Abstract
Background
The purpose of this study was to compare the turnaround time for liquid culturing and primary anti-tuberculous drug susceptibility testing (DST) performed using the mycobacteria growth indicator tube (MGIT) 960 system (Becton Dickinson, USA) with that for conventional culturing and DST (by the absolute concentration method) performed using solid culture medium and to determine the concordance rates of DST results obtained using these 2 methods.
Methods
In this retrospective study, we compared the turnaround times from receiving the request for mycobacterial culture to reporting the DST results before and after the introduction of the MGIT 960 system. Further, we determined the concordance between DST results for isoniazid and rifampin for Mycobacterium tuberculosis isolates obtained using the MGIT 960 system and the absolute concentration method, which was conducted at the Korean Institute of Tuberculosis.
Results
The overall turnaround time for mycobacterial culturing and DST was 27 days for liquid culturing and DST using the MGIT 960 system versus approximately 70 days for culturing on solid medium and DST with the absolute concentration method (P<0.001). There was a good concordance between findings of DST obtained with the 2 methods (97.2%, kappa coefficient=0.855 for rifampin; and 95.6%, kappa coefficient=0.864 for isoniazid), for 1,083 clinical isolates.
Conclusions
The automated MGIT 960 system for culturing and DST of M. tuberculosis was successfully introduced in a hospital laboratory setting in Korea with significant shortening of the turnaround time.
Keywords: Drug resistance, Microbial sensitivity tests, Mycobacterium tuberculosis, Rifampin, Isoniazid
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB), which is tuberculosis (TB) resistant to at least isoniazid and rifampin, is a major threat to TB control worldwide, including Korea [1-6]. Early detection of MDR-TB allows initiation of an appropriate treatment; this directly and positively impacts the control of the disease [7, 8]. Conventional culturing methods and drug susceptibility testing (DST) using solid egg- or agar-based media is a standard technique that is still utilized in many countries, including Korea [9, 10]. However, this technique is relatively time-consuming; the median interval from initiation of anti-TB treatment to receipt of DST results is as long as 2-3 months [11, 12]. Recently, rapid liquid culture-based techniques have been designed that can detect growth-dependent changes such as CO2 production (BACTEC 460; Becton Dickinson, Sparks, MD, USA) or oxygen consumption (mycobacteria growth indicator tube [MGIT]; Becton Dickinson) [7-9].
In 2007, WHO recommended the widespread adoption of liquid culture techniques and DST, even in low- and middle-income countries [13]. Therefore, conventional culturing and DST performed using solid media has been replaced by automated liquid culture systems such as the MGIT 960 system (Becton Dickinson), which makes it possible to perform DST for first-line drugs using prepared kits [7, 8].
In Korea, MDR-TB strains account for 2.7-3.9% of new TB cases and 14.0-27.2% of previously treated cases [14, 15]. Liquid culture systems such as the MGIT 960 system are now increasingly used in Korea [16]. However, to date, liquid culture systems for DST have not been established in routine clinical practice in Korea [17-19]. The present study aimed to compare the turnaround time for liquid culturing and primary anti-tuberculous DST performed with the MGIT 960 system (defined as MGIT 960 method in the present study) with that of solid culturing and DST by the absolute concentration method (defined as the AC method in the present study). Further, the concordance rates of DST results obtained by these 2 methods were determined.
METHODS
1. Study setting
This study was conducted at the Samsung Medical Center, a 1,960-bed tertiary referral hospital in Seoul, Korea. Prior to January 1, 2009, 3% Ogawa solid media (Shinyang, Seoul, Korea) was used for mycobacterial culturing at this institution, and all specimens were processed and pretreated, as described elsewhere [15]. All isolates of Mycobacterium tuberculosis were referred for DST to the Korean Institute of Tuberculosis, a WHO-designated Supranational Reference Laboratory. Our institution had an automatic system for requesting DST of M. tuberculosis isolates obtained from every patient for whom previous DST results were not available. If multiple isolates were obtained from the same patient, only the first isolate was used for DST. In the Korean Institute of Tuberculosis, DST for isoniazid and rifampin was performed by the AC method using the Löwenstein-Jensen (LJ) medium, and the critical concentrations of isoniazid and rifampin were 0.2 µg/mL and 40.0 µg/mL, respectively [14].
Starting on January 1, 2009, the MGIT 960 method was introduced at our institution. Therefore, both solid and liquid media were used to culture mycobacteria. All isolates of M. tuberculosis were tested for resistance to isoniazid and rifampin using the MGIT 960 system and were referred to the Korean Institute of Tuberculosis for conventional DST. The drug concentrations used for the MGIT 960 system were 0.1 µg/mL for isoniazid and 1.0 µg/mL for rifampin. Therefore, primary anti-TB DST by both the MGIT 960 and AC method were performed in parallel for all M. tuberculosis isolates, and all results were available.
2. Study design
This study compared the turnaround times from receiving the request of mycobacterial culture to reporting the DST results before and after the introduction of the MGIT 960 system. We reviewed the data from the mycobacterial laboratory from January to June 2008 (culturing and DST performed using solid media) and from January to June 2010 (culturing and DST performed using liquid media).
Further, we determined the rates of concordance of DST results for isoniazid and rifampin for M. tuberculosis isolates obtained by the MGIT 960 system and the AC method using LJ medium, which was conducted at the Korean Institute of Tuberculosis. All patients with culture-confirmed TB who were diagnosed over the 2-yr period between January 2009 and December 2010 were identified, and their DST results were analyzed.
3. Statistical analysis
Results presented in the text and tables are expressed as median values + the interquartile range (IQR) or as the number (percentage). Categorical variables were analyzed using the Pearson χ2-test or Fisher's exact test. Continuous variables were analyzed using the t-test. All P values were 2-sided; P less than 0.05 was considered to be statistically significant. Kappa correlation statistics for the concordance of DST results for the 2 techniques were performed. Analyses were conducted using the Predictive Analytics Software (PASW, version 19.0 for Windows; SPSS Inc., Chicago, IL, USA).
4. Ethics statement
The study protocol was approved by the institutional review board of the Samsung Medical Center (IRB No. 2011-09-070). Informed consent was waived by the institutional review board.
RESULTS
1. Turnaround times for reporting positive cultures and DST results for clinical isolates of M. tuberculosis
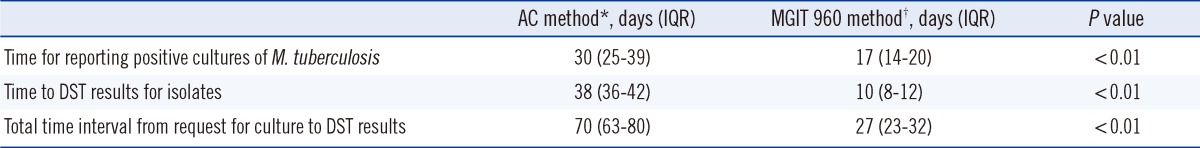
The median time for reporting positive cultures was 17 days for liquid culturing with the MGIT 960 system (for the 262 isolates identified as M. tuberculosis) and 30 days for culturing on the solid Ogawa medium (for the 247 isolates identified as M. tuberculosis) (Table 1). DST of the isolates required an additional 10 days for the MGIT 960 system after liquid culturing and an additional 38 days for the AC method after solid culturing. Therefore, the overall turnaround time, for both mycobacterial culturing and DST, was 27 days for the MGIT 960 method and approximately 70 days for the AC method using the LJ medium.
Table 1.
Time (days) to report positive cultures and drug susceptibility testing results for M. tuberculosis clinical isolates
*Culture on Ogawa medium and DST by absolute concentration method using Löwenstein-Jensen (LJ) medium (obtained between January and June 2008); †Liquid culture and DST using MGIT 960 system (obtained between January and June 2010).
Abbreviations: IQR, interquartile range; MGIT, mycobacteria growth indicator tube; DST, drug susceptibility testing; AC, absolute concentration.
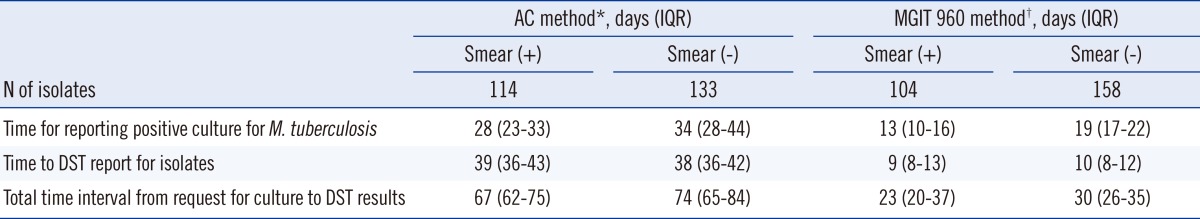
For smear-positive specimens, the overall turnaround times from culturing to DST results were 23 days and 67 days for the MGIT 960 and AC methods, respectively (Table 2). For smear-negative specimens, the corresponding overall turnaround times were 30 days and 74 days. The differences in the turnaround times between smear-positive and smear-negative specimens obtained for both the MGIT 960 and AC methods were statistically significant (P<0.001).
Table 2.
Time (days) to report positive cultures and drug susceptibility testing results for M. tuberculosis clinical isolates, according to smear status
*Culture on Ogawa medium and DST by absolute concentration method using Löwenstein-Jensen (LJ) medium (obtained between January and June 2008); †Liquid culture and DST using MGIT 960 system (obtained between January and June 2010).
Abbreviations: IQR, interquartile range; MGIT, mycobacteria growth indicator tube; DST, drug susceptibility testing; AC, absolute concentration.
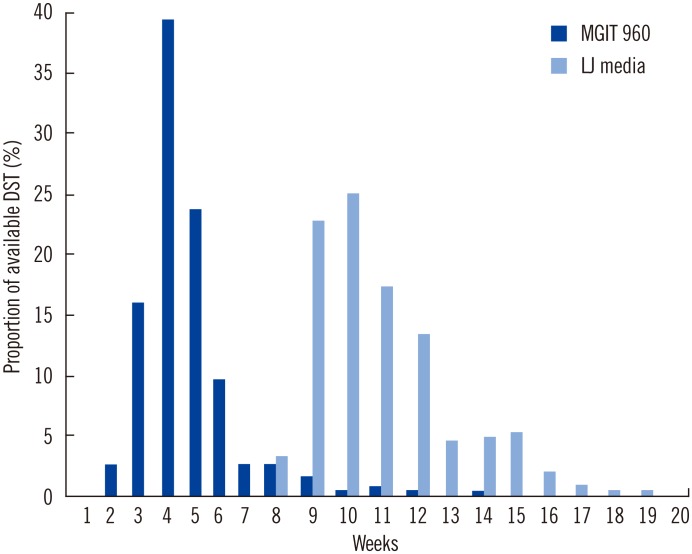
By the end of the first 4 weeks of anti-TB treatment, DST results were reported for 58% of the patients using the MGIT 960 method, whereas no DST results for clinical isolates using the AC method were reported by this time (Fig. 1). By the end of the first 8 weeks of treatment, DST results were available for 97% of the patients using the MGIT 960 method and for only 3% of the patients using the AC method (P<0.001).
Fig. 1.
Weekly availability of the results of drug susceptibility tests after request of mycobacterial culture.
Abbreviations: DST, drug susceptibility testing; MGIT, mycobacteria growth indicator tube; LJ, Löwenstein-Jensen.
2. Rate of concordance of DST results obtained by the AC and MGIT 960 methods
During the 2-yr study period, 1,083 M. tuberculosis isolates were tested for drug susceptibility using both the MGIT 960 and AC methods. Of these, 183 (16.9%) and 225 (20.8%) isolates were identified to be resistant to isoniazid by the AC and MGIT methods, respectively. Further, 138 (12.7%) LJ-grown isolates and 122 (11.3%) MGIT-grown isolates showed resistance to rifampin. Of these 1,083 isolates, 115 (10.6%) and 112 (10.3%) isolates were identified as MDR-TB by the AC and MGIT methods, respectively.
There was good concordance between DST results obtained using the 2 methods, with an agreement of 97.2% (kappa coefficient=0.855, 95% confidence interval [CI], 0.816-0.895) for rifampin resistance and 95.6% (kappa coefficient=0.864, 95% CI, 0.817-0.912) for isoniazid resistance (Table 3). Eighteen TB isolates (1.7%) were found to be rifampin-resistant using the AC method but not using the MGIT 960 method. Conversely, 12 (1.1%) isolates were identified as rifampin-susceptible by the AC method performed on the LJ medium, but as rifampin-resistant using the MGIT 960 method. Forty five of the isolates (4.2%) that were identified as susceptible to isoniazid using the AC method performed on the LJ medium were identified as resistant to isoniazid using the MGIT 960 method. Only 3 isolates (0.3%) identified as susceptible to isoniazid using the MGIT 960 method were identified as resistant by the AC method.
Table 3.
The results of drug susceptibility testing of M. tuberculosis clinical isolates for isoniazid and rifampin obtained using the MGIT 960 system and the absolute concentration method on the LJ medium
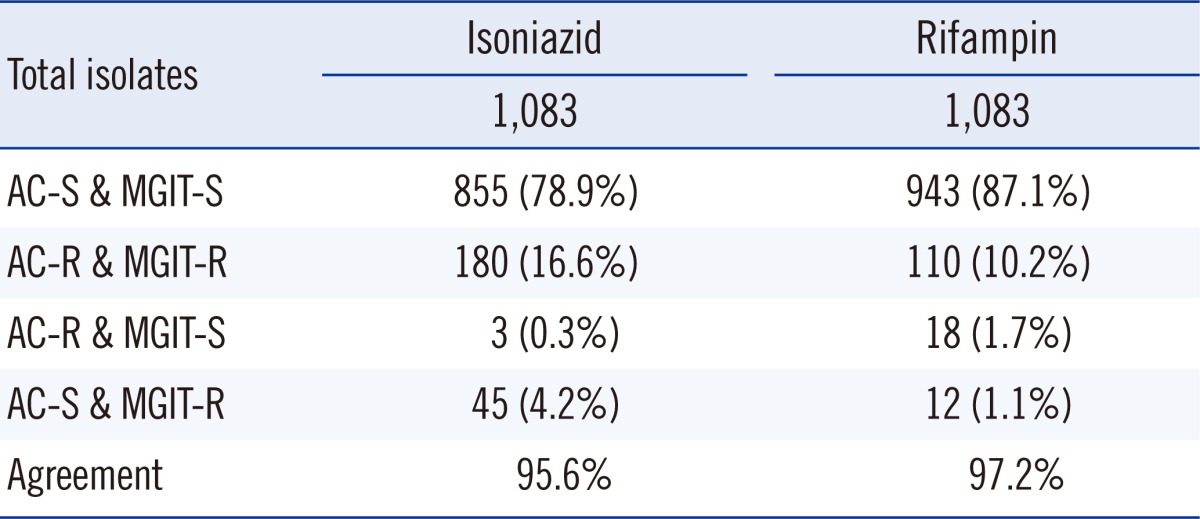
Kappa coefficient between the 2 methods was 0.855 (95% confidence interval [CI], 0.816-0.895) for isoniazid and 0.864 (95% CI, 0.817-0.912) for rifampin.
Abbreviations: LJ, Löwenstein-Jensen; AC, absolute concentration (culture on Ogawa medium and drug susceptibility testing by absolute concentration method using Löwenstein-Jensen medium); MGIT, mycobacteria growth indicator tube (liquid culture and drug susceptibility testing using MGIT 960 system); S, susceptible; R, resistant.
DISCUSSION
Rapid diagnosis is essential for treating and preventing transmission of MDR-TB. Choosing the appropriate treatment early is an essential determinant of a favorable outcome, and rapid determination of drug resistance can allow a customized approach for providing treatment early in the course of the disease [1, 2]. In addition, early diagnosis of drug resistance is also important for patients with drug-susceptible TB; ethambutol can be discontinued when DST shows the infecting organism to be drug-susceptible [20]. In addition, pyrazinamide can be withdrawn after 2 months of intensive treatment [20]. The long turnaround time of DST conducted using the solid media method could prolong the use of these potentially toxic drugs because clinicians may continue these drugs until confirmed results for drug resistance are available.
The conventional culturing and DST method uses solid media and requires long waiting periods to yield results [11, 12]. However, this method is still used in many laboratories in Korea because it is inexpensive and easily accessible. A previous study demonstrated that the average time from the initiation of treatment to confirmation of DST was 80 days, and DST results for only 15% of the patients were available to clinicians by the end of a 2-month intensive phase of anti-TB treatment [21].
Several new approaches such as liquid culture systems have been developed to fasten detection of MDR-TB. The most commonly used and commercially available automated liquid culture DST system is the MGIT 960 system. In our study, introduction of the MGIT 960 method can significantly decrease the overall turnaround times for DST results to 27 days, compared to approximately 70 days using the AC method. In addition, DST results were available for 58% and 97% of the patients using the MGIT 960 method but for only 0% and 3% of the patients using the AC method at the end of the first 4 and 8 weeks of anti-TB treatment, respectively.
Accurate diagnosis of isoniazid and rifampin resistance is very important. The second purpose of this study was to evaluate the reliability of the newly introduced MGIT 960 system for susceptibility testing of the two most important anti-TB drugs. Although the overall DST agreement rate and kappa coefficient value was high between the MGIT and AC methods, discrepant results were obtained for 4.5% of the isolates for isoniazid and for 3.0% of the isolates for rifampin. The discrepancies of DST results between the two methods may be caused by several factors.
In our study, 45 (4.2%) strains were identified as susceptible to isoniazid by the AC method performed using the LJ medium but as resistant to this drug by the MGIT 960 method. In a previous study [22], of the 30 strains which were resistant to isoniazid by the MGIT 960 system but susceptible by the proportion method when the Ogawa medium was used, 28 strains (93%) were identified as resistant to isoniazid (minimum inhibitory concentration [MIC] 0.4-0.8 µg/mL) by the proportion method when Middlebrook agar plates were used. Furthermore, these 28 isolates had katG and inhA gene mutations. In addition, 18 strains were identified as susceptible to rifampin by the MGIT 960 method but as resistant by the AC method. A previous study reported that low level rifampin resistance, linked to specific rpoB mutations, is sometimes missed by standard growth-based methods, particularly automated broth-based systems such as MGIT 960 [23].
The important limitation of the present study is lack of genotypic DST data for the isolates with discordant results between solid and liquid DSTs. Several molecular or genotypic DST methods are currently available for rapid diagnosis of MDR-TB. These genotypic DST methods are based on identification of resistance-conferring mutations in the bacillary genome. However, the main problem is that these mutations have not been well characterized; this may explain why genotypic DST methods fail to document mutations in phenotypically resistant strains [24]. Correlation between phenotypic and genotypic DST data remains problematic due to our insufficient knowledge of the mutations underlying drug resistance [24]. Further studies using both phenotypic and genotypic techniques will, therefore, be required to confirm the accuracy of DST by the MGIT method.
In addition, the results of DST for isoniazid and rifampin can differ, depending on DST methods [18]. In the AC method using LJ medium, resistance is defined as growth of more than 20 colony-forming units at a particular drug concentration, which generally is the critical concentration. However, the variations in the number of bacilli in the inoculums can alter the interpretation of the test results. Moreover, the reproducibility of the anti-TB DST results can be influenced by the physicochemical environment [9]. A previous study reported that efficiency scores varied from 90 to 99% (mean, 95%) for isoniazid and 77 to 100% (mean, 94%) for rifampin, even though these had been obtained by 16 national reference laboratories [25]. The participating laboratories used their own DST methods, the AC method, the resistance ratio method, or the proportion method.
In contrast, the MGIT 960 system is a semi-automated technique with standardized media and reagents and therefore, can provide standardized data and reduce transcription errors. However, until recently, the Food and Drug Administration (FDA) had cleared the use of the MGIT 960 system for initial susceptibility testing for only the primary anti-tuberculous drugs.
In conclusion, the MGIT 960 liquid culture system is a rapid and reliable tool, having the advantage of an automated non-radiometric system for the diagnosis of TB and MDR-TB. The MGIT 960 method was successfully introduced into a routine laboratory setting in a referral hospital in Korea. High rates of MDR-TB in Korea make the introduction of rapid DST assays particularly useful.
Acknowledgements
The authors thank Mr. Yuyean Hwang and Onkyun Kang for their help in data collection and analysis.
This study was supported by a grant of the Korea Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100027) and by the Mid-career Researcher Program through an National Research Foundation of Korea (NRF) grant funded by the MEST (2011-0015546).
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 2.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 4.Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008;178:1075–1082. doi: 10.1164/rccm.200801-132OC. [DOI] [PubMed] [Google Scholar]
- 6.Jeon DS, Shin DO, Park SK, Seo JE, Seo HS, Cho YS, et al. Treatment outcome and mortality among patients with multidrug-resistant tuberculosis in tuberculosis hospitals of the public sector. J Korean Med Sci. 2011;26:33–41. doi: 10.3346/jkms.2011.26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis. 2010;14:131–140. [PubMed] [Google Scholar]
- 8.O'Grady J, Maeurer M, Mwaba P, Kapata N, Bates M, Hoelscher M, et al. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr Opin Pulm Med. 2011;17:134–141. doi: 10.1097/MCP.0b013e3283452346. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005;25:564–569. doi: 10.1183/09031936.05.00111304. [DOI] [PubMed] [Google Scholar]
- 10.Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, et al. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–1873. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 11.Schaberg T, Reichert B, Schulin T, Lode H, Mauch H. Rapid drug susceptibility testing of Mycobacterium tuberculosis using conventional solid media. Eur Respir J. 1995;8:1688–1693. doi: 10.1183/09031936.95.08101688. [DOI] [PubMed] [Google Scholar]
- 12.Muralidhar S, Srivastava L. Evaluation of three methods to determine the antimicrobial susceptibility of Mycobacterium tuberculosis. Indian J Med Res. 2004;120:463–467. [PubMed] [Google Scholar]
- 13.World Health Organization (WHO), editors Use of liquid TB culture and drug susceptibility testing (DST) in low- and medium-income settings. Summary report of the Expert Group Meeting on the Use of Liquid Culture Media. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 14.Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007;11:571–576. [PubMed] [Google Scholar]
- 15.Choi JC, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Drug Resistance Rates of Mycobacterium tuberculosis at a Private Referral Center in Korea. J Korean Med Sci. 2007;22:677–681. doi: 10.3346/jkms.2007.22.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae E, Im JH, Kim SW, Yoon NS, Sung H, Kim MN, et al. Evaluation of combination of BACTEC mycobacteria growth indicator tube 960 system and Ogawa media for mycobacterial culture. Korean J Lab Med. 2008;28:299–306. doi: 10.3343/kjlm.2008.28.4.299. [DOI] [PubMed] [Google Scholar]
- 17.Kim BJ, Lee IH, Lee DH, Bai GH, Kong SJ, Lee SH, et al. The current status of multidrug-resistant tuberculosis in Korea. Tuberc Respir Dis. 2006;60:404–411. [Google Scholar]
- 18.Oh SH, Kim YJ, Park SK, Hwang SH, Kim HH, Lee EY, et al. Comparison of anti-mycobacterial drug susceptibility test results by institutes and methods. Korean J Clin Microbiol. 2008;11:43–48. [Google Scholar]
- 19.Han MD, Im JS, Yim J, Oh DK. A study on the drug susceptibility test of multi-drug resistant tuberculosis patients. Korean J Epidemiol. 2008;30:301–308. [Google Scholar]
- 20.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 21.Joh JS, Lee CH, Lee JE, Park YK, Bai GH, Kim EC, et al. The interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J Korean Med Sci. 2007;22:26–29. doi: 10.3346/jkms.2007.22.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe C, Kobayashi I, Mitarai S, Wada M, Kawabe Y, Takashima T, et al. Biological and molecular characteristics of Mycobacterium tuberculosis clinical isolates with low-level resistance to isoniazid in Japan. J Clin Microbiol. 2008;46:2263–2268. doi: 10.1128/JCM.00561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, et al. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol. 2009;47:3501–3506. doi: 10.1128/JCM.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis. 2010;14:131–140. [PubMed] [Google Scholar]
- 25.Bai GH, Kim SJ, Chang CL. Proficiency analysis of drug susceptibility testing by national-level tuberculosis reference laboratories from 1995 to 2003. J Clin Microbiol. 2007;45:3626–3630. doi: 10.1128/JCM.00784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]