The intestinotropic hormone GLP-2 increases IGF-I mRNA levels in a dose-, time-, and PI3-K/Akt-dependent manner in murine jejunal subepithelial myofibroblasts.
Abstract
IGF-I, a known secretory product of intestinal subepithelial myofibroblasts (ISEMFs), is essential for the intestinotropic effects of glucagon-like peptide-2 (GLP-2). Furthermore, GLP-2 increases IGF-I mRNA transcript levels in vitro in heterogeneous fetal rat intestinal cultures, as well as in vivo in the rodent small intestine. To determine the mechanism underlying the stimulatory effect of GLP-2 on intestinal IGF-I mRNA, murine ISEMF cells were placed into primary culture. Immunocytochemistry showed that the ISEMF cells appropriately expressed α-smooth muscle actin and vimentin but not desmin. The cells also expressed GLP-2 receptor and IGF-I mRNA transcripts. Treatment of ISEMF cells with (Gly2)GLP-2 induced IGF-I mRNA transcripts by up to 5-fold of basal levels after treatment with 10−8 m GLP-2 for 2 h (P < 0.05) but did not increase transcript levels for other intestinal growth factors, such as ErbB family members. Immunoblot revealed a 1.6-fold increase in phospho (p)-Akt/total-(t)Akt with 10−8 m GLP-2 treatment (P < 0.05) but no changes in cAMP, cAMP-dependent β-galactosidase expression, pcAMP response element-binding protein/tcAMP response element-binding protein, pErk1/2/tErk1/2, or intracellular calcium. Furthermore, pretreatment of ISEMF cells with the phosphatidylinositol 3 kinase (PI3K) inhibitors, LY294002 and wortmannin, abrogated the IGF-I mRNA response to GLP-2, as did overexpression of kinase-dead Akt. The role of PI3K/Akt in GLP-2-induced IGF-I mRNA levels in the murine jejunum was also confirmed in vivo. These findings implicate the PI3K/Akt pathway in the stimulatory effects of GLP-2 to enhance intestinal IGF-I mRNA transcript levels and provide further evidence in support of a role for IGF-I produced by the ISEMF cells in the intestinotropic effects of GLP-2.
Glucagon-like peptide-2 (GLP-2) is a 33-amino acid hormone synthesized by the intestinal L cell and released into the circulation in response to nutrient ingestion (1). In the physiological setting, the major biological action of GLP-2 is to promote adaptive regrowth of the intestine after nutrient deprivation, through enhancement of proliferation and inhibition of apoptosis (2–4). Both GLP-2 and its degradation-resistant analog, (Gly2)GLP-2, also increase intestinal growth, as well as intestinal digestive and absorptive capacity and barrier function, when administered at pharmacological levels to normal rodents (5–11). Importantly, many of these biological actions of GLP-2 have been recapitulated in humans with short bowel syndrome (12), leading to clinical trials of human (h)(Gly2)GLP-2 (teduglutide; Gattex) for the treatment of this condition (13, 14). Findings of protective effects of GLP-2 in rodent models of inflammatory bowel disease (15–17) have similarly led to clinical trials in patients with Crohn's disease (18).
The tropic actions of GLP-2 are restricted to the intestinal mucosa through tissue-specific expression of the G protein-coupled GLP-2 receptor (GLP-2R) (19). However, findings that the GLP-2R is expressed by scattered enteroendocrine cells, enteric neurons, and intestinal subepithelial myofibroblasts (ISEMFs), but not by the crypt cells (20–25), have led to suggestions that the proliferative effects of GLP-2 must be mediated indirectly, through another growth factor (20). Consistent with this hypothesis, we and others have recently demonstrated that the intestinotropic actions of GLP-2 are dependent upon the presence of IGF-I (3, 11). Similarly, we have found that the acute effects of GLP-2 to enhance nuclear translocation of the proliferative marker, β-catenin, in crypt cells requires both IGF-I and the IGF-I receptor (IGF-IR) (26). However, because these studies were conducted using models of global IGF-I/IGF-IR elimination, the question remains as to the specific cell type(s) whereby the IGF-I-dependent intestinal actions of GLP-2 are mediated.
ISEMF cells form a syncytium underlying the epithelial layer and are known to play important roles in the paracrine regulation of intestinal epithelial cell growth and differentiation (27, 28). These actions are mediated, at least in part, through production of a number of growth factors, including IGF-I (27–30). Because GLP-2 treatment of rodents increases intestinal IGF-I mRNA transcript levels (11, 31, 32), these findings implicate the ISEMF cells in the tropic effects of GLP-2. Previous studies on ISEMF cell cultures have demonstrated these cells to be responsive to a number of growth factors, including IGF-I, GH, TNF-α, and epidermal growth factor (EGF), as well as to express mRNA transcripts for IGF-I (29, 30, 33, 34). We have therefore evaluated primary murine ISEMF cells in culture to determine the effects and mechanism of action of GLP-2 on IGF-I mRNA transcript levels in this specific intestinal cell compartment.
Materials and Methods
Jejunal segments (approximately 8 cm of midsmall intestine) were collected from 6- to 8-wk-old male and female C57BL/6 mice (Charles River Canada, St. Constant, Quebec, Canada), as well as from cAMP response element (CRE)-LacZ transgenic animals (35). The Animal Care Committee of the University of Toronto approved all animal protocols. Primary ISEMF and CRE-LacZ-ISEMF cultures were prepared using an established protocol (30, 34). In brief, jejunal segments were cut into 2-cm pieces, washed eight times in high-glucose DMEM with penicillin 50 U/ml and streptomycin 50 μg/ml (P/S) at 24 C, and then incubated in high-glucose DMEM containing P/S, 0.1 mg/ml dispase, and 300 U/ml collagenase I (Worthington Biochemicals, Freehold, NJ) for 25 min at 20 C with gentle rocking at 80 cycles/min. After centrifugation to remove residual tissue fragments, the supernatant was incubated in T75 flasks at 37 C with 5% CO2 [passage (P)1]. The medium was aspirated after 2 d, and high-glucose DMEM containing 5% fetal bovine serum and P/S was added. After 2 wk in culture, cells were collected by trypsinization and replated (P2). The different ISEMF cell lines (Ls) used in the present study are shown in Table 1; L1 was a kind gift from J. G. Simmons (University of North Carolina). With the exception of the calcium studies, all experiments were performed in a minimum of two different Ls at multiple Ps between P5 and P15. BHK cells were grown as previously reported (36).
Table 1.
ISEMF Ls used
| L no. | Mouse strain |
|---|---|
| L1 | SK800 |
| L2 | C57BL/6 |
| L3 | C57BL/6 |
| L4 | C57BL/6 |
| L5 | C57BL/6 |
| L6 | C57BL/6 CRE-LacZ |
| L7 | C57BL/6 CRE-LacZ |
| L8 | C57BL/6 CRE-LacZ |
ISEMF cells were plated onto six-well (for mRNA) or 12-well (for immunoblot) plates until 70–85% confluent, serum starved in low-glucose DMEM containing 0.5% BSA and P/S for 24 h, and then incubated with or without GLP-2 at various concentrations (10−12 to 10−6 m), for different times (20 min to 24 h). The degradation-resistant human analog of GLP-21-33 [h(Gly2)GLP-2] (Peptidec Technologies, Pierrefonds, Quebec, Canada) was used for all experiments; peptide purity has previously been confirmed by mass spectrometry, amino acid sequence analysis, and N-terminal Edman sequencing (2). We have previously reported that the EC50 of this GLP-2 analog for the mouse GLP-2R is identical to that of rat GLP-21-33 for both the rat GLP-2R and hGLP-2R, indicating a high degree of similarity between the biological activity of GLP-2 and h(Gly2)GLP-2 in different species (2). Human (Gly2)GLP-2 has also been used extensively with in vitro murine intestinal models of GLP-2 signaling as well as in vivo in mice to induce both chronic intestinal growth and acute crypt cell signaling responses (2, 4, 10, 11, 26, 37). In some experiments, cells were treated with the phosphatidylinositol 3 kinase (PI3K) inhibitors, wortmannin (500 nm; Sigma-Aldrich, Inc., Oakville, Ontario, Canada) or LY294002 (50 μm; Calbiochem, EMD Chemicals, Inc., Mississauga, Ontario, Canada). Other cells were infected with 109 PFU/ml adenovirus (Adv)-expressing green fluorescent protein (GFP) (control) or kinase-dead Akt (Myc-His-tagged protein kinase B-α-K179M) (38) in serum-free low-glucose DMEM for 2 h and then washed and incubated in high-glucose DMEM with 5% fetal bovine serum and P/S for 2 d before treatment with GLP-2.
Overnight fasted mice were injected ip at t = 0 min with 0.5 μg/g h(Gly2)GLP-2 or PBS (vehicle) and segments of the jejunum collected and flash frozen at t = 90 min. Some mice were pretreated at t = −30 min with wortmannin [1.5 mg/kg in 4% (vol/vol) methanol in saline] or with vehicle alone, as previously reported (26).
Total RNA was extracted from ISEMF, intact jejunum, jejunal mucosal scrapes, and liver using the QIAGEN, Inc. RNeasy kit with the QIAGEN, Inc. RNase-Free DNase kit (QIAGEN, Inc., Mississauga, Ontario, Canada). RT-PCR was conducted using the QIAGEN, Inc. One-Step RT-PCR kit with the following primers (Integrated DNA Technologies, Coralville, IA) and conditions: murine IGF-I, 5′-GCTGAGCTGGTGGATGCTCTTCAGTTC-3′ and 5′-CTTCTGAGTCTTGGGCATGTCAGTGTG-3′ at 65 C for 30 cycles (11); murine GLP-2R, 5′-TCATCTCCCTCTTCTTGGCTCTTAC-3′ and 5′-TCTGACAGATATGACATCCATCCAC-3′ at 60 C for 30 cycles (2); and murine IGF-2, 5′-CACGCTTCAGTTTGTCTGTT-3′ and 5′-CGGGGTCTTTGGGTGGTAAC-3′ at 58 C for 30 cycles (11). Negative (water) controls were run in the absence of template. Amplified products were run on a 1.2% agarose gel and visualized using ethidium bromide. Semi-quantitative (q) real-time RT-PCR was performed by reverse-transcription of total RNA, followed by TaqMan Gene Expression assay (Applied Biosystems, Inc., Foster City, CA) using the following murine primer kits: IGF-I (Mm00439559_m1), GLP-2R (exons 3–4, Mm01329473_m1; and exons 11–12, Mm00558835_m1), IGF-IR (Mm00802837_m1), the ErbB ligands epiregulin (Mm00514794_m1), amphiregulin (Mm00437583_m1) and heparin binding (HB)-EGF (betacellulin; Mm00439307_m1), and the ErbB receptors ErbB1 (Mm01187858_m1) and ErbB2 (Mm00658541_m1); h18S RNA (Hs99999901_sl) was used as the internal control, as previously validated (11). Quantitative RT- PCR primers corresponded to coding sequences within exons 1 and 2 of the mouse IGF-I gene, which amplify isoform I, the major splicing variant expressed in rat nonhepatic tissues (39), as well as isoforms IIA and IIB. Expression of the target gene was calculated relative to 18S rRNA expression using the δ C(t) method (40).
For immunoblot analysis, ISEMF cells were lysed, and total protein was quantified by Bradford assay (Bio-Rad Laboratories Ltd., Mississauga, Ontario, Canada); 50 μg of protein were run on 8% acrylamide gels, transferred onto polyvinylidene fluoride membranes, and immunoblotted using rabbit antisera directed toward phospho-AktSer473 (pAkt), total-Akt (tAkt), phospho-p44/42 MAPKThr202/Tyr204 (pErk1/2), total-p44/42 MAPK antiserum (tErk1/2); pCRE-binding protein (CREB) and tCREB (all at 1:1000; all from Cell Signaling, Danvers, MA), or actin (1:5000; Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada). A horseradish peroxidase-conjugated antirabbit secondary antibody (1:2000; Cell Signaling) was then used, and bands were visualized using Amersham enhanced chemiluminescence Western Detection Reagent (Amersham Pharmacia Biotech, Piscataway, NJ).
For immunocytochemistry, ISEMF cells were plated onto eight-well glass chamber slides, allowed to reach 50–70% confluence, washed with Hank's buffered salt solution, and then fixed with −20 C methanol. Normal mouse jejunum was fixed in formalin for immunohistochemistry, paraffin embedded, sectioned, and rehydrated in a graded ethanol series. Cells and tissues were then washed, blocked with normal serum, as appropriate, and stained with primary antisera/antibodies directed against α-smooth muscle actin (αSMA) (prediluted; αSMA, mouse antihuman; Dako Canada, Inc., Mississauga, Ontario, Canada), vimentin (prediluted; mouse antihuman; Dako Canada, Inc.), desmin (prediluted; mouse antiswine; Dako Canada, Inc.), sucrase-isomaltase (1:200; goat antirat; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), pAkt (1:100, rabbit antimouse; Cell Signaling), or β-catenin (1:100; mouse antimouse; BD Biosciences, Mississauga, Ontario, Canada). Staining was visualized using secondary antisera linked to Alexa Fluor 488 or 555 (1:300; goat antimouse or goat antirabbit; Invitrogen Canada, Inc., Burlington, Ontario, Canada), TX Red (1:300; donkey antimouse; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), or Cy2 (1:300; donkey antigoat; Jackson ImmunoResearch Laboratories, Inc.). Primary antibodies were omitted from the negative controls. Nuclei were visualized using mounting medium with 4–6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlington, Ontario). Digital images were obtained using a Zeiss AxioPlan fluorescence microscope (Carl Zeiss Canada, Don Mills, Ontario, Canada).
For cAMP analyses, ISEMF cells were treated for 20 min with or without GLP-2 in fresh medium containing 100 μm 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, Inc.); 100 μm forskolin (Sigma-Aldrich, Inc.) with 100 μm IBMX was used as a positive control. After treatment, the medium was removed, a chilled solution of 80% ethanol, Hank's buffered salt solution, and 20 μm IBMX was added to each well, and the plates were kept at −80 C overnight. Cells were then scraped into extraction medium containing 65% ethanol, 1 m formic acid, and 100 μm IBMX. Cell debris was collected by centrifugation, and the supernatant was analyzed for cAMP content by RIA (Biomedical Technologies, Inc., Stoughton, MA). cAMP levels were calculated relative to total protein, as determined by the Bradford method (Bio-Rad Laboratories Ltd.).
For cAMP-dependent β-galactosidase analyses, CRE-LacZ-ISEMF cells were plated on four-well chamber slides (VWR, Mississauga, Ontario, Canada) until confluent and then treated for 3 h with or without GLP-2; 10 μm forskolin/10 μm IBMX was used as a positive control. Cells were then fixed in 2% paraformaldehyde/0.2% glutaraldehyde (pH 7.4) for 15 min at room temperature, rinsed with PBS, and incubated overnight at 37 C in the dark with PBS containing 0.02% (vol/vol) IPEGAL, 0.01% (vol/vol) sodium dodecyl sulfate, 2 mm MgCL2, 5 mm K3FeCN, 5 mm K4FeCN, and 2.5% (vol/vol) X-gal in N,N-dimethylformamide. After rinsing with PBS, cells were counterstained with Fast Red, and the number of positively stained cells was counted using a Zeiss AxioPlan fluorescence microscope.
Intracellular calcium imaging was conducted using an established protocol (41). In brief, ISEMFs were loaded with 5 μm fura 2 and then treated with GLP-2; carbachol (100 μm, muscarinic agonist) and ionomycin (1 μm, Ca2+ ionophore) were used as positive controls. For fura 2 imaging, alternating excitation wavelengths (355 ± 5/396 ± 5 nm) were provided at one excitation pair per second in conjunction with a 495-nm dichroic mirror and a 510 ± 20 nm emission filter (Chroma Technology Corp. Rockingham, VT). Fluorescent ratio values (355 ± 5/396 ± 5 nm) were determined for each cell.
All data are presented as mean ± se. The results were analyzed using Student's unpaired t test, one-way ANOVA or two-way ANOVA, as appropriate, followed by appropriate post hoc comparisons. Significance was set at P < 0.05.
Results
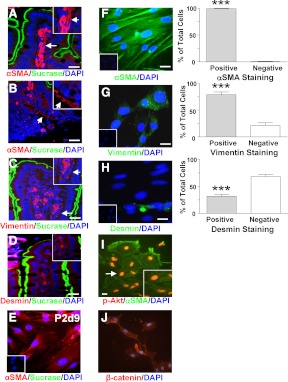
Human and rodent ISEMFs have been reported to express αSMA and vimentin but not desmin, whereas intestinal smooth muscle cells are immunopositive for αSMA and desmin but not for vimentin (30, 34, 42). Consistent with the localization of ISEMF cells to the subepithelial compartment of the lamina propria, immunostaining of murine jejunal sections for αSMA, vimentin, and desmin, with costaining for sucrase to mark the differentiated epithelial cells, demonstrated the presence of subepithelial cells with positive staining for αSMA in the villus compartment as well as in the pericryptal region (Fig. 1, A and B). Positive staining for vimentin, but not for desmin, was also detected in the murine jejunal sections (Fig. 1, C and D). Immediately upon placement of primary murine ISEMF cells in culture (e.g. P1, d 2–7), immunostaining for both αSMA and sucrase was evident (data not shown). However, positive staining for sucrase was no longer detectable in cells from P2, indicative of loss of the differentiated epithelial cells with time in culture (Fig. 1E). Furthermore, once the ISEMF cultures were established (e.g. P5–P15), 99 ± 1 and 79 ± 5% of the cells were immunopositive for αSMA and vimentin, respectively (P < 0.001), with only 32 ± 4% of the cells found to express desmin (Fig. 1, F–H). Collectively, these findings indicate that approximately 70% of the cells in the cultures were representative of the ISEMF phenotype.
Fig. 1.
Murine ISEMF cells show enrichment of cells with a SEMF phenotype. A–D, Representative double-immunohistochemical staining of mouse jejunum for αSMA (A and B), vimentin (C), or desmin (D) (red) with sucrase-isomaltase (green). Nuclei were identified by DAPI staining (blue). Arrows indicate potential ISEMF cells in the main panels and the magnified insets. Photomicrographs were taken with identical microscope settings. Scale bar, 20 μm. E, Double-immunocytochemical staining of ISEMF cells on d 9 of P2 for αSMA (red) and sucrase-isomaltase (green). Nuclei were identified by DAPI staining (blue). Inset shows the negative control. Scale bar, 20 μm. F–H, Representative immunocytochemical pictures of ISEMF stained for αSMA (F), vimentin (G), or desmin (H) (green). Nuclei were identified by DAPI staining (blue). Insets show negative controls. Scale bar, 20 μm. The percentage of cells that were positively and negatively stained for each marker was determined from more than 100 cells from L1 and L2, at P5, P7, and P11, counted to make each n = 1, for a total of n = 3–4; ***, P < 0.001 vs. negative cells. I, Representative immunocytochemical picture of ISEMF cells stained for αSMA (green) and pAkt (red). Nuclei were identified by DAPI staining (blue) (data not shown). Inset shows magnification of cell indicated by arrow. Greater than 100 cells from L5, at P10, P13, and P15, was counted to make n = 1, for a total of n = 4. J, Representative immunocytochemical staining of ISEMF cells for β-catenin (red). Nuclei were identified by DAPI staining (blue).
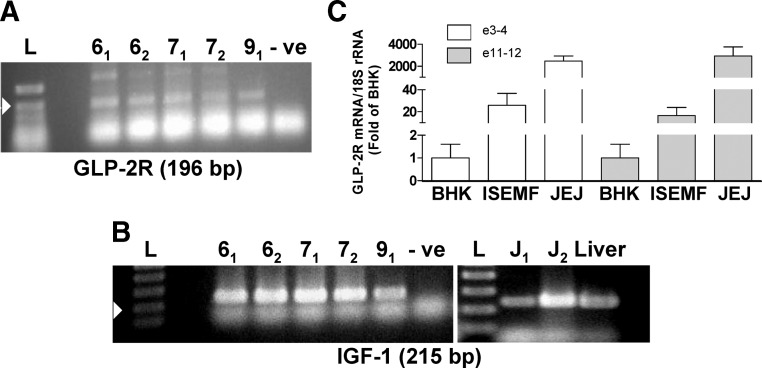
To determine whether ISEMF cells express the machinery necessary for an IGF-I response to GLP-2, cells were examined for the presence of GLP-2R and IGF-I mRNA transcripts (Fig. 2). RT-PCR demonstrated the presence of both transcripts in multiple Ps of the cultures. IGF-2 mRNA transcripts were also detected in all Ps (data not shown). Further analysis of the GLP-2R transcripts by qRT-PCR using primers directed against exons 3–4 and exons 11–12 demonstrated a 26 ± 11- and 17 ± 7-fold enrichment (P < 0.05), respectively, compared with BHK cells that are not known to express the GLP-2R (and which, therefore, represent background levels of amplification). In contrast, the levels of GLP-2R mRNA in the ISEMF cells were found to be approximately two orders of magnitude lower than those of whole mouse jejunum.
Fig. 2.
Murine ISEMF cells express GLP-2R and IGF-I mRNA transcripts. A and B, RT-PCR for GLP-2R (A) and IGF-I (B) mRNA transcripts in ISEMF cells from L2 and L3, at P6, P7, and P9. The subscript indicates two independent samples. The arrowhead indicates the 200-bp marker on the molecular weight ladder (L); -ve indicates the water control. Mouse jejunum (J) and liver were used as positive controls for the IGF-I mRNA transcript. C, GLP-2R mRNA transcript levels were determined by real-time RT-PCR in BHK cells (negative control), ISEMF cells, and normal mouse jejunum (positive control). Expression was normalized to 18S rRNA levels (n = 4–6; L5 and L6, at P5–P7 and P9).
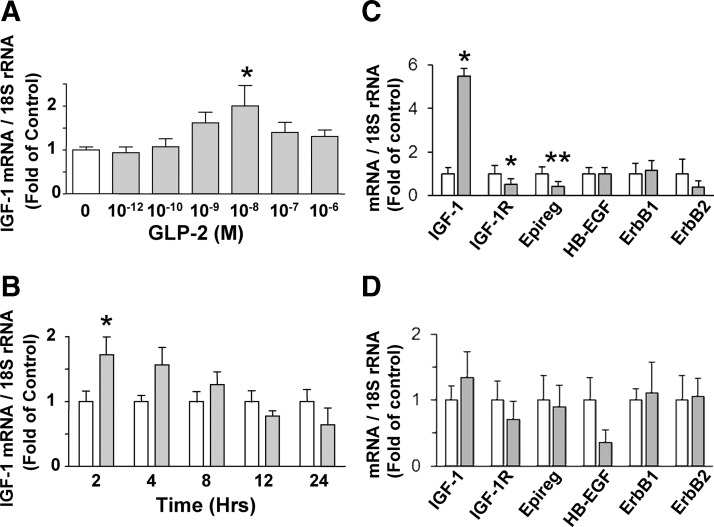
To establish whether GLP-2 modulates IGF-I mRNA transcript levels in ISEMF cells, cultures were subjected to both a dose- and time-dependent analysis. Treatment of the cells for 2 h with 10−12 to 10−6 m GLP-2 induced a maximal increase to 2.0 ± 0.5-fold of basal IGF-I mRNA transcript levels at 10−8 m GLP-2 (P < 0.05), with a decline in the response observed at higher doses (Fig. 3A). A similar response was observed when cells were treated with 10−8 m GLP-2 for 2–24 h, with the maximal effect observed at 2 h (P < 0.05) (Fig. 3B). Because GLP-2 has been reported by others to induce rapid changes in ErbB ligand and receptor transcript levels (4, 10), ISEMF cells were also examined for GLP-2-induced changes in the ErbB ligands, epiregulin, HB-EGF, and amphiregulin, as well as for the ErbB1 and ErbB2 receptors at the 2-h time point (Fig. 3C). IGF-IR receptor transcript levels were concomitantly examined. Interestingly, although GLP-2 increased IGF-I transcript levels to 5.5 ± 0.3-fold of basal values (P < 0.05), decreases were observed in the transcript levels for both IGF-IR (to 0.5 ± 0.3-fold; P < 0.05) and epiregulin (to 0.4 ± 0.2-fold; P < 0.01); no changes in the levels of any of the other transcripts could be detected. Furthermore, no significant changes in any of the transcript levels were detectable at the 30-min time point (Fig. 3D). Amphiregulin transcripts were not detectable in the ISEMF cells (data not shown).
Fig. 3.
GLP-2 treatment of murine ISEMF cells increases IGF-I mRNA transcript levels. ISEMF cells were treated with media alone (control; open bars) or with h(Gly2)GLP-2 (closed bars) at (A) 10−12 to 10−6 m for 2 h (n = 6–18; L2–L5, at P5, P7, P8, P11, P12, and P15), (B) 10−8 m for 2–24 h (n = 4–11; L1–L5, at P5, P7, P7, P11, P12, and P15), (C) 10−8 m for 2 h (n = 5; L2 and L5, at P6, P8, and P9), and (D) 10−8 m for 30 min (n = 6–8; L2 and L3, at P7–P8). Total RNA was analyzed by qRT-PCR for the indicated transcripts, and data are expressed relative to 18S rRNA levels; *, P < 0.05; **, P < 0.01 vs. control.
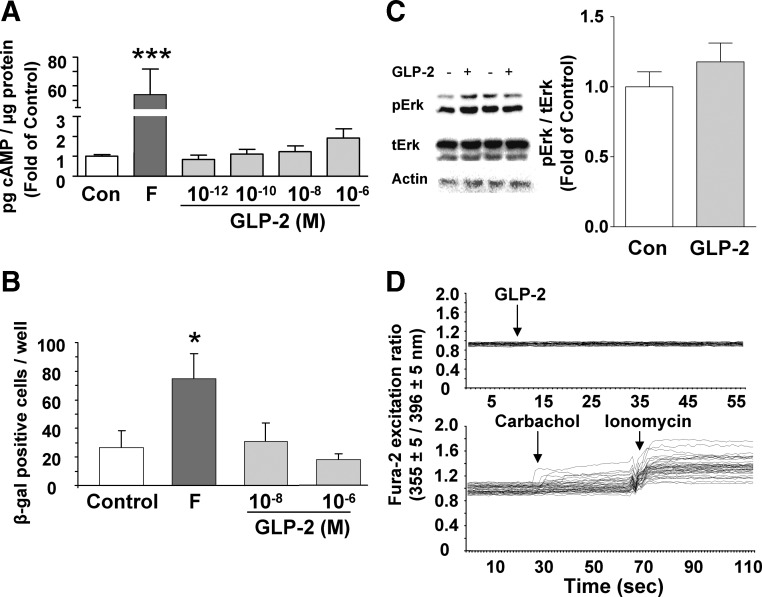
ISEMF cells were next examined for changes in cAMP, Erk1/2, and calcium to determine the signaling pathways activated by GLP-2. Although forskolin treatment increased cAMP levels to 54 ± 18-fold of control (P < 0.001), concentrations of GLP-2 ranging from 10−12 to 10−6 m had no effect (Fig. 4A). Similarly, forskolin significantly increased the number of cells expressing β-galactosidase in the CRE-LacZ-ISEMF cells (P < 0.05), but no effects of either 10−8 or 10−6 m GLP-2 treatment were detectable (Fig. 4B). Finally, 10−8 m GLP-2 had no effect on pErk/tErk (Fig. 4C) or on intracellular calcium at concentrations ranging from 10−12 to 10−6 m, although both carbachol and ionomycin did increase intracellular calcium levels (Fig. 4D).
Fig. 4.
GLP-2 does not stimulate cAMP, Erk1/2 phosphorylation, or intracellular calcium in murine ISEMF cells. A, ISEMF cells were treated with media alone [control (Con)], forskolin (F) (100 μm) or h(Gly2)GLP-2 (10−12 to 10−6 m) for 20 min in the presence of IBMX (100 μm), and cell lysates were collected for determination of cAMP by RIA. ***, P < 0.001 vs. control (n = 12–16; L1–L5, at P8, P9, and P11). B, ISEMF cells from CRE-LacZ transgenic animals were treated with media alone (control), forskolin plus IBMX (10 μm each), or h(Gly2)GLP-2 (10−8 and 10−6 m with 10 μm IBMX) for 3 h and then stained for β-galactosidase expression. *, P < 0.05 vs. control (n = 6; L6–L8, at P7 and P9). C, ISEMF cells were treated with 10−8 m GLP-2 for 30 min, and cell lysates were analyzed by Western blotting for the levels of pErk1/2 (pErk) and tErk1/2 (tErk); actin was used as the loading control. A representative blot is shown for two different experiments; graph shows results from densitometry analysis. *, P < 0.05 (n = 6; L2–L6, at P10–P15). D, ISEMF cells were loaded with fura 2 and then treated with 10−10 to 10−6 m h(Gly2)GLP-2. Positive controls included carbachol (100 μm; muscarinic agonist) and ionomycin (1 μm; Ca2+ ionophore). Fluorescent ratio values (355 ± 5/396 ± 5 nm) = (bound Ca2+/free Ca2+) were determined for each cell (n = 98–114 cells; L1, at P5). Arrows indicate addition of treatment; representative experiments are shown.
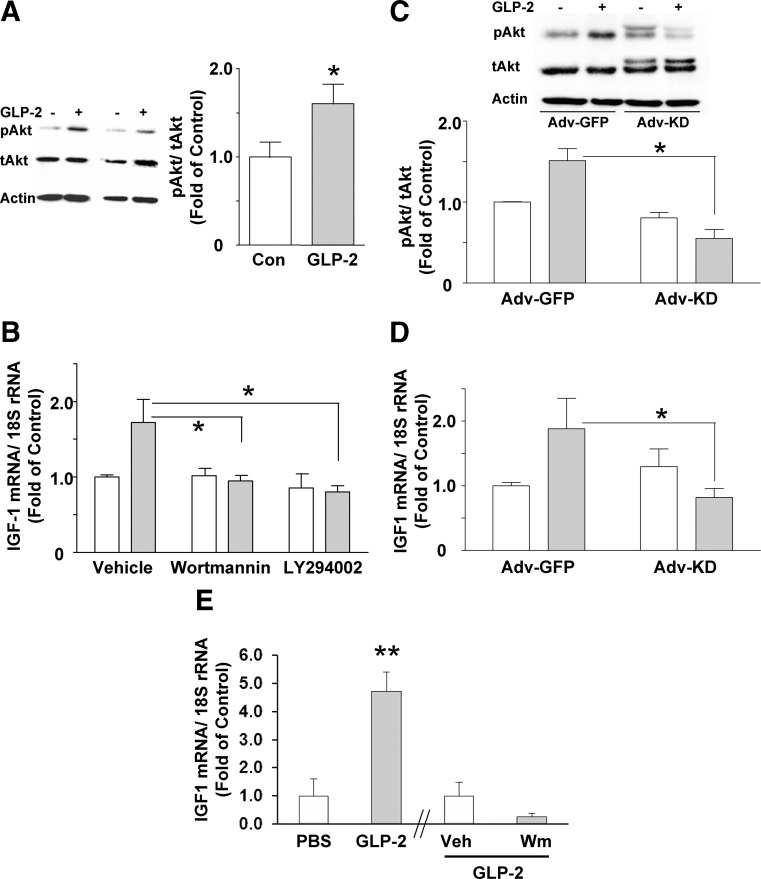
In marked contrast to the findings with cAMP, Erk1/2, and calcium, treatment of the ISEMF cells with 10−8 m GLP-2 increased pAkt/tAkt levels, to 1.6 ± 0.2-fold of control (P < 0.05) (Fig. 5A). To determine whether this pathway was required for the induction of IGF-I mRNA transcripts by GLP-2, cells were coincubated with GLP-2 and either wortmannin or LY294002 to inhibit PI3K. Both inhibitors were found to prevent the stimulatory effects of GLP-2 on IGF-I mRNA transcript levels (Fig. 5B). To confirm these finding using an alternative approach, ISEMF cells were also infected with Adv constructs expressing either GFP (control) or Myc-His-tagged kinase-dead Akt. Immunoblot of the infected cells demonstrated the presence of the kinase-dead Akt, present as a higher molecular weight band (Fig. 5C). Expression of this construct not only prevented the stimulatory effect of GLP-2 on Akt phosphorylation in these cells but also abrogated the GLP-2-induced rise in IGF-I mRNA transcript levels (Fig. 5, C and D).
Fig. 5.
GLP-2 stimulates IGF-I mRNA transcript levels in murine ISEMF cells through a PI3K- and Akt-dependent mechanism. A, ISEMF cells were treated with 10−8 m h(Gly2)GLP-2 for 30 min, and cell lysates were analyzed by Western blotting for the levels of pAkt and tAkt; actin was used as the loading control. A representative blot is shown for two different experiments; graph shows results from densitometry analysis. *, P < 0.05 (n = 6; L2 and L3, at P10–P15). B, ISEMF cells were treated with 10−8 m h(Gly2)GLP-2 for 2 h in the absence (open bars) or presence (closed bars) of either vehicle alone, wortmannin (500 nm), or LY294002 (50 μm). Total RNA was analyzed by qRT-PCR for IGF-I mRNA transcript levels, and data are expressed relative to 18S rRNA levels. *, P < 0.05 vs. GLP-2 in the absence of inhibitor (n = 4–12; L2–L5, at P10–P15). C, ISEMF cells were infected with either Adv-GFP (control) or Adv-kinase-dead-Akt (Adv-KD) at 109 PFU/ml for 2 h. Two days later, cells were treated with 10−8 m GLP-2 for 30 min, and cell lysates were analyzed by Western blotting for the levels of pAkt and tAkt; actin was used as the loading control; a representative blot is shown, with the graph showing results from densitometry analysis. *, P < 0.05 vs. GLP-2 with Adv-GFP (n = 4; L2–L4, at P10–P15). D, Total RNA from Adv-GFP- and Adv-KD-infected ISEMF cells was analyzed by qRT-PCR for IGF-I mRNA transcript levels. *, P < 0.05 vs. GLP-2 with Adv-GFP (n = 4–7; L2–L4, at P10–P15). E, Mice were fasted overnight and then injected with vehicle (PBS) or 0.5 μg/g h(Gly2)GLP-2, and jejunal samples were collected at t = 90 min for analysis of IGF-I mRNA transcript levels in mucosal scrapes by qRT-PCR. In a separate study, animals were pretreated with wortmannin (Wm) [1.5 mg/kg in 4% (vol/vol) methanol in saline] or with vehicle (Veh) alone 30 min before administration of GLP-2 and analysis, as above. **, P < 0.01 (PBS vs. GLP-2, n = 5–6; Veh vs. Wm, n = 8–9).
The PI3K dependence of the effects of GLP-2 on intestinal IGF-I mRNA levels was also confirmed in mice in vivo. Compared with vehicle-injected mice, animals treated with GLP-2 demonstrated an increase in IGF-I mRNA transcript levels in jejunal mucosal scrapes, to 4.7-fold of vehicle controls (P < 0.01) at t = 90 min (Fig. 5E). We have previously reported that GLP-2 administration in vivo increases pAkt levels in the jejunum in a dose-dependent fashion and that pretreatment with wortmannin abrogates this response (26). Therefore, in a separate experiment, mice were given GLP-2 with and without wortmannin pretreatment Compared with vehicle pretreated animals, wortmannin reduced the IGF-I mRNA response to 0.25-fold of GLP-2-treated controls.
Finally, to investigate the potential downstream mechanisms by which GLP-2 enhances IGF-I mRNA transcript levels, ISEMF cells were examined for changes in the subcellular localization of both pAkt and β-catenin. Interestingly, all of the pAkt was found to be localized in the nuclear compartment under basal conditions (Fig. 1I), with no change in distribution observed during the 2-h treatment with GLP-2 (data not shown). Conversely, preliminary studies demonstrated that β-catenin was predominantly localized in the cell membrane and cytoplasm (Fig. 1J), but again, no change in distribution was found with up to 2 h of GLP-2 treatment (data not shown). Finally, pCREB levels were undetectable in the ISEMF cells and did not demonstrate any increase in response to a 15- to 120-min treatment with GLP-2 (data not shown).
Discussion
The exact mechanism of action of GLP-2 to stimulate intestinal growth has proven enigmatic, because the GLP-2R is not expressed by the proliferative crypt cells that respond to GLP-2 treatment in vivo. The specific cell type involved in the tropic response to GLP-2 could include enteroendocrine cells, enteric neurons, and/or ISEMF cells, based on the known distribution of the GLP-2R (20–25). However, findings of increased intestinal IGF-I mRNA transcripts in response to GLP-2 treatment (11, 31, 32), as well as of an essential role for IGF-I and the IGF-IR in the crypt cell responses to GLP-2 (11, 26), have implicated the IGF-I-expressing ISEMF cells in the intestinotropic actions of GLP-2 (1). The results of the present study confirm that primary murine ISEMF cells are responsive to GLP-2, which acts through a PI3K/Akt-dependent pathway to increase IGF-I mRNA transcript levels.
Previous studies have demonstrated expression of the GLP-2R in rat and human ISEMF cells in situ (22, 43). Consistent with these findings, GLP-2R mRNA transcripts were found to be expressed by cells with the key characteristics of ISEMF, namely expression of αSMA and vimentin, and lack of expression of desmin (30, 34, 42). The Ls also did not express the enterocyte marker, sucrase, consistent with a lack of differentiated epithelial cells in the cultures. Furthermore, previous studies have demonstrated that these Ls do exhibit typical ISEMF functions, such as production of collagen I and of tissue inhibitor of metalloproteinase-1 (27, 28, 30, 34). Because ISEMFs, but not myofibroblasts, have also been reported to express the GLP-2R (22, 23), these findings suggest that it is the ISEMF cells in these cultures that are responsive to GLP-2. However, although the GLP-2R transcript levels were clearly greater than those of background, as determined by comparison with BHK cells, the levels were markedly lower than found in whole mouse jejunum, the tissue source of the ISEMF used in the present study. Given the relative enrichment of ISEMF cells in the cultures (e.g. ∼70% of the total cells), these findings were somewhat surprising. Nonetheless, previous studies on human ISEMF cultures have similarly noted that IGF-I mRNA transcript levels are also low compared with in vivo expression (29). The reasons for this are not clear but may relate to the placement of the cells in monolayer culture without any neighboring cell populations for support and/or the requirement for multiple Ps of the cells before use for experimental purposes. Regardless, the finding of GLP-2R expression in these cells, as well as of functional responses to GLP-2, clearly demonstrates the utility of this model for our investigations.
IGF-I mRNA transcript levels in the ISEMF cells were modulated by h(Gly2)GLP-2 in a dose-dependent manner, with the greatest stimulation observed at 10−8 m. Although the peak magnitude of the IGF-I response to 10−8 m GLP-2 differed somewhat between studies (i.e. between a 2- and 5.5-fold increase) (Fig. 3, A–C), different combinations of mouse strains, Ls, and P numbers were used in each study. Nonetheless, the findings were consistent in that, regardless of the L, the ISEMF cells responded to treatment with GLP-2 with a significant increase in IGF-I mRNA transcript levels. Furthermore, the jejunal mucosal IGF-I mRNA response to GLP-2 in vivo was found to be similar in magnitude (4.7-fold). Similar changes in small intestinal IGF-I mRNA transcript levels have also been reported for the parenterally fed rat treated with GLP-2 for 7 d (31). Hence, collectively, these studies indicate that IGF-I mRNA transcripts represent a novel target of GLP-2 action in murine jejunum.
Interestingly, a progressive but complete loss of effectiveness of GLP-2 was observed at concentrations greater than 10−8 m. The lack of response to high doses of GLP-2 is consistent with observations made in other primary intestinal models expressing the endogenous GLP-2R, such as rat mucosal cells, isolated mouse small intestine muscle strips, and fetal rat intestinal cells, which demonstrated maximal responses to h(Gly2)GLP-2 at 10−11, 10−10, and 10−9 m GLP-2, respectively, with marked loss of the response at higher concentrations (2, 11, 37). Furthermore, a similar loss of effectiveness of GLP-2 was observed at higher doses in mice in vivo with respect to the stimulation of jejunal Akt phosphorylation (26). However, these findings are in contrast to the dose-response curves typically seen in cell systems in which GLP-2R is overexpressed, in which maximal responses reach a plateau or are only slightly diminished at high concentrations of the ligand (e.g. 10−6 m) (2, 44–46). The loss of GLP-2R responsiveness at higher concentrations of GLP-2 in all of the primary cell models is strongly suggestive of desensitization of the endogenous GLP-2R. Consistent with this theory, preincubation of rat mucosal cells with 10−9 m h(Gly2)GLP-2 prevents the subsequent response to the same concentration of h(Gly2)GLP-2 (37). Such homologous desensitization has been demonstrated to involve unique signaling pathways that are lipid raft dependent but clathrin-, dynamin-, and β-arrestin independent, at least in heterologous cells transfected with the GLP-2R (46, 47).
The time course of the IGF-I transcript response to 10−8 m GLP-2 demonstrated a maximal increase at t = 2 h. Similar rapid induction of IGF-I mRNA has also been reported for the actions of GH on C2C12 myocytes and of insulin on hepatocytes, such that IGF-I mRNA transcript levels were increased within 1–2 h of treatment (48, 49). In contrast, IGF-I mRNA levels were not found to be increased in murine jejunum at either 30 min or 4–12 h after administration of GLP-2 (10). Notwithstanding, we have previously reported that the jejunal pAkt response to GLP-2 in vivo is rapid, with a peak at 30 min and complete loss of this response by 4 h (26), which is consistent with the time frame observed in the present study for the induction of IGF-I mRNA transcript levels by GLP-2 in the ISEMF cells.
Unexpectedly, despite the stimulatory effect on GLP-2 on IGF-I mRNA, transcript levels for the IGF-IR and for members of the ErbB signaling system, including epiregulin, HB-EGF, and both ErbB1 and ErbB2, were unaffected or even decreased by GLP-2 treatment. Recent studies have implicated this family of growth factors in the tropic responses to GLP-2 in the intestine, such that GLP-2 treatment of mice in vivo increases jejunal mRNA transcript levels of the ErbB ligands, epiregulin, HB-EGF, and amphiregulin within 30 min to 1 h (4, 10). However, these analyses were conducted using whole-thickness intestinal segments, which precluded cell-specific localization of the responses. Epiregulin has been localized to intestinal epithelial cells, as well as to scattered cells in the lamina propria (50), whereas HB-EGF has only been found in the epithelial layer (51), and amphiregulin mRNA transcripts were not even detectable in the ISEMF cultures. The results of the present studies therefore suggest that the GLP-2-induced increases in ErbB family member transcript levels in vivo (4, 10) may not be localized to the ISEMF cell population.
Given previous reports of GLP-2R coupling to cAMP-dependent signaling (2, 11, 19, 37, 52), it was surprising that no effects of GLP-2 on either cAMP or cAMP-dependent β-galactosidase gene expression could be detected in the ISEMF cells. However, previous studies on HeLa cervical cancer cells have similarly demonstrated a lack of induction of cAMP levels by the endogenously expressed GLP-2R, although overexpression of the receptor in these cells was coupled to GLP-2-dependent increases in cAMP (45). GLP-2 was also found to increase Erk1/2 phosphorylation in HeLa cells transfected with the GLP-2R (45). Although, again, no such effect was found with the endogenous GLP-2R in either the ISEMF or primary rat intestinal mucosal cells (37). It currently remains unclear as to whether these differences are consequent to the relatively low levels of endogenous expression of the GLP-2R found for the ISEMF, HeLa, and primary mucosal cells or whether differences exist in the signaling pathway(s) used by the GLP-2R in different cell types.
In contrast to the differences observed between cell models for cAMP and Erk1/2 signaling, a consistent lack of effect on intracellular calcium levels has been found for the endogenous receptor in ISEMF cells and in both primary rat mucosal cells and BHK cells expressing the GLP-2R (37, 52). Taken together, these findings suggest that calcium signaling may not be a major pathway used by the GLP-2R.
GLP-2 has been demonstrated to activate intestinal Akt signaling in the intestinal epithelium in vivo, although this is presumed to be mediated through a paracrine factor, such as IGF-I or Erb1/ErbB2 ligands (4, 26, 53). Consistent with this possibility, GLP-2 has not been previously reported to activate PI3K/Akt signaling in BHK cells overexpressing the GLP-2R (52, 54, 55). Nonetheless, significant effects of GLP-2 on Akt phosphorylation were detected in the ISEMF cells in the current study. Furthermore, inhibition of either PI3K or Akt signaling completely prevented the stimulatory effects of GLP-2 on IGF-I mRNA transcript levels, both in vitro and in vivo, indicating that the activation of Akt was associated with functional downstream effects on IGF-I expression. Although these findings clearly implicate Akt in the mechanism of action of GLP-2 to increase IGF-I mRNA transcript levels, the exact mechanisms underlying induction of IGF-I gene expression currently remain unclear. For example, cAMP/protein kinase A signaling has been demonstrated to regulate IGF-I gene transcription in fetal rat bone cell cultures, through a noncanonical CRE within the 5′-untranslated region of exon 1 (56). However, although CREB is a known downstream target of Akt (57), pCREB levels were not elevated in response to GLP-2, suggesting that this transcription factor does not play a role in GLP-2-induced IGF-I mRNA in ISEMF cells. Furthermore, although GLP-2 has been found to increase nuclear translocation of the cWnt transcription factor, β-catenin, in crypt epithelial cells (26), no such effect was observed in the ISEMF cells. However, other transcription factors, such as CCAAT/enhancer binding protein α, liver-enriched activating protein, and hepatocyte nuclear factor 1α, have been shown to enhance IGF-I promoter 1 activity, at least in human hepatoma Hep3b cells (58, 59). Whether these or other factors play a role in the stimulation of IGF-I mRNA transcript levels by GLP-2 currently remains unclear. Finally, changes in IGF-I mRNA levels might also be modulated by alterations in transcript stability (60). Furthermore, a microRNA binding site has been detected in the 3′-untranslated region of the IGF-I gene (61). Notwithstanding, the finding that pAkt is localized predominantly within the nucleus of the ISEMF cells lends support to the notion that Akt is a primary regulator of IGF-I gene expression. Indeed, activated Akt has been demonstrated in the nucleus of a number of different cell types (62, 63), where it has been shown to inhibit forkhead box subfamily O3a-induced transcription (62), although how such an effect might increase IGF-I mRNA transcript levels remains unclear.
The primary intestinal cell models tested to date (2, 11, 37), including the ISEMF cells used in the present study, have largely demonstrated sensitivity to GLP-2 at concentrations that are higher than circulating levels of endogenous GLP-2, which are approximately 1–3 × 10−11 m in normal humans (64). Nonetheless, levels of exogenously administered h(Gly2)GLP-2 in humans have been reported to be in the range of 0.3–1.7 × 10−9 m (65). These findings are consistent with the dose effectiveness of this peptide observed in vitro and suggest that findings made in primary cell models are clinically relevant.
Finally, although preliminary analyses were conducted to determine the effect of GLP-2 on IGF-I secretion by the ISEMF cells, any interpretation of the findings was precluded due to extremely high levels of constitutive, basal release of IGF-I in this model (e.g. 96 ± 1% of the total cell content over a 2-h period) (data not shown). In previous studies of IGF-I release using heterogeneous fetal rat intestinal cells in culture, GLP-2 was found to stimulate IGF-I release, although again, basal secretion was extremely high (e.g. 80 ± 1% of the total cell content over a 2-h period), and the stimulatory effect of GLP-2 was correspondingly limited (11). Because IGF-I release is so rapid and can be complicated by the presence of IGF binding proteins (66), future studies to examine this possibility will be required. Notwithstanding, because of such difficulties in accurately determining the levels of free, bioactive IGF-I, it has been previously reported that IGF-I mRNA levels can be used as a reliable indicator of changes in IGF-I production (67).
In summary, the results of the present study demonstrate that murine jejunal ISEMF cells are a direct target of GLP-2 action, increasing the levels of IGF-I mRNA transcripts in a dose-, time-, and PI3K/Akt-dependent fashion. These studies therefore implicate IGF-I produced by the ISEMF cells in the intestinotropic actions of GLP-2.
Acknowledgments
We thank Dr. J. G. Simmons (University of North Carolina) for the gift of the SK800 ISEMF cells.
This work was supported by a Margaret J. Santolo fellowship from the Department of Physiology, University of Toronto. K.J.R. and P.E.D. were supported by graduate studentships from the Canadian Institutes of Health Research/Digestive Diseases Foundation and P.L.B. by the Canada Research Chairs Program. This work was also supported by the Canadian Institutes of Health Research Operating Grant MOP-9940 and by an equipment grant from the Banting and Best Diabetes Centre, University of Toronto.
Disclosure Summary: J.L.S.L., A.I., C.U., K.J.R., P.E.D., S.G., S.P.H., C.J.R., D.R.S., and P.K.L. have nothing to disclose. P.LB. has received a consulting fee from Glaxo-Smith-Kline.
Footnotes
- Adv
- Adenovirus
- CRE
- cAMP response element
- CREB
- CRE-binding protein
- DAPI
- 4–6-diamidino-2-phenylindole
- EGF
- epidermal growth factor
- GFP
- green fluorescent protein
- GLP-2
- glucagon-like peptide-2
- GLP-2R
- GLP-2 receptor
- HB
- heparin binding
- h(Gly2)GLP-2
- human (Gly2)GLP-2
- IBMX
- 3-isobutyl-1-methylxanthine
- IGF-IR
- IGF-I receptor
- ISEMF
- intestinal subepithelial myofibroblast
- L
- cell line
- P
- passage
- pAkt
- phospho-AktSer473
- PI3K
- phosphatidylinositol 3 kinase
- P/S
- penicillin 50 U/ml and streptomycin 50 μg/ml
- q
- quantitative
- αSMA
- α-smooth muscle actin
- tAkt
- total-Akt.
References
- 1. Dubé PE, Brubaker PL. 2007. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 293:E460–E465 [DOI] [PubMed] [Google Scholar]
- 2. Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. 2005. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology 128:1340–1353 [DOI] [PubMed] [Google Scholar]
- 3. Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. 2008. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol 294:R1175–R1184 [DOI] [PubMed] [Google Scholar]
- 4. Bahrami J, Yusta B, Drucker DJ. 2010. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138:2447–2456 [DOI] [PubMed] [Google Scholar]
- 5. Drucker DJ, Erlich P, Asa SL, Brubaker PL. 1996. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 93:7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brubaker PL, Izzo A, Hill M, Drucker DJ. 1997. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol Endocrinol Metab 272:E1050–E1058 [DOI] [PubMed] [Google Scholar]
- 7. Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. 2000. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 47:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scott RB, Kirk D, MacNaughton WK, Meddings JB. 1998. GLP-2 augments the adaptive response to massive intestinal resection in rats. Am J Physiol Gastrointest Liver Physiol 275:G911–G921 [DOI] [PubMed] [Google Scholar]
- 9. Koopmann MC, Nelson DW, Murali SG, Liu X, Brownfield MS, Holst JJ, Ney DM. 2008. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. J Parenter Enteral Nutr 32:254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. 2009. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 137:986–996 [DOI] [PubMed] [Google Scholar]
- 11. Dubé PE, Forse CL, Bahrami J, Brubaker PL. 2006. Essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131:589–605 [DOI] [PubMed] [Google Scholar]
- 12. Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, Gregory J, Tappenden KA, Holst J, Mortensen PB. 2005. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 54:1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeppesen PB, Lund P, Gottschalck IB, Nielsen HB, Holst JJ, Mortensen J, Poulsen SS, Quistorff B, Mortensen PB. 2009. Short bowel patients treated for two years with glucagon-like peptide 2 (GLP-2): compliance, safety, and effects on quality of life. Gastroenterol Res Pract 2009:425759:1–425759:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeppesen PB, Lund P, Gottschalck IB, Nielsen HB, Holst JJ, Mortensen J, Poulsen SS, Quistorff B, Mortensen PB. 2009. Short bowel patients treated for two years with glucagon-like peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol Res Pract 2009:616054:1–616054:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boushey RP, Yusta B, Drucker DJ. 1999. Glucagon-like peptide 2 decreases mortality and reduces the severity of indomethacin-induced murine enteritis. Am J Physiol Endocrinol Metab 277:E937–E947 [DOI] [PubMed] [Google Scholar]
- 16. L'Heureux M-C, Brubaker PL. 2003. Glucagon-like peptide-2 and common therapeutics in a murine model of ulcerative colitis. J Pharm Exp Ther 306:347–354 [DOI] [PubMed] [Google Scholar]
- 17. Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. 2007. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293:G211–G221 [DOI] [PubMed] [Google Scholar]
- 18. Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG. 2010. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn's disease. Inflamm Bowel Dis 16:962–973 [DOI] [PubMed] [Google Scholar]
- 19. Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. 1999. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96:1569–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. 2000. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119:744–755 [DOI] [PubMed] [Google Scholar]
- 21. Bjerknes M, Cheng H. 2001. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA 98:12497–12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramsanahie A, Duxbury MS, Grikscheit TC, Perez A, Rhoads DB, Gardner-Thorpe J, Ogilvie J, Ashley SW, Vacanti JP, Whang EE. 2003. Effect of GLP-2 on mucosal morphology and SGLT1 expression in tissue-engineered neointestine. Am J Physiol Gastrointest Liver Physiol 285:G1345–G1352 [DOI] [PubMed] [Google Scholar]
- 23. Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. 2005. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124:105–112 [DOI] [PubMed] [Google Scholar]
- 24. Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell DL, Nichols BL, Burrin DG. 2006. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130:150–164 [DOI] [PubMed] [Google Scholar]
- 25. Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. 2007. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 148:1954–1962 [DOI] [PubMed] [Google Scholar]
- 26. Dubé PE, Rowland KJ, Brubaker PL. 2008. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149:291–301 [DOI] [PubMed] [Google Scholar]
- 27. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. 1999. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277:C1–C9 [DOI] [PubMed] [Google Scholar]
- 28. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. 1999. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 277:C183–C201 [DOI] [PubMed] [Google Scholar]
- 29. Simmons JG, Pucilowska JB, Lund PK. 1999. Autocrine and paracrine actions of intestinal fibroblast-derived insulin-like growth factors. Am J Physiol Gastrointest Liver Physiol 276:G817–G827 [DOI] [PubMed] [Google Scholar]
- 30. Fruchtman S, Simmons JG, Michaylira CZ, Miller ME, Greenhalgh CJ, Ney DM, Lund PK. 2005. Suppressor of cytokine signaling-2 modulates the fibrogenic actions of GH and IGF-I in intestinal mesenchymal cells. Am J Physiol Gastrointest Liver Physiol 289:G342–G350 [DOI] [PubMed] [Google Scholar]
- 31. Liu X, Murali SG, Holst JJ, Ney DM. 2008. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am J Physiol Regul Integr Comp Physiol 295:R1794–R1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garrison AP, Dekaney CM, von Allmen DC, Lund PK, Henning SJ, Helmrath MA. 2009. Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. Am J Physiol Gastrointest Liver Physiol 296:G643–G650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jobson TM, Billington CK, Hall IP. 1998. Regulation of proliferation of human colonic subepithelial myofibroblasts by mediators important in intestinal inflammation. J Clin Invest 101:2650–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theiss AL, Simmons JG, Jobin C, Lund PK. 2005. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 280:36099–36109 [DOI] [PubMed] [Google Scholar]
- 35. Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. 1996. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron 16:973–982 [DOI] [PubMed] [Google Scholar]
- 36. Dhanvantari S, Seidah NG, Brubaker PL. 1996. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol Endocrinol 10:342–355 [DOI] [PubMed] [Google Scholar]
- 37. Walsh NA, Yusta B, DaCambra MP, Anini Y, Drucker DJ, Brubaker PL. 2003. Glucagon-like peptide-2 receptor activation in the rat intestinal mucosa. Endocrinology 144:4385–4392 [DOI] [PubMed] [Google Scholar]
- 38. Dickson LM, Lingohr MK, McCuaig J, Hugl SR, Snow L, Kahn BB, Myers MG, Jr, Rhodes CJ. 2001. Differential activation of protein kinase B and p70(S6)K by glucose and insulin-like growth factor 1 in pancreatic beta-cells (INS-1). J Biol Chem 276:21110–21120 [DOI] [PubMed] [Google Scholar]
- 39. Hoyt EC, Van Wyk JJ, Lund PK. 1988. Tissue and development specific regulation of a complex family of rat insulin-like growth factor I messenger ribonucleic acids. Mol Endocrinol 2:1077–1086 [DOI] [PubMed] [Google Scholar]
- 40. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu S, He J, Ho WT, Ramineni S, Thal DM, Natesh R, Tesmer JJ, Hepler JR, Heximer SP. 2007. Unique hydrophobic extension of the RGS2 amphipathic helix domain imparts increased plasma membrane binding and function relative to other RGS R4/B subfamily members. J Biol Chem 282:33064–33075 [DOI] [PubMed] [Google Scholar]
- 42. Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. 2002. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 126:829–836 [DOI] [PubMed] [Google Scholar]
- 43. Iakoubov R, Izzo A, Yeung A, Whiteside CI, Brubaker PL. 2007. Essential role of protein kinase Czeta in oleic acid-induced secretion of glucagon-like peptide-1. Endocrinology 148:1089–1098 [DOI] [PubMed] [Google Scholar]
- 44. Thulesen J, Knudsen LB, Hartmann B, Hastrup S, Kissow H, Jeppesen PB, Orskov C, Holst JJ, Poulsen SS. 2002. The truncated metabolite GLP-2 (3–33) interacts with the GLP-2 receptor as a partial agonist. Reg Pep 103:9–15 [DOI] [PubMed] [Google Scholar]
- 45. Koehler JA, Yusta B, Drucker DJ. 2005. The HeLa cell glucagon-like peptide-2 receptor is coupled to regulation of apoptosis and ERK1/2 activation through divergent signaling pathways. Mol Endocrinol 19:459–473 [DOI] [PubMed] [Google Scholar]
- 46. Estall JL, Yusta B, Drucker DJ. 2004. Lipid raft-dependent glucagon-like peptide-2 receptor trafficking occurs independently of agonist-induced desensitization. Mol Biol Cell 15:3673–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Estall JL, Koehler JA, Yusta B, Drucker DJ. 2005. The glucagon-like peptide-2 receptor C terminus modulates beta-arrestin-2 association but is dispensable for ligand-induced desensitization, endocytosis, and G-protein-dependent effector activation. J Biol Chem 280:22124–22134 [DOI] [PubMed] [Google Scholar]
- 48. Böni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. 1991. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol 260:E846–E851 [DOI] [PubMed] [Google Scholar]
- 49. Frost RA, Nystrom GJ, Lang CH. 2002. Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinology 143:492–503 [DOI] [PubMed] [Google Scholar]
- 50. Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. 2004. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol 24:8907–8916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakamura Y, Handa K, Iwamoto R, Tsukamoto T, Takahasi M, Mekada E. 2001. Immunohistochemical distribution of CD9, heparin binding epidermal growth factor-like growth factor, and integrin alpha3beta1 in normal human tissues. J Histochem Cytochem 49:439–444 [DOI] [PubMed] [Google Scholar]
- 52. Yusta B, Somwar R, Wang F, Munroe D, Grinstein S, Klip A, Drucker DJ. 1999. Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibroblasts expressing the rat GLP-2 receptor. J Biol Chem 274:30459–30467 [DOI] [PubMed] [Google Scholar]
- 53. Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. 2007. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab 292:E281–E291 [DOI] [PubMed] [Google Scholar]
- 54. Yusta B, Boushey RP, Drucker DJ. 2000. The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J Biol Chem 275:35345–35352 [DOI] [PubMed] [Google Scholar]
- 55. Yusta B, Estall J, Drucker DJ. 2002. Glucagon-like peptide-2 receptor activation engages Bad and glucagon synthase kinase-3 in a protein kinase A-dependent manner and prevents apoptosis following inhibition of phosphatidylinositol 3-kinase. J Biol Chem 277:24896–24906 [DOI] [PubMed] [Google Scholar]
- 56. Thomas MJ, Umayahara Y, Shu H, Centrella M, Rotwein P, McCarthy TL. 1996. Identification of the cAMP response element that controls transcriptional activation of the insulin-like growth factor-I gene by prostaglandin E2 in osteoblasts. J Biol Chem 271:21835–21841 [DOI] [PubMed] [Google Scholar]
- 57. Du K, Montminy M. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273:32377–32379 [DOI] [PubMed] [Google Scholar]
- 58. Nolten LA, van Schaik FM, Steenbergh PH, Sussenbach JS. 1994. Expression of the insulin-like growth factor I gene is stimulated by the liver-enriched transcription factors C/EBP alpha and LAP. Mol Endocrinol 8:1636–1645 [DOI] [PubMed] [Google Scholar]
- 59. Nolten LA, Steenbergh PH, Sussenbach JS. 1995. Hepatocyte nuclear factor 1 alpha activates promoter 1 of the human insulin-like growth factor I gene via two distinct binding sites. Mol Endocrinol 9:1488–1499 [DOI] [PubMed] [Google Scholar]
- 60. Goya L, de la Puente A, Ramos S, Martin MA, Escrivá F, Alvarez C, Pascual-Leone AM. 2001. Regulation of IGF-I and -II by insulin in primary cultures of fetal rat hepatocytes. Endocrinology 142:5089–5096 [DOI] [PubMed] [Google Scholar]
- 61. Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX, Lin SG, Li Y. 2008. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun 376:548–552 [DOI] [PubMed] [Google Scholar]
- 62. Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. 2006. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang R, Brattain MG. 2006. AKT can be activated in the nucleus. Cell Signal 18:1722–1731 [DOI] [PubMed] [Google Scholar]
- 64. Xiao Q, Boushey R, Drucker DJ, Brubaker PL. 1999. Secretion of the intestinotropic hormone glucagon-like peptide-2 is differentially regulated by nutrients in humans. Gastroenterology 117:99–105 [DOI] [PubMed] [Google Scholar]
- 65. Marier JF, Beliveau M, Mouksassi MS, Shaw P, Cyran J, Kesavan J, Wallens J, Zahir H, Wells D, Caminis J. 2008. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol 48:1289–1299 [DOI] [PubMed] [Google Scholar]
- 66. Lund PK. 1994. Insulin-like growth factors. In: Walsh JH, Dockray GJ. eds. Gut peptides: biochemistry and physiology. New York: Raven Press; 587–614 [Google Scholar]
- 67. Lund PK, Ulshen MH, Rountree DB, Selub SE, Buchan AM. 1990. Molecular biology of gastrointestinal peptides and growth factors: relevance to intestinal adaptation. Digestion 46(Suppl 2):66–73 [DOI] [PubMed] [Google Scholar]