Abstract
Objective
This study aimed to assess the surgical morbidity and mortality of thoracic endovascular repair (TEVAR) as compared with open surgical repair (OSR) for isolated descending thoracic aortic disease.
Materials and Methods
From January 1, 2006 through May 31, 2010, a total of 68 patients with isolated descending thoracic aortic disease were retrospectively reviewed for the presence of perioperative complication, 30-day mortality, and clinical success. The patients were divided into two groups (group 1, OSR, n = 40 vs. group 2, TEVAR, n = 28) and these groups were compared for major variables and late outcomes.
Results
The mean age was 58 years (group I = 54 vs. group II = 63 years, p = 0.011). Significant perioperative complications occurred in 12 patients: 8 (20%) in group I and 4 (13%) in group II (p = 0.3). There were five 30 day mortalities of which 4 occurred in group I and 1 in group II (p = 0.23). Clinical success (effective aortic remodeling and complete false lumen obliteration or thrombosis) was achieved in 20 patients (71%). Mean Kaplan-Meier survival rate at 1 year was similar for both groups (group 1 = 87% vs. group 2 = 80%, p = 0.65).
Conclusion
Thoracic endovascular repair for isolated thoracic aortic disease shows comparable results to OSR. However, the potential for endoleak or rupture remains a challenge that needs to be addressed in the future. Therefore, close follow-up study is needed for the evaluation of satisfactory long-term outcomes.
Keywords: Descending aorta disease, Thoracic endovascular repair, Open surgical repair
INTRODUCTION
Recently, the incidence of degenerative thoracic aortic disease has increased substantially (1). Conventional open surgery has historically been the mainstay of therapy for this condition (2). After the first successful repair of descending thoracic aortic aneurysm by DeBakey and Cooley using an artificial vascular graft in 1953 (3), similar surgical techniques were subsequently used to treat thoracic aortic dissection. Although the open surgical approach is established and durable, it is often associated with significant mortality and morbidity such as neurologic complications (4, 5), and given a high surgical risk of acute type B dissection, the propriety of advocating open surgical repair (OSR) especially for this condition remains controversial.
With increasing incidence of thoracic aortic disease, there has been a steady demand for thoracic aortic endovascular intervention which has been perceived as a similarly effective but less invasive and safer method of treating isolated descending thoracic aortic aneurysmal disease compared to OSR.
With the successful demonstration of endovascular stent grafting for abdominal aortic aneurysm in 1991, significant advances have since been made leading to a steady application of this technology to disorders that include not only the abdominal aorta but also thoracic aortic aneurysms and dissections (6-9). Since the approval of thoracic stent graft (GORE TAG endovascular prosthesis: WL Gore & Associates, Newark, DE, USA) usage in the United States in 2005, its use has progressively expanded to include hybrid procedures in the treatment of various complex thoracic aortic diseases as well. However, complications such as endoleakage, graft migration, aneurysm rupture (10), atheroembolic events, and concerns regarding the uncertainty of long term durability of this treatment modality has remaind an issue for the future.
The purpose of this study was to compare the midterm outcomes of thoracic endovascular repair (TEVAR) with OSR with respect to durability, morbidity and mortality after treatment of isolated descending thoracic aortic disease.
MATERIALS AND METHODS
Patients receiving surgical and endovascular repair for isolated descending thoracic aortic disease between January 2006 and May 2010 were prospectively entered into our database and reviewed retrospectively. Inclusion criteria for disease extent was defined as the portion of the aorta that was below the left subclavian artery (LSCA) and above the diaphragm. The patients were divided into two groups according to their treatment modality: patients receiving open surgical repair (OSR, n = 40) were placed in group I and those receiving endovascular repair were placed in group II (n = 28), which included patients not only undergoing TEVAR but also hybrid procedures aided by left subclavian artery debranching.
The aortic pathologies varied from aortic aneurysm, dissection, penetrating aortic ulceration (PAU) and intramural hematoma (IMH) (Fig. 1). In principle, acute and complicated cases were treated by OSR while traumatic aortic disorders presenting with multiple high surgical risk factors were referred to endovascular repair. Suitability of TEVAR was based on patient co-morbidities, characteristics of available devices, anatomic features of the lesion, and the quality of the vascular access. The treatment decision based on the final assessment was made collaboratively by the surgeons and interventional radiologists that were involved in the care of the patient. Computed tomographic (CT) scans were performed preoperatively as the main diagnostic modality and for immediate postoperative baseline assessment prior to discharge. Follow up CT scans were performed 6 and 12 months after stent-grafting and annually thereafter. All TEVAR procedures were performed with S & G SEAL endovascular stent-grafts (S & G Biotech, Seongnam, Korea) (11).
Fig. 1.
Disease categories. AD = aortic dissection, IMH = intramural hematoma, PAU = penetrating aortic ulceration
Procedure Techniques
Open Surgical Repair
Open surgical repair was performed routinely via a left thoracotomy. After general anesthesia, the patient was placed in the right lateral decubitus position for cerebrospinal fluid (CSF) drainage in the third or fourth lumbar space after which the CSF pressure was maintained at or below 15 mm Hg during the surgery. The most important consideration in the decision to perform CSF drainage was the level of distal anastomosis, which if it was below the 8th thoracic vertebral spine level or if the preoperative magnetic resonance imaging (MRI) showed a significant anterior spinal artery (the artery of Adamkiewicz) that was at risk of being sacrificed, a CSF drainage catheter was inserted. CSF drainage in this study was performed in 12 surgical cases.
A left thoracotomy incision was made over the left subscapular area. As the incision was deepened to enter the thoracic cavity, the left lung was deflated and the fourth or fifth rib, if needed, was excised at its base. The incision was extended anteriorly and the costal cartilage was divided with heavy scissors. A self-retaining retractor was then inserted, and the aorta was inspected under direct vision. During partial cardiopulmonary bypass, which was performed via the femoral vascular access, the aortic clamps were applied a few centimeters above and below the disease margins. The proximal graft anastomosis was performed first after which the distal anastomosis was performed sequentially.
Endovascular Repair
In the endovascular group, all procedures were performed in the operating room under fluoroscopic guidance with fixed imaging equipments. General anesthesia was used in all of the cases. Vascular access for angiograms was obtained percutaneously usually through the left femoral artery. CSF drainage was also performed in cases where the preoperative magnetic resonance imaging (MRI) showed a significant anterior spinal artery (the artery of Adamkiewicz) that was at risk of being occluded by the endovascular stent graft. Patients were injected with unfractionated heparin to maintain the activated clotting times above 250 seconds. The femoral vascular access was then isolated for device delivery. Device positioning and deployment were guided by angiographic landmarks. After the procedure, the access vessel was repaired, and protamine was administered for heparin reversal. Follow-up was 100% complete and the median length of follow-up was 17.2 months.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences, version 18.0 (SPSS, Inc, Chicago, IL, USA). Dichotomous variables were evaluated using chi-square (χ2) analysis and continuous variables using student t test. Multivariate models (logistic regression for dichotomous variables and linear regression for continuous variables) were constructed using a forward selection process to identify factors that were independently associated with each of the outcomes of interest. Survival analysis and freedom from re-intervention were analyzed by life table methods. All results with probability less than 0.05 were considered statistically significant. Early outcomes included 30-day mortality, reoperation for bleeding, neurologic complications including seizure, motor weakness, paralysis, paresis, renal dysfunction, respiratory complications and endoleakage were investigated. Late outcomes included presence of newly onset or persistent endoleakage, need for aortic re-intervention such as operation, re-endovascular repair, and survival. Clinical success was defined as the complete obliteration or thrombosis of the false lumen at follow-up. The size of the false lumen was said to be decreased if there was at least a 5 mm reduction in the diameter of the false lumen at the level of the lesion (11). All results with probability of less than 0.05 were considered statistically significant.
RESULTS
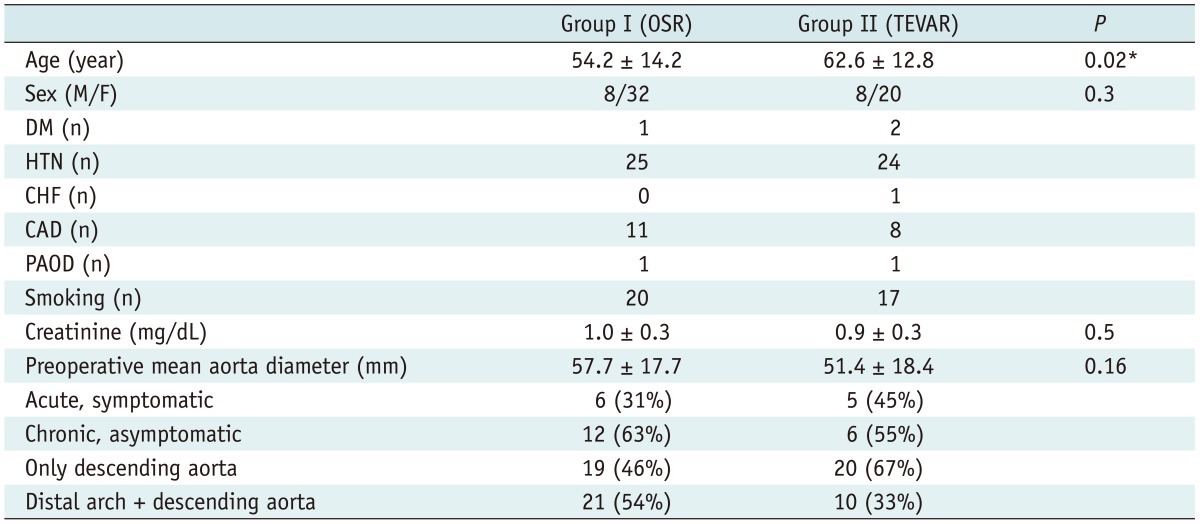
Follow-up completed 100% and the median length of follow-up was 17.2 months. The baseline characteristics of the two groups are shown in Table 1. The mean age in group II was approximately 8 years more advanced than in group I (group I, 54.2 ± 14.2 years and group II, 62.6 ± 12.3 years. p < 0.02). However, the gender, incidence of diabetes mellitus, hypertension, coronary artery disease, peripheral artery disease, heart failure, and preoperative creatinine levels were similar between the two groups (all p > 0.05).
Table 1.
Patient Demographics and Baseline Characteristics
Note.- *p < 0.05. CAD = coronary artery disease, CHF = congestive heart failure, DM = diabetes mellitus, HTN = hypertension, OSR = open surgical repair, PAOD = peripheral artery occlusive disease, TEVAR = thoracic endovascular repair
According to etiology, there were 29 type III aortic dissections (AD III), 32 aortic aneurysms (AA), 5 penetrating aortic ulcers (PAU) and 2 intramural hematomas (IMH). There were no significant inter group differences in the disease categories investigated. Among the AD III patients, 6 (31%) in group I and 5 (45%) in group II were acute and 12 (63%) in group I and 6 (55%) cases in group II were chronic. All acute symptomatic dissections were treated emergently or urgently, while chronic asymptomatic dissections were treated electively. As for aortic aneurysms, all cases were diagnosed coincidentally by computed tomography (CT) as a result of which no patient received emergency open surgery or TEVAR. Disease extent involving the distal arch was noted in 21 (54%) patients in group I and 10 (33%) in group II (Table 2), of which subclavian artery debranching was necessary to secure an adequate landing zone in the latter. OSR was performed more frequently in aortic disease with distal arch involvement to avoid the adjunctive surgical procedures necessary to secure an adequate landing zone with TEVAR.
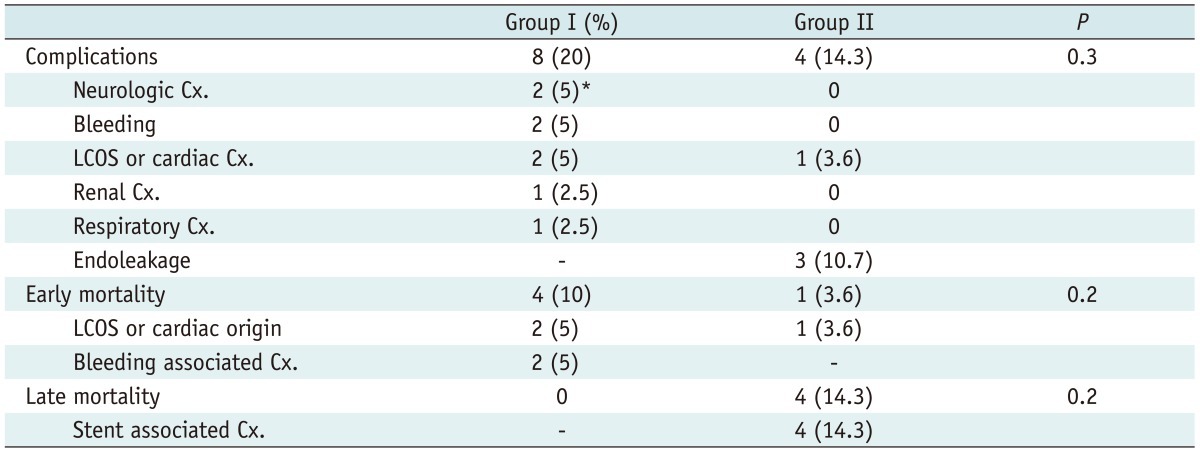
Table 2.
Morbidities and Mortalities of All Cohorts
Note.- *1. Seizure, 2. Rt. side weakness. Cx. = complications, LCOS = low cardiac output syndrome
In TEVAR the right femoral artery was usually used for inserting the stent graft and the left femoral artery to perform the angiogram. Twenty-two TEVAR cases were performed via the right femoral artery. The majority of the cases were performed by using the Seldinger technique (n = 18) and the remaining 10 cases were performed through a direct cut down. With the cut down method the femoral artery was exposed and purse string sutured with a 5-0 prolene for puncturing. There were no significant differences in pre-procedural complications between the two groups. Major complications were not observed in the elective cases either, such as multi-organ failure. However, among the acute open surgical cases, major complications were noted in 2 cases. One patient with non-oliguric renal dysfunction preoperatively suffered postoperative renal failure necessitating continuous renal replacement therapy. The other significant preprocedural complication was noted in a patient undergoing emergency surgery for multiple organ trauma that had systemic malperfusion. In the endovascular repair group, no preoperative complication was observed.
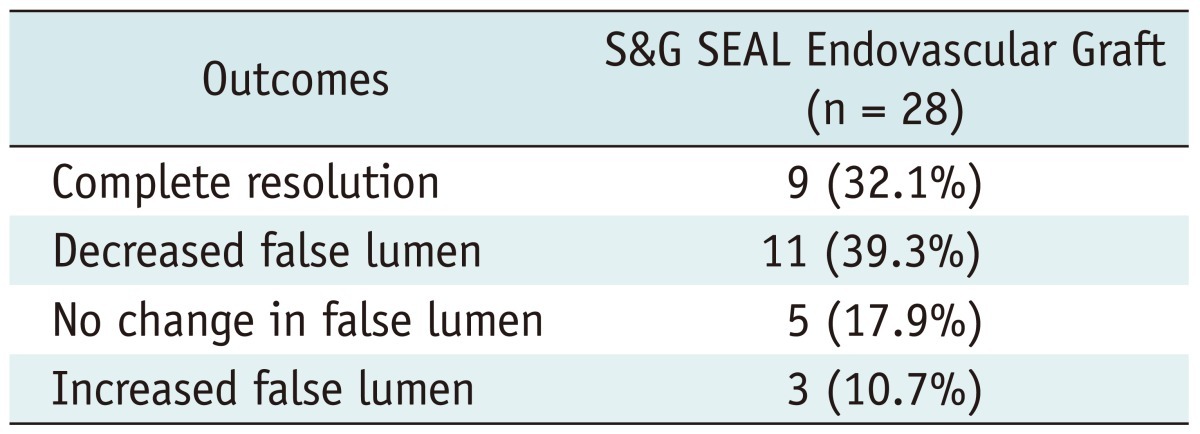
Complications occurred in 12 cases for the entire cohort: 8 (20%) in group I and 4 (13.3%) in group II (p = 0.3). Transient neurologic complications occurred in 2 cases in group I (right side weakness, partial seizure) (Table 2). More importantly, there were no cases of spinal cord ischemia in either group. All surgical graft interpositions were performed with woven Dacron graft (Hemashield, Maquet Inc.) which resulted in excellent aortic remodeling with false lumen thrombosis in the acute type III dissections. In surgery of aortic aneurysms, the graft size determined the postoperative aortic dimension. However in endovascular repair, the aortic dimensions were dependant on the progression of aortic remodeling. In the present study, the overall aortic dimensions decreased from 51.5 ± 18.5 mm to 50.3 ± 21.8 mm in size. Clinical success in the endovascular repair group was achieved in 20 (71.4%) patients. During the follow-up, complete resolution of the false lumen in chronic type B dissection was seen in 9 (32.1%) while a decrease in false lumen diameter was seen in 11 (39.3%) patients (Table 3).
Table 3.
Outcomes of Repair with S&G SEAL Endovascular Graft
There were 5 (7%) thirty-day mortalities of which 4 (10%) were in the OSR group and the remaining one (3.3%) was in the endovascular group. Two mortalities in group I was associated with uncontrolled postoperative bleeding which occurred from the fragile dissection tissue that eventually led to massive transfusion related complications and death. The two other cases in group I were associated low cardiac output syndrome. One patient in group II died from sustained ventricular arrhythmia a few hours post procedurally. Statistically there were no significant intergroup differences (p = 0.2).
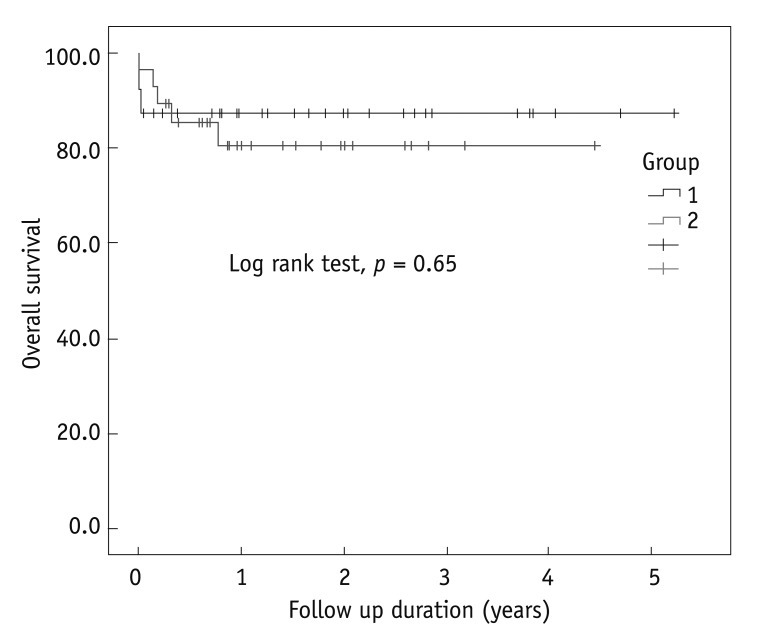
Survival assessment by the Kaplan-Meier method showed similar 1 year survival rates of 87% vs. 80% in groups I and II, respectively (p = 0.65) (Fig. 2). In the TEVAR group, 4 deaths were attributed to stent graft related complications including endoleakage and abrupt rupture. Although no statistically significant intergroup differences were found, a tendency for higher early mortality was noted in group I and a higher late mortality rate was noted in group II.
Fig. 2.
Overall survival in all cohorts.
DISCUSSION
Patients with descending thoracic aortic disease often present with either advanced age or significant co-morbid conditions that increase the risk of open surgical repair. The introduction of endovascular repair which was described as a less invasive method of treating descending thoracic aortic disease was a significant advance in the care of these high risk patients. Shim et al. (12) reported the feasibility and safety of stent-grafting type B dissections with a 93% technical success rate (13). White et al. recently reported acceptable 30-day and 1 year mortality and morbidity rates after emergency TEVAR for complicated type B aortic dissections (i.e. patients with malperfusion or aortic rupture) (14). Thoracic aortic stent grafting has only recently been introduced in Korea, as a result of which studies comparing the mid-to long term outcomes with those of OSR are relatively limited. The present study showed acceptable perioperative and midterm results after either open surgical repair or TEVAR over a wide variety of aortic pathologies. Although OSR compared with TEVAR showed a tendency for higher early perioperative mortality, the midterm survival outcomes after OSR tended to be superior.
Numerous reports have already shown thoracic aortic stent grafting to be a safe and feasible alternative to conventional repair (15, 16). The safety and feasibility of TEVAR in both low- and high-risk patients has been well reported previously (16, 17). The multicenter phase II trial of the Gore TAG endoprosthesis (performed in acceptable surgical candidates) documented a mortality rate of 1.5% along with a 7% neurologic complication rate (4% stroke, 3% transient or permanent paralysis).
Despite improving outcomes, adverse neurologic complications after either open or endovascular thoracic aortic repair continues to be the focus of concern and debate (17-20). Studies reporting the outcomes of TEVAR have mainly centered on the benefits of reducing the incidence of comparable surgery related complications such as severe bleeding or other catastrophic adverse postoperative outcomes related to surgery in high risk patients. Although TEVAR has been shown to be effective in reducing the hemodynamic and physical stress of surgery, issues unique to stent grafting such as the need to cover a greater length of the thoracic aorta to secure an adequate landing zone as well as the inability to reattach the intercostals remain challenges which undermine the potential benefits of TEVAR, especially in relation to spinal cord injury (4). Furthermore, the necessity of manipulating large-bore sheaths and guide wires through often heavily calcified or atheromatous segments of both the arch and descending aorta may significantly increase the risk of adverse atheroembolic events. In this study however, the only two cases of neurologic complications for the entire cohort occurred in group I. The lower incidence of neurologic complications in group II may be attributed to improvements in device design and delivery mechanisms such as precurved hydrophilic sheaths designed to better simulate the natural curvature of the aortic arch. S & G SEAL endovascular stent-grafts (S & G Biotech, Seongnam, Korea) are composed of a nickel titanium alloy (Nitinol) wire frame and polyester fabric. The enhanced biocompatibility of the polyester fabric wrapped around the self-expandable nitinol allowed the development of a simplified deployment system for optimizing endovascular repair to be possible. The low profile introducing system obviates the need for an arteriotomy and allows the procedure to be performed percutaneously. The device incorporates a delayed deployment system based on a unique design which safeguards against immediate deployment after intravascular insertion. The stent graft deploys by releasing a special key on the introducer that only the operating physician can release. Hence, a "wind-sock" type stent migration resulting from the forward pulsating force of the aortic flow is minimized and the need to lower the blood pressure during deployment is eliminated. In addition, the provision to undergo precise repositioning allows the stent graft deployment to be more exact. A further design advantage of this particular stent lies in preventing arch and cerebral vessel occlusion by incorporating a proximal bare metal stent and fabric covered portion.
Despite the demonstrated safety of TEVAR, the issue of long-term durability remains relatively uncertain. Currently FDA approval for stent grafts covers a duration of just 10 years. Although the results thus far have shown surgical repair to be a safe and durable therapeutic option with low failure rates (21), endoleakage after TEVAR potentially predisposes patients to catastrophic adverse events such as aneurysm progression and mortality (22). In our study, major stent graft-related complications occurred in four cases (10%) in which three were due to endoleakage (all of type III, junctional) and one to abrupt rupture. However, no statistically significant intergroup differences were noted with regards to midterm survival (p = 0.65).
The relatively short follow-up duration and the small study population are limitations of this study. Consequently, attempts to derive a strong meaningful conclusion based on the statistical analysis of the present data may be underpowered. Intergroup comparison was limited by the heterogeneous etiology of the patient population. However, this study was focused more on safety issues and feasibility of endovascular treatment as a viable option to OSR for treating isolated descending thoracic aortic disease. From this perspective, the present study showed TEVAR to be an excellent treatment modality with a nearly 100% procedural success rate and early results that were significantly superior to open surgical repair. However, further long term investigation over a larger cohort is warranted to better understand the clinical implications of stent grafting as a viable short-term and a long term alternative to open surgical repair.
In conclusion, the present study showed thoracic endovascular repair to be a safe alternative treatment to OSR in the treatment of a wide variety of thoracic aortic diseases with acceptable early morbidity and mortality rates. However, the potential for developing late adverse aortic events such as endoleakage or acute aortic events including ruptures and dissections remain unresolved. Subsequently continued close follow-up monitoring with appropriate imaging studies at regular intervals are warranted to ensure satisfactory long-term results.
Footnotes
This work was supported by a grant from the Asan Institute for Life Science (Grant number 2011-459 to SJC).
References
- 1.Ehrlich M, Grabenwoeger M, Cartes-Zumelzu F, Grimm M, Petzl D, Lammer J, et al. Endovascular stent graft repair for aneurysms on the descending thoracic aorta. Ann Thorac Surg. 1998;66:19–24. doi: 10.1016/s0003-4975(98)00390-7. discussion 24-25. [DOI] [PubMed] [Google Scholar]
- 2.Galloway AC, Schwartz DS, Culliford AT, Ribakove GH, Grossi EA, Esposito RA, et al. Selective approach to descending thoracic aortic aneurysm repair: a ten-year experience. Ann Thorac Surg. 1996;62:1152–1157. doi: 10.1016/0003-4975(96)00475-4. [DOI] [PubMed] [Google Scholar]
- 3.De Bakey ME, Cooley DA. Successful resection of aneurysm of thoracic aorta and replacement by graft. J Am Med Assoc. 1953;152:673–676. doi: 10.1001/jama.1953.03690080017005. [DOI] [PubMed] [Google Scholar]
- 4.Patel HJ, Williams DM, Upchurch GR, Jr, Shillingford MS, Dasika NL, Proctor MC, et al. Long-term results from a 12-year experience with endovascular therapy for thoracic aortic disease. Ann Thorac Surg. 2006;82:2147–2153. doi: 10.1016/j.athoracsur.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv. 2008;1:395–402. doi: 10.1016/j.jcin.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546–1552. doi: 10.1056/NEJM199905203402004. [DOI] [PubMed] [Google Scholar]
- 7.Leurs LJ, Bell R, Degrieck Y, Thomas S, Hobo R, Lundbom J EUROSTAR; UK Thoracic Endograft Registry collaborators. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg. 2004;40:670–679. doi: 10.1016/j.jvs.2004.07.008. discussion 679-680. [DOI] [PubMed] [Google Scholar]
- 8.Sun Z, Mwipatayi BP, Allen YB, Hartley DE, Lawrence-Brown MM. Multislice CT angiography of fenestrated endovascular stent grafting for treating abdominal aortic aneurysms: a pictorial review of the 2D/3D visualizations. Korean J Radiol. 2009;10:285–293. doi: 10.3348/kjr.2009.10.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu WC, Kwak BK, Kim KN, Kim SY, Woo JJ, Chung DJ, et al. Tuberculous aneurysm of the abdominal aorta: endovascular repair using stent grafts in two cases. Korean J Radiol. 2000;1:215–218. doi: 10.3348/kjr.2000.1.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaer RA, Makaroun MS. Late failure after endovascular repair of descending thoracic aneurysms. Semin Vasc Surg. 2009;22:81–86. doi: 10.1053/j.semvascsurg.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Park S, Min PK, Joung B, Ko YG, Choi D, Jang Y, et al. Comparison of a percutaneous separate stent endograft and a conventional thoracic stent-graft for endovascular repair of type B aortic dissection. J Endovasc Ther. 2004;11:378–384. doi: 10.1583/04-1199.1. [DOI] [PubMed] [Google Scholar]
- 12.Shim WH, Koo BK, Yoon YS, Choi D, Jang Y, Lee DY, et al. Treatment of thoracic aortic dissection with stent-grafts: midterm results. J Endovasc Ther. 2002;9:817–821. doi: 10.1177/152660280200900615. [DOI] [PubMed] [Google Scholar]
- 13.Won JY, Lee DY, Shim WH, Chang BC, Park SI, Yoon CS, et al. Elective endovascular treatment of descending thoracic aortic aneurysms and chronic dissections with stent-grafts. J Vasc Interv Radiol. 2001;12:575–582. doi: 10.1016/s1051-0443(07)61478-x. [DOI] [PubMed] [Google Scholar]
- 14.White RA, Miller DC, Criado FJ, Dake MD, Diethrich EB, Greenberg RK, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg. 2011;53:1082–1090. doi: 10.1016/j.jvs.2010.11.124. [DOI] [PubMed] [Google Scholar]
- 15.Cambria RP, Brewster DC, Lauterbach SR, Kaufman JL, Geller S, Fan CM, et al. Evolving experience with thoracic aortic stent graft repair. J Vasc Surg. 2002;35:1129–1136. doi: 10.1067/mva.2002.123323. [DOI] [PubMed] [Google Scholar]
- 16.Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9. doi: 10.1016/j.jvs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Criado FJ, Abul-Khoudoud OR, Domer GS, McKendrick C, Zuzga M, Clark NS, et al. Endovascular repair of the thoracic aorta: lessons learned. Ann Thorac Surg. 2005;80:857–863. doi: 10.1016/j.athoracsur.2005.03.110. discussion 863. [DOI] [PubMed] [Google Scholar]
- 18.Bortone AS, De Cillis E, D'Agostino D, de Luca Tupputi Schinosa L. Endovascular treatment of thoracic aortic disease: four years of experience. Circulation. 2004;110(11 Suppl 1):II262–II267. doi: 10.1161/01.CIR.0000138977.54611.3b. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg RK, O'Neill S, Walker E, Haddad F, Lyden SP, Svensson LG, et al. Endovascular repair of thoracic aortic lesions with the Zenith TX1 and TX2 thoracic grafts: intermediate-term results. J Vasc Surg. 2005;41:589–596. doi: 10.1016/j.jvs.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Katzen BT, Dake MD, MacLean AA, Wang DS. Endovascular repair of abdominal and thoracic aortic aneurysms. Circulation. 2005;112:1663–1675. doi: 10.1161/CIRCULATIONAHA.105.541284. [DOI] [PubMed] [Google Scholar]
- 21.Estrera AL, Miller CC, 3rd, Chen EP, Meada R, Torres RH, Porat EE, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg. 2005;80:1290–1296. doi: 10.1016/j.athoracsur.2005.02.021. discussion 1296. [DOI] [PubMed] [Google Scholar]
- 22.Hansen CJ, Bui H, Donayre CE, Aziz I, Kim B, Kopchok G, et al. Complications of endovascular repair of high-risk and emergent descending thoracic aortic aneurysms and dissections. J Vasc Surg. 2004;40:228–234. doi: 10.1016/j.jvs.2004.03.051. [DOI] [PubMed] [Google Scholar]