Biological thiols are essential for maintaining the appropriate redox status of proteins, cells, and organisms.[1] Cysteine (Cys) is an essential amino acid that is involved in protein synthesis, detoxification, and metabolism. Elevated levels of Cys have been associated with neurotoxicity,[2] and Cys deficiency is involved in slowed growth rate, hair depigmentation, edema, lethargy, liver damage, muscle and fat loss, skin lesions, and weakness.[3] Homocysteine (Hcy) has been implicated in various types of vascular and renal diseases. Elevated Hcy in the blood (>12 µm) is a well-known risk factor for cardiovascular[4a] and Alzheimer’s disease,[4b] neutral tube defects, complications during pregnancy, inflammatory bowel disease, and osteoporosis.[4c] Because Cys and Hcy levels are associated with different diseases despite their similar structures, their discrimination is necessary.
Significant effort has gone into the development of colorimetric,[5] phosphorescent,[6] and fluorescent probes[7–10] for these thiol-containing amino acids to achieve high sensitivity, low cost, and ease of detection. To date, most of the indicators or dosimeters are based on the strong nucleophilicity of the thiol group, and various mechanisms have been employed, including the Michael addition,[8] cleavage reactions,[6b, 9] and others.[10] Though these probes show high sensitivity toward thiol-containing compounds, the direct detection of Cys (or Hcy) is hampered because of interference from other thiols.
In 2004, we discovered a fluorophore with an appended aldehyde to serve as a fluorescent probe for both Cys and Hcy.[11] It was based on the well-known cyclization of Cys (or Hcy) with aldehydes to form thiazolidines (or thiazinanes). Because both the sulfhydril and the amino groups contribute to the cyclization, it enables selectivity for Cys and Hcy over other common thiols such as glutathione (GSH). Since the aminothiol moieties of Cys and Hcy have similar reactivities towards aldehydes in general, discriminating between them is challenging when using heterocyle formation.[12] Later, research in this area was extended and some probes that were selective for either Cys or Hcy have been developed.[6a, 12d] However, the simultaneous determination of Cys or Hcy using a single probe remains a significant challenge. This challenge arises because of the structural similarity of Cys and Hcy which differ by a single methylene unit in their side chains (see Figure S1 in the Supporting Information). Herein we report the design of a probe that achieves the detection of Cys and Hcy simultaneously with excellent selectivity.
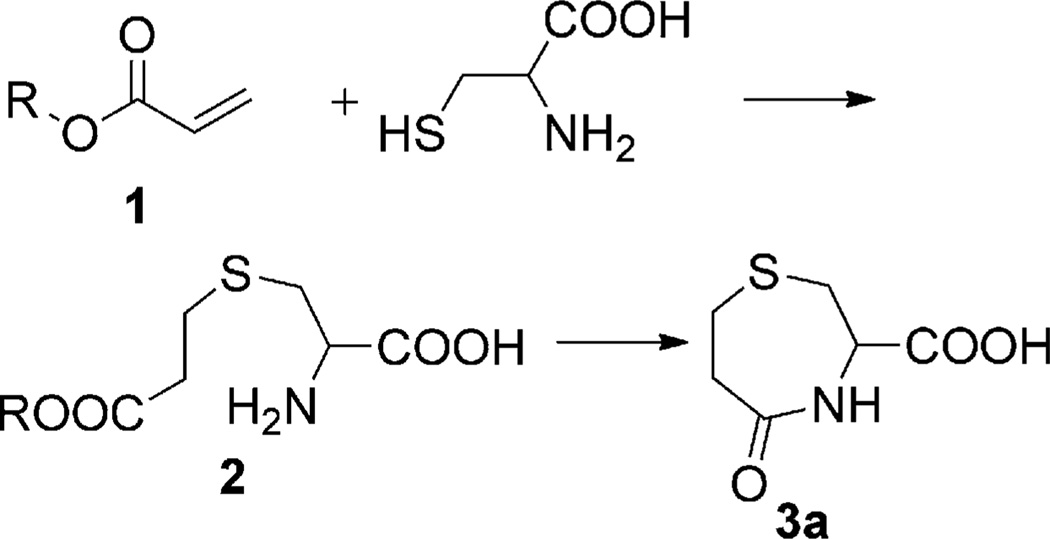
It has been known for more than 40 years that the condensation of acrylates with Cys can be used for the preparation of substituted 1,4-thiazepines.[13] The reaction involves the conjugate addition of Cys to acrylates (1) to generate thioethers (2), which can additionally undergo an intramolecular cyclization to yield the desired compound 3a, as illustrated in Scheme 1.
Scheme 1.
The formation of 3-carboxy-5-oxoperhydro-1,4-thiazepine (3a) from the condensation of acrylates (R = alkyl) and Cys.
As for Hcy, the analogous thioether should be generated readily.[14] We propose that the intramolecular cyclization reaction to form an eight-membered ring should be kinetically disfavored relative to the formation of seven-membered ring that would result from Cys.[15] Therefore, it occurred to us to design a fluorescent probe for the discrimination of Cys and Hcy based on their different relative rates of intramolecular cyclization.
It is well known that 2-(2′-hydroxyphenyl)benzothiazole (HBT) can undergo an excited-state intramolecular photontransfer (ESIPT) process upon photoexcitation whereby rapid photoinduced proton transfer results in tautomerization (see Figure S2 in the Supporting Information). Accordingly, HBT exhibits dual emission bands which originate from its enol and keto tautomeric forms.[16] Modification of the hydroxy group of HBT thus blocks ESIPT and results exclusively in enol-like emission.[17] Generation of the free hydroxy group results in dual emission bands associated with keto–enol tautomerism. This signal transduction mechanism has been successfully applied in probe design for fluoride[17a,b] and phosphatase.[17c]
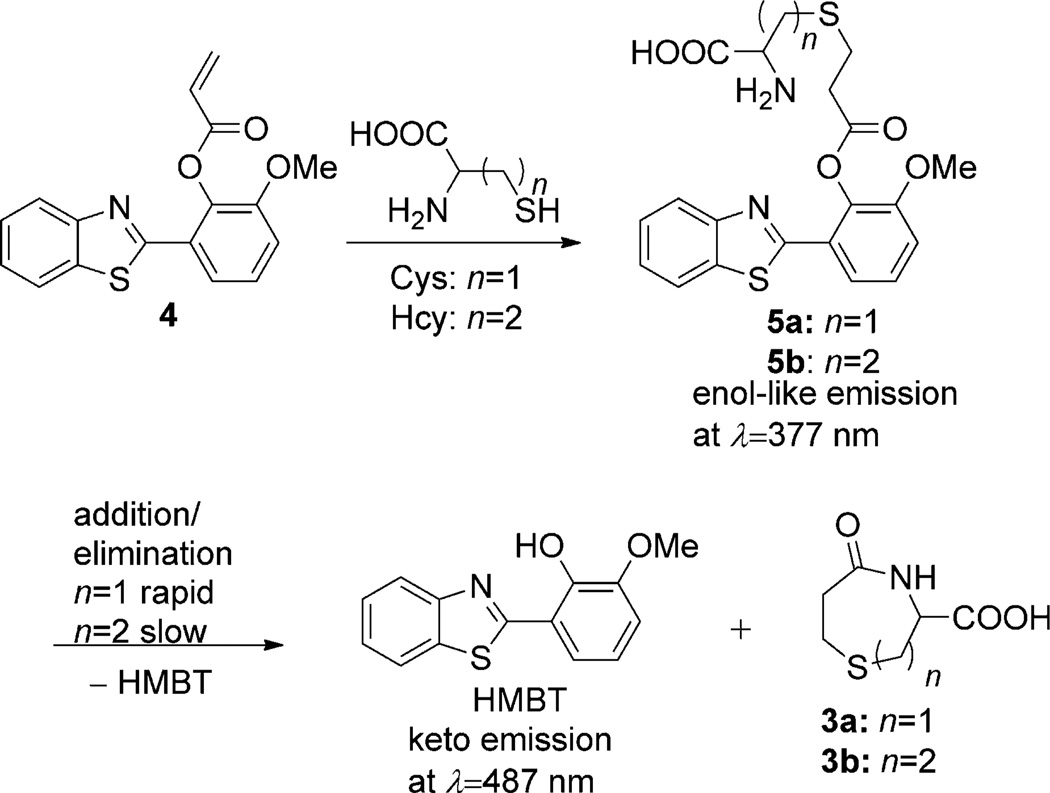
We hypothesize that masking the hydroxy group in the fluorophore of 2-(2′-hydroxy-3′-methoxyphenyl)benzothiazole (HMBT) with an α,β-unsaturated carbonyl moiety could generate the probe 4 for the selective dual emission differentiation of Cys and Hcy based on their different reaction rates in the process depicted in Scheme 2. In the case of HMBT, the enol (λ = 377 nm) and keto (λ = 487 nm) emission bands facilitate monitoring the conjugate addition of Cys (or Hcy) and the ensuing intramolecular cyclization reaction.
Scheme 2.
The reaction sequence that enables the sensing of Cys and Hcy when using 4.
To demonstrate the above hypothesis, we first synthesized 4 in two steps (see Scheme S1 in the Supporting Information). The reaction of 2-aminothiophenol and o-vanillin in EtOH affords HMBT in 79% yield. HMBT is acylated with acryloyl chloride to get 4 in 71% yield. As expected, 4 is weakly fluorescent because the fluorophore is quenched by the carbon–carbon double bond through a photoinduced electron-transfer (PET) process.[8e,g]
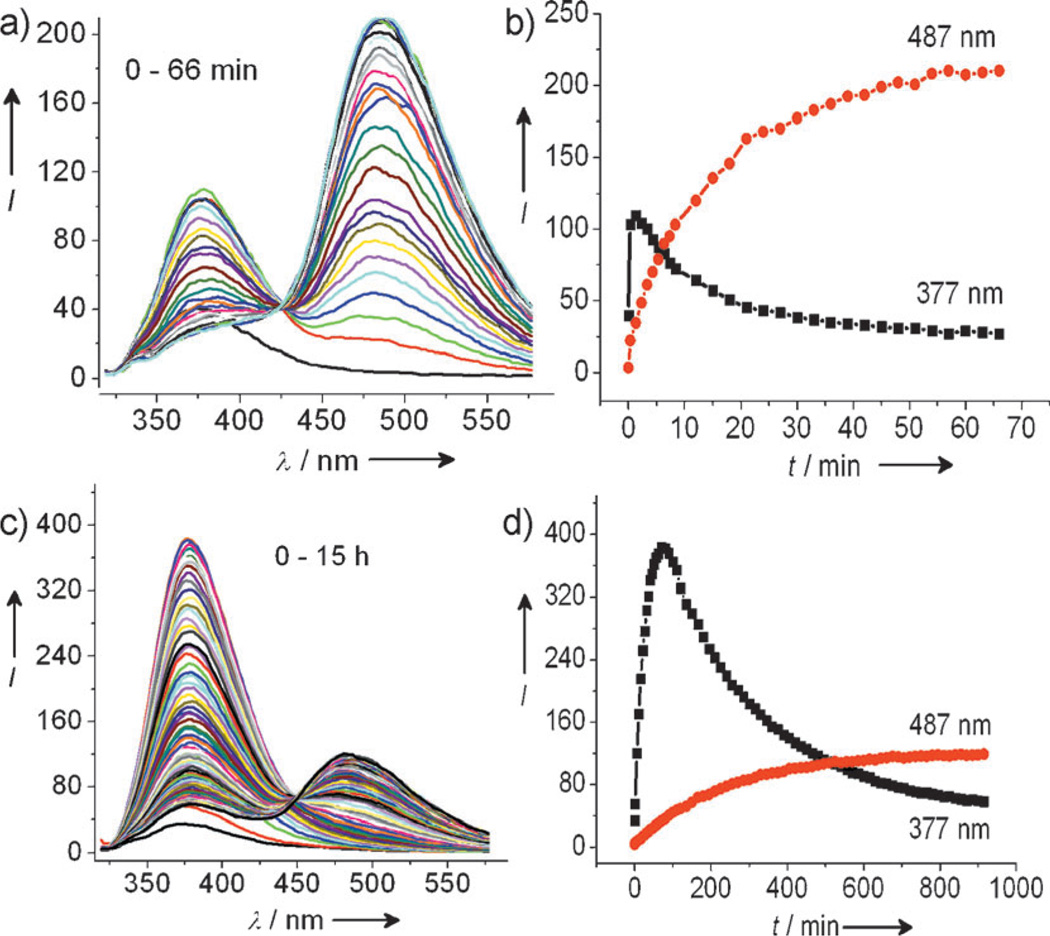
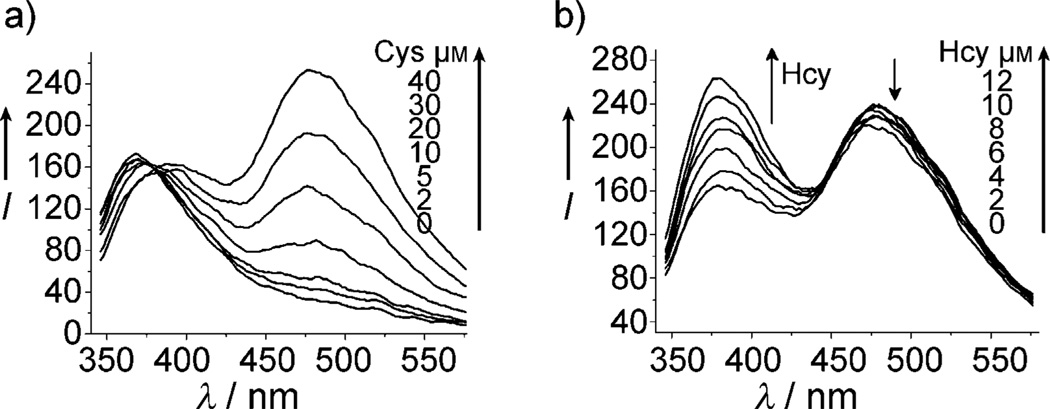
Initially the fluorescence sensing behavior of 4 toward Cys was investigated using a 20 µm solution of 4 in an EtOH/H2O (2:8, v/v) solution buffered at pH 7.4 (phosphate buffer, 20 mm). Upon addition of Cys (1 equiv), the emission at λ = 377 nm increases initially as a result of the conjugate addition which removes the alkene-induced PET quenching (see Figure S3-a in the Supporting Information). The emission band at λ = 377 nm then decreases with concomitant ingrowth of the keto band at λ = 487 nm (Figure 1a). A well-defined isoemissive point appears at λ = 427 nm (see Figure S3-b). The latter spectral change is due to lactam formation which results in the formation of HMBT exhibiting ESIPT.
Figure 1.
Time-dependent fluorescence spectral changes of 4 [20 µm; EtOH/phosphate buffer (20 mm, pH 7.4, 2:8 v/v)] with 1 equiv of either a) Cys or c) Hcy. Time-dependent fluorescence intensity changes of 4 (20 µm) in the presence of 1 equiv of either b) Cys or d) Hcy; λex = 304 nm.
In the case of Hcy, the conjugate addition reaction leads to thioether 3b. However, the rate for the subsequent eight-membered-ring lactam formation (3b) is relatively slow as expected (see below). One can observe the emission at λ = 377 nm steadily increasing over time (see Figure S4-a in the Supporting Information), with a subsequent decrease after 56 minutes and an accompanying increase of the emission at λ = 487 nm (Figure 1c). Scheme 2 summarizes the reaction pathway and associated signaling mechanisms.
On the basis of the kinetic differences in the intramolecular cyclization reactions of 5a and 5b, the presence of Cys and Hcy can be simultaneously determined over a time course. It can be observed from Figure 1b that the reaction of 4 with Cys is nearly complete within 40 minutes, whereas for Hcy, only conjugate addition adduct 5b is formed and a small peak for the emission of HMBT is observed. Therefore, Cys gives emission mainly at λ = 487 nm (keto form of HMBT) and Hcy at λ = 377 nm (enol form of HMBT) at 40 minutes (see below for improvements in the reaction rate). The significant difference in emission wavelengths, up to λ = 110 nm, enables the peaks corresponding to keto and enol forms to afford simultaneous determination of Cys and Hcy.
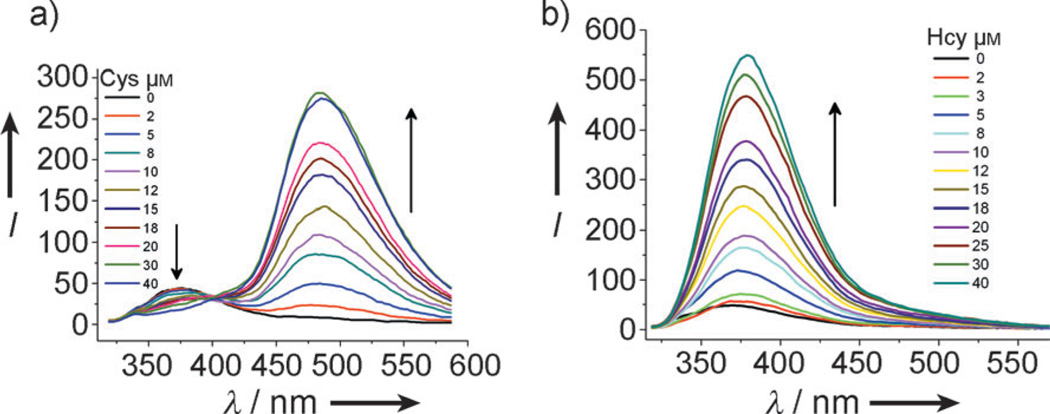
Figures 2a and b shows the fluorescence changes with increasing amounts of Cys and Hcy in EtOH/phosphate buffer (20 mm, pH 7.4; 2:8, v/v) at 40 minutes, respectively. It can be observed that the fluorescence intensity at λ = 487 nm (or λ = 377 nm) increases with increasing Cys (or Hcy) concentration. The fluorescent intensity is linearly proportional to the amount of Cys from 0 to 20 µm and 0 to 25 µm for Hcy (see Figure S19 in the Supporting Information). The detection limits of Cys and Hcy are 0.11 and 0.18 µm, respectively, which is below the requisite detection limits for Cys and Hcy assays in human plasma samples.[18] The assay can distinguish concentration changes on the order of 2–3 µm. Such sensitivity readily enables sensitivity distinguishing, for instance, normal (5–12 µm) Hcy levels, hyperhomocysteinemia (16–100 µm, indicating cardiovascular risk), and homocysteinuria (>100 µm, a severe inherited metabolic disorder associated with mental retardation, a multisystemic disorder of the connective tissue, muscles, central nervous system, and cardiovascular system).[19]
Figure 2.
Fluorescence spectra of 4 [20 µm; EtOH/phosphate buffer (20 mm, pH 7.4; 2:8 v/v)] after 40 min in the presence of increasing concentrations of either a) Cys or b) Hcy; λex = 304 nm.
Additional evidence for the proposed reaction mechanism comes from the formation of HMBT, which is observed in the 1H NMR spectra of the product mixture (see Figure S23 in the Supporting Information). The formation of 3a and 3b is confirmed by 1H NMR, 13C NMR, and 1H-13C COSY NMR spectroscopy, as well as HRMS (Figures S28–S34).[13a] The data clearly shows that an intramolecular cyclization is involved in the signaling event.
Control experiments were also carried out to prove that the amino group of Cys is needed in the selective cyclization reaction. First, cysteamine was introduced to a solution of 4. Similar fluorescence changes are observed as for Cys under analogous reaction conditions (see Figure S5 in the Supporting Information). However, 3-mercaptopropanoic acid (MPA) exhibits fluorescence emission centered at λ = 377 nm as a result of the formation of the conjugate addition product only (Figure S6). Lastly, N-acetyl-l-cysteine (NAC) affords a similar result to that of MPA (Figure S7), that is, formation of the conjugate addition adduct. The latter product is evidenced by HRMS data (ESI-FTMS m/z = 473.0850 [M–H]−, calc. 473.0841 for C22H21N2O6S2; Figure S24). The above experiments prove that the amino group is indeed involved in the intramolecular cyclization reaction.
To evaluate the selectivity of the present probe for Cys and Hcy, changes in the fluorescence intensity of 4 caused by other analytes, such as leucine, proline, arginine, histidine, valine, methionine, threonine, glutamine, alanine, aspartic acid, norleucine, isoleucine, lysine, cystine, and homocystine were also tested. It can be seen that only Cys and Hcy promote significant fluorescence intensity changes at λ = 487 and 377 nm, respectively, whereas other amino acids cause no fluorescence intensity changes under the same conditions (see Figure S8 in the Supporting Information).
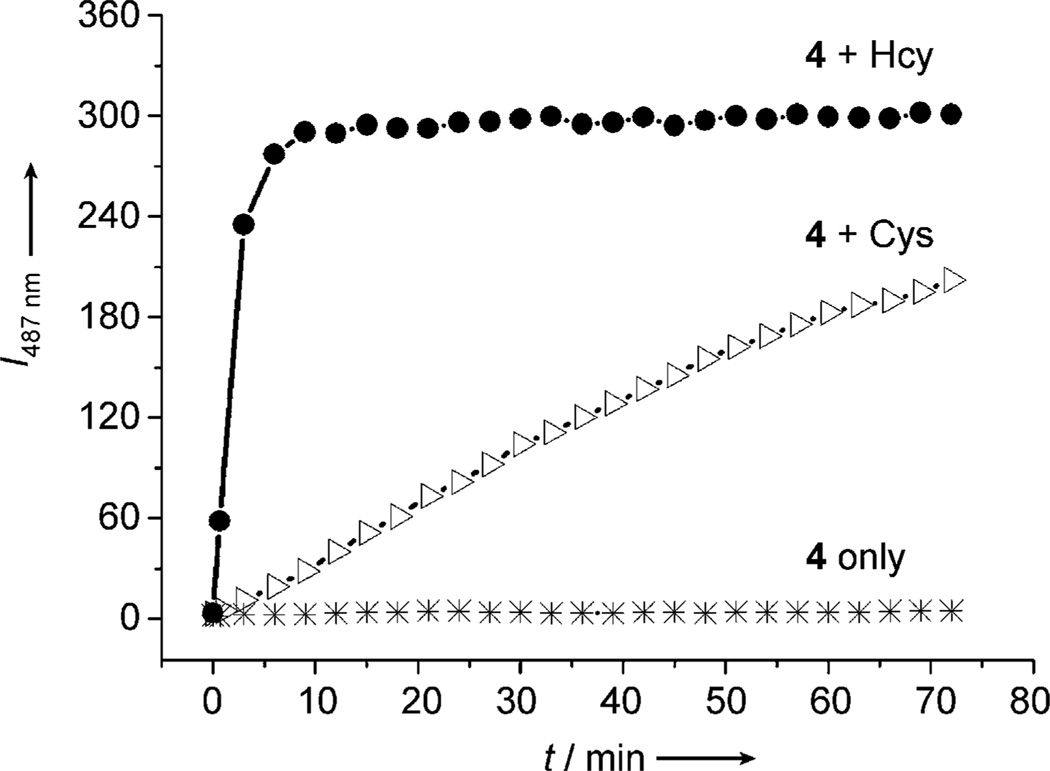
However, GSH can also give rise to enol-like emission because of the conjugate addition reaction with 4 (see Figure S9 in the Supporting Information). To overcome interference from GSH and other sulfydrils, we use cetyltrimethylammonium bromide (CTAB) micellar media, as this significantly enhances reaction rates (Figures S10–S12). It is precedented that analogous cyclocondensation reactions of aminothiols are catalyzed by surfactants.[20] As shown in Figure 3, the formation of HMBT from 4 and Cys is complete within 9 minutes. In the case of Hcy, almost no free HMBT emission can be observed in 9 minutes.
Figure 3.
Time-dependent fluorescence intensity changes of 4 (10 µm) at λ = 487 nm upon adding either Cys or Hcy (both 20 µm) in CTAB media (1.0 mm) buffered at 7.4 (phosphate buffer, 20 mm).
λex = 304 nm.
Thus, Cys can be measured through a stable signal appearing at λ = 487 nm after 9 minutes. GSH and non-amino thiols can be measured through a stable signal at λ = 377 nm after 9 minutes. Hcy beginning at 9 minutes is the only analyte that causes a proportional change of the signals at λ = 377 nm and 487 nm as it promotes formation of HMBT. In this way, Hcy can be monitored selectively over time after the respective signals resulting from Cys (λ = 487 nm) and other sulfhydrils (λ = 377 nm) stabilize after 9 minutes.
The detection of Cys in diluted (10%) deproteinized human plasma[21] was carried out successfully. A concentration-dependent fluorescence increase at λ = 483 nm (Figure 4a) was observed, with a reaction time similar to that observed in the buffered solution. Moreover, the fluorescence increase at λ = 378 nm can still be observed for Hcy even in the presence of excess of Cys (40 µm) under the above-mentioned conditions (Figure 4b). In addition GSH also exhibits no significant interference (see Figure S22 in the Supporting Information) with the Hcy assay at GSH levels that are found in plasma to be proportional to those of Hcy.[22] These results are additional evidence of the potential utility of 4 in clinical diagnosis.
Figure 4.
a) Fluorescence spectra of 4 (50 µm) and Cys (0–40 µm) in 10% deproteinized human plasma. b) Fluorescence spectra of 4 (50 µm) and Hcy (0–12 µm) in the presence of 40 µm Cys in 10% deproteinized human plasma. The plasma was diluted with EtOH/phosphate buffer (20 mm, pH 7.4; 2:8, v/v) and the reaction monitored at 40 min. λex = 330 nm. This data shows that physiologically relevant sensitivity and limits of detection, even upon sample dilution, can be obtained in detecting Cys and Hcy in blood plasma. Moreover, Hcy can be detected in the presence of excess Cys.
In summary, we have presented an optical method to discriminate Cys and Hcy from other amino acids and thiols at physiological pH. The discrimination of Cys and Hcy is attributed to different rates of intramolecular cyclizations of their respective thioether adducts derived from 4. Spectral and kinetic modes may be used for the simultaneous determination of Cys and Hcy. This is a unique example of a single fluorescent probe that can effectively discriminate between Cys and Hcy. Studies are underway to optimize the sensing method.
Supplementary Material
Footnotes
Support from the National Institutes of Health through award RO1 EB002044 is gratefully acknowledged. X.F.Y. acknowledges financial support from the China Scholarship Council.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201103759.
Contributor Information
Xiaofeng Yang, Email: strongin@pdx.edu, Department of Chemistry, Portland State University 1719 SW 10 Ave, Portland, OR 97201 (USA) Homepage: http://www.pdx.edu/chem/profile/dr-robert-strongin; Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Institute of Analytical Sciences College of Chemistry & Materials Science, Northwest University Xi’an 710069 (P.R. China).
Yixing Guo, Department of Chemistry, Portland State University 1719 SW 10 Ave, Portland, OR 97201 (USA) Homepage: http://www.pdx.edu/chem/profile/dr-robert-strongin.
Robert M. Strongin, Email: xfyang@nwu.edu.cn, Department of Chemistry, Portland State University 1719 SW 10 Ave, Portland, OR 97201 (USA) Homepage: http://www.pdx.edu/chem/profile/dr-robert-strongin.
References
- 1.a) Wood ZA, Schröder E, Harris JR, Poole LB. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]; b) Schulz J, Lindenau J, Seyfried J, Dichgans J. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang XF, Cynader MS. J. Neurosci. 2001;21:3322–3331. doi: 10.1523/JNEUROSCI.21-10-03322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahrokhian S. Anal. Chem. 2001;73:5972–5978. doi: 10.1021/ac010541m. [DOI] [PubMed] [Google Scholar]
- 4.a) Refsum H, Ueland PM, Nygård O, Vollset SE. Annu. Rev. Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]; b) Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PWF, Wolf PA. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]; c) Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM. Clin. Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 5.a) Huo F-J, Sun Y-Q, Su J, Chao J-B, Zhi H-J, Yin C-X. Org. Lett. 2009;11:4918–4921. doi: 10.1021/ol901951h. [DOI] [PubMed] [Google Scholar]; b) Zeng Y, Zhang GX, Zhang DQ. Anal. Chim. Acta. 2008;627:254–257. doi: 10.1016/j.aca.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 6.a) Chen HL, Zhao Q, Wu YB, Li FY, Yang H, Yi T, Huang CH. Inorg. Chem. 2007;46:11075–11081. doi: 10.1021/ic7010887. [DOI] [PubMed] [Google Scholar]; b) Ji SM, Guo HM, Yuan XL, Li XH, Ding HD, Gao P, Zhao CX, Wu WT, Wu WH, Zhao JZ. Org. Lett. 2010;12:2876–2879. doi: 10.1021/ol100999j. [DOI] [PubMed] [Google Scholar]
- 7.a) Chen XQ, Zhou Y, Peng XJ, Yoon J. Chem. Soc. Rev. 2010;39:2120–2135. doi: 10.1039/b925092a. [DOI] [PubMed] [Google Scholar]; b) Yao ZY, Feng XL, Li C, Shi GQ. Chem. Commun. 2009:5886–5888. doi: 10.1039/b912811e. [DOI] [PubMed] [Google Scholar]; c) Lee JH, Lim CS, Tian YS, Han JH, Cho BR. J. Am. Chem. Soc. 2010;132:1216–1217. doi: 10.1021/ja9090676. [DOI] [PubMed] [Google Scholar]; d) Zhu BC, Zhang XL, Li YM, Wang PF, Zhang HY, Zhuang XQ. Chem. Commun. 2010;46:5710–5712. doi: 10.1039/c0cc00477d. [DOI] [PubMed] [Google Scholar]
- 8.a) Kwon H, Lee K, Kim HJ. Chem. Commun. 2011;47:1773–1775. doi: 10.1039/c0cc04092d. [DOI] [PubMed] [Google Scholar]; b) Jung HS, Ko KC, Kim GH, Lee AR, Na YC, Kang C, Lee JY, Kim JS. Org. Lett. 2011;13:1498–1501. doi: 10.1021/ol2001864. [DOI] [PubMed] [Google Scholar]; c) Liu Y, Yu Y, Lam JWY, Hong YN, Faisal M, Yuan WZ, Tang BZ. Chem. Eur. J. 2010;16:8433–8438. doi: 10.1002/chem.200902505. [DOI] [PubMed] [Google Scholar]; d) Jiang W, Fu QQ, Fan HY, Ho J, Wang W. Angew. Chem. 2007;119:8597–8600. Angew. Chem. Int. Ed.2007, 46, 8445–8448; [Google Scholar]; e) Yi L, Li HY, Sun L, Liu LL, Zhang CH, Xi Z. Angew. Chem. 2009;121:4094–4097. Angew. Chem. Int. Ed.2009, 48, 4034–4037; [Google Scholar]; f) Lin WY, Yuan L, Cao ZM, Feng YM, Long LL. Chem. Eur. J. 2009;15:5096–5103. doi: 10.1002/chem.200802751. [DOI] [PubMed] [Google Scholar]; g) Matsumoto T, Urano Y, Shoda T, Kojima H, Nagano T. Org. Lett. 2007;9:3375–3377. doi: 10.1021/ol071352e. [DOI] [PubMed] [Google Scholar]; h) Guo X-F, Wang H, Guo Y-H, Zhang H-S. Anal. Chim. Acta. 2009;633:71–75. doi: 10.1016/j.aca.2008.11.028. [DOI] [PubMed] [Google Scholar]; i) Chen XQ, Ko S-K, Kim MJ, Shin I, Yoon J. Chem. Commun. 2010;46:2751–2753. doi: 10.1039/b925453f. [DOI] [PubMed] [Google Scholar]; j) Sreejith S, Divya KP, Ajayaghosh A. Angew. Chem. 2008;120:8001–8005. doi: 10.1002/anie.200803194. Angew. Chem. Int. Ed.2008, 47, 7883–7887; [DOI] [PubMed] [Google Scholar]; k) Hong V, Kislukhin AA, Finn MG. J. Am. Chem. Soc. 2009;131:9986–9994. doi: 10.1021/ja809345d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Shiu HY, Chong HC, Leung YC, Wong MK, Che CM. Chem. Eur. J. 2010;16:3308–3313. doi: 10.1002/chem.200903121. [DOI] [PubMed] [Google Scholar]; b) Long LL, Lin WY, Chen BB, Gao WS, Yuan L. Chem. Commun. 2011;47:893–895. doi: 10.1039/c0cc03806g. [DOI] [PubMed] [Google Scholar]; c) Shao JY, Guo HM, Ji SM, Zhao JZ. Biosens. Bioelectron. 2011;26:3012–3017. doi: 10.1016/j.bios.2010.12.004. [DOI] [PubMed] [Google Scholar]; d) Li X, Qian SJ, He QJ, Yang B, Li J, Hu YZ. Org. Biomol. Chem. 2010;8:3627–3630. doi: 10.1039/c004344c. [DOI] [PubMed] [Google Scholar]; e) Maeda H, Matsuno H, Ushida M, Katayama K, Saeki K, Itoh N. Angew. Chem. 2005;117:2982–2985. doi: 10.1002/anie.200500114. Angew. Chem. Int. Ed.2005, 44, 2922–2925; Angew. Chem. Int. Ed.2005, 44, 2922–2925; [DOI] [PubMed] [Google Scholar]; f) Wang S-P, Deng W-J, Sun D, Yan M, Zheng H, Xu J-G. Org. Biomol. Chem. 2009;7:4017–4020. doi: 10.1039/b909760k. [DOI] [PubMed] [Google Scholar]; g) Bouffard J, Kim Y, Swager TM, Weissleder R, Hilderbrand SA. Org. Lett. 2008;10:37–40. doi: 10.1021/ol702539v. [DOI] [PubMed] [Google Scholar]; h) Jiang W, Fu QQ, Fan HY, Ho J, Wang W. Angew. Chem. 2007;119:8597–8600. Angew. Chem. Int. Ed.2007, 46, 8445–8448; Angew. Chem. Int. Ed.2007, 46, 8445–8448; [Google Scholar]; i) Shibata A, Furukawa K, Abe H, Tsuneda S, Ito Y. Bioorg. Med. Chem. Lett. 2008;18:2246–2249. doi: 10.1016/j.bmcl.2008.03.014. [DOI] [PubMed] [Google Scholar]; j) Tang B, Yin LL, Wang X, Chen ZZ, Tong LL, Xu KH. Chem. Commun. 2009:5293–5295. doi: 10.1039/b909542j. [DOI] [PubMed] [Google Scholar]; k) Tang B, Xing YL, Li P, Zhang N, Yu FB, Yang GW. J. Am. Chem. Soc. 2007;129:11666–11667. doi: 10.1021/ja072572q. [DOI] [PubMed] [Google Scholar]; l) Yang X-F, Su Z, Liu CH, Qi HP, Zhao ML. Anal. Bioanal. Chem. 2010;396:2667–2674. doi: 10.1007/s00216-010-3475-4. [DOI] [PubMed] [Google Scholar]
- 10. Zhang M, Yu MX, Li FY, Zhu MW, Li MY, Gao YH, Li L, Liu ZQ, Zhang JP, Zhang DQ, Yi T, Huang CH. J. Am. Chem. Soc. 2007;129:10322–10323. doi: 10.1021/ja073140i. Huang S-T, Ting K-N, Wang K-L. Anal. Chim. Acta. 2008;620:120–126. doi: 10.1016/j.aca.2008.05.006. c) Ref. [7c]; Pires MM, Chmielewski J. Org. lett. 2008;10:837–840. doi: 10.1021/ol702769n. Yang Y-K, Shim S, Tae J. Chem. Commun. 2010;46:7766–7768. doi: 10.1039/c0cc02381g.
- 11.a) Rusin O, Luce NNS, Agbaria RA, Escobedo JO, Jiang S, Warner IM, Dawan FB, Lian K, Strongin RM. J. Am. Chem. Soc. 2004;126:438–439. doi: 10.1021/ja036297t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang WH, Rusin O, Xu XY, Kim KK, Escobedo JO, Fakayode SO, Fletcher KA, Lowry M, Schowalter CM, Lawrence CM, Fronczek FR, Warner IM, Strongin RM. J. Am. Chem. Soc. 2005;127:15949–15958. doi: 10.1021/ja054962n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li HL, Fan JL, Wang JY, Tian MZ, Du JJ, Sun SG, Sun PP, Peng XJ. Chem. Commun. 2009:5904–5906. doi: 10.1039/b907511a. Lee K-S, Kim T-K, Lee JH, Kim H-J, Hong J-I. Chem. Commun. 2008:6173–6175. doi: 10.1039/b814581d. Tanaka F, Mase N, Barbas CF., III Chem. Commun. 2004:1762–1763. doi: 10.1039/b405642f. d) Ref. [12a]; Duan LP, Xu YF, Qian XH, Wang F, Liu JW, Cheng TY. Tetrahedron Lett. 2008;49:6624–6627. Kim T-K, Lee D-N, Kim H-J. Tetrahedron Lett. 2008;49:4879–4881. Zhang M, Li MY, Zhao Q, Li FY, Zhang DQ, Zhang JP, Yi T, Huang CH. Tetrahedron Lett. 2007;48:2329–2333. Zhang XJ, Ren XS, Xu Q-H, Loh KP, Chen Z-K. Org. Lett. 2009;11:1257–1260. doi: 10.1021/ol802979n. Lim S, Escobedo JO, Lowry M, Xu XY, Strongin R. Chem. Commun. 2010;46:5707–5709. doi: 10.1039/c0cc01398f.
- 13.a) Blondeau P, Gauthier R, Berse C, Gravel D. Can. J. Chem. 1971;49:3866–3876. [Google Scholar]; b) Leonard NJ, Ning RY. J. Org. Chem. 1966;31:3928–3935. doi: 10.1021/jo01350a011. [DOI] [PubMed] [Google Scholar]
- 14.Khatik GL, Kumar R, Chakraborti AK. Org. Lett. 2006;8:2433–2436. doi: 10.1021/ol060846t. [DOI] [PubMed] [Google Scholar]
- 15.a) Illuminati G, Mandolini L. Acc. Chem. Res. 1981;14:95–102. [Google Scholar]; b) Galli C, Mandolini L. Eur. J. Org. Chem. 2000:3117–3125. [Google Scholar]; c) Galli C, Illuminati G, Mandolini L, Tamborra P. J. Am. Chem. Soc. 1977;99:2591–2597. [Google Scholar]
- 16.a) Lochbrunner S, Wurzer AJ, Riedlea E. J. Chem. Phys. 2000;112:10699–10702. [Google Scholar]; b) Brewer WE, Martinez ML, Chou P-T. J. Phys. Chem. 1990;94:1915–1918. [Google Scholar]
- 17.a) Hu R, Feng J, Hu DH, Wang SQ, Li SY, Li Y, Yang GQ. Angew. Chem. 2010;122:5035–5038. Angew. Chem. Int. Ed.2010, 49, 4915–4918; [Google Scholar]; b) Yang X-F, Qi HP, Wang LP, Su Z, Wang G. Talanta. 2009;80:92–97. doi: 10.1016/j.talanta.2009.06.030. [DOI] [PubMed] [Google Scholar]; c) Kim T-I, Kang HJ, Han G, Chung SJ, Kim Y. Chem. Commun. 2009:5895–5897. doi: 10.1039/b911145j. [DOI] [PubMed] [Google Scholar]
- 18.Total Hcy concentration in healthy human plasma is approximately 5–12 µm. Cys concentration is approximately 20–30 times that of Hcy (See Ref. [4a,b] and [11a]).
- 19.Carmel R, Jacobsen DW. In: Homocysteine in Health and Disease. Vollset SE, Refsum H, Nygård O, Ueland PM, editors. Vol. 28. Cambridge: Cambridge University Press; 2001. pp. 341–355. [Google Scholar]
- 20.Sharma G, Kumar R, Chakraborti AK. Tetrahedron Lett. 2008;49:4269–4271. [Google Scholar]
- 21.The human plasma samples were deproteinized by using the procedure as described in the literature: Ma JG, Stoter G, Verweij J, Schellens JHM. Cancer Chemother. Pharmacol. 1996;38:391–394. doi: 10.1007/s002800050501.
- 22.Beutler E, Gelbart T. J. Lab. Clin. Med. 1985;105:581–584. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.