setyawati MI, Tan EC, Zhao X, Xiong S, Fang W, Leong DT, Loo JS. Biomaterials. 32(32):8218–8225. doi: 10.1016/j.biomaterials.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 49.Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Int J Antimicrob Agents. 2009;33(6):587–590. doi: 10.1016/j.ijantimicag.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Raffi M, Mehrwan S, Bhatti TM, Akhter JI, Hameed A, Yawar W, Hasan MU. Annl Microb. 2010;60(1):75–80. [Google Scholar]
- 51.Date AA, Joshi MD, Patravale VB. Adv Drug Deliv Rev. 2007;59(6):505–521. doi: 10.1016/j.addr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Huang L, Liu Y. Annu Rev Biomed Eng. 13:507–530. doi: 10.1146/annurev-bioeng-071910-124709. [DOI] [PubMed] [Google Scholar]
- 53.McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. Atherosclerosis. 209(1):18–27. doi: 10.1016/j.atherosclerosis.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mou Y, Chen B, Zhang Y, Hou Y, Xie H, Xia G, Tang M, Huang X, Ni Y, Hu Q. Int J Nanomedicine. 6:1779–1786. doi: 10.2147/IJN.S23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Dong H, Pacheco V, Willbold D, Zhang Y, Offenhausser A, Hartmann R, Weirich TE, Ma P, Krause HJ, Gu Z. J Phys Chem B. doi: 10.1021/jp2066138. [DOI] [PubMed] [Google Scholar]
- 56.Liu HL, Chen PY, Yang HW, Wu JS, Tseng IC, Ma YJ, Huang CY, Tsai HC, Chen SM, Lu YJ, Hua MY, Ma YH, Yen TC, Wei KC. J Magn Reson Imaging. doi: 10.1002/jmri.22697. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz S, Wong JE, Bornemann J, Hodenius M, Himmelreich U, Richtering W, Hoehn M, Zenke M, Hieronymus T. Nanomedicine. doi: 10.1016/j.nano.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Quan Q, Xie J, Gao H, Yang M, Zhang F, Liu G, Lin X, Wang A, Eden HS, Lee S, Zhang G, Chen X. Mol Pharm. 8(5):1669–1676. doi: 10.1021/mp200006f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C, Kim JY, Lee WI, Nelson KL, Yoon J, Sedlak DL. Environ Sci Technol. 2008;42(13):4927–4933. doi: 10.1021/es800408u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatterjee S, Bandyopadhyay A, Sarkar K. J Nanobiotechnology. 9:34. doi: 10.1186/1477-3155-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianco A, Kostarelos K, Prato M. Curr Opin Chem Biol. 2005;9(6):674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Rosen Y, Elman NM. Expert Opin Drug Deliv. 2009;6(5):517–530. doi: 10.1517/17425240902865579. [DOI] [PubMed] [Google Scholar]
- 63.Prajapati VK, Awasthi K, Gautam S, Yadav TP, Rai M, Srivastava ON, Sundar S. J Antimicrob Chemother. 66(4):874–879. doi: 10.1093/jac/dkr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bottini M, Rosato N, Bottini N. Biomacromolecules. 12(10):3381–3393. doi: 10.1021/bm201020h. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Bai Y, Yan B. Drug Discov Today. 15(11–12):428–435. doi: 10.1016/j.drudis.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He L, Wang GL, Zhang Q. Int J Pharm. 2003;250(1):45–50. doi: 10.1016/s0378-5173(02)00478-7. [DOI] [PubMed] [Google Scholar]
- 67.Seki J, Sonoke S, Saheki A, Fukui H, Sasaki H, Mayumi T. Int J Pharm. 2004;273(1–2):75–83. doi: 10.1016/j.ijpharm.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 68.Santos-Magalhaes NS, Pontes A, Pereira VM, Caetano MN. Int J Pharm. 2000;208(1–2):71–80. doi: 10.1016/s0378-5173(00)00546-9. [DOI] [PubMed] [Google Scholar]
- 69.GL, PP Die Pharmazie. 1997;52:642–643. [Google Scholar]
- 70.Yang S, Zhu J, Lu Y, Liang B, Yang C. Pharm Res. 1999;16(5):751–757. doi: 10.1023/a:1018888927852. [DOI] [PubMed] [Google Scholar]
- 71.Davidson RN, Croft SL, Scott A, Maini M, Moody AH, Bryceson AD. Lancet. 1991;337(8749):1061–1062. doi: 10.1016/0140-6736(91)91708-3. [DOI] [PubMed] [Google Scholar]
- 72.Guaglianone P, Chan K, DelaFlor-Weiss E, Hanisch R, Jeffers S, Sharma D, Muggia F. Invest New Drugs. 1994;12(2):103–110. doi: 10.1007/BF00874439. [DOI] [PubMed] [Google Scholar]
- 73.Gabizon A, Peretz T, Sulkes A, Amselem S, Ben-Yosef R, Ben-Baruch N, Catane R, Biran S, Barenholz Y. Eur J Cancer Clin Oncol. 1989;25(12):1795–1803. doi: 10.1016/0277-5379(89)90350-7. [DOI] [PubMed] [Google Scholar]
- 74.Kayser O, Olbrich C, Yardley V, Kiderlen AF, Croft SL. Int J Pharm. 2003;254(1):73–75. doi: 10.1016/s0378-5173(02)00686-5. [DOI] [PubMed] [Google Scholar]
- 75.Manandhar KD, Yadav TP, Prajapati VK, Kumar S, Rai M, Dube A, Srivastava ON, Sundar S. J Antimicrob Chemother. 2008;62(2):376–380. doi: 10.1093/jac/dkn189. [DOI] [PubMed] [Google Scholar]
- 76.Merisko-Liversidge E, Sarpotdar P, Bruno J, Hajj S, Wei L, Peltier N, Rake J, Shaw JM, Pugh S, Polin L, Jones J, Corbett T, Cooper E, Liversidge GG. Pharm Res. 1996;13(2):272–278. doi: 10.1023/a:1016051316815. [DOI] [PubMed] [Google Scholar]
- 77.Zara GP, Cavalli R, Bargoni A, Fundaro A, Vighetto D, Gasco MR. J Drug Target. 2002;10(4):327–335. doi: 10.1080/10611860290031868. [DOI] [PubMed] [Google Scholar]
- 78.Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. J Control Release. 1999;59(3):299–307. doi: 10.1016/s0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 79.Shenoy DB, Amiji MM. Int J Pharm. 2005;293(1–2):261–270. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Molpeceres J, Chacon M, Guzman M, Berges L, del Rosario Aberturas M. Int J Pharm. 1999;187(1):101–113. doi: 10.1016/s0378-5173(99)00177-5. [DOI] [PubMed] [Google Scholar]
- 81.Radwan MA, Zaghloul IY, Aly ZH. Eur J Pharm Sci. 1999;8(2):95–98. doi: 10.1016/s0928-0987(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 82.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 83.Rhaese S, von Briesen H, Rubsamen-Waigmann H, Kreuter J, Langer K. J Control Release. 2003;92(1–2):199–208. doi: 10.1016/s0168-3659(03)00302-x. [DOI] [PubMed] [Google Scholar]
- 84.Wartlick H, Spankuch-Schmitt B, Strebhardt K, Kreuter J, Langer K. J Control Release. 2004;96(3):483–495. doi: 10.1016/j.jconrel.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 85.Chauhan AS, Jain NK, Diwan PV, Khopade AJ. J Drug Target. 2004;12(9–10):575–583. doi: 10.1080/10611860400010655. [DOI] [PubMed] [Google Scholar]
- 86.Tripathi PK, Khopade AJ, Nagaich S, Shrivastava S, Jain S, Jain NK. Pharmazie. 2002;57(4):261–264. [PubMed] [Google Scholar]
- 87.Hussain M, Shchepinov M, Sohail M, Benter IF, Hollins AJ, Southern EM, Akhtar S. J Control Release. 2004;99(1):139–155. doi: 10.1016/j.jconrel.2004.06.009. [DOI] [PubMed] [Google Scholar]